SUMMARY
SETTING
Fifteen human immunodeficiency virus (HIV) clinics in Nyanza Region, Western Kenya.
OBJECTIVE
To describe routine tuberculosis (TB) screening and diagnostic practices among newly enrolled people living with HIV (PLHIV) prior to the implementation of World Health Organization recommended TB intensified case finding.
DESIGN
Retrospective chart abstraction of PLHIV aged ⩾7 years who were newly enrolled in HIV care in July and August 2009, and who had not received antiretroviral treatment in the preceding 2 years or been diagnosed with TB in the previous year. Factors associated with evidence of TB diagnostic evaluation among symptomatic PLHIV were assessed.
RESULTS
Of 1020 patients included in the analysis, 995 (98%) were screened for TB at enrolment and 613 (62%) reported TB symptoms. Ninety-six (16%) patients with symptoms had evidence of referral for TB diagnostic evaluation, including patients at large clinics, those with advanced HIV disease and those reporting multiple TB symptoms. Among the 43 (45%) with documented evaluation results, 26 (60%) were diagnosed with TB.
CONCLUSION
Although most PLHIV were screened for TB, very few underwent an evaluation, and the proportion diagnosed with TB was very low. Efforts to improve TB screening should focus on standardizing the intensified case finding algorithm and linkage to, and adequate infrastructure for, TB diagnostic evaluation.
Keywords: TB case finding, screening, TB-HIV
GLOBALLY, 9 million new cases of tuberculosis (TB) were reported in 2013, of whom 13% were infected with the human immunodeficiency virus (HIV).1 Three quarters of the TB patients infected with HIV worldwide are in sub-Saharan Africa, where TB remains the leading cause of morbidity and mortality among persons living with HIV (PLHIV).2–4 To reduce the burden of the TB-HIV syndemic (synergistically interacting epidemics),5 the World Health Organization (WHO) recommends implementing intensified case finding (ICF), isoniazid preventive therapy (IPT) and infection control. ICF involves regular screening for symptoms and signs of TB among all PLHIV, followed by prompt diagnosis and treatment, and is the cornerstone for delivering IPT and maintaining TB infection control.6 However, implementation remains lower than desired; among the 62 countries reporting on TB screening among PLHIV in 2013, only 5.5 million PLHIV were screened. Furthermore, data required to calculate IPT coverage were often missing or considered inaccurate.1,7
Kenya, one of the world’s 22 high TB burden countries, has a TB epidemic that has been substantially exacerbated by HIV.7–9 In 2010, Kenya’s Division of Leprosy Tuberculosis and Lung Diseases (DLTLD) adopted the WHO’s ‘Three I’s’ recommendations.8 As it has the highest TB and HIV burden in the country, with 70% of TB patients infected with HIV compared to the national average of 44% in 2009, Nyanza Province was prioritized as the first region to implement these activities.10 TB screening was recommended for all HIV-infected persons at initial enrollment into HIV care and at every subsequent visit thereafter, with referral to the nearest TB diagnostic center. At those clinics without TB diagnostic capacity, sputum samples would be sent to other clinics; however, patients had to travel to clinics with radiographic capacity. Irrespective of HIV status, patients whose cough symptoms were unresponsive to a course of antibiotics were also to be assessed for TB.11,12
We describe routine TB screening and diagnostic practices among PLHIV newly enrolled into HIV care in Nyanza Province prior to implementation of the enhanced TB ICF activities.
METHODS
Study design and setting
We sampled those HIV care and treatment clinics in public health facilities in Siaya and Kisumu Counties that had a patient enrollment of >200, and stratified them as low volume (200–1000 PLHIV enrolled in care at the time of selection, n = 14), and high volume (patient enrollment >1000, n = 10). Using probability-proportionate-to-size sampling (PPS), we randomly selected six high-volume clinics (three district hospitals and three subdistrict hospitals) and nine low-volume clinics (health centers).
Study population
We reviewed the HIV clinic patient files and registers of all PLHIV enrolled in HIV care at the selected clinics between 1 July and 31 August 2009. We included patients aged ⩾7 years who had not been diagnosed with TB in the previous year, and who had not received cotrimoxazole prophylaxis for opportunistic infections, antiretroviral drugs for HIV treatment or prevention of mother-to-child transmission in the previous 2 years, as this might have impacted the presence or absence of TB symptoms.
Data collection
A standardized data collection tool was used by trained data abstractors to obtain data from paper-based medical records. A referral source was defined as ‘community-based’ if a patient was tested for HIV through a home-based HIV testing and counseling program; ‘facility-based’ if a patient was tested for HIV in an in-patient ward, general out-patient department, voluntary counseling and testing center, TB clinic or any other provider-initiated testing and counseling location; and ‘unknown’ if the referral source indicated was documented as missing. All patients, irrespective of referral source, were screened for TB at enrollment into HIV care at the facility they attended to receive care. Clinics were described either as large clinics when the facility was a district hospital or as small clinics when the facility was a subdistrict hospital or a health center, to reflect the level of complexity of health services provided at these facilities.12 When a medical record indicated that a patient was referred for a TB diagnostic evaluation or anti-tuberculosis treatment, the patient’s demographic and clinical details were used to locate the patient in the onsite TB clinic medical records to obtain further information about the anti-tuberculosis treatment given and the clinical outcome.
Clinical care
Evidence of clinical TB screening included any documentation of symptom-based screening. A patient was considered to have screened positive for TB symptoms if he/she was documented as having any TB symptom (cough, fever, lymphadenopathy, weight loss, night sweats, chest pain or breathlessness) or close contact with a known TB case documented in the TB screening forms or in the medical charts. Patients were described as having been referred for diagnostic evaluation if at the enrollment visit, or any visit within 3 months of enrollment, there was documentation of referral or of results of a TB diagnostic evaluation in their medical charts. TB diagnostic evaluation included sputum microscopy, chest radiography (CXR) or both. Time to TB diagnosis was defined as time from date of recorded TB symptom screen to date of documented TB diagnosis only for symptomatic patients diagnosed with TB.
Data analysis
Data collection forms were scanned into a Microsoft Access database (MicroSoft, Redmond, WA, USA) and imported into SAS version 9.2 software (Statistical Analysis System, Cary, NC, USA). The study population was characterized using simple descriptive statistics. Persons screening positive for TB symptoms who were referred for TB diagnostic evaluation were compared with those who were not referred, and patients who completed a TB diagnostic evaluation were compared with those who did not using the Kruskal-Wallis test or χ2 test, as appropriate. Multivariate analyses were conducted using conditional logistic regression, with clinic size being treated as a fixed effect; data comparing large and small clinics are therefore not shown. A P value of ≤0.25 in univariate analysis was used to identify variables for inclusion in the multivariate model, and a backward elimination procedure was used to first select the relevant variables, and a P value of ≤0.1 to retain variables.13,14
Ethics approval
Ethics approval was obtained from the Kenya Medical Research Institute Ethics Review Committee (KEMRI; Nairobi, Kenya) and the US Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA) Institutional Review Board.
RESULTS
Patients
Of 1681 PLHIV enrolled in HIV care during the study period, 661 (39%) did not meet the study’s eligibility criteria and were excluded from the analysis (Figure). Of the remaining 1020 PLHIV, the majority (n = 995, 98%) had documented evidence of TB screening at enrollment using different formatted TB screening tools (n=992, 97%). Table 1 shows the demographic and clinical characteristics of the 995 patients screened for TB at enrollment into HIV care. The majority (n = 601, 60%) were female, aged 15–49 years (n = 881, 89%), enrolled at large clinics (n = 688, 69%) and were referred from health facilities (n = 935, 94%). The majority (n = 613, 62%) of PLHIV screened for TB had documented symptoms: cough was the most commonly reported symptom (n = 472, 77%), followed by weight loss (n = 451, 73%), fever (n = 324, 51%) and night sweats (n = 235, 38%); less than one fifth (n = 117, 19%) had documentation of other symptoms (data not shown).
Figure.
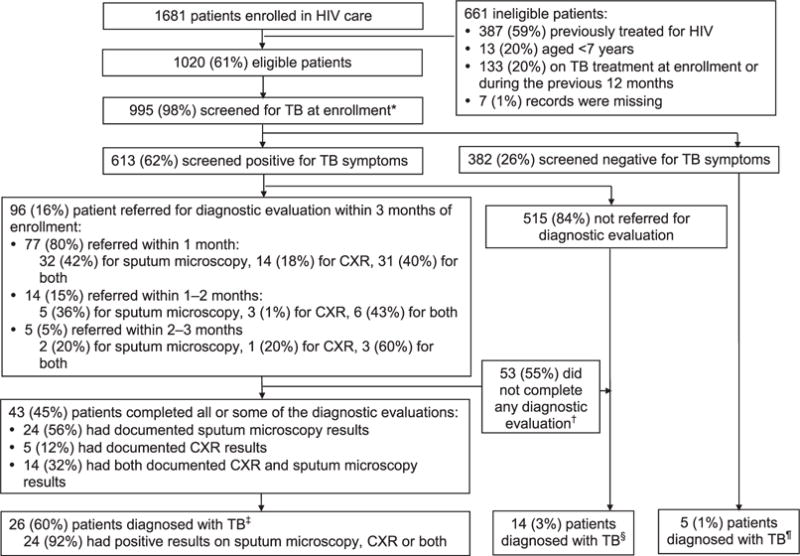
TB screening and evaluation at enrollment into HIV care. * Different sets of symptoms were used for screening at enrollment; some patients were screened based only on their presenting complaints. † 19 of these patients did not return to the clinic after referral for TB diagnostic evaluation. ‡ 2 patients had negative results. § These 14 patients had no TB diagnostic evaluation but were started on anti-tuberculosis treatment. ¶ Clinicians noted that these patients were started on anti-tuberculosis treatment. One patient had a negative sputum microscopy documented, 2 patients had no documented sputum microscopy or CXR results. HIV = human immunodeficiency virus; TB = tuberculosis; CXR = chest X-ray.
Table 1.
Demographic and clinical characteristics of patients screened for TB at enrollment in human immunodeficiency virus care (n = 995)
| All n (%) |
Documented as having TB symptoms n (%) |
Not documented as having TB symptoms n (%) |
P value | |
|---|---|---|---|---|
| Total | 995 | 613 | 382 | |
| Age, years, median [IQR] | 32 [26–41] | 33 [26–42] | 31 [25–40] | 0.0076 |
| Age group, years | ||||
| <15 | 20 (2) | 13 (2) | 7 (2) | 0.7527 |
| ⩾15 | 975 (98) | 600 (98) | 375 (98) | |
| Sex | ||||
| Female | 601 (60) | 376 (61) | 225 (51) | 0.4446 |
| Male | 394 (40) | 237 (39) | 157 (49) | |
| Type of facility | ||||
| Large clinics | 688 (69) | 400 (65) | 288 (75) | 0.0008 |
| Small clinics | 307 (31) | 213 (35) | 94 (25) | |
| Referral source | ||||
| Facility-based | 935 (94) | 571 (93) | 364 (95) | 0.2042 |
| Community-based | 46 (5) | 34 (6) | 12 (3) | |
| Unknown | 14 (1) | 8 (1) | 6 (2) | |
| WHO category* | (n = 929) | (n =594) | (n = 335) | |
| WHO Stage I & II | 586 (63) | 340 (57) | 246 (73) | <0.0001 |
| WHO Stage III & IV | 343 (37) | 254 (43) | 89 (27) |
WHO HIV staging at enrollment was available for 929 patients, of whom 594 were symptomatic and 335 were asymptomatic.
TB =tuberculosis; IQR =interquartile range; WHO = World Health Organization; HIV =human immunodeficiency virus.
Evaluation of PLHIV with TB symptoms
PLHIV with documented TB symptoms were slightly older than those with no documentation of symptoms (33 vs. 31 years, P < 0.01) (Table 1). PLHIV with documented TB symptoms were more likely to be from large clinics (65% vs. 35%, P <0.01) and to present with WHO HIV Stage I and II conditions (57% vs. 43%, P < 0.01) (Table 1).
Among the 613 symptomatic PLHIV with documented TB symptoms, only 96 (16%) had documentation of referral for TB diagnostic evaluation within 3 months of enrolment; 77 (80%) of these were referred for TB diagnostic evaluation within 1 month, 14 (15%) within 1–2 months and 5 (5%) within 2–3 months of enrollment into HIV care (Figure). Symptomatic PLHIV were more likely to have documented referral for TB diagnostic evaluation if they were male (P = 0.04), had multiple symptoms, or a WHO HIV Stage III or IV condition at enrollment (P < 0.01; Table 2). Patients who were from large clinics were more likely to be referred for TB diagnostic evaluations (odds ratio [OR] 2.3, 95%CI 1.3–3.8, P < 0.01) (data not shown).
Table 2.
Factors associated with referral for TB diagnostic evaluation among patients who screened positive for TB symptoms
| Patient characteristic | Referred for TB diagnostic evaluation/total (96/613, 16%) n/N (%) |
OR (95%CI) | P value | aOR (95%CI) | P value |
|---|---|---|---|---|---|
| Age, years | |||||
| <15 | 3/13 (23) | 1.6 (0.4–5.9) | 0.49 | ||
| ⩾15 | 93/600 (16) | Reference | |||
| Sex | |||||
| Female | 48/376 (13) | Reference | |||
| Male | 48/237 (21) | 1.7 (1.7–2.7) | 0.01 | 1.7 (1.0–2.7) | 0.04 |
| Referral source | |||||
| Facility-based | 92/571 (16) | Reference | |||
| Community-based | 3/34 (9) | 0.9 (0.3–3.2) | 0.85 | ||
| Unknown | 1/8 (13) | 0.9 (0.1–7.1) | 0.88 | ||
| WHO category* | |||||
| WHO Stage I & II | 25/340 (7) | Reference | |||
| WHO Stage III & IV | 68/254 (27) | 4.3 (2.6–7.0) | <0.01 | 3.8 (2.3–6.4) | <0.01 |
| TB symptoms | |||||
| Any one symptom | 16/238 (7) | Reference | |||
| ⩾2 symptoms | 80/375 (21) | 43.9 (2.7–8.7) | <0.01 | 5.2 (2.8–9.7) | <0.01 |
WHO HIV staging at enrollment was available for 594 patients (93 were referred for TB diagnostic evaluation and 501 were not referred).
TB=tuberculosis; OR=odds ratio; CI=confidence interval; aOR = adjusted OR; WHO = World Health Organization; HIV = human immunodeficiency virus.
Of the 613 symptomatic patients, 88 (14%) received antibiotics and none had a documented referral for TB evaluation. Compared to symptomatic patients with documentation of referral for TB diagnostic evaluation, patients who received antibiotics were less likely to present with WHO Stage III and IV conditions (44% vs. 72%, P < 0.01) and to have a CD4 count of <200 cells/ml (23% vs. 52%, P < 0.01) (data not shown).
Among the 96 PLHIV with documented referral for TB diagnostic evaluation, 43 (45%) had documented results of TB diagnostic evaluation: 24 (56%) had smear microscopy results, 5 (12%) had CXR results and 14 (32%) had both (Figure). The results of the diagnostic evaluations were not documented for the remaining 53 (55%), 19 (36%) of whom were referred for diagnostic evaluation at enrollment but did not have evidence of having returned to the clinic. Male patients were more likely to complete their TB diagnostic evaluation (OR 2.9, 95%CI 1.2–6.9, P < 0.01), as were patients from large clinics (OR 6.9, 95%CI 1.8–26.7, P < 0.01) (data not shown).
TB was diagnosed in 26 (60%) of the 43 patients who were evaluated for TB and an additional 14 (3%) who were screened for TB symptoms but did not have documentation of TB evaluation; 5 (1%) who did not screen positive for TB were also started on anti-tuberculosis treatment, bringing the total number of patients treated for TB to 45 (4% of all 1020 PLHIV included in the evaluation) (Figure). The majority of the TB patients (n = 39, 9%) were treated for TB at the onsite TB or HIV clinic, as evidenced by documentation in their medical charts or on-site TB registers (data not shown).
Time to TB diagnosis
Of the 40 patients with TB symptoms who were diagnosed with TB within 3 months of enrollment into HIV care, 30 (75%) were diagnosed within 1 month, 5 (13%) within 1–2 months, and 5 (13%) within 2–3 months. The overall median time to TB diagnosis was 10 days (interquartile range [IQR] 1.5–34), 5.5 days (IQR 0–11) in the 10 patients diagnosed on smear microscopy only, 8 days (IQR 2–15) for the 5 patients diagnosed by a combination of smear microscopy and CXR, 11 days (IQR 4–15) for the 8 patients diagnosed on CXR only, and 29 days (IQR 3–53) for the 17 patients diagnosed on clinical suspicion alone (data not shown).
The median time to TB diagnosis was significantly shorter for patients who presented with WHO Stage III and IV conditions than patients who presented with WHO Stage I and II conditions (7 days, IQR 0–22 vs. 51 days, IQR 9–78, P = 0.03), and for patients who presented with multiple TB symptoms than those who presented with one TB symptom (16 days, IQR 6–49 vs. 45 days, IQR 15–85 days, P < 0.01). The median time to TB diagnosis did not differ by age group, sex, site strata or referral source (data not shown).
DISCUSSION
Documentation of TB screening was found for nearly all of the PLHIV included in the evaluation, and symptoms were documented in almost two thirds of those screened. Only about one in six of PLHIV with TB symptoms had documentation of referral for TB evaluation, and over half of those referred for TB diagnostic evaluation had no evidence of having been evaluated. In this retrospective review of TB screening practices among PLHIV prior to implementation of enhanced TB ICF activities in Western Kenya, only 3% of PLHIV who presented were diagnosed with TB. This compares poorly to evidence from a prospective study on standardized TB ICF activities conducted after this retrospective review at the same clinics, which suggests that 11–15% of PLHIV entering HIV care will have TB disease (Cavanaugh J, personal communication).3 TB is the most common cause of death in PLHIV, and it is likely substantially underdiagnosed in Western Kenya; incomplete evaluation of patients and the low sensitivity of readily available diagnostic tests (sputum smear microscopy and CXR) may be a key reason for this.4,15,16 Routine case finding therefore presents a key opportunity to save lives.17,18
Our study was conducted before the implementation of nationally standardized TB screening and referral algorithms. Clinicians may therefore have prioritized referral for patients who were more ill and those deemed more likely to have TB, as suggested by the higher likelihood of referring patients with multiple TB symptoms and WHO Stage III/IV.3,4,19–22 This may also have led to the high proportion of patients who were evaluated (60%) being diagnosed with TB, which appeared to be higher than in other studies.20
Lack of onsite diagnostic capacity and the cost of the diagnostic tests may have contributed to the incomplete evaluation of referred patients, particularly at lower-level facilities.23 In studies in Africa, South America and Asia, TB notification rates were higher at HIV care and treatment sites where CXR, sputum microscopy and culture were available and offered free of charge than at sites where patients had to pay for these evaluations or the evaluations were unavailable.24,25 It is also possible that clinicians were not sufficiently trained to clinically screen all PLHIV routinely and refer all symptomatic patients for diagnostic evaluation.
As the facilities included in this evaluation were selected from two high TB and HIV burden counties in Western Kenya, they may not be representative of all clinics in Kenya. These findings may also not apply to PLHIV receiving antiretroviral therapy. Our findings are limited by the completeness and accuracy of documentation of these records, as some TB investigations may not have been documented in the medical charts.
In conclusion, there was a high rate of symptom screening for TB among PLHIV at enrollment into HIV care, but an alarmingly low rate of referrals for diagnostic testing and of completion of diagnostic evaluations. It is thus very likely that TB was substantially under-diagnosed. Kenya implemented a standardized TB screening and evaluation algorithm in 2010, and prospective research is required to evaluate the impact of these practices both at enrollment into HIV care and during follow-up. An appropriate monitoring and evaluation tool for the HIV cascade of care is recommended. Continued mentorship is required to ensure that quality TB screening is occurring regularly and that all patients with symptoms suggestive of TB receive diagnostic testing for TB immediately. The availability of rapid and accurate diagnostic tests such as the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA), which was endorsed by the WHO in 2010 as part of TB case-finding activities for PLHIV, increases the TB diagnostic yield by 45%, and its use is currently being expanded in Kenya in more health facilities, which would increase TB diagnosis and reduce delays.26–29 To further optimize TB diagnosis among PLHIV, clinicians need to be trained and mentored, laboratory capacity needs to be enhanced with a wider and more efficient network of TB diagnostic testing, diagnostic tests need to be made accessible and affordable and documentation/feedback of results needs to be improved.
Acknowledgments
The authors wish to thank the health care workers and people living with the human immunodeficiency virus (HIV) at the HIV care and treatment sites described in this evaluation; study coordinators B Ochuka and C Ogwang, the field site supervisors and the entire data team; the Kenya Ministry of Health, including the Division of Leprosy, Tuberculosis and Lung Disease (Nairobi, Kenya) and the National AIDS and STI Control Programme, Nairobi, Kenya, and the Director of the Kenya Medical Research Institute for their collaboration in this study.
Funding for this study was provided by the US President’s Emergency Plan for AIDS Relief (Washington DC, USA) through the US Centers for Disease Control and Prevention (CDC, cooperative agreement No 5U19GH000041).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US CDC.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2014 WHO/HTM/TB/2013.11. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 2.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLOS MED. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Eng J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 4.Reid MJA, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Program collaboration and service intergration (PCSI) at NCHHSTP. Atlanta, GA, USA: CDC; 2012. http://www.cdc.gov/nchhstp/programintegration/definitions.htm Accessed October 2015. [Google Scholar]
- 6.World Health Organization. WHO three I’s meeting: intensified case finding (ICF), isoniazid preventative therapy (IPT) and TB infection control (IC) for people living with HIV. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 7.World Health Organization. Global tuberculosis report, 2013 WHO/HTM/TB/2013–11. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 8.Division of Leprosy Tuberculosis and Lung Diseases, Kenya Annual Report 2010. Nairobi, Kenya: Division of Leprosy Tuberculosis and Lung Diseases; 2010. [Google Scholar]
- 9.World Health Organization. Tuberculosis country profiles. Geneva, Switzerland: WHO; 2015. http://www.stoptb.org/countries/tbdata.asp Accessed October 2015. [Google Scholar]
- 10.Division of Leprosy Tuberculosis and Lung Disease. TB Quarterly Bulletin. I. I. Nairobi, Kenya: DLTLD; 2014. http://www.nltp.co.ke/docs/Q3_NTLD-U_bulletin.pdf. Accessed October 2015. [Google Scholar]
- 11.Kenyan Division of Leprosy Tuberculosis and Lung Disease. Guidelines on management of leprosy and tuberculosis. Nairobi, Kenya: Ministry of Public Health and Sanitation; 2009. [Google Scholar]
- 12.Ministry of Health. Essential package for health at the community level. Nairobi, Kenya: Ministry of Health; 2007. Reversing the trends, the second national health sector strategic plan of Kenya: community strategy and implementation guidelines for managers of the Kenya. [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York, NY, USA: Wiley; 2000. [Google Scholar]
- 14.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn S, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLOS MED. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLOS ONE. 2014;9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Wood R, Kaplan R, Bekker L, Lawn SD. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLOS ONE. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenner L, Forster M, Boulle A, et al. Tuberculosis in HIV programmes in lower-income countries: practices and risk factors. Int J Tuberc Lung Dis. 2011;15:620–627. doi: 10.5588/ijtld.10.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenner L, Ballif M, Graber C, et al. Tuberculosis in antiretroviral treatment programs in lower-income countries: availability and use of diagnostics and screening. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0077697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawa LG, Reid T, Edington ME, et al. Diagnostic management and outcomes of pulmonary tuberculosis suspects admitted to a central hospita in Malawi. Public Health Action. 2011;1:2–5. doi: 10.5588/pha.11.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field N, Murray J, Wong M, et al. Missed opportunities in TB diagnosis: a TB process-based performance review tool to evaluate and improve clinical care. BMC Public Health. 2011;11:127. doi: 10.1186/1471-2458-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos J, Smalbraak L, Macomed AC, et al. TB diagnostic process management of patients in a referral hospital in Mozambique in comparison with the 2007 WHO recommendations for the diagnosis of smear-negative pulmonary TB and extrapulmonary TB. Int Health. 2003;5:302–308. doi: 10.1093/inthealth/iht025. [DOI] [PubMed] [Google Scholar]
- 23.Day JH, Charalambous S, Fielding KL, Hayes RJ, Grant AD. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis. 2006;10:523–529. [PubMed] [Google Scholar]
- 24.Lukas F, Marie B, Claire G, et al. Tuberculosis in antiretroviral treatment programs in lower income countries: availability and use of diagnostics and screening. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0077697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukas F, Mathieu F, Andrew B, et al. Tuberculosis in HIV programmes in lower-income countries: practices and risk factors. Int J Tuberc Lung Dis. 2011;15:620–627. doi: 10.5588/ijtld.10.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins MD, Roscigino G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942–943. doi: 10.1016/S0140-6736(06)68386-4. [DOI] [PubMed] [Google Scholar]
- 27.Storla DG, Yimer S, Bjune AG. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8 doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golub JE, Bur S, Cronin WA, et al. Impact of Empiric antibiotics and chest radiographs on delays in the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2005;9:392–397. [PubMed] [Google Scholar]
- 29.Wang M, Fitzgerald JM, Richardson K, et al. Is the delay in diagnosis of pulmonary tuberculosis related to exposure to fluoroquinolones or any antibiotic? Int J Tuberc Lung Dis. 2011;15:1062–1068. doi: 10.5588/ijtld.10.0734. [DOI] [PubMed] [Google Scholar]


