Abstract
Liver allocation policies are evaluated by how they impact waitlisted patients, without considering broader outcomes for all patients with end-stage liver disease (ESLD) not on the waitlist. We conducted a retrospective cohort study using two nationally-representative databases: HealthCore (2006–2014) and 5-state Medicaid (CA, FL, NY, OH, and PA; 2002–2009). UNOS linkages enabled ascertainment of waitlist and transplant-related outcomes. We included patients aged 18–75 with ESLD (decompensated cirrhosis or hepatocellular carcinoma) using validated ICD-9-based algorithms. Among 16,824 ESLD HealthCore patients, 3-year incidences of waitlisting and transplantation were 15.8% (95% CI: 15.0–16.6%) and 8.1% (7.5–8.8%), respectively. Among 67,706 ESLD Medicaid patients, 3-year incidences of waitlisting and transplantation were 10.0% (9.7–10.4%) and 6.7% (6.5–7.0%), respectively. In HealthCore, the absolute ranges in states’ waitlist mortality and transplant rates were larger than corresponding ranges among all ESLD patients (waitlist mortality: 13.6–38.5%, ESLD 3-year mortality: 48.9–62.0%; waitlist transplant rates: 36.3–72.7%, ESLD transplant rates: 4.8–13.4%). States’ waitlist mortality and ESLD population mortality were not positively correlated: ρ=−0.06, p-value=0.83 (HealthCore); ρ=−0.87, p-value=0.05 (Medicaid). Waitlist and ESLD transplant rates were weakly positively correlated in Medicaid (ρ=0.36, p-value=0.55), but were positively correlated in HealthCore (ρ=0.73, p-value=0.001). Compared to population-based metrics, waitlist-based metrics overestimate geographic disparities in access to liver transplantation.
INTRODUCTION
The Physician Charter of the American Board of Internal Medicine (ABIM) identifies a “commitment to a just distribution of finite resources” as one of the core professional responsibilities of physicians.1 This a key principle to transplant physicians who are charged with equitably and efficiently allocating the gift of donor organs.2–4 Indeed, the stated mission of the United Network for Organ Sharing (UNOS), the organization that manages all aspects of solid-organ transplantation in the US, is to “promote long, healthy and productive lives for persons with organ failure by promoting maximized organ supply, effective and safe care, and equitable organ allocation and access to transplantation.”5
Despite the priority of equitable allocation practices, also valued by the public6, current UNOS policies for allocating donor organs and strategies for evaluating these policies only consider patients who are fortunate enough to be placed on transplant waitlists. The current waitlist-based approach facilitates evaluations of allocation policies’ impacts on these selected patients, yet it may fail to promote the needs of the broader population of patients with end-stage organ disease if few patients are ever waitlisted, which is an important consideration in the context of the broader goals of UNOS to serve “persons with organ failure.” For example, much time and energy is currently being invested in resolving geographic disparities in access to transplant care. However, these concerns are fueled only by data documenting disparities among waitlisted patients,7–9 despite some evidence that many patients who could benefit from transplantation are never waitlisted.2,10
More nuanced evaluations of the impact of liver allocation policies on all patients with ESLD and not simply waitlisted patients have been limited by the inability to identify all patients with end-stage liver disease (ESLD). The United States Renal Data System establishes a relevant “denominator” of patients eligible for kidney transplant,10,11 but previous attempts to define ESLD patients in the community have focused only on those receiving benefits from the Veterans Health Administration.2 Therefore, it remains unknown what proportion of all Americans with ESLD is waitlisted, whether there are geographic differences in waitlist rates, and whether waitlist-based metrics of allocation success and geographic disparities align with population-based metrics. We sought to address these questions by leveraging two distinct, nationally representative datasets to identify patients with ESLD, and linking them with UNOS transplant data.
METHODS
We conducted a retrospective cohort study using two nationally representative databases: HealthCore Integrated Research Database (HealthCore) from 2006–2014; and 5-state Medicaid (California, Florida, New York, Ohio, and Pennsylvania), which includes 40% of all Medicaid beneficiaries from 2002–2009. The primary analyses focused on the HealthCore patients who are representative of commercially insured patients in the US, while the Medicaid cohort provided a secondary cohort of patients, enriched with chronic liver disease, to validate the primary analyses.
HealthCore is a wholly owned subsidiary of Anthem, Inc., which, through its multiple Blue Cross/Blue Shield insurance plans, serves members residing in all 50 states. HealthCore has data on 25 million patients with medical and pharmacy coverage with at least one year of continuous coverage, and contains comprehensive longitudinal medical and pharmacy claims, with laboratory data on a subset of patients. Patients included in the HealthCore database are insured through a Blue Cross/Blue Shield insurance plan, and are representative of other commercially insured patients in the US.12,13 HealthCore data used in this study included beneficiaries from January 1, 2006 through September 30, 2014.
Medicaid data included Medicaid beneficiaries from California, Florida, New York, Ohio, and Pennsylvania with Medicaid service dates from February 27, 2002–September 2, 2009. The Medicaid population from these five states comprised approximately 22 million active enrollees, or nearly 40% of the national Medicaid population. The study cohort was created using the inpatient and outpatient Medicaid files, and the Medicare file of dual enrollees in Medicaid and Medicare.
The outcomes of waitlisting, transplantation, and waitlist mortality were assessed by linking HealthCore and Medicaid data to that from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Linkages were based on patient name, date of birth and social security number. For HealthCore and Medicaid patients, waitlisting or transplantation outcomes were assessed through March 5, 2015 and September 30, 2013, respectively.
Study sample and inclusion criteria
The study cohort included all HealthCore and Medicaid patients with ESLD, defined as the presence of one (or more) of the three main clinical criteria defining a patient as having liver disease severe enough to be waitlisted14: decompensated cirrhosis (cirrhosis with at least one complication of portal hypertension including ascites, variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis, and hepatorenal syndrome); hepatocellular carcinoma (HCC); or laboratory evidence of significant hepatocellular dysfunction (Model for End-Stage Liver Disease (MELD) score ≥15 and/or jaundice defined as a serum bilirubin ≥3mg/dL).14 Patients with decompensated cirrhosis were identified using a validated algorithm using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (inpatient or outpatient) for cirrhosis plus a complication of portal hypertension, while HCC diagnosis was based on ICD-9-CM codes for HCC concurrently with an ICD-9-CM code for cirrhosis. These algorithms are validated for identifying patients with decompensated cirrhosis and/or HCC with high positive predictive values of >85%15–17 and have been used in prior work.2 Laboratory criteria for ESLD were only used in the absence of codes for decompensated cirrhosis or HCC, and were only available for approximately 20% of the HealthCore cohort.
We limited cohort inclusion to patients 18–75 years old at the date of ESLD diagnosis because patients under 18 receive organs using different allocation criteria, and patients older than 75 represented only 0.1% of waitlist additions in the US from 2002–2013.18–20 Because American Association for the Study of Liver Diseases guidelines recommend that all patients with a prior extrahepatic malignancy receive definitive treatment with “adequate” tumor-free survival prior to listing, we excluded patients receiving their first diagnosis of any extrahepatic malignancy (excluding non-melanoma skin cancer, using validated ICD-9-based algorithms21) in the 365 days prior to the ESLD index date.14 Although cancer stage cannot be ascertained using administrative data, we reasoned that a 365-day time-horizon provided appropriate balance between our goals of excluding those with active cancers and including those with previously cured early-stage cancers. Secondary analyses excluded all patients with a diagnosis of any extrahepatic malignancy (excluding non-melanoma skin cancer) prior to the ESLD index date.
Study outcomes
The primary outcome was waitlisting for transplant. We calculated 3-year incidence rates of waitlisting from the ESLD index date given the variable follow-up time of patients in HealthCore and Medicaid. The outcome of death was ascertained using Medicaid, UNOS, and HealthCore data, all supplemented with Social Security Death Master File data.
We assessed waitlist mortality and transplant rates because these measures are routinely used to define geographic disparities in transplantation, and are widely used measures of transplant center performance.7,8,22 Patients were considered to have died on the waitlist if they actually did so or were removed from the waitlist for being ‘too sick’ and died within 90 days of removal.2,23–25 The waitlist transplant rate were calculated as: (HealthCore or Medicaid waitlist candidates transplanted) / (HealthCore or Medicaid ESLD patients on the waitlist during study period)7. The waitlist mortality rate was calculated as (HealthCore or Medicaid patients removed from the waitlist for death or clinical deterioration) / (HealthCore or Medicaid patients on the waitlist during study period).
Finally, we assessed corresponding metrics of transplant and mortality rates among the overall HealthCore and Medicaid ESLD populations. These population-level transplant and mortality rates were estimated using Kaplan-Meier methods, accounting for variable follow-up time of patients within HealthCore and Medicaid. Patients were censored at the time of loss of insurance or end of follow-up. Survival time included pre-and post-transplant time to account for the increased survival time associated with transplantation. Unadjusted ESLD mortality and transplant rates were also calculated to enable comparisons with the aforementioned, unadjusted metrics of waitlist outcomes. Cox regression models were also fit to evaluate the association between waitlisting and survival in the HealthCore population, due to limited medical co-morbidity data available in Medicaid. These models accounted for patient age, gender, initial indication for ESLD (decompensated cirrhosis, HCC, or liver synthetic dysfunction), etiology of liver disease, and medical co-morbidities as measured by ICD-9 codes.
Comparison of waitlist and population-level metrics
Among-state comparisons were evaluated using chi-square tests for binary outcomes, and log-rank tests for time-to-event outcomes. We grouped patients by state of residence because it enabled larger sample sizes across clusters. Similarly, among-state comparisons were limited to states with at least 100 ESLD patients during the study period (n=16) to promote the precision of state-specific estimates. Per our contract with HealthCore, state names are not provided in the results to retain anonymity for individual insurance plans.
Outcomes of Medicaid beneficiaries were compared across the five states because contracting with transplant centers occurs at the state level. Given the non-normal distributions of data, we used Spearman correlations to evaluate relationships among waitlist and population-level mortality, and among waitlist and population-level transplant rates.
The study was approved by the Institutional Review Board at the University of Pennsylvania, and linkages of Medicaid and HealthCore data with the UNOS dataset were approved by CMS, HealthCore, and HRSA. Stata 14.0 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
From 2006–2014, there were 16,904,409 unique patients aged 18–75 years registered in the researchable HealthCore population with at least one year of continuous coverage, of whom 16,824 (0.10%) met our inclusion criteria for transplant-eligible ESLD. From 2002–2009, there were 40,290,278 unique patients aged 18–75 years enrolled in Medicaid in the five states, with 67,706 (0.17%) being diagnosed with ESLD. The demographic characteristics of the ESLD patients were similar in the two cohorts, as were the initial manifestations of ESLD (decompensated cirrhosis vs HCC; Table 1). The etiologies of liver disease differed, with viral hepatitis being more common among Medicaid beneficiaries (p<0.001).
Table 1.
Baseline demographics and clinical characteristics of HealthCore and Medicaid beneficiaries with end-stage liver disease
| HealthCore N=16,824 |
Medicaid N=67,706 |
|
|---|---|---|
| Male gender, No. (%) | 10,515 (62.5) | 43,491 (64.2) |
| Age at ESLD diagnosis, years | 60 (52–67) | 53 (47–61) |
| Initial indication for waitlisting, No. (%) | ||
| Decompensated cirrhosis | 14,643 (87.0) | 60,402 (89.2) |
| Hepatocellular carcinoma | 1,853 (11.0) | 7,304 (10.8) |
| Laboratory evidence liver dysfunction only* | 328 (2.0) | ----- |
| Etiology of liver disease, No. (%) | ||
| Hepatitis C | 5,145 (30.6) | 26,340 (38.9) |
| Alcohol | 6,798 (40.4) | 23,246 (34.3) |
| Hepatitis B | 273 (1.6) | 3,205 (4.7) |
| Primary biliary cirrhosis | 380 (2.3) | 1,131 (1.7) |
| Primary sclerosing cholangitis | 279 (1.6) | 156 (0.2) |
| Other/cryptogenic† | 3,949 (23.5) | 13,628 (20.1) |
Defined as Model for End-Stage Liver Disease (MELD) score ≥15 and/or total serum bilirubin ≥3.0mg/dL
Includes patients who potentially had non-alcoholic steatohepatitis because there is no specific ICD-9-CM code for NASH-related cirrhosis
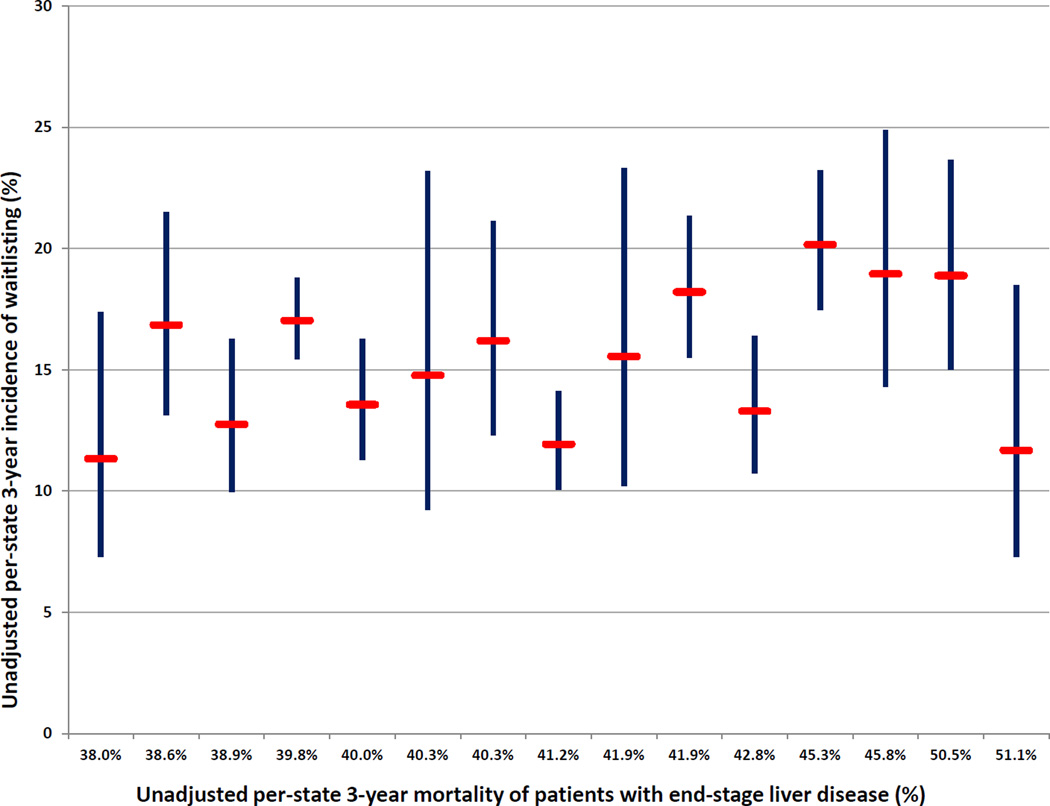
The 3-year incidence of waitlisting and transplantation in HealthCore was 15.8% (95% CI: 15.0–16.6%) and 8.1% (7.5–8.8%), respectively. Corresponding values in Medicaid were 10.0% (9.7–10.4%) and 6.7% (6.5–7.0%). The 16 states with at least 100 HealthCore patients with ESLD displayed significant variability in the 3-year incidence of waitlisting, ranging from 11.3% (95% CI: 7.3–17.4%) to 23.1% (95% CI: 14.3–35.9%) of patients with ESLD (Figure 1). The overall 3-year incidence of waitlisting in HealthCore with exclusion of all patients with an extrahepatic malignancy prior to the ESLD index date (n=1,198 excluded) was 16.4% (95% CI: 15.6–17.3%), ranging from 11.2% (95% CI: 6.7–18.3%) to 24.0% (95% CI: 14.8–37.4%) in the 16 states with at least 100 HealthCore patients with ESLD.
Figure 1.
- Figure Legend: *Red bars represent the estimated unadjusted 3-year incidence of waitlisting; blue bands represent 95% confidence intervals
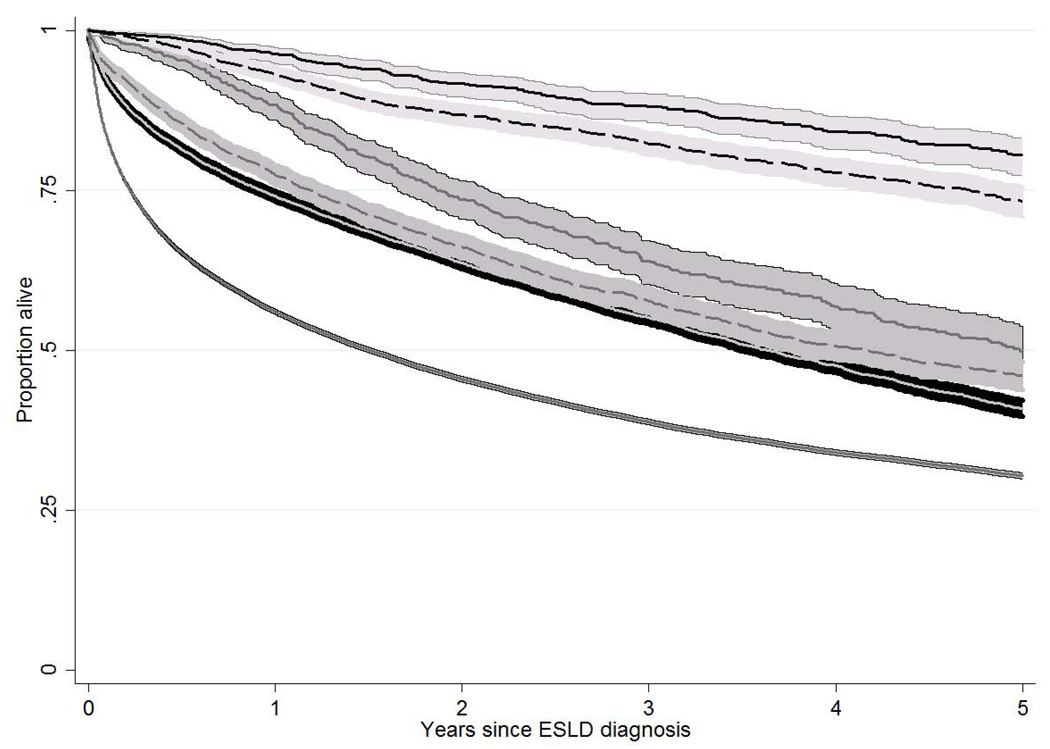
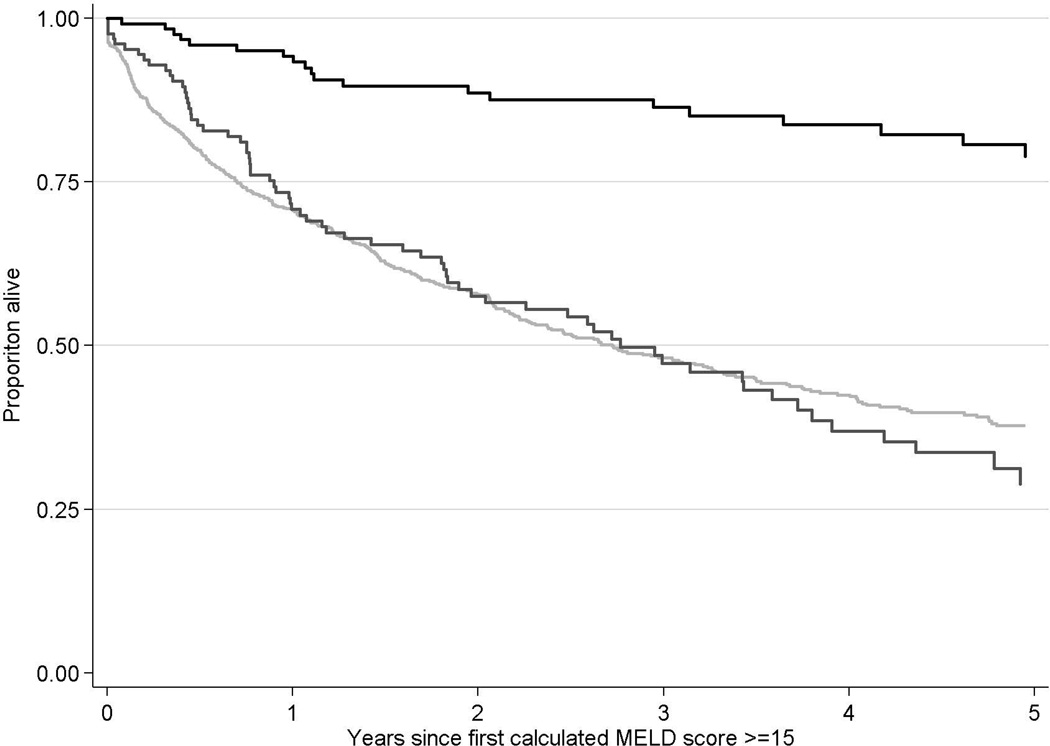
The overall unadjusted survival from diagnosis of ESLD was significantly longer for HealthCore patients (log-rank test p<0.001 compared to Medicaid). In both cohorts, the unadjusted survival from the index date of ESLD diagnosis was significantly longer among patients who were waitlisted and not transplanted, or waitlisted and transplanted, compared with those who were never waitlisted (both p<0.001; Figure 2a). For example, in HealthCore, the 3-year unadjusted survival rates from ESLD diagnosis among patients who were never waitlisted, listed but not transplanted, and listed and transplanted were 55.2%, 63.9%, and 88.1%, respectively. The improved survival among waitlisted patients, independent of transplantation, persisted when restricted to HealthCore patients with calculated MELD scores ≥15 during the index period (Figure 2b; n=1,202). These differences in overall survival persistent in multivariable Cox regression models among HealthCore patients (not waitlisted as reference in both models): 1) all HealthCore ESLD patients: waitlisted and not transplanted HR: 0.76, 95% CI: 0.68–0.84; waitlisted and transplanted HR: 0.22, 95% CI: 0.19–0.26; 2) HealthCore ESLD patients with calculated MELD scores ≥15 during the index period : waitlisted and not transplanted HR: 0.82, 95% CI: 0.63–1.07; waitlisted and transplanted HR: 0.17, 95% CI: 0.12–0.26.
Figure 2 (two panels).
- a: Unadjusted patient survival of patients with end-stage liver disease in HealthCore and Medicaid, stratified by waitlisting and transplant status
-
Figure legend
- Risk table
Years since ESLD diagnosis 0 1 2 3 4 5 Healthcore; never listed 15,045 7,992 5,014 3,199 1,985 1,196 Medicaid; never listed 61,303 28,148 19,476 13,507 9,017 5,590 HealthCore; listed, not transplanted 897 747 544 393 294 200 Medicaid; listed, not transplanted 3,615 2,214 1,581 1,100 711 438 HealthCore; listed, transplanted 882 829 722 624 493 395 Medicaid; listed, transplanted 2,788 2,149 1,706 1,312 924 588
-
- b: Unadjusted patient survival of patients with end-stage liver disease in HealthCore with a calculated MELD score ≥15
-
Figure legend
- Risk table
Years since ESLD diagnosis 0 1 2 3 4 5 Never listed 949 496 321 216 162 108 Listed, not transplanted 128 81 56 38 23 12 Listed, transplanted 125 105 84 71 57 44
-
Among-state comparisons and correlation of waitlist and population metrics
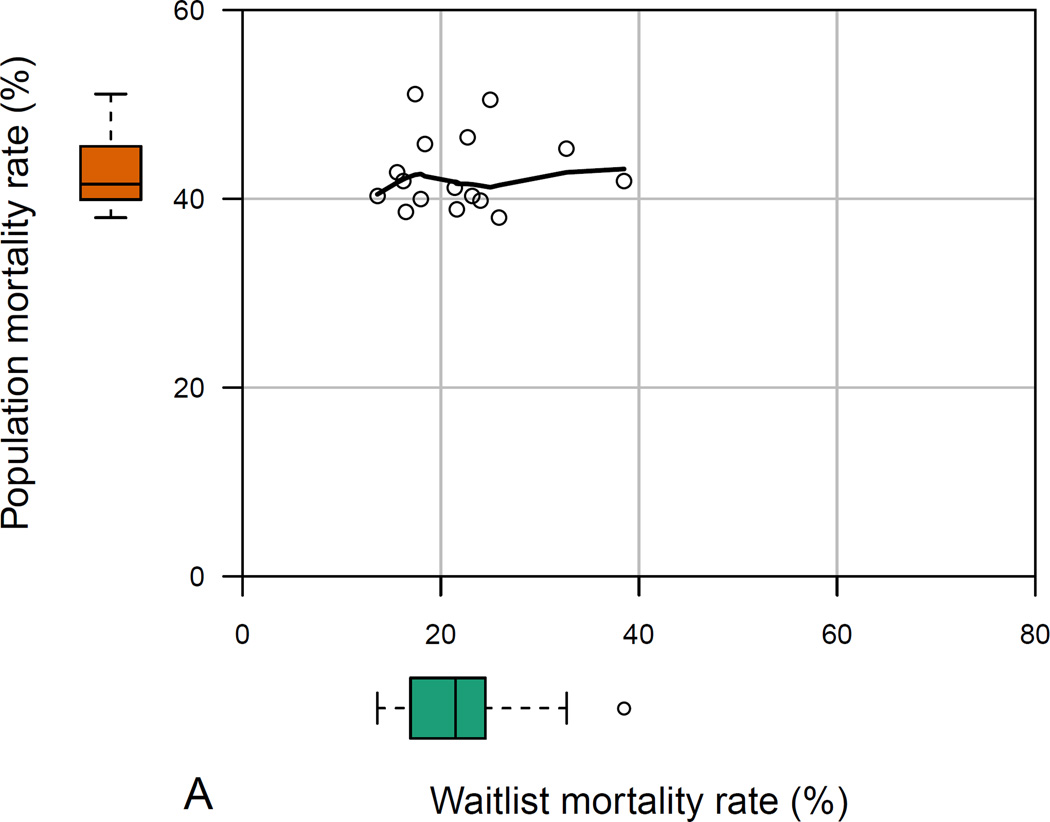
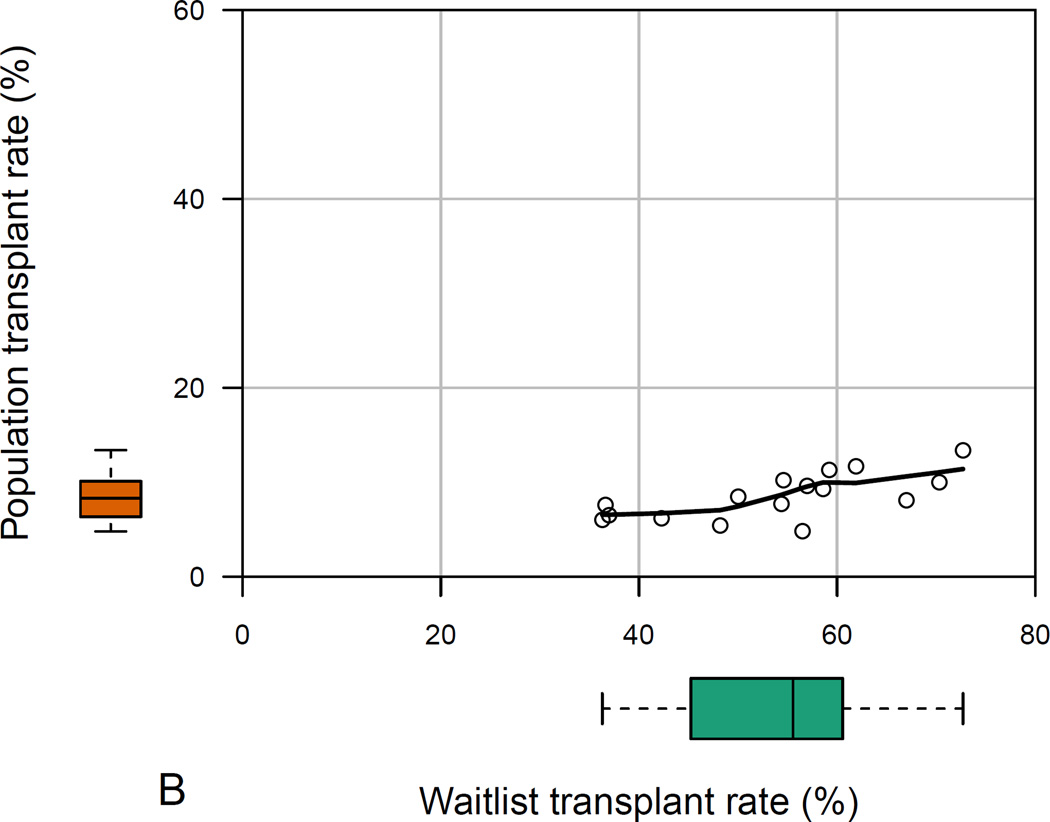
The 16 states with at least 100 HealthCore patients with ESLD displayed significant variability in all measured population- and waitlist-level metrics (p<0.001 for all among-state comparisons). In HealthCore, the absolute ranges of waitlist mortality and transplant rates were larger than corresponding ranges among all ESLD patients (Figures 3a and 3b). Waitlist mortality rates varied from 13.6–38.5% (absolute range 24.9%), whereas 3-year ESLD mortality ranged from 38.0–51.1% (absolute range 13.1%). And waitlist transplant rates ranged from 36.3–72.7% (absolute range 36.4%), compared with 3-year ESLD transplant rates which varied from 4.8–13.4% (absolute range 8.6%).
Figure 3 (two panels).
- a: Among-state measures of waitlist- and population-level mortality in HealthCore patients with ESLD*
- Footnote: * Data reported for the 16 states with ≥100 HealthCore patients with end-stage liver disease. The main plot demonstrates the correlation between waitlist- and population-level mortality rates, while the box-and-whisker plots demonstrate the absolute range in waitlist-and population-level mortality rates among the 16 sampled states.
- b: Among-state measures of waitlist- and population-level transplant rates in HealthCore patients with ESLD*
- Footnote: * Data reported for the 16 states with ≥100 HealthCore patients with end-stage liver disease. The main plot demonstrates the correlation between waitlist- and population-level transplant rates, while the box-and-whisker plots demonstrate the absolute range in waitlist-and population-level transplant rates among the 16 sampled states.
In Medicaid, similar among-state differences were found for waitlist- versus population-level metrics. The unadjusted 3-year waitlisting rates ranged from 8.0–11.9%. Waitlist mortality rates ranged from 15.2–25.0% (absolute range 9.8%) versus population-based ESLD mortality rates from 54.1–63.3% (absolute range 9.2%). Waitlist transplant rates ranged from 34.0–67.6% (absolute range 33.6%), compared with population-based ESLD transplant rates which ranged from 6.0–8.5% (absolute range 2.5%).
In neither cohort were states’ waitlist mortality and ESLD population mortality rates positively correlated: ρ=−0.06, p-value=0.83 (HealthCore; Figure 3a); ρ=−0.87, p-value=0.05 (Medicaid). Waitlist and ESLD transplant rates were weakly positively correlated in Medicaid (ρ=0.36, p-value=0.55), but were positively correlated in HealthCore (ρ=0.73, p-value=0.001; Figure 3b).
DISCUSSION
This study of more than 80,000 patients with ESLD from two large, nationally representative databases shows that fewer than 1 in 6 patients with ESLD is ever waitlisted for a liver transplant, and fewer than 1 in 12 is transplanted. Although some of the ESLD patients in the population may not benefit from transplantation, these data indicate that the scarcity of transplantable livers is far greater than is commonly appreciated by simply examining the waitlist. Furthermore, the finding that survival was significantly better for patients who were waitlisted but not transplanted in unadjusted and adjusted analyses of HealthCore patients, suggests that there may be a potential benefit to waitlisting itself, because even in the absence of transplantation, patients who were waitlisted lived longer than patients who were never waitlisted. Although this finding may in part reflect selection of healthier patients for waitlisting, it also raises the possibility that care is improved by the greater access to hepatology and transplant specialists conferred by waitlisting, as described among patients hospitalized with complications of liver disease.26,27 These findings require further study and validation, but if confirmed, highlight that waitlisting itself may confer a survival benefit because of access to specialized care, and that policies to increase access to the waitlist may improve outcomes in patients with ESLD, even if this does not occur in the setting of more transplants.
A second important finding is that waitlist-based metrics of regional performance (waitlist mortality and waitlist transplant rates) are not correlated with corresponding population-based measures of access, with the exception of a positive correlation between waitlist and ESLD transplant rates in HealthCore. Furthermore, the absolute differences in measures of access to transplant care, and corresponding disparities based on geography, were much larger when viewed from the perspective of the waitlist rather than the overall ESLD population. This is understandable when considering our third key finding of dramatic differences among regions in the proportions of patients with ESLD who are ever waitlisted. Together, these data suggest limits to the current approach of evaluating liver allocation policies entirely based on their impacts on waitlisted patients without understanding the potential downstream consequences on the broader population who could benefit from a transplant but are not waitlisted. Waitlist-based approaches may fail to capture the impacts of such policies on the broader population of patients who could benefit from liver transplantation. Furthermore, using waitlist data alone to define and measure geographic disparities in access to transplantation may be misleading, as true, population-level differences across regions are much smaller. This is not to say that population-based metrics should be the determining factor in developing and evaluating allocation policies, as the immediate direct impact of allocation is on the waitlist population. However, the transplant community should not exclude the broader population with end-stage organ disease who could be waitlisted when considering changes to allocation or distribution. Thus, recent proposals aimed at reducing geographic differences in access to transplant care should consider not only waitlist data in order to ensure that such policies may not exacerbate geographic disparities in access to transplant care.
UNOS states that, “Our vision is to promote long, healthy and productive lives for persons with organ failure.” Based on this view, we would argue that transplant physicians should work to improve access to transplant care. This could include initiatives to increase access for patients who have less access due to persistent racial and/or socioeconomic disparities in access to healthcare, as well as patients who are geographically isolated. Such measures could include increasing outreach clinics or using Telehealth or other mobile technologies to improve access to specialized transplant care. In liver transplantation, where transplant hepatologists care for patients with chronic liver disease outside of the transplant setting, such measures are possible and already being executed, as such physicians have the bandwith to reach the broader population with ESLD.28–30 Furthermore, future organ allocation proposals should consider the potential impact on the broader ESLD population to ensure that changes in organ allocation don’t exacerbate geographic disparities in patients with ESLD. This could include the development of registries, akin to SEER and USRDS that identify patients with ESLD in order to measure access to transplant care on the broader perspective. Second, transplant policymakers can consider other measures of mortality from ESLD beyond just waitlist data (i.e., CDC cause-of-death data) to monitor outcomes in the larger population of patients with ESLD who are not waitlisted. Third, metrics of transplant center performance should not disincentivize centers to waitlist patients for fear of increasing their waitlist mortality (due to a finite organ supply), given the potential benefit to waitlisting because of providing access to specialized care for patients with ESLD.
Organ allocation methods and transplant-center performances have historically been evaluated based on what happens to waitlisted patients. This is not surprising as it is much easier to measure the waitlisted population than the community-based population of patients with ESLD. However, increasing access to large data sources such as those used in this study may help surmount that limitation. Incorporating population-based metrics into such decisions would require a change in perspective. The findings of limited access to transplant waitlist and lifesaving transplantation are not novel to liver transplant, and reflect recent data evaluating access to the kidney transplant waitlist for dialysis patients in Georgia.10 However, the specific comparisons of waitlist versus population-level outcomes are novel and have not been explored in other fields of transplantation. As a transplant community, it is important to consider the problems with respect to access to transplant care, and how allocation policies may impact the broader population. However, not until we begin to view access to transplant care from the perspective of the broader population can we develop interventions to improve access and evaluate the consequences of our current transplant system on the broader population in need of a lifesaving transplant.
There remains a role for considering waitlist-based metrics in evaluating transplant centers and optimizing organ allocation. But this study suggests that the tendency to consider such metrics in isolation may spur the development or maintenance of allocation policies that do not optimally address geographic and other disparities in access to liver transplantation, and may improve outcomes for waitlisted patients while exacerbating outcomes and access to transplant care for the larger population with ESLD. For example, UNOS is currently considering a proposal to redraw US maps for liver distribution, at projected costs of greater than $50 million. Although motivated by “…significant geographic differences in access to liver transplantation,31” this proposal is expected to achieve modest improvements in access among patients already on the liver transplant waitlist.7,32 Further, the present data suggest far smaller geographic differences in mortality among all patients with ESLD. For example, although the absolute difference in transplant rates among HealthCore beneficiaries who were waitlisted in 16 states was 36.4%, the absolute difference was only 8.6% among all HealthCore patients with ESLD in these same 16 states. Thus, instead of simply shifting organs between states by redrawing maps, greater improvements may emanate from efforts to address the well-documented barriers to waitlisting2,11,33 and to increasing the number of organs available for transplantation, while also considering ways to normalize geographic disparities.10,33–36 These data are not meant to imply that variable access to waitlists is the sole driver of variability in ESLD mortality, and differences in healthcare delivery and access to healthcare at a broader level contribute to differences in mortality from ESLD. Nevertheless, these data highlight how population and waitlist metrics evaluate two different outcomes, and both are important to consider in the context of access to transplantation and transplant care.
These conclusions are supported by several strengths of this study. First, the long-term follow-up of more than 100,000 patients with ESLD in two distinct, nationally representative administrative datasets provides a much larger and broader portrayal of outcomes among patients with chronic liver disease than do prior studies. Second, the similarity of the results in the two datasets provides much greater confidence in the veracity of the results than could be obtained in any single sample. Third, the inclusion of commercially insured patients as well as beneficiaries of state-sponsored Medicaid programs provided substantial diversity in socioeconomic status among the sample. And although commercially insured patients may not fully represent patients with public insurance, the among-state differences in these outcomes would not be expected to differ substantially, especially for patients with Medicare (the second largest cohort of patients on the LT waitlist after commercially insured patients) for whom their insurance is managed centrally by the Centers for Medicaid and Medicare Services. Lastly, although these analyses focused on identified populations with ESLD in HealthCore and Medicaid, the overarching findings reflect previously presented data that suggest, based on cause-of-death data from the CDC, that liver-related mortality at the statewide level does not reflect mortality among waitlisted patients.37
The study also has limitations. Due to the nature of the administrative data, we had limited laboratory data, and could only calculate the MELD score on a small fraction of the study cohort. Yet this lack of risk adjustment is unlikely to bias either among-state comparisons or overall comparisons between waitlist and population metrics. Second, we could not identify all medical co-morbidities that influence patients’ eligibility for waitlisting. Although this likely led us to overestimate the total transplant-eligible population, and thereby underestimate the proportion of truly eligible patients who were waitlisted, this too would be unlikely to explain our among-state or waitlist versus population comparisons. Additionally, our goal was to identify all patients with ESLD who might benefit from transplant, not solely those who would be waitlisted under current practice patterns. However, data suggest that the patients who were not waitlisted did not have significantly more medical co-morbidities based on the Charlson co-morbidity index (excluding points for liver disease) at the index data of ESLD diagnosis—in the non-waitlisted cohort, 42.4% had a score of 0, while 18.7% had a score of 1 or 2, while 36.2% of waitlisted patients had a Charlson score of 0, and 14.6% who had a score of 1 or 2. Lastly, due to the geographic distribution of patients, with small sample sizes of ESLD patients in certain states, we were unable to predict the impact of current redistricting proposals on ESLD population.
In conclusion, the number of patients in need of liver transplantation exceeds the supply of donor organs by a much greater degree than is apparent by only examining the waitlist. Given the dedication of UNOS to providing equitable access to transplant care while optimizing use of this scarce resource, our data suggest the need to re-examine existing and recently proposed allocation policies to evaluate their impact not only on waitlisted patients, but the broader population in need of a lifesaving transplant in order to ensure that such policies are in the best interests of both waitlisted patients as well as all potential beneficiaries of transplantation. Fortunately, such work now appears feasible by using approaches such as ours that merge population-representative claims data with UNOS data to estimate population-based demand and access to this lifesaving therapy.
Acknowledgments
Dr. Goldberg was funded by the National Institutes of Health (K08-DK098272). This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
Abbreviations
- ESLD
End-stage liver disease
- ABIM
American Board of Internal Medicine
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
- HCC
Hepatocellular carcinoma
- MELD
Model for End-Stage Liver Disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
Footnotes
Disclaimer
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Medical professionalism in the new millennium: a physician charter. Annals of internal medicine. 2002;136(3):243–246. doi: 10.7326/0003-4819-136-3-200202050-00012. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. Jama. 2014;311(12):1234–1243. doi: 10.1001/jama.2014.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern SD, Ubel PA, Caplan AL. Solid-organ transplantation in HIV-infected patients. The New England journal of medicine. 2002;347(4):284–287. doi: 10.1056/NEJMsb020632. [DOI] [PubMed] [Google Scholar]
- 4.Ubel PA, Caplan AL. Geographic favoritism in liver transplantation--unfortunate or unfair? The New England journal of medicine. 1998;339(18):1322–1325. doi: 10.1056/NEJM199810293391811. [DOI] [PubMed] [Google Scholar]
- 5.Bajpayee M, Dhawan A, Parmar D, Pandey AK, Mathur N, Seth PK. Gender-related differences in basal DNA damage in lymphocytes of a healthy Indian population using the alkaline Comet assay. Mutat Res. 2002;520(1–2):83–91. doi: 10.1016/s1383-5718(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 6.Ubel PA, Loewenstein G. Distributing scarce livers: the moral reasoning of the general public. Social science & medicine (1982) 1996;42(7):1049–1055. doi: 10.1016/0277-9536(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 7.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12(11):3085–3093. doi: 10.1111/j.1600-6143.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 10.Patzer RE, Plantinga LC, Paul S, et al. Variation in Dialysis Facility Referral for Kidney Transplantation Among Patients With End-Stage Renal Disease in Georgia. Jama. 2015;314(6):582–594. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. Jama. 2008;299(2):202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 12.Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network--improving the evidence of medical-product safety. N Engl J Med. 2009;361(7):645–647. doi: 10.1056/NEJMp0905338. [DOI] [PubMed] [Google Scholar]
- 13.Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. Jama. 2010;303(14):1401–1409. doi: 10.1001/jama.2010.410. [DOI] [PubMed] [Google Scholar]
- 14.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V., 3rd Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiol Drug Saf. 2013;22(1):103–107. doi: 10.1002/pds.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V., 3rd Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiology and drug safety. 2012;21(7):765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chacra WRD, Yang J, Gordon S. Development of an algorithm based on ICD-9 codes to identify patients with decompensated cirrhosis. Hepatology. 2013;(S1):92A–207A. [Google Scholar]
- 18.Network OPaT. According to OPTN/UNOS Data as of June 3, 2013 [Google Scholar]
- 19.According to OPTN data as of September 30 [Google Scholar]
- 20.Schwartz JJ, Pappas L, Thiesset HF, et al. Liver transplantation in septuagenarians receiving meld exception points for hepatocellular carcinoma: The national experience. Liver Transpl. 2012 doi: 10.1002/lt.23385. [DOI] [PubMed] [Google Scholar]
- 21.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 22.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4(6):920–927. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology. 2014;146(5):1256.e1251–1265.e1251. doi: 10.1053/j.gastro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bittermann T, Niu B, Hoteit MA, Goldberg D. Waitlist priority for hepatocellular carcinoma beyond milan criteria: a potentially appropriate decision without a structured approach. Am J Transplant. 2014;14(1):79–87. doi: 10.1111/ajt.12530. [DOI] [PubMed] [Google Scholar]
- 25.Recipients SRoT. Composite Pre-Transplant Metric (CPM) [Accessed April 18, 2014]; http://transplantpro.org/wp-content/uploads/Updated-CPM-FAQ_DEC-2011.pdf.
- 26.Ko CW, Kelley K, Meyer KE. Physician specialty and the outcomes and cost of admissions for end-stage liver disease. Am J Gastroenterol. 2001;96(12):3411–3418. doi: 10.1111/j.1572-0241.2001.05343.x. [DOI] [PubMed] [Google Scholar]
- 27.Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34(6):1089–1095. doi: 10.1053/jhep.2001.29204. [DOI] [PubMed] [Google Scholar]
- 28.Thomson M, Volk M, Kim HM, Piette JD. An Automated Telephone Monitoring System to Identify Patients with Cirrhosis at Risk of Re-hospitalization. Dig Dis Sci. 2015;60(12):3563–3569. doi: 10.1007/s10620-015-3744-3. [DOI] [PubMed] [Google Scholar]
- 29.Ertel AE, Kaiser T, Shah SA. Using Telehealth to Enable Patient-Centered Care for Liver Transplantation. JAMA Surg. 2015;150(7):674–675. doi: 10.1001/jamasurg.2015.0676. [DOI] [PubMed] [Google Scholar]
- 30.Perumalswami PV, Factor SH, Kapelusznik L, et al. Hepatitis Outreach Network: A practical strategy for hepatitis screening with linkage to care in foreign-born communities. Journal of Hepatology. 2013;58(5):890–897. doi: 10.1016/j.jhep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 32.Gentry SE, Segev DL, Kasiske BL, Mulligan DC, Hirose R. Robust Models Support Redistricting Liver Allocation to Reduce Geographic Disparity. Transplantation. 2015;99(9):e159–e160. doi: 10.1097/TP.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9(9):2092–2101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaral S, Patzer R. Disparities, race/ethnicity and access to pediatric kidney transplantation. Current opinion in nephrology and hypertension. 2013;22(3):336–343. doi: 10.1097/MNH.0b013e32835fe55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95(2):309–318. doi: 10.1097/TP.0b013e31827191d4. [DOI] [PubMed] [Google Scholar]
- 36.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho HC, Jung HY, Sinn DH, et al. Mortality after surgery in patients with liver cirrhosis: comparison of Child-Turcotte-Pugh, MELD and MELDNa score. Eur J Gastroenterol Hepatol. 2011;23(1):51–59. doi: 10.1097/MEG.0b013e3283407158. [DOI] [PubMed] [Google Scholar]