Abstract
Homeobox genes, including HOX and non-HOX genes, have been identified to be expressed aberrantly in solid tumors. In gastrointestinal (GI) cancers, most studies have focused on the function of non-HOX genes including caudal-related homeobox transcription factor 1 (CDX1) and CDX2. CDX2 is a crucial factor in the development of pre-cancerous lesions such as Barrett’s esophagus or intestinal metaplasia in the stomach, and its tumor suppressive role has been investigated in colorectal cancers. Recently, several HOX genes were reported to have specific roles in GI cancers; for example, HOXA13 in esophageal squamous cell cancer and HOXB7 in stomach and colorectal cancers. HOXD10 is upregulated in colorectal cancer while it is silenced epigenetically in gastric cancer. Thus, it is essential to examine the differential expression pattern of various homeobox genes in specific tumor types or cell lineages, and understand their underlying mechanisms. In this review, we summarize the available research on homeobox genes and present their potential value for the prediction of prognosis in GI cancers.
Keywords: Homeobox genes, HOX genes, Caudal-related homeobox transcription factor 2, Gastrointestinal cancers, HOXB7
Core tip: Aberrant up- or downregulation of homeobox genes may play pivotal roles in the development and progression of gastrointestinal (GI) cancers. Core research in GI cancers has focused on non-HOX genes including caudal-related homeobox transcription factor 2. However, recent studies have demonstrated significant functions of specific HOX genes, including HOXB7, HOXA13, and HOXD10, in GI cancers. Here, we review the major research data concerning the deregulation of homeobox genes in GI cancers and their underlying mechanisms.
INTRODUCTION
The homeobox genes were first discovered in Drosophila melanogaster where their mutation led to malformations of body parts[1]. As the name implies, homeobox genes play crucial roles in the development of the embryo along the anterior-posterior axis. The human genome contains about 235 functional homeobox genes, most of which are dispersed throughout the genome and contain a highly conserved 180 nucleotide sequence (homeobox) encoding 60 amino acids along the DNA-binding protein domain (homeodomain)[2]. A typical characteristic of the homeodomain is its DNA-binding nature; it functions as a transcription factor by binding to the promoter of various target genes. Several cofactors, such as pre-B-cell leukemia transcription factor 2 (PBX2) or myeloid ecotropic viral integration site (MEIS), interact with homeobox genes to form a protein complex and facilitate the specificity and stability of homeobox genes by binding to promoter DNA[3].
Homeobox genes are generally classified as class I (HOX) and class II (non-HOX). In humans, 39 HOX genes have been identified. They cluster into 4 groups named A, B, C, and D, located in 7p15.3, 17q21.3, 12q13.1, and 2q31, respectively[4]. Each HOX gene in a cluster is arranged from the 3’ to 5′ end and named from 1 to 13. HOX genes located at the 3′ end are expressed early in development and in anterior tissues, while HOX genes at the 5′ end are expressed later and in posterior tissues[5].
Numerous studies have revealed that various homeobox genes have either tumor-suppressive or tumor-promoting effects according to their aberrant expression patterns in certain organs. In terms of their oncogenetic properties, homeobox genes are normally expressed during the embryonic period and are reactivated in tumors, while being downregulated in normal differentiated adult tissues. In contrast, certain homeobox genes are expressed in normal differentiated adult tissues, but are downregulated in tumors[1]. This aberrant reduced or enhanced expression of homeobox genes is regulated by several mechanisms, such as loss of heterozygosity, gene amplification, CpG island promoter hypermethylation, or histone deacetylation, and consequently contributes to the development and progression of cancer.
Interestingly, a homeobox gene may have both tumor-promoting and tumor-suppressing properties depending on the specific organs or cell lineages where it is expressed. For example, HOXA9 is downregulated in lung cancer tissues compared to that in surrounding non-cancerous tissues by an epigenetic silencing mechanism, whereas it is upregulated in acute lymphocystic leukemia[6,7]. HOXB13 is another example that is upregulated in breast cancer but downregulated in prostate cancer compared to surrounding normal tissues[8,9]. Several long and short non-coding RNAs are also involved in the regulation of transcription or expression of homeobox genes. For example, HOX transcript antisense intergenic RNA (HOTAIR), a long non-coding RNA, is located in the HOXC locus near the 5′ end, and recruits polycomb repressive complex 2 to lead epigenetic silencing of the HOXD locus[10]. MicroRNAs (miRNAs), including miR-10a, miR-10b, miR-196a, and miR-196b, are also located within the HOX clusters and target multiple HOX genes to regulate their expression post-transcriptionally[4]. Therefore, it is important to understand the aberrant expression pattern of homeobox genes in specific cancer types or cell lineages, and their underlying mechanisms for carcinogenesis and invasion of certain types of cancer.
In this editorial, we summarize the outcomes of previous studies of homeobox genes that showed valid influences on solid tumors in the gastrointestinal (GI) tract, including esophageal, gastric, and colorectal cancers (CRCs). This article provides information on the underlying molecular mechanisms, aberrant expression in GI cancer tissues, and the potential value of various homeobox genes for early recognition or prediction of prognosis in GI cancers.
ESOPHAGEAL CANCER
Most studies of homeobox genes in Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC) have focused on non-HOX genes, especially caudal-related homeobox transcription factor 2 (CDX2). Generally, acid and bile reflux at the esophagogastric junction promotes dedifferentiation of the basal layer of the esophageal squamous epithelium. This is where secretion of CDX2 is increased, and morphogenic and metaplastic changes occur, eventually leading to the development of intestinal-type squamous to columnar metaplasia[11]. Indeed, CDX2 plays a crucial role in the development of BE, a major precursor of EAC. In addition, several previous studies showed that mRNA and protein expression of CDX2 was increased significantly in BE and EAC compared to normal esophageal tissues, although no significant difference could be found between BE and EAC[12,13]. The expression of CDX2 protein was well-conserved in an EAC cell line, but was not detected in esophageal squamous cell carcinoma (ESCC) cells. Furthermore, demethylation or exposure of esophageal squamous epithelial cells to acid or bile induced CDX2 as well as other intestinal markers. These findings suggested that CDX2 is a key modulator of intestinal metaplasia of esophageal squamous cells in response to acid or bile reflux[14]. In terms of HOX genes, a previous well-designed study showed that mid-cluster HOXB genes (HOXB5, B6, and B7) were upregulated in BE tissue as well as in dysplasia and EAC. However, no significant difference was observed between BE with dysplasia and EAC. Furthermore, these mid-cluster HOXB genes induced several intestinal markers including KRT20, Muc2, and villin in esophageal cells in a CDX2-independent manner[15].
A previous study using the reverse transcriptase-polymerase chain reaction (RT-PCR) showed that HOXA7, A9, and C6 mRNAs were overexpressed significantly in ESCC tissues compared to non-cancerous surrounding tissues[16]. A microarray study showed that the mRNA expression of several HOX genes, including HOXA5, A10, B13, C6, C10, C13, and D3, was upregulated significantly in ESCC tissues compared to normal esophageal mucosa, and these genes were differentially expressed according to the T stage; expression was the highest in T2[17]. This study also showed that several non-HOX genes, including CDX1 and CDX2, were expressed at higher levels in ESCC than normal esophageal mucosa. However, another crucial study demonstrated that most of the expression of CDX2 in ESCC cell lines and tissues was governed by an epigenetic silencing mechanism that was not found in EAC, CRC, or normal esophageal tissues[18]. This suggested that aberrant inactivation of CDX2 is an important step toward the development of ESCC.
Among the HOX genes, HOXA13 has been most actively investigated in ESCC. A previous pivotal study very nicely showed the tumorigenic effect of HOXA13 in vivo, and that there was a significant association between HOXA13 and both median and disease-free survival[19]. Chen and his colleagues, using knockdown of HOXA13 in ESCC cell lines and 2-dimmensional electrophoresis, suggested that annexinA2, manganese superoxide dismutase (MnSOD) and endoplasmic reticulum-associated amyloid β-binding protein (ERAB) were crucial target genes of HOXA13[20]. These researchers also used ESCC tissues to show that co-expression of HOXA13 with annexinA2 and SOD was significantly associated with poor prognosis[21]. Other HOX genes, such as HOXA9 and B7, were also upregulated in ESCC at advanced T or N stages, and in patients with poor prognosis[22,23]. Meanwhile, a recent study demonstrated that MEIS1, a non-HOX homeobox gene, was downregulated in ESCC patients and was associated inversely with advanced TNM stage. The mechanism was thought to be mediated by upregulation of SRY (sex determining region Y)-box 2 (SOX2) in ESCC cells[24] (Table 1).
Table 1.
Aberrant expression of HOX and non-HOX genes in esophageal cancer
| Homeobox gene | Change | Underlying mechanism | Ref. |
| BE/EAC | |||
| CDX2 | ↑ in BE/EAC | Concomitant decrease of PITX1 | [12,13] |
| No difference between BE and EAC | Association with β-catenin | ||
| HOXB5, B6, B7 | ↑ in BE/dysplasia/EAC | Induction of intestinal markers such as KRT20, Muc2 and villin | [15] |
| ESCC | |||
| CDX2 | ↓ in a ESCC cell line and tissues | Promoter hypermethylation | [18] |
| MEIS | ↓ in ESCC, inversely related with nodal status and high tumor stage | Concomitant increase of SOX2 | [24] |
| HOXA7, A9, C6 | ↑ in ESCC | Not presented | [16] |
| HOXA5, A10, B13, C6, C10, C13, D3 | ↑ in BE/EAC, highest in T2 stage | Not presented | [17] |
| HOXA13 | ↑ in ESCC, associated with OS and DFS | Targeting annexinA2, MnSOD, ERAB | [19-21] |
| HOXB7 | ↑ in ESCC, associated with T/N stage and DFS | Not presented | [23] |
BE: Barrett’s esophagus; EAC: Esophageal adenocarcinoma; ESCC: Esophageal squamous cell carcinoma; MnSOD: Manganese superoxide dismutase; OS: Overall survival; DFS: Disease free survival.
STOMACH CANCER
The most extensively researched homeobox genes in stomach cancer are CDX2 and CDX1. These genes are closely involved in the development of intestinal metaplasia of the gastric mucosa. A previous pivotal study demonstrated the causal role of CDX2 in the development of intestinal metaplasia in the stomach by using a Cdx2-expressing transgenic mouse model[25]. The Cdx1 transgenic mouse also exhibited significant intestinal metaplasia, although the characteristics were somewhat different from the Cdx2 transgenic mouse; the former replaced the gastric mucosa with intestinal metaplasia involving all four intestinal epithelial cell types (absorptive enterocytes, goblet, enteroendocrine, and Paneth cells), whereas only pseudopyloric gland metaplasia was observed in the Cdx2 transgenic mouse[26]. This phenomenon suggested that a different mechanism and roles between CDX1 and CDX2 may exist in the differentiation of intestinal metaplasia.
In human stomach, ectopic expression of CDX1 and CDX2 was observed frequently in intestinal metaplasia tissues. However, only CDX2 was an independent factor of intestinal type gastric adenocarcinoma[27]. Another study showed that the expression of CDX2 in gastric cancer was governed mainly by promoter hypermethylation. This suggested that aberrant downregulation of CDX2 might promote gastric carcinogenesis[28]. Liu et al[29] suggested that CDX2 was associated mainly with the formation of intestinal metaplasia of gastric mucosa, and was less involved in dysplasia and cancer, by demonstrating that the expression of CDX2 protein was highest in complete type intestinal metaplasia, followed by incomplete intestinal metaplasia, dysplasia, and the lowest in gastric cancer tissues. The exact molecular characteristics of CDX2 in the development of intestinal metaplasia and gastric cancer should be further evaluated. Indeed, the unique characteristics of CDX2 are associated with both oncogenic and tumor-suppressive functions, and these ambivalent roles of CDX2 might be tissue- or site-specific. At present, CDX2 appears to be involved in the initiation of the process leading to intestinal type gastric neoplasia such as induction of intestinal metaplasia[30].
Several other non-HOX homeobox genes, including intestine-specific homeobox (ISX), prospero homeobox 1 (PROX1), paired-related homeobox 1 (PRRX1), iroquois-class homeodomain (IRX1), and pancreatic-duodenal homeobox 1 (PDX1), have been investigated for their relationship with intestinal metaplasia and gastric cancer. Among these genes, ISX, PROX1, and PRRX1 were associated with the promotion of gastric cancer, suggesting their oncogenetic roles[31-33]. Specifically, ISX was upregulated in intestinal metaplasia and its levels correlated significantly with CDX2 expression in mice with chronic Helicobacter felis infections. However, ISX also enhanced cyclin D1 (a G1 → S cell cycle modulator) and CD44 (a stem cell marker of gastric cancer) expression, and its protein expression was increased significantly in undifferentiated-type gastric cancer, unlike CDX2[31]. PROX1 promoted cellular proliferation, angiogenesis, and epithelial-mesenchymal transition (EMT) in vitro. Furthermore, its tissue expression was significantly associated with advanced stage, undifferentiated type, lymph node metastasis, and poor prognosis[32]. PRRX1 also showed EMT-promoting functions via inducing the Wnt/β-catenin pathway, and was significantly associated with advanced-stage and distant metastasis[33]. In contrast, several in vitro studies showed that the expression of IRX1 and PDX1 mRNA was downregulated in gastric cancer cells by an epigenetic silencing mechanism via promoter hypermethylation, suggesting their tumor-suppressive functions[34-36]. Another study demonstrated that PDX1 expression was associated with the pseudopyloric gland of intestinal metaplasia tissues, and was decreased in patients with advanced stage and lymph node metastasis, compared to early stage gastric cancer[37]. However, a few studies demonstrated a significant relationship between various non-HOX homeobox genes and clinicopathological parameters such as TNM stage, differentiation, overall and disease-free survival rate of gastric cancer patients. The nature of this relationship requires further study.
Recently, investigations into the role of HOX genes in gastric carcinogenesis and progression have been performed. One notable study used microarray analysis to reveal the global expression patterns of 39 human HOX genes among 12 pairs of gastric cancer and non-cancerous tissues. The authors showed that the expression of HOXA1, A4, A10, A13, B7, and C10 was increased significantly in cancer tissues. Among these genes, upregulation of HOXA13 was associated significantly with T stage, M stage, advanced UICC stage, histologic differentiation and relapse. Furthermore, patient with positive HOXA13 expression had a lower overall survival and disease-free survival compared with patients with negative HOXA13 expression. The contribution of HOXA13 towards tumorigenesis and aggressive biologic behavior in gastric cancer might be associated with downregulation of tumor growth factor-β (TGF-β) and its downstream target of Runt-related transcription factor 3 by antagonizing Smad3[38]. Concurrent researches on individual HOX genes in gastric cancer have been conducted. An in vitro study showed that HOXB5 promoted migration and invasion of gastric cancer cells by binding directly to the CTNNB1 promoter and thus activating the Wnt/β-catenin signaling pathway[39]. Another pivotal study showed that HOXD10 mRNA expression was downregulated significantly in stomach cancer tissues compared to normal surrounding tissues. This downregulation was caused by promoter hypermethylation, and the aberrant reduction of HOXD10 expression led to proliferation, migration, invasion, and tumorigenesis in gastric cancer cells[40]. We reported recently that HOXB7, one of the most widely investigated oncogenic HOX genes, was highly expressed in primary or metastatic gastric cancer tissues compared to chronic gastritis or intestinal metaplasia tissues. This suggested that HOXB7 might be involved in the progression rather than initiation process of gastric cancer[41]. This phenomenon has been validated by in vitro studies showing that overexpression of HOXB7 in gastric cancer cells promoted cellular invasion and migration, and inhibited apoptosis, whereas silencing HOXB7 showed the opposite effects[41,42].
The main target of HOXB7, and the mechanism involved in the upregulation of HOXB7 in cancer, are still under controversy. We suggested that HOXB7 regulates Akt/PTEN signaling to induce migration and invasion of gastric cancer cells, by using transient transfection of a HOXB7-expressing plasmid and HOXB7 siRNA. A recent well-designed study demonstrated that HOXB7 promoted the EMT and invasiveness of breast cancer cells by regulating the TGF β2-SMAD3 axis[43]. Several receptor tyrosine kinase signaling pathways, including beta fibroblast growth factor and epidermal growth factor receptor, were also reported to be activated by HOXB7 in breast cancer cells[44-46]. Thus, HOXB7 might be simultaneously involved in various key molecular signaling pathways involving cancer progression, which supports the potential value of HOXB7 as a promising therapeutic target. Several miRNAs, including miR-196a and miR-196b, were suggested as key regulators of HOXB7 expression in other types of cancer[47,48]. Further investigations to reveal the mechanisms underlying the induction of HOXB7 and its targets in gastric cancer are needed (Table 2).
Table 2.
Aberrant expression of HOX and non-HOX genes in gastric cancer
| Homeobox gene | Change | Underlying mechanism | Ref. |
| CDX2 | ↑ in complete IM > incomplete IM > dysplasia > GC | Promoter hypermethylation in GC | [27-29] |
| Associated with differentiated type GC | Decreased intake of green tea or cruciferous vegetables | ||
| ISX | ↑ in IM and GC | Increase of cyclin D1 and CD44 | [31] |
| Upregulated in undifferentiated type GC | |||
| PROX1 | ↑ in GC | Inhibition of apoptosis, promoting lymphangiogenesis and angiogenesis | [32] |
| Associated with undifferentiated type, advanced stage and poor OS | |||
| PRRX1 | ↑ in GC | Induction of Wnt/β-catenin | [33] |
| Associated with advanced stage and distant metastasis | |||
| IRX1 | ↓ in GC | Promoter hypermethylation | [35] |
| PDX1 | ↓ in GC | Promoter hypermethylation, histone hypoacetylation | [34,36,37] |
| ↑ in pseudopyloric gland IM | |||
| Inversely related with advanced T/ N stage and undifferentiated type GC | |||
| HOXA13 | ↑ in GC | Not presented | [38] |
| Associated with advanced TNM stage, undifferentiated type and poor response to chemotherapy | |||
| HOXB5 | ↑ in GC | Upregulation of β-catenin | [39] |
| HOXB7 | ↑ in primary or metastatic cancer than chronic gastritis or IM | Modulation of PI3K/Akt/PTEN axis | [41,42] |
| Associated with advanced TNM stage and undifferentiated type GC | |||
| HOXD10 | ↓ in GC | Promoter hypermethylation | [40] |
| Induction of IGFBP3 |
Note: PROX1, PRRX1, HOXA13 and HOXB7 are associated with advanced TNM stage, while PDX1 is inversely associated; ISX, PROX1, HOXA13 and HOXB7 are associated with undifferentiated type GC. IM: Intestinal metaplasia; GC: Gastric cancer; OS: Overall survival; PI3K: Phosphatidylinositol-3 kinase; IGFBP3: Insulin like growth factor binding protein 3.
CRC
Similar to esophageal and gastric cancer, HOX and non-HOX homeobox genes have been investigated for their unique roles in the development and progression of CRC. Among these genes, CDX2 in colon cancer cells has been researched extensively and reported to regulate the expression of cell junctional proteins. These proteins include liver-intestine cadherin (LI-cadherin)[49] or protocadherin Mucdhl[50]. Loss of CDX2 in colon cancer cells downregulated Mucdhl, thereby eliminating the latter’s inhibition of Wnt/β-catenin activity. Inflammatory cytokines, such as tumor necrosis factor-α, mediated this process (loss of CDX2 and induction of Wnt/β-catenin signaling) in CaCo2 colon cancer cells[51]. Furthermore, significant tumor formation was observed when heterozygous Cdx2+/-, but not wild-type mice, were treated with the DNA mutagen azosymethane. This indicated that CDX2 had a tumor-suppressive function in CRC[52]. At the tissue level, reduced expression of CDX2 in colorectal adenoma or cancer was associated significantly with right side tumors, poorly differentiated or high-grade carcinomas, advanced stage, poor prognosis, CpG island methylator phenotype, and mismatch repair-deficient tumors[53-55].
Previous pivotal studies showed that CDX1 inhibited the proliferation of colon cancer cells by regulating the cyclin D1 or β-catenin/T-cell factor (TCF) pathways[56,57], and that tissue expression of CDX1 was increased significantly in adenomatous polyps but abolished in adenocarcinomas. Furthermore, a novel in vitro study showed that CDX1 was governed by miR-215 to promote differentiation and inhibit stemness in colon cancer cells[58]. These data suggested that CDX1 might play a crucial role in the transformation of benign adenomas to malignant tumors.
Other types of non-HOX homeobox genes have been investigated for their roles in CRC. The expression of the airstaless-like homeobox-4 gene (ALX4) was aberrantly reduced in colorectal dysplasia or adenocarcinoma compared with normal colonic mucosa, through DNA methylation[59]. In addition, PROX1 promoted neoplastic transformation, tumorigenesis, and the EMT via induction of the β-catenin/TCF pathway and inhibition of E-cadherin activity[60,61].
Relatively few data concerning HOX genes have been presented in terms of CRC compared to other GI cancers. A previous quantitative RT-PCR study showed that the expression of several HOX genes, including HOXA9, B3, B8, and B9, was increased significantly in left side colon cancer tissues compared to surrounding normal tissues. In contrast, the expression of HOXB2, B13, D1, D3, D4, D8, and D12 was significantly decreased[62]. A recent gene microarray and immunohistochemical study showed that the expression of HOXA4 and HOXD10 was significantly increased in CRC tissues compared to that in normal tissues. Furthermore, the expression of these genes was clustered in the crypt bottom rather than the top or middle of the crypt where the stem cell niche was overpopulated[63]. Remarkably, HOX genes showed a tendency to be differentially expressed in colon tumors according to their location. Specifically, several HOX genes, including HOXA5, A9, A10, and C6, were expressed at higher levels in the proximal colon, and gradually decreased in the distal colon and rectum. HOXB13 was an exception that showed the opposite pattern[64]. Previous studies showed that expression pattern of HOXB13 was site-specific, which was mainly confined to prostate, rectum and distal colon[65], and HOXB13 inhibited the β-catenin/TCF signaling pathway as post-translational manner, which was downregulated in colorectal tumors[66].
A previous pivotal study demonstrated the prognostic value of HOXB7 in CRC. Patients in the high HOXB7 CRC group had a poorer prognosis than those in the low HOXB7 group. In addition, the tumorigenic and anti-apoptotic effects of HOXB7 in colon cancer cells were mediated by the phosphatidylinositol-3-kinase/Akt and mitogen-activated protein kinase pathways[67] (Table 3).
Table 3.
Aberrant expression of HOX and non-HOX genes in colorectal cancer
| Homeobox gene | Change | Underlying mechanism | Ref. |
| CDX1 | ↑ in adenomatous polyp, ↓ in CRC | Regulation of cyclin D1 and β-catenin/TCF pathway | [55,56,58] |
| Regulated by miR-215 | |||
| CDX2 | ↓ in adenoma and CRC | Loss of Mucdhl | [50-55] |
| Inversely associated with right side tumor, poorly differentiated type, advanced stage, poor prognosis, CIMP, MMR-deficient tumor | Induction of Wnt/β-catenin axis | ||
| ALX4 | ↓ in dysplasia and CRC | Promoter hypermethylation | [59] |
| PROX1 | ↑ in CRC | Induction of β-catenin/TCF axis | [60,61] |
| Associated with advanced stage and lymph node metastasis | Inhibition of E-cadherin activity | ||
| HOXA4, D10 | ↑ in CRC | Stem cell overpopulation and crypt renewal | [63] |
| HOXA5, A9, A10, C6 | Proximal colon tumor > distal colon tumor | Not presented | [64] |
| HOXB13 | Distal colon tumor > proximal colon tumor | Not presented | [64] |
| HOXB7 | ↑ in CRC | Activation of PI3K/Akt and MAPK pathways | [67] |
| Associated with advanced stage, T stage, distant metastasis and poor OS |
CRC: Colorectal cancer; TCF: β-catenin/T-cell factor; CIMP: CpG island methylation phenotype; OS: Overall survival; PI3K: Phosphatidylinositol-3 kinase; MAPK: Mitogen-activated protein kinase.
CONCLUSION
Several homeobox genes are expressed aberrantly in various types of cancers, and the GI tract is no exception. Previous studies focused mainly on the roles of non-HOX genes, such as CDX1 and CDX2, in GI cancers. Recently, several HOX genes have been investigated and shown to have specific roles in the development and invasion of GI cancers (Figure 1). However, intensive understanding of the underlying mechanisms including their transcriptional target genes, and co-factors or downstream effectors of homeobox genes in GI cancers are still lacking. Moreover, current knowledge of the homeobox genes in GI cancer could not reach the clinical efficacy of therapeutic targets or biomarkers, which need to be fulfilled in the future research.
Figure 1.
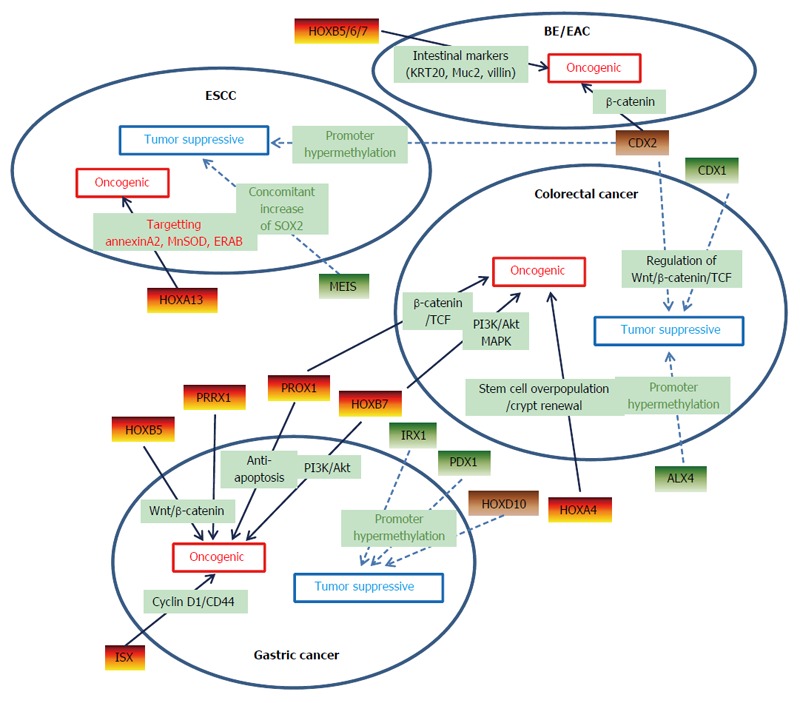
Schematic diagram of homeobox genes which have diverse effects on gastrointestinal cancers. Solid arrow indicates upregulated homeogox genes and dashed arrow indicates downregulated ones. Note that CDX2 shows tumor suppressive function in colorectal and esophageal squamous cell cancer whereas oncogenic effect on the formation of Barrett’s esophagus and esophageal adenocarcinoma. HOXD10 also shows dual function, which has tumor suppressive effect on gastric cancer whereas oncogenic effect on colorectal cancer. BE: Barrett’s esophagus; EAC: Esophageal adenocarcinoma; ESCC: Esophageal squamous cell carcinoma.
Recent studies demonstrated the significant contribution of several HOX genes to chemoresistance. For example, downregulation of HOXA1 under regulation of HOTAIR or miR-100 enhance chemoresistance in pancreas cancer and small cell lung cancer[68,69]. An improved understanding of the mechanism of this effect may reveal a means to create tailored, precision medicine of GI cancers. Meanwhile, the regulation of homeobox genes by several non-coding RNAs, including miRNAs, may provide a means to restore the aberrant expression of homeobox genes in GI cancers. Finally, the differential expression pattern of homeobox genes in various cancers may provide valuable information for the diagnosis of challenging cases of GI tumors.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None of the authors have potential conflicts (financial, professional, or personal) that are relevant to this publication.
Peer-review started: July 6, 2016
First decision: July 29, 2016
Article in press: August 23, 2016
P- Reviewer: Mocellin S, Tarnawski AS, Wargovich MJ S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Haria D, Naora H. Homeobox Gene Deregulation: Impact on the Hallmarks of Cancer. Cancer Hallm. 2013;1:67–76. doi: 10.1166/ch.2013.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawat VP, Humphries RK, Buske C. Beyond Hox: the role of ParaHox genes in normal and malignant hematopoiesis. Blood. 2012;120:519–527. doi: 10.1182/blood-2012-02-385898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Errico MC, Felicetti F, Bottero L, Mattia G, Boe A, Felli N, Petrini M, Bellenghi M, Pandha HS, Calvaruso M, et al. The abrogation of the HOXB7/PBX2 complex induces apoptosis in melanoma through the miR-221& amp; 222-c-FOS pathway. Int J Cancer. 2013;133:879–892. doi: 10.1002/ijc.28097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platais C, Hakami F, Darda L, Lambert DW, Morgan R, Hunter KD. The role of HOX genes in head and neck squamous cell carcinoma. J Oral Pathol Med. 2016;45:239–247. doi: 10.1111/jop.12388. [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 6.Rauch T, Wang Z, Zhang X, Zhong X, Wu X, Lau SK, Kernstine KH, Riggs AD, Pfeifer GP. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc Natl Acad Sci USA. 2007;104:5527–5532. doi: 10.1073/pnas.0701059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faber J, Krivtsov AV, Stubbs MC, Wright R, Davis TN, van den Heuvel-Eibrink M, Zwaan CM, Kung AL, Armstrong SA. HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood. 2009;113:2375–2385. doi: 10.1182/blood-2007-09-113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Dahiya S, Provencher H, Muir B, Carney E, Coser K, Shioda T, Ma XJ, Sgroi DC. The prognostic biomarkers HOXB13, IL17BR, and CHDH are regulated by estrogen in breast cancer. Clin Cancer Res. 2007;13:6327–6334. doi: 10.1158/1078-0432.CCR-07-0310. [DOI] [PubMed] [Google Scholar]
- 9.Jung C, Kim RS, Lee SJ, Wang C, Jeng MH. HOXB13 homeodomain protein suppresses the growth of prostate cancer cells by the negative regulation of T-cell factor 4. Cancer Res. 2004;64:3046–3051. doi: 10.1158/0008-5472.can-03-2614. [DOI] [PubMed] [Google Scholar]
- 10.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–G218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 12.Lord RV, Brabender J, Wickramasinghe K, DeMeester SR, Holscher A, Schneider PM, Danenberg PV, DeMeester TR. Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett’s esophagus and Barrett’s-associated adenocarcinoma. Surgery. 2005;138:924–931. doi: 10.1016/j.surg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Vaninetti N, Williams L, Geldenhuys L, Porter GA, Guernsey DL, Casson AG. Regulation of CDX2 expression in esophageal adenocarcinoma. Mol Carcinog. 2009;48:965–974. doi: 10.1002/mc.20549. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–496. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- 15.di Pietro M, Lao-Sirieix P, Boyle S, Cassidy A, Castillo D, Saadi A, Eskeland R, Fitzgerald RC. Evidence for a functional role of epigenetically regulated midcluster HOXB genes in the development of Barrett esophagus. Proc Natl Acad Sci USA. 2012;109:9077–9082. doi: 10.1073/pnas.1116933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KN, Gu ZD, Ke Y, Li JY, Shi XT, Xu GW. Expression of 11 HOX genes is deregulated in esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1044–1049. [PubMed] [Google Scholar]
- 17.Takahashi O, Hamada J, Abe M, Hata S, Asano T, Takahashi Y, Tada M, Miyamoto M, Kondo S, Moriuchi T. Dysregulated expression of HOX and ParaHOX genes in human esophageal squamous cell carcinoma. Oncol Rep. 2007;17:753–760. [PubMed] [Google Scholar]
- 18.Guo M, House MG, Suzuki H, Ye Y, Brock MV, Lu F, Liu Z, Rustgi AK, Herman JG. Epigenetic silencing of CDX2 is a feature of squamous esophageal cancer. Int J Cancer. 2007;121:1219–1226. doi: 10.1002/ijc.22828. [DOI] [PubMed] [Google Scholar]
- 19.Gu ZD, Shen LY, Wang H, Chen XM, Li Y, Ning T, Chen KN. HOXA13 promotes cancer cell growth and predicts poor survival of patients with esophageal squamous cell carcinoma. Cancer Res. 2009;69:4969–4973. doi: 10.1158/0008-5472.CAN-08-4546. [DOI] [PubMed] [Google Scholar]
- 20.Shen LY, Chen KN. Exploration of target genes of HOXA13 in esophageal squamous cell carcinoma cell line. Cancer Lett. 2011;312:18–23. doi: 10.1016/j.canlet.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Ma RL, Shen LY, Chen KN. Coexpression of ANXA2, SOD2 and HOXA13 predicts poor prognosis of esophageal squamous cell carcinoma. Oncol Rep. 2014;31:2157–2164. doi: 10.3892/or.2014.3088. [DOI] [PubMed] [Google Scholar]
- 22.Lv J, Cao XF, Ji L, Zhu B, Wang DD, Tao L, Li SQ. Association of β-catenin, Wnt1, Smad4, Hoxa9, and Bmi-1 with the prognosis of esophageal squamous cell carcinoma. Med Oncol. 2012;29:151–160. doi: 10.1007/s12032-010-9816-5. [DOI] [PubMed] [Google Scholar]
- 23.Xie X, Zhang SS, Wen J, Yang H, Luo KJ, Yang F, Hu Y, Fu JH. Prognostic value of HOXB7 mRNA expression in human oesophageal squamous cell cancer. Biomarkers. 2013;18:297–303. doi: 10.3109/1354750X.2013.773380. [DOI] [PubMed] [Google Scholar]
- 24.Rad A, Farshchian M, Forghanifard MM, Matin MM, Bahrami AR, Geerts D, A’rabi A, Memar B, Abbaszadegan MR. Predicting the molecular role of MEIS1 in esophageal squamous cell carcinoma. Tumour Biol. 2016;37:1715–1725. doi: 10.1007/s13277-015-3780-9. [DOI] [PubMed] [Google Scholar]
- 25.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 26.Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416–1423. doi: 10.1136/gut.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55. doi: 10.1016/s0304-3835(01)00753-4. [DOI] [PubMed] [Google Scholar]
- 28.Yuasa Y, Nagasaki H, Akiyama Y, Sakai H, Nakajima T, Ohkura Y, Takizawa T, Koike M, Tani M, Iwai T, et al. Relationship between CDX2 gene methylation and dietary factors in gastric cancer patients. Carcinogenesis. 2005;26:193–200. doi: 10.1093/carcin/bgh304. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Teh M, Ito K, Shah N, Ito Y, Yeoh KG. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol. 2007;20:1286–1297. doi: 10.1038/modpathol.3800968. [DOI] [PubMed] [Google Scholar]
- 30.Yan LH, Wei WY, Xie YB, Xiao Q. New insights into the functions and localization of the homeotic gene CDX2 in gastric cancer. World J Gastroenterol. 2014;20:3960–3966. doi: 10.3748/wjg.v20.i14.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sue S, Shibata W, Kameta E, Sato T, Ishii Y, Kaneko H, Miwa H, Sasaki T, Tamura T, Kondo M, et al. Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis. J Gastroenterol. 2016 doi: 10.1007/s00535-016-1176-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Park KJ, Cho SB, Park YL, Kim N, Park SY, Myung DS, Lee WS, Kweon SS, Joo YE. Prospero homeobox 1 mediates the progression of gastric cancer by inducing tumor cell proliferation and lymphangiogenesis. Gastric Cancer. 2016 doi: 10.1007/s10120-015-0592-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Guo J, Fu Z, Wei J, Lu W, Feng J, Zhang S. PRRX1 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer. Med Oncol. 2015;32:393. doi: 10.1007/s12032-014-0393-x. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Wang JD, Zhang WJ, Zou B, Chen WJ, Lam CS, Chen MH, Pang R, Tan VP, Hung IF, et al. Promoter hypermethylation and histone hypoacetylation contribute to pancreatic-duodenal homeobox 1 silencing in gastric cancer. Carcinogenesis. 2010;31:1552–1560. doi: 10.1093/carcin/bgq140. [DOI] [PubMed] [Google Scholar]
- 35.Guo X, Liu W, Pan Y, Ni P, Ji J, Guo L, Zhang J, Wu J, Jiang J, Chen X, et al. Homeobox gene IRX1 is a tumor suppressor gene in gastric carcinoma. Oncogene. 2010;29:3908–3920. doi: 10.1038/onc.2010.143. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Chen M, Wang J, Xia HH, Zhu S, Liang Y, Gu Q, Qiao L, Dai Y, Zou B, et al. Pancreatic duodenal homeobox-1 (PDX1) functions as a tumor suppressor in gastric cancer. Carcinogenesis. 2008;29:1327–1333. doi: 10.1093/carcin/bgn112. [DOI] [PubMed] [Google Scholar]
- 37.Sakai H, Eishi Y, Li XL, Akiyama Y, Miyake S, Takizawa T, Konishi N, Tatematsu M, Koike M, Yuasa Y. PDX1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut. 2004;53:323–330. doi: 10.1136/gut.2003.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Tu WW, Wen YG, Li DP, Qiu GQ, Tang HM, Peng ZH, Zhou CZ. Identification and validation that up-expression of HOXA13 is a novel independent prognostic marker of a worse outcome in gastric cancer based on immunohistochemistry. Med Oncol. 2013;30:564. doi: 10.1007/s12032-013-0564-1. [DOI] [PubMed] [Google Scholar]
- 39.Hong CS, Jeong O, Piao Z, Guo C, Jung MR, Choi C, Park YK. HOXB5 induces invasion and migration through direct transcriptional up-regulation of β-catenin in human gastric carcinoma. Biochem J. 2015;472:393–403. doi: 10.1042/BJ20150213. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Chen S, Xue M, Zhong J, Wang X, Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al. Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med. 2012;18:389–400. doi: 10.2119/molmed.2011.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joo MK, Park JJ, Yoo HS, Lee BJ, Chun HJ, Lee SW, Bak YT. The roles of HOXB7 in promoting migration, invasion and anti-apoptosis in gastric cancer. J Gastroenterol Hepatol. 2016 doi: 10.1111/jgh.13330. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Cai JQ, Xu XW, Mou YP, Chen K, Pan Y, Wu D. Upregulation of HOXB7 promotes the tumorigenesis and progression of gastric cancer and correlates with clinical characteristics. Tumour Biol. 2016;37:1641–1650. doi: 10.1007/s13277-015-3948-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Jin K, Hui Y, Fu J, Jie C, Feng S, Reisman D, Wang Q, Fan D, Sukumar S, et al. HOXB7 promotes malignant progression by activating the TGFβ signaling pathway. Cancer Res. 2015;75:709–719. doi: 10.1158/0008-5472.CAN-14-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caré A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Parmiani G, Peschle C, Colombo MP. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–4851. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 46.Jin K, Kong X, Shah T, Penet MF, Wildes F, Sgroi DC, Ma XJ, Huang Y, Kallioniemi A, Landberg G, et al. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the EGFR pathway. Proc Natl Acad Sci USA. 2012;109:2736–2741. doi: 10.1073/pnas.1018859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braig S, Mueller DW, Rothhammer T, Bosserhoff AK. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci. 2010;67:3535–3548. doi: 10.1007/s00018-010-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.How C, Hui AB, Alajez NM, Shi W, Boutros PC, Clarke BA, Yan R, Pintilie M, Fyles A, Hedley DW, et al. MicroRNA-196b regulates the homeobox B7-vascular endothelial growth factor axis in cervical cancer. PLoS One. 2013;8:e67846. doi: 10.1371/journal.pone.0067846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002;123:1565–1577. doi: 10.1053/gast.2002.36598. [DOI] [PubMed] [Google Scholar]
- 50.Hinkel I, Duluc I, Martin E, Guenot D, Freund JN, Gross I. Cdx2 controls expression of the protocadherin Mucdhl, an inhibitor of growth and β-catenin activity in colon cancer cells. Gastroenterology. 2012;142:875–885.e3. doi: 10.1053/j.gastro.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 51.Coskun M, Olsen AK, Bzorek M, Holck S, Engel UH, Nielsen OH, Troelsen JT. Involvement of CDX2 in the cross talk between TNF-α and Wnt signaling pathway in the colon cancer cell line Caco-2. Carcinogenesis. 2014;35:1185–1192. doi: 10.1093/carcin/bgu037. [DOI] [PubMed] [Google Scholar]
- 52.Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard MP, Kedinger M, Beck F, Freund JN, Domon-Dell C. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut. 2003;52:1465–1471. doi: 10.1136/gut.52.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knösel T, Chen Y, Hotovy S, Settmacher U, Altendorf-Hofmann A, Petersen I. Loss of desmocollin 1-3 and homeobox genes PITX1 and CDX2 are associated with tumor progression and survival in colorectal carcinoma. Int J Colorectal Dis. 2012;27:1391–1399. doi: 10.1007/s00384-012-1460-4. [DOI] [PubMed] [Google Scholar]
- 54.Olsen J, Eiholm S, Kirkeby LT, Espersen ML, Jess P, Gögenür I, Olsen J, Troelsen JT. CDX2 downregulation is associated with poor differentiation and MMR deficiency in colon cancer. Exp Mol Pathol. 2016;100:59–66. doi: 10.1016/j.yexmp.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Baba Y, Nosho K, Shima K, Freed E, Irahara N, Philips J, Meyerhardt JA, Hornick JL, Shivdasani RA, Fuchs CS, et al. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665–4673. doi: 10.1158/1078-0432.CCR-09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynch J, Keller M, Guo RJ, Yang D, Traber P. Cdx1 inhibits the proliferation of human colon cancer cells by reducing cyclin D1 gene expression. Oncogene. 2003;22:6395–6407. doi: 10.1038/sj.onc.1206770. [DOI] [PubMed] [Google Scholar]
- 57.Guo RJ, Huang E, Ezaki T, Patel N, Sinclair K, Wu J, Klein P, Suh ER, Lynch JP. Cdx1 inhibits human colon cancer cell proliferation by reducing beta-catenin/T-cell factor transcriptional activity. J Biol Chem. 2004;279:36865–36875. doi: 10.1074/jbc.M405213200. [DOI] [PubMed] [Google Scholar]
- 58.Jones MF, Hara T, Francis P, Li XL, Bilke S, Zhu Y, Pineda M, Subramanian M, Bodmer WF, Lal A. The CDX1-microRNA-215 axis regulates colorectal cancer stem cell differentiation. Proc Natl Acad Sci USA. 2015;112:E1550–E1558. doi: 10.1073/pnas.1503370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebert MP, Model F, Mooney S, Hale K, Lograsso J, Tonnes-Priddy L, Hoffmann J, Csepregi A, Röcken C, Molnar B, et al. Aristaless-like homeobox-4 gene methylation is a potential marker for colorectal adenocarcinomas. Gastroenterology. 2006;131:1418–1430. doi: 10.1053/j.gastro.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 60.Petrova TV, Nykänen A, Norrmén C, Ivanov KI, Andersson LC, Haglund C, Puolakkainen P, Wempe F, von Melchner H, Gradwohl G, et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. 2008;13:407–419. doi: 10.1016/j.ccr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 61.Lu MH, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res. 2012;18:6416–6425. doi: 10.1158/1078-0432.CCR-12-0832. [DOI] [PubMed] [Google Scholar]
- 62.Kanai M, Hamada J, Takada M, Asano T, Murakawa K, Takahashi Y, Murai T, Tada M, Miyamoto M, Kondo S, et al. Aberrant expressions of HOX genes in colorectal and hepatocellular carcinomas. Oncol Rep. 2010;23:843–851. [PubMed] [Google Scholar]
- 63.Bhatlekar S, Addya S, Salunek M, Orr CR, Surrey S, McKenzie S, Fields JZ, Boman BM. Identification of a developmental gene expression signature, including HOX genes, for the normal human colonic crypt stem cell niche: overexpression of the signature parallels stem cell overpopulation during colon tumorigenesis. Stem Cells Dev. 2014;23:167–179. doi: 10.1089/scd.2013.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanz-Pamplona R, Cordero D, Berenguer A, Lejbkowicz F, Rennert H, Salazar R, Biondo S, Sanjuan X, Pujana MA, Rozek L, et al. Gene expression differences between colon and rectum tumors. Clin Cancer Res. 2011;17:7303–7312. doi: 10.1158/1078-0432.CCR-11-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung C, Kim RS, Zhang HJ, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004;64:9185–9192. doi: 10.1158/0008-5472.CAN-04-1330. [DOI] [PubMed] [Google Scholar]
- 66.Jung C, Kim RS, Zhang H, Lee SJ, Sheng H, Loehrer PJ, Gardner TA, Jeng MH, Kao C. HOXB13 is downregulated in colorectal cancer to confer TCF4-mediated transactivation. Br J Cancer. 2005;92:2233–2239. doi: 10.1038/sj.bjc.6602631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao WT, Jiang D, Yuan J, Cui YM, Shi XW, Chen CM, Bian XW, Deng YJ, Ding YQ. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17:3569–3578. doi: 10.1158/1078-0432.CCR-10-2533. [DOI] [PubMed] [Google Scholar]
- 68.Fang S, Gao H, Tong Y, Yang J, Tang R, Niu Y, Li M, Guo L. Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab Invest. 2016;96:60–68. doi: 10.1038/labinvest.2015.123. [DOI] [PubMed] [Google Scholar]
- 69.Xiao F, Bai Y, Chen Z, Li Y, Luo L, Huang J, Yang J, Liao H, Guo L. Downregulation of HOXA1 gene affects small cell lung cancer cell survival and chemoresistance under the regulation of miR-100. Eur J Cancer. 2014;50:1541–1554. doi: 10.1016/j.ejca.2014.01.024. [DOI] [PubMed] [Google Scholar]


