Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States and represents an increasingly important etiology of hepatocellular carcinoma (HCC) with annual cumulative incidence rates ranging from 2% to 12% in cohorts of NAFLD cirrhosis. While the risk of progression of NAFLD to HCC remains higher among patients with fibrosis or cirrhosis, an increasing amount of literature describes NAFLD-HCC as a disease that can occur in the absence of cirrhosis. Efforts to characterize the pathogenesis of NAFLD-HCC have suggested mechanisms that strongly associate with states of hyperinsulinemia and chronic inflammation, cellular mechanisms including adaptive immune responses and hepatic progenitor cell populations, and genetic polymorphisms including mutations of PNPLA3. Current literature describes NAFLD-HCC mostly as a disease of late presentation with lower rates of receipt of curative therapy and worse prognosis. However, a growing body of evidence has reported comparable and potentially more favorable disease-free and overall survival rates among patients with NAFLD-HCC after receipt of curative treatment. This review summarizes current evidence of epidemiology, pathophysiology, disease presentation, demand and receipt of curative therapy, post-treatment outcomes, and overall survival of NAFLD-associated HCC.
Keywords: Fatty liver, Nonalcoholic steatohepatitis, Hepatocellular carcinoma, Liver cirrhosis, Liver cancer
Core tip: This review summarizes the epidemiology, pathogenesis, disease presentation, demand and receipt of curative treatment, and post-treatment outcomes of hepatocellular carcinoma (HCC) in nonalcoholic fatty liver disease (NAFLD). The review highlights the developing understanding of NAFLD-HCC pathogenesis, which has broadened to include genetic polymorphisms, adaptive immune responses, and cellular regenerative pathways using hepatic progenitor cell populations. While NAFLD-HCC has been described to have poorer prognosis as compared with other HCC etiologies, this review features summarized evidence that disease-free and survival rates among patients with NAFLD-HCC are comparable and potentially favorable after receipt of curative treatment.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and third most common cause of cancer-related mortality worldwide[1-3]. In the United States, the overall incidence of HCC has increased approximately four-fold from 1.5 cases per 100000 in 1973 to 6.2 cases per 100000 in 2011[3]. Rates of mortality are similar to rates of incidence of HCC in the United States[1,3]. Based on recent analysis using the Surveillance, Epidemiology, and End Results (SEER) 18 database, the estimated annual incidence of HCC is 6.2 cases per 100000 and the incidence-based mortality rate is 5 cases per 100000[3]. A recent United States report revealed that while the overall rate of cancer-related deaths has declined, mortality from liver cancer has increased at the highest rate as compared with all other reported cancers from 2003 to 2012[4]. While most cases of HCC in the United States are attributable to chronic hepatitis C virus (HCV) infection, there has been growing evidence to suggest that nonalcoholic fatty liver disease (NAFLD) is projected to become a leading cause of HCC incidence and mortality[5-7]. A recent study by Younossi et al[7] revealed that the annual incidence of NAFLD-HCC based on a SEER- Medicare cohort has grown by 9% per year from 2004 to 2009.
The prevalence of NAFLD-HCC is expected to rise in concurrence with the proportion of NAFLD among adults in the United States population. In the Western world, NAFLD is already the most common chronic liver condition with an estimated prevalence of 30% in the United States, although a previous cohort has reported prevalence as high as 46%[6,8-10].
The prevalence of NAFLD will continue to grow in a setting of increasing rates of obesity, diabetes, dyslipidemia, and other disorders associated with the metabolic syndrome which are closely related to NAFLD[6,8-16].
The risk of progression to cirrhosis among patients with NAFLD is approximately four to 20% depending on the severity of necrosis and fibrosis[6,17]. Although the risk of developing NAFLD-HCC from NAFLD cirrhosis is not clearly delineated, the cumulative incidence of HCC from NAFLD cirrhosis has been reported as 2.4% and 12.8% over a median follow-up of 3.2 to 7.2 years[16,18,19]. A large single-center cohort study reported an annual HCC incidence of 2.6% among patients with NAFLD cirrhosis[18].
NAFLD-HCC without cirrhosis
While previous studies have described fibrosis progression in NAFLD and its risk of cirrhosis and HCC, recent reports increasingly recognize NAFLD-HCC as an entity that can occur in the absence of cirrhosis[6,16,20-27]. In fact, up to 50% of incident NAFLD-HCC may occur without cirrhosis[6,28]. In a recent Veterans Administration (VA) study, 34.6% of patients with NAFLD-HCC did not have diagnostic evidence of cirrhosis[23].
Notably, liver biopsy was performed in 52.4% of the study cohort[23]. While only 8% of patients in this cohort had NAFLD, the study demonstrated that patients with NAFLD had the greatest odds of developing HCC in the absence of cirrhosis in comparison to other etiologies including chronic hepatitis B virus (HBV), HCV, and alcoholic liver disease (ALD) (adjusted odds ratio 3.9 when compared with HCV)[23]. Another large United States study comprised of three tertiary care centers identified 157 patients, who underwent surgical resection for HCC, with histological evidence of non-cirrhotic hepatic steatosis after excluding patients with viral hepatitis, alcohol abuse, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, alpha-1-antitrypsin deficiency, and Wilson disease[20]. The study revealed that 80% of cases were associated with stage 0 liver fibrosis and only 15% had steatohepatitis[20].
PATHOPHYSIOLOGY
Although our understanding of the pathogenesis of NAFLD-HCC remains limited, several proposed mechanisms have been described including chronic inflammation in the setting of hyperinsulinemia or metabolic syndrome, cellular mechanisms including hepatic progenitor cells and adaptive immune responses, and genetic polymorphism including variations of patatin-like phospholipase domain-containing protein 3 (PNPLA3) (Figure 1).
Figure 1.
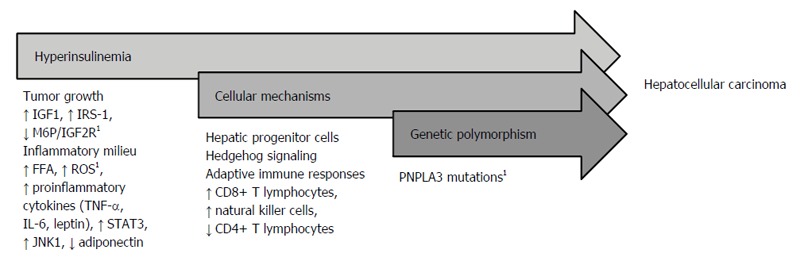
Proposed mechanisms of nonalcoholic fatty liver disease-hepatocellular carcinoma. 1Mechanism has been identified in nonalcoholic fatty liver disease in the absence of cirrhosis. IGF-1: Insulin-like growth factor-1; IRS-1: Insulin receptor substrate-1; M6P/IGF2R: Mannose 6-phosphate/insulin-like growth factor-2 receptor; FFA: Free fatty acids; ROS: Reactive oxidative species; TNF-α: Tumor necrosis factor-alpha; IL-6: Interleukin-6; STAT3: Signal transducer and activator of transcription; JNK1: c-Jun amino-terminal kinase 1; PNPLA3: Patatin-like phospholipase domain-containing protein 3.
Hyperinsulinemia
Carcinogenic features of the metabolic syndrome including uninhibited tumor growth, chronic inflammation, increased production of proinflammatory cytokines like c-Jun amino-terminal kinase 1 (JNK1), and reduction of anti-inflammatory proteins like adiponectin are mechanisms seen in NAFLD. NAFLD is a hepatic manifestation of the metabolic syndrome and considered a mechanism through which metabolic syndrome could lead to HCC[29].
Tumor growth: Uninhibited and dysregulated cell growth in the setting of hyperinsulinemia has been described as a function of insulin-like growth factor-1 (IGF-1), mannose 6-phosphate/insulin-like growth factor-2 receptor (M6P/IGF2R), and insulin receptor substrate-1 (IRS-1)[14,30,31]. IGF-1, a peptide hormone upregulated in hyperinsulinemia, facilitates cellular proliferation and inhibits cell death[14]. The M6P/IGF2R, a tumor- suppressor gene, regulates cell growth by inhibiting cell proliferation and promoting apoptosis via transforming growth factor-beta and insulin-like growth factor-2, respectively[31]. Interestingly, mutations of M6P/IGF2R are seen in HCC and have been identified even in the absence of viral hepatitis and liver cirrhosis[31]. IRS-1, an intracellular protein that promotes cell growth via cytokine signaling, is overexpressed (> 200%) in HCC tumors, which induces a downstream signaling effect that promotes tumor cell growth and enhances tumor progression[30].
Inflammatory cascade: Hyperinsulinemia also triggers an inflammatory milieu involving free fatty acids (FFA), proinflammatory cytokines, reactive oxidative species (ROS), JNK1, and adiponectin[14,32-35]. A high insulin state promotes release of FFA from adipocytes, which promotes steatosis, and in the setting of inflammation fostered by proinflammatory cytokines, can lead to cirrhosis and HCC[14].
Accelerated production of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and leptin, promotes a chronic cycle of hepatocyte injury, apoptosis, and compensatory proliferation in conditions of inflammation and oxidative stress that can lead to mutations, dysplasia, and eventually carcinogenesis[14]. A study by Park et al[33] described HCC development in the setting of obesity as a function of enhanced TNF-α and IL-6 expression. In addition to causing hepatic inflammation, both cytokines activate signal transducer and activator of transcription 3 (STAT3), an oncogenic transcription factor, which can enhance proliferation and progression of hepatocytes that can acquire oncogenic mutations that lead to tumor development[33]. Interestingly, leptin has been described in liver carcinogenesis via upregulation of telomerase reverse transcriptase leading to immortalization of tumor cells in HCC[34].
ROS are implicated in carcinogenesis through dysregulation of the cell cycle[35]. A mouse model demonstrated that mitochondria in fatty livers have accelerated production of superoxide anions as compared with mitochondria in normal livers[35]. A case report from Japan described a patient without cirrhosis and with NAFLD-associated HCC that had localized markers of oxidative cellular injury (e.g., anti- oxidized phosphatidylcholine) in tumorous and non-tumorous steatotic hepatocytes, which suggests that the prevalence and persistence of oxidative injury in the setting of steatosis is related to the development of HCC[36]. Therefore, the presence of increased oxidative species as a suspected function of chronic cell stress is associated with cell injury, apoptosis, and compensatory proliferation, which can enhance mutation formation and cancer cell growth.
JNK1: The combination of proinflammatory cytokines, ROS, and FFA activates JNK1, a protein kinase that has increased activity in the setting of obesity and insulin resistance, and in turn phosphorylates IRS-1[32]. JNK1 has been demonstrated to promote development and proliferation of HCC through epigenetic mechanisms[37]. Chang et al[37] described the association of JNK1 activity with alterations in histone H3 methylation in HCC, which leads to sustained expression of genes that promote cell proliferation and repression of genes responsible for cell differentiation and tumor suppression.
Adiponectin: A decreased amount of adiponectin, an anti-inflammatory polypeptide, in the setting of obesity has also been described in HCC pathogenesis[14,38]. Adiponectin has been identified as a negative regulator of angiogenesis and inhibitor of primary tumor growth via decreased neovascularization and enhancement of tumor cell apoptosis[38]. Repression of adiponectin activity in the setting of hyperinsulinemia allows uninhibited tumor cell growth, which can subsequently lead to HCC in NAFLD.
Cellular mechanisms
Hepatic progenitor cell populations: NAFLD-related hepatocyte injury induces Hedgehog signaling, a complex cellular pathway for tissue repair and regeneration. One of the main mechanisms activated through Hedgehog signaling includes mobilization of hepatic progenitor cell populations to replace damaged hepatocytes. While essential for liver repair, aberrations in liver progenitor population activation can lead to impaired repair and dysregulated proliferation of hepatocytes which can potentiate carcinogenesis[39,40].
Current data suggest that greater duration and degree of cell injury as seen in NAFLD lead to overstimulation of Hedgehog signaling, which can result in dysregulated cellular repair and malignant transformation[40,41]. The development of HCC has been described as a function of aberrant Hedgehog hyperactivity as progenitor cells activated through Hedgehog could survive independently from regulation of nuclear localization of nuclear factor-kappa B (NF-κB) and thereby less susceptible to NF-κB-driven apoptosis[42].
Adaptive immune responses: Recent studies have revealed the potential role of the adaptive immune system, specifically the roles of CD8+ T lymphocytes, natural killer cells, and CD4+ T lymphocytes in the development of NAFLD-associated HCC[43,44]. From feeding mouse models with choline-deficient and high-fat diets, Wolf et al[44] described the metabolic activation of intrahepatic CD8+ T lymphocytes and natural killer cells, which in turn, synergistically with inflammatory cytokines, cause liver damage and induce carcinogenesis. Ma et al[43] demonstrated in mouse models of NAFLD-HCC that selective CD4+ T lymphocyte depletion results from NAFLD and leads to enhanced carcinogenesis. Hepatocytes derived from NAFLD-HCC mice exhibited increased linoleic acid secretion, which promoted selective CD4+ T lymphocyte death through increased mitochondrial-derived ROS generation[43]. While deregulated lipid metabolism and inflammation have been characterized in NAFLD, the role of hepatocyte-derived lipid secretion causing selective CD4+ T lymphocyte loss highlights a novel function of the adaptive immune system in this disease.
Genetic polymorphism
Variation in PNPLA3: Another mechanism that has been associated with the pathogenesis of NAFLD-HCC focuses on genetic risk, specifically variation in PNPLA3. A genome-wide association analysis of 9229 genetic variants of PNPLA3 revealed that homozygous carriers of the I148M variant protein of PNPLA3 had a > 2-fold higher hepatic fat content than noncarriers[45]. Furthermore, the I148M variant protein was more prevalent in the group at highest risk of NAFLD[45]. Similarly, a study by Liu et al[46] identified that the PNPLA3 rs738409 C→→G single nucleotide polymorphism, which encodes the I148M variant protein, demonstrated a gene-dosage effect in which an increased number of G alleles present (i.e., homozygous G allele) was associated with an increased incidence of NAFLD-HCC with an odds ratio as high as 12.19 when compared with the general UK population. Interestingly, the PNPLA3 rs738409 C→→G polymorphism was associated with NAFLD-HCC risk independent of the presence of cirrhosis[46].
PRESENTATION AT DIAGNOSIS
Patients with NAFLD-HCC generally have a more advanced presentation of HCC at the time of diagnosis[14,28,47,48]. A VA cohort study by Mittal et al[47] revealed that in comparison to HCV-related HCC, fewer cases of NAFLD-HCC were diagnosed in early HCC stages or Barcelona Clinic Liver Cancer (BCLC) stage A (5.8% vs 15.7%, P = 0.04) and more cases were diagnosed in terminal stages of HCC or BCLC stage D (19.2% vs 16.1%, P = 0.04). A recent study by Piscaglia et al[28] also revealed a significantly greater proportion of patients with NAFLD-HCC who presented in advanced stages as compared with patients with HCV-HCC (BCLC stage C 33.1% vs 23.9%, respectively, P = 0.033).
Advanced stage at presentation among patients with NAFLD-HCC is likely attributable to delayed diagnosis in the setting of suboptimal HCC surveillance among patients with cirrhosis and no surveillance among patients without cirrhosis or unrecognized cirrhosis[28,47]. In an Italian cohort study, a significantly lower proportion of patients with NAFLD-HCC underwent surveillance as compared with those with HCV-HCC (47.6% vs 63.3%, P = 0.001)[28]. In the United States, Mittal et al[47] reported that fewer patients with NAFLD-HCC had surveillance within three years before their HCC diagnosis as compared with patients with ALD and HCV. Approximately 43% in the NAFLD-HCC group had HCC surveillance within three years of diagnosis as compared with 60% and 87% in the ALD- and HCV-related HCC groups, respectively[47].
CURATIVE TREATMENTS AND OUTCOMES
Demand and receipt of curative treatment
Recipients of curative treatment, which includes orthotopic liver transplantation (OLT), resection, and radiofrequency ablation (RFA), have favorable survival outcomes[3]. In concurrence with the growing incidence of NAFLD-HCC, studies have reported an increasing demand for curative treatment, especially OLT and resection[49,50]. NAFLD has become the second leading etiology among OLT cases for HCC since 2006[49]. Wong et al[49] reported that OLT for NAFLD-HCC has increased four-fold in the United States from 2002 to 2012. Over the past decade, the proportion of resections of HCC in the setting of NAFLD has also increased[50]. A study by Cauchy et al[50] demonstrated a continuous increase in the number of patients undergoing resection for HCC from 2.5% in 2000 to more than 15% in 2011.
Interestingly, patients with NAFLD-HCC have been recognized as less likely recipients of curative therapy[7,47]. In a VA cohort, only 10.8% of the NAFLD-HCC group received curative treatment as compared with 21.9% of the HCV-related HCC group[47].
Furthermore, in comparison to the HCV-related group, more patients in the NAFLD- HCC group received no treatment (77.5% vs 61.5%)[47]. An Italian study showed that fewer patients with NAFLD-HCC received resection as compared with patients with HCV-HCC (19% vs 11%, P = 0.002)[28]. A large United States population-based study using SEER data revealed that 5.7% of patients with NAFLD-HCC received OLT as compared with 11.3%, 6.4%, and 6% of patients with HCC secondary to HCV, HBV, and ALD, respectively[7]. Similarly, a European cohort revealed that none of its 45 cases of NAFLD-HCC received OLT as primary therapy[48].
Survival outcomes from curative treatment
Data on outcomes of patients who receive curative therapy for NAFLD-HCC remain scarce as most available studies are limited to small sample sizes, surrogate diagnoses of NAFLD (i.e., metabolic syndrome, cryptogenic cirrhosis), or grouped treatment outcomes (i.e., OLT, resection, RFA combined). Based on the available data, the recurrence and survival rates of NAFLD-HCC appear similar to other etiologies of HCC who undergo treatment with curative intent[28,50-56] (Table 1).
Table 1.
Summary of survival outcomes for presumed nonalcoholic fatty liver disease-hepatocellular carcinoma undergoing curative therapies
| First author | Study design | Study period | Study location | Number of NAFLD-HCC cases | Treatment1 |
Survival outcomes |
|
| Overall survival | Disease-free survival | ||||||
| Reddy | Retrospective cohort | 2000-2010 | Single-centered (United States) | 52 | OLT, resection, RFA2 | 1-yr: 90%3 | 1-yr: 84%3 |
| 3-yr: 72%3 | 3-yr: 70%3 | ||||||
| 5-yr: 65%3 | 5-yr: 60%3 | ||||||
| Wong | Retrospective cohort | 2002-2012 | Multi-centered (United States) | NA4 | OLT | 1-yr: 87.5% | NA |
| 3-yr: 79.8% | |||||||
| 5-yr: 65.5% | |||||||
| Piscaglia | Prospective cohort | 2010-2012 | Multi-centered (Italy) | 49 | OLT, resection, RFA | 1-yr: 90%-95%3 | NA |
| 3-yr: 85%-90%3 | |||||||
| Malik | Retrospective cohort | NA | Single-centered (United States) | 17 | OLT | 1-yr: 85%-90%3 | NA |
| Hernandez-Alejandro | Retrospective cohort | 2000-2011 | Single-centered (Canada) | 17 | OLT | NA | 1-yr: 95%3 |
| 3-yr: 95%3 | |||||||
| 5-yr: 85%3 | |||||||
| Cauchy | Retrospective cohort | 2000-2011 | Single-centered (France) | 625 | Resection | 1-yr: 83% | 1-yr: 83% |
| 3-yr: 75% | 3-yr: 70% | ||||||
| Wakai | Retrospective cohort | 1990-2007 | Single-centered (Japan) | 17 | Resection | 5-yr: 59% | 5-yr: 66% |
| Takuma | Retrospective cohort | 1992-2009 | Single-centered (Japan) | 366 | Resection, RFA, PEI, MCT | 1-yr: 94% | 1-yr: 89% |
| 3-yr: 85% | 3-yr: 68% | ||||||
| 5-yr: 54% | 5-yr: 54% | ||||||
Total cohort underwent one treatment unless otherwise specified;
Four patients had both RFA and resection;
Survival rates were not reported in the text;
Total number of NAFLD-HCC cases was not reported as analysis of outcomes among HCC cases secondary to NAFLD was done in a subanalysis. Total HCC cases in the cohort was 5326;
Metabolic syndrome was used as a surrogate for NAFLD after exclusion of chronic hepatitis B or C infection, excessive alcoholic consumption, alcoholic liver disease, and hemochromatosis;
Crytogenic cirrhosis was used as a surrogate for NAFLD after exclusion of chronic hepatitis B or C infection, alcohol consumption, primary biliary cholangitis, autoimmune hepatitis, primary sclerosing cholangitis, alpha 1-antitrypsin deficiency, Wilson’s disease, and hemochromatosis. Estimates are determined from the Kaplan Meier figures. NAFLD: Nonalcoholic fatty liver disease; HCC: Hepatocellular carcinoma; OLT: Orthotopic liver transplantation; RFA: Radiofrequency ablation; PEI: Percutaneous ethanol injection; MCT: Microwave coagulation therapy; NA: Data not available.
OLT for NAFLD-HCC: A United States cohort study of patients who underwent curative treatment for HCC identified 20 patients who received OLT for NAFLD-HCC and 83 who received OLT for HCC secondary to HCV and/or ALD[52]. No significant difference existed in disease-free survival and overall survival in patients who received OLT for HCC over a median follow-up of 50 mo[52].
In a retrospective cohort using United Network for Organ Sharing data from 2002 to 2012, Wong et al[54] reported comparable survival rates at one, three, and five years between those who received OLT for HCC secondary to NAFLD, HCV, ALD, or HCV and ALD (P = 0.99). Survival at one year post-OLT for HCC was 87.5% for nonalcoholic steatohepatitis (NASH) (vs 88.5% for HCV, 90.2% for ALD, 88.7% for HCV and ALD)[54]. At three years, survival for NAFLD-HCC was 79.8% as compared with 73.9%, 73.3%, and 75% for HCV, ALD, and HCV and ALD, respectively[54]. Five- year survival data remained similar between all etiologies of HCC in the cohort: 65.5% for NAFLD, 65.7% for HCV, 63.9% for ALD, and 65.7% for HCV and ALD[54]. Rates of graft survival were also not significantly different between all HCC etiologies, and patient survival was similar across groups (84.3%, 77%, and 63.1% at year one, three, and five, respectively for NAFLD-HCC)[54].
Another United States cohort study by Malik et al[51] retrospectively reviewed 17 patients with NASH-related cirrhosis who underwent OLT with and without known HCC. When compared with the group of patients with NASH-related cirrhosis without HCC, the group with NAFLD-HCC had similar survival (88%) over a mean follow-up period of 2.5 years[51].
A Canadian retrospective cohort study described disease-free survival in 81 cases of liver transplant recipients, which included 64 with HCV-related HCC and 17 patients with NAFLD-HCC[55]. Over a 10-year follow-up period, disease-free survival in the HCV and NAFLD groups were approximately 65% and 85%, respectively (P = 0.11)[55]. While the NAFLD group had a similar number and cumulative size of tumors as compared with the HCV group, the NAFLD group had a significantly lower proportion of patients with vascular invasion and poorly differentiated HCC[55]. Therefore, NAFLD-HCC can have favorable, and potentially better tumor recurrence rates than their HCV counterparts as demonstrated by their trend towards greater disease-free survival (85% vs 65%) in the setting of less aggressive HCC presentation at diagnosis.
OLT for NAFLD-cirrhosis: Literature on post-OLT outcomes among those who undergo transplantation for NAFLD-HCC remains limited. Given the comparable rates of post-OLT survival between those who undergo transplantation for NALFD-related cirrhosis without HCC and those with HCC, inclusion of a few relevant studies that assess post-OLT outcomes in the setting of NASH and not specifically HCC can provide supplementary information[57-59].
A retrospective cohort study at a single large transplant center in the United States compared post-OLT outcomes for NASH cirrhosis and alcoholic cirrhosis[57]. Estimates of patient survival at nine years post-OLT were comparable between those who received transplantation for alcoholic cirrhosis or biopsy- or ultrasound-confirmed NASH (P = 0.30)[57]. Survival at one, three, five, and nine years were 78%, 78%, 78%, and 52%, respectively, in the NASH group vs 92%, 86%, 86%, and 76%, respectively, in the alcoholic cirrhosis group. Rates of graft failure were similar between both groups (24% in NASH vs 18% in alcoholic cirrhosis group, P value 0.40)[57]. Acute rejection (41% vs 23%) and recurrent steatohepatitis (33% vs 0%) were significantly more common in the NASH group as compared with the alcohol group; however, neither complication was associated with higher rates of re-transplantation[57].
A United States cohort study by Yalamanchili et al[58] retrospectively identified patients who underwent OLT for cryptogenic cirrhosis (n = 239) or NASH-related cirrhosis (n = 18) after biopsy confirmation and exclusion of HCV and alcoholic cirrhosis. In comparison to a miscellaneous group that included other indications for OLT, survival at one, five, 10, and 20 years was similar (85.6% vs 86.3%, 71.4% vs 69.9%, 56.5% vs 52.7%, 12.6% vs 20.6%, respectively between the cryptogenic/NASH group vs miscellaneous group)[58]. Post-OLT mortality was more likely from cardiovascular disease (21.2% vs 14.1% of deaths) and less likely from recurrent liver disease (0.7% vs 10.2%) in patients with cryptogenic/NASH cirrhosis as compared with those who underwent transplantation for other indications[58].
An analysis of outcomes for primary OLT from the Scientific Registry of Transplant Recipients database from 2001 to 2009 compared 1959 patients who underwent OLT for NASH with 33822 patients who had OLT for other indications including cryptogenic cirrhosis, HCV, ALD, HBV, primary biliary cholangitis, primary sclerosing cholangitis, and autoimmune hepatitis[59]. One- and three-year survival was not significantly different between post-OLT patients who underwent transplant for NASH (84%, 78%) vs cryptogenic cirrhosis (86%, 79%) vs others (87%, 78%) (P = 0.67)[59]. Graft survival remained similar and comparable between all groups (76% for NASH at three years)[59].
Liver resection for NAFLD-HCC: In addition to OLT, outcomes of liver resections have been studied for NAFLD-HCC[50,53]. Cauchy et al[50] assembled a cohort of 62 patients with HCC in the setting of metabolic syndrome as a surrogate for NAFLD after exclusion of other etiologies of HCC including HCV, HBV, ALD, hemochromatosis, and autoimmune liver disease who underwent surgical resection. Overall survival and disease-free survival rates were 83% and 83%, respectively, at one year and 75% and 70%, respectively, at three years[50].
In a Japanese cohort study, a group of 17 patients with NAFLD was compared with a group with HCV or HBV who underwent surgical resection for HCC[53]. Overall survival was 59%, 57%, and 63% in the patients with NAFLD-HCC, HCV-related HCC, and HBV-related HCC, respectively, after five years post-resection, and was not significantly different among the three groups[53]. On the contrary, five-year disease- free survival was significantly better in the NAFLD-HCC group as compared with the HCV and HBV groups (66% vs 29% vs 39%)[53].
Another Japanese study by Takuma et al[56] described disease-free and overall survival among HCC cases secondary to cryptogenic cirrhosis as a surrogate for NAFLD vs HCV All patients received some form of curative treatment for HCC except for OLT, which included RFA, percutaneous ethanol injection, or microwave coagulation therapy[56]. Over a mean follow-up of 49 mo, those with NAFLD-HCC had significantly lower tumor recurrence rates (39% vs 71%, P < 0.001) and significantly higher five-year survival rates (80% vs 61%, P = 0.02) as compared with their HCV counterparts[56]. While the NAFLD-HCC group had a significantly greater proportion of patients with larger tumors (2.0 cm vs 2.8 cm) than the HCV-related HCC group, all were within Milan criteria[56]. Therefore, patients with NAFLD-HCC can have improved and better disease-free and overall survival as compared with patients with HCV-associated HCC contingent that they present with early-stage HCC.
Postoperative complications
While long-term disease-free and overall survival among patients with NAFLD-HCC who receive curative therapy is favorable, some studies have reported increased rate of postoperative morbidity and mortality post-hepatectomy in the setting of higher NAFLD activity score (NAS) or cirrhosis[50,53].
In a study by Cauchy et al[50], outcome comparisons were made between: (1) patients without severe fibrosis and NAS < 2; and (2) patients with severe fibrosis (stage F3 or F4) or with a NAS ≥ 2. Aside from having a higher body mass index (BMI) in the latter group (median BMI 31.1 kg/m2 vs 28.4 kg/m2), no preoperative clinical characteristics or operative characteristics differed between the two groups[50]. The group with severe fibrosis or higher NAS had significantly higher rates of 90-d mortality (18% vs 0%), liver-related complications (32% vs 4%), and cardiorespiratory complications (37% vs 13%)[50]. Interestingly, multivariate analysis showed that severe underlying fibrosis was not a risk factor for major complications; however, a NAS of two or greater was associated with increased major complications[50].
Wakai et al[53] reported significantly higher rates of postoperative morbidity and 30-d mortality among patients with NAFLD-HCC as compared with those of other HCC etiologies including HBV or HCV. Postoperative morbidity was seen in 59% of the NAFLD group vs 31% and 28% of the HCV and HBV groups, respectively[53]. In the NAFLD group of 10 patients who had postoperative complications, the most common complication was hepatic insufficiency, which was observed in four patients[53].
Mortality at 30 d postoperatively was 12%, 0.7%, and 3.3% in the NAFLD, HCV, and HBV groups, respectively[53]. The two patients who died in the NAFLD group had underlying NAFLD-related cirrhosis[53].
Therefore, postoperative morbidity and mortality may be enhanced in the setting of inflammatory processes as suggested by the association between postoperative complications and higher NAS and cirrhosis.
OVERALL SURVIVAL
Patients with NAFLD-HCC are recognized to have worse prognosis related to advanced stage of HCC at presentation and lower eligibility for curative treatment[7,28]. In a recent SEER cohort study, patients with NAFLD-HCC appeared to have poorer prognosis than patients with viral HCC as demonstrated by a shorter survival time and poorer one year survival rate from time of diagnosis (61% vs 50%, P < 0.0001)[7].
A large Italian study by Piscaglia et al[28] showed that survival was significantly shorter in NAFLD-HCC as compared with HCV-related HCC (25.5 mo vs 33.7 mo, P = 0.017) which was mainly attributable to later stage of HCC at time of diagnosis.
Interestingly, in the same study, survival difference disappeared after matching NAFLD-HCC and HCV-related HCC by curative treatment (34.2 mo vs 40.8 mo, respectively, P value 0.073)[28].
A study by Reddy et al[52] assessed severity of liver dysfunction and stage of HCC at diagnosis and long-term survival in patients with NAFLD-HCC vs HCV- and/or ALD- HCC. At time of HCC diagnosis, patients with NAFLD-HCC had significantly better hepatic function as compared with the HCV- and ALD-related HCC group[52]. There were no significant differences in previous HCC therapy and tumor characteristics at time of diagnosis, including largest tumor size, presence of satellite lesions, stage T3-4 disease (based on American Joint Committee on Cancer staging), and vascular invasion[52]. Subsequently, no differences existed between receipt of curative treatment between the two groups (NAFLD vs HCV and/or ALD)[52]. While recurrence-free survival was not significantly different between patients with NAFLD- and HCV/ALD- related-HCC, those with NAFLD-HCC had longer overall survival[52]. While having less liver dysfunction at baseline may have contributed to overall improved survival in the NAFLD-HCC group, multivariate analysis adjusting for clinical factors and curative treatment continued to demonstrate that patients with NAFLD-HCC had longer overall survival[52]. Therefore, after controlling for disease presentation and receipt of curative therapy, patients with NAFLD-HCC experienced better long-term survival.
A European cohort study showed a non-significant difference of overall survival between patients with NAFLD-HCC vs those with non-NAFLD-HCC (median 11.28 mo vs 15.5 mo, P = 0.287)[48]. In this cohort, there was no significant difference in receipt of curative treatment and no difference of BCLC stage at time of HCC diagnosis[48]. Similarly, a compilation of case reports of NAFLD-HCC of predominantly early-stage HCC (single tumors approximately 3cm in largest size) revealed that all cases that received a form of curative therapy had no tumor recurrence or death over a 5- to 50-mo follow-up period[60]. Therefore, patients with NAFLD-HCC can have favorable survival contingent that they are diagnosed at early stages of HCC and receive curative treatment.
FUTURE DIRECTIONS
Our review highlights recent updates on NAFLD-HCC epidemiology, pathophysiology, disease presentation, demand and receipt of curative treatment, outcomes from curative treatment as compared with other etiologies of HCC, and overall survival. Current literature demonstrate that the incidence of NAFLD-associated HCC is increasing, may often occur in the absence of cirrhosis, and present at a more advanced stage, and thereby patients with NAFLD-HCC are less likely to be candidates of curative treatment modalities relative to other etiologies of HCC. However, OLT for NAFLD-associated HCC has increased four-fold over the past decade, and post-transplant survival appears to be similar to other HCC-based indications for OLT.
Although the literature addressing NAFLD-HCC is growing, there are several areas which represent high priorities for further research. More robust epidemiological studies to identify high-risk groups for NAFLD-HCC incidence and NAFLD-related mortality may help inform future surveillance and treatment strategies. Additional investigation into mechanisms and determinants of HCC development in non-cirrhotic NAFLD vs NASH may provide critical insight to support evidence-based guidelines on HCC surveillance. Further studies addressing surgical and transplant outcomes among patients with NAFLD and NASH-associated HCC may also guide clinicians and the transplant community on optimal organ allocation and post- transplant management. Significant opportunity exists to address key deficits in knowledge regarding epidemiology, pathogenesis, surveillance, treatment, and surgical outcomes of NAFLD-associated HCC, which remains a rapidly growing global public health problem.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There is no conflict of interest associated with any of the authors.
Peer-review started: May 4, 2016
First decision: July 13, 2016
Article in press: August 23, 2016
P- Reviewer: Grieco A, Higuera-de la Tijera M, Lalor P, Lee HC Tarantino G S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Goh GB, McCullough AJ. Natural History of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1226–1233. doi: 10.1007/s10620-016-4095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 15.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 18.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 19.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 21.Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 22.Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, Gotoh K, Yamada T, Tomita Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 23.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124–131.e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Perumpail RB, Wong RJ, Ahmed A, Harrison SA. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig Dis Sci. 2015;60:3142–3148. doi: 10.1007/s10620-015-3821-7. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e1-9; quiz e39-40. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasdelli AS, Brodosi L, Marchesini G. NAFLD-Associated Hepatocellular Carcinoma: a Threat to Patients with Metabolic Disorders. Curr Hepatology Rep. 2016;15:103–112. [Google Scholar]
- 28.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 29.Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217–9228. doi: 10.3748/wjg.v20.i28.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- 31.Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA. 1997;94:10351–10355. doi: 10.1073/pnas.94.19.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 33.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10:442. doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 36.Ikura Y, Mita E, Nakamori S. Hepatocellular carcinomas can develop in simple fatty livers in the setting of oxidative stress. Pathology. 2011;43:167–168. doi: 10.1097/PAT.0b013e32834274ec. [DOI] [PubMed] [Google Scholar]
- 37.Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, Castranova V, Shi X, Chen F. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol. 2009;50:323–333. doi: 10.1016/j.jhep.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohinc BN, Diehl AM. Mechanisms of disease progression in NASH: new paradigms. Clin Liver Dis. 2012;16:549–565. doi: 10.1016/j.cld.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Zheng X, Zeng W, Gai X, Xu Q, Li C, Liang Z, Tuo H, Liu Q. Role of the Hedgehog pathway in hepatocellular carcinoma (review) Oncol Rep. 2013;30:2020–2026. doi: 10.3892/or.2013.2690. [DOI] [PubMed] [Google Scholar]
- 42.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C & gt; G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 47.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601.e1. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinmann A, Alt Y, Koch S, Nelles C, Düber C, Lang H, Otto G, Zimmermann T, Marquardt JU, Galle PR, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. doi: 10.1186/s12885-015-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 50.Cauchy F, Zalinski S, Dokmak S, Fuks D, Farges O, Castera L, Paradis V, Belghiti J. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- 51.Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800–806. doi: 10.1016/j.cgh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 53.Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450–1458. doi: 10.1007/s11605-011-1540-8. [DOI] [PubMed] [Google Scholar]
- 54.Wong RJ, Chou C, Bonham CA, Concepcion W, Esquivel CO, Ahmed A. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002-2012 United Network for Organ Sharing data. Clin Transplant. 2014;28:713–721. doi: 10.1111/ctr.12364. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez-Alejandro R, Croome KP, Drage M, Sela N, Parfitt J, Chandok N, Marotta P, Dale C, Wall W, Quan D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18:4145–4149. doi: 10.3748/wjg.v18.i31.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takuma Y, Nouso K, Makino Y, Gotoh T, Toshikuni N, Morimoto Y, Shimomura H, Yamamoto H. Outcomes after curative treatment for cryptogenic cirrhosis-associated hepatocellular carcinoma satisfying the Milan criteria. J Gastroenterol Hepatol. 2011;26:1417–1424. doi: 10.1111/j.1440-1746.2011.06775.x. [DOI] [PubMed] [Google Scholar]
- 57.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 58.Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 59.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 60.Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13:631–647. doi: 10.1016/j.cld.2009.07.007. [DOI] [PubMed] [Google Scholar]


