Abstract
Broiler breast (pectoralis major) meat was submitted to salting with NaCl + NaNO3 followed by a drying process to produce jerky-type chicken. The final product (raw broiler charqui) was desalted and then cooked using grilled, roasted, fried and sous-vide techniques. Sous-vide cooked samples showed lowest results of moisture loss compared to roasted and fried ones. Fatty acid profile suffered minor changes after cooking of broiler charqui. Regarding to protein oxidation, tryptophan fluorescence, protein carbonylation and disulphide bonds formation of chicken charqui were affected by cooking temperature while free thiol groups, Schiff base formation and hardness were mostly impacted by the length of cooking. Instrumental color of broiler charqui was affected by the type of cooking, being closely related with Maillard products formation. In conclusion, sous-vide technique seems to be the most advantageous cooking method to obtain high-quality ready-to-eat chicken charqui.
Keywords: Carbonylation, Cross-linking, Color, Cooking methods, Jerky-like meat, Tryptophan depletion, Texture
Introduction
The application of hurdle technologies to fresh meat enables the production of safe, shelf-stable and appetizing meat products. Charqui is a jerky-type muscle food largely produced and consumed by tradition in diverse South American countries. Charqui production dates from the pre-Columbian era when Quechua tribe natives preserved meat by manufacturing ch’arki, which means ‘to burn (meat)’ (Gade 2000). Nowadays, charqui is traditionally elaborated from beef by combining heavy salting and a succeeding drying stage (T < 45 °C) until the water activity of the meat lowers down to 0.75 (Shimokomaki et al. 1998). The final product can be stored at room temperature and is regarded as a good source of animal protein (>30 g/100 g product) while still contains considerably high salt levels (≈15 %) (Shimokomaki et al. 1998; Rocha Garcia et al. 2003). Hence, charqui meat is always desalted by immersion in water and subsequently cooked prior to consumption. Beef charqui has been characterized in terms of chemical, physical, microbiological and sensory properties (Shimokomaki et al. 1998; Torres et al. 1994). Recently, charqui produced from chicken and spent hen meat has also been studied for their composition and oxidative stability (Rocha Garcia et al. 2003; Silva et al. 2015). While these previous works have also covered the physicochemical changes occurred in the meat as a result of the severe processing conditions (salting/drying), the impact of the subsequent desalting and cooking stages are still to be studied. The coverage of these aspects is essential as the nutritional value and eating quality (color/texture) of the ready-to-eat product are dependent on these final stages. Charqui is typically cooked by frying or by immersion in hot water (≈boiled). Other methods of interest for being widely used in meat cooking (i.e. roasting or grilling) or for being of innovative application in restoration (i.e. sous-vide) are scarcely applied to charqui meat.
Meat proteins are known to be oxidized during culinary preparation (Soladoye et al. 2015). Traore et al. (2012) observed increased carbonylation, aggregation and impaired water holding capacity (WHC) in pork subjected to a heat treatment (100 °C/30 min) simulating roasting conditions. According to the same authors (Traore et al. 2012) the formation of protein carbonyls may alter the ability of proteins to interact chemically with water molecules while aggregation stresses the impaired WHC by restraining the available surface for water binding. Similar oxidative deterioration has been reported to occur in proteins from cooked chicken (Xiao et al. 2011) and beef (Gatellier et al. 2010). In a recent study, Roldán et al. (2014) found an effect of the combination of time and temperature on the extent of protein carbonylation in sous-vide cooked lamb and hypothesized whether the oxidative damage to meat proteins may have an impact on the color and texture of the final product. The occurrence of protein oxidation in processed meats has also been linked to a loss of nutritional value (Soladoye et al. 2015). In addition to the loss of essential amino acids as a result of their irreversible oxidative degradation, oxidized proteins may be less digestible than intact ones (Liu and Xiong 2000). In particular, Santé-Lhoutellier et al. (2008) observed a significant correlation between protein carbonylation and aggregation and a decreased activity of pepsin on oxidized myofibrillar proteins. These authors also observed that the effect of digestive enzymes on meat proteins depended on the severity of the cooking treatment (Santé-Lhoutellier et al. 2008).
In the present work, we study for the first time, the depletion of tryptophan (trp) and the formation of specific protein carbonyls and Schiff bases (SB) in chicken charqui subjected to four different cooking procedures. In addition, the impact of the cooking procedures on the physical properties (color and texture) of the final product is discussed.
Materials and methods
Chemicals
All chemicals were supplied from Panreac (Panreac Quimica, S.A., Barcelona, Spain), Merck (Merck, Darmstadt, Germany), Sigma Chemicals (Sigma-Aldrich, Steinheim, Germany) and Scharlau (Gillman, South Australia). Water used was purified by passage through a Milli-Q system (Millipore Corp., Bedford, MA, USA).
Production of chicken charqui and sample preparation
Chicken charqui processing was performed as follows. Briefly, twenty-four broiler breasts (pectoralis major) were submitted to dry-salting (72 h) using coarse marine salt and 50 mg kg−1 of sodium nitrite, followed by a drying phase (45 °C) in a drying chamber (Excalibur®, Food dehydrator 3000, Sacramento, USA) until the moisture and water activity (Aw) levels reached values below 50 g/100 g and 0.75, respectively. After processing, chicken charqui samples were sliced into 30 mm thick steaks and divided into six groups. One group was used as a control (raw charqui) and the other groups were desalted in distillated water at 4 °C for 24 h (5 L of distillated water/kg charqui). The water was changed at each 8 h. Four desalted samples were separated for further analysis (desalted charqui). Afterwards, four types of heat treatment were performed in the remained desalted samples using the following methods: (A) grilled at 180–200 °C for 5 min at each surface using an electrical griddle (Repagas, 550 series, Madrid, Spain); (B) roasted at 200 °C for 15 min using an electrical oven (Unox®, Mod. GN2.1, Cadonegue, Italy); (C) fried using 15 mL olive oil at 160–180 °C for 5 min at each surface; and (D) sous-vide cooked in a thermostatized water bath at 65 °C for 8 h. A heating treatment was considered completed when samples reached an internal temperature of 75 °C, which was monitored during cooking using a digital probe thermometer (Testo thermocouple, Mod. 735-1, Lenzkirch, Germany). After cooking and cooling, samples were vacuum-packed and stored at 80 °C until laboratory analysis was carried out.
Analytical methods
Chemical composition
Moisture, protein, ash and sodium chloride (NaCl) of chicken charqui were determined according to AOAC (2000) methods. Lipid content was determined following Folch et al. (1957) procedure.
Fatty acid profile
Fatty acid methyl esters (FAMEs) were prepared by trans-esterification using methanolic solution according to Utrera et al. (2012). FAMEs were separated on a gas chromatograph (Hewlett-Packard, HP-5890A, USA) equipped with a flame-ionized detector and using a capillary column (Supelco®, 50 m × 0.32 mm internal diameter × 1.0 µm film). Detector gas flow rates were 35 mL min−1 for the hydrogen, 15 mL min−1 for the helium and 400 mL min−1 for the synthetic air. Helium was used as the carrier gas (flow rate 1.7 mL min−1). The initial column temperature was programed to 180 °C for 5 min, which was then increased to 210 °C at 1.0 °C min−1 and held the maximum temperature for 30 min. Afterwards, the temperature was increased to 250 °C at 4 °C min−1 and held the maximum temperature for 20 min, resulting in a total run time of 55 min. The Supelco® 37 Component FAME Mix (Sigma-Aldrich®, USA) was used as a reference standard to identify the fatty acid peaks.
Maillard products (MRPs) quantification
The extraction of colored material formed by cooking was carried out in accordance to the following protocol. Methanol was used for the extraction of colored material. Tubes containing 0.5 g of the sample and 4.0 mL of methanol were hermetically closed and placed in an orbital shaker. After 1 h, tubes were centrifuged at 5000g for 20 min. Pellets were discarded and the absorbances of supernatants were measured at 420 nm. The concentration of MRPs was calculated using a molar extinction coefficient (1.0 ± 0.03 L mmol−1 cm−1) described by Martins and Van Boekel (2003).
Tryptophan fluorescence measurements
Fluorescence spectroscopy was used to assess the loss of natural tryptophan fluorescence in charqui samples (Utrera and Estévez 2012). Sample homogenization was carried out according to the process described by Utrera and Estévez (2012). Emission spectra of tryptophan were recorded from 300 to 400 nm with the excitation wavelength established at 283 nm (LS 55 Perkin Elmer luminescence spectrometer, MA, USA). Tryptophan content was calculated from a standard curve of N-acetil-L-tryptophan amide (NATA) and the results were expressed as mg of NATA per g of protein.
Determination of σ-aminoadipic (AAS) and γ-glutamic (GGS) semialdehydes
Samples (5 mg of protein) were derivatized with 50 mM aminobenzoic acid (ABA) and subsequently hydrolyzed with 6 N HCl according to the procedure described by Utrera et al. (2011). Hydrolysates were dried in vacuo, reconstituted with 200 μL of Milli-Q water, and filtered through a PVDF syringe filter (0.45 μm pore size, Pall Corp., New York, USA). Samples were injected in a HPLC using a Cosmosil (Phenomenex, California, USA) C18-AR-II RP-HPLC column (5 μm, 150 × 4.6 mm) and a guard column (10 × 4.6 mm) filled with the same material. The Shimadzu “Prominence” HPLC apparatus (Shimadzu Corp., Kyoto, Japan) was equipped with a quaternary solvent delivery system (LC-20 AD), a DGU-20AS online degasser, an SIL-20A autosampler, an RF-10A XL fluorescence detector, and a CBM-20A system controller. Fifty millimolar sodium acetate buffer (pH 5.4, eluent A) and acetonitrile (ACN, eluent B) were used as eluents. A low-pressure gradient program was used, varying eluent B concentration from 0 % (min 0) to 8 % (min 20). The injection volume was 1 μL, the flow rate was kept at 1 mL min−1, and the temperature of the column was maintained constant at 30 °C. Excitation and emission wavelengths were set at 283 and 350 nm, respectively. Identification of the derivatized semialdehydes in the FLD chromatograms was carried out by comparing their Rt with those from a standard compound injected and analyzed under the above-mentioned conditions (Utrera et al. 2011). Peaks corresponding to AAS-ABA and GGS-ABA were manually integrated from FLD chromatograms and resulting areas plotted against an ABA standard curve (ranging from 0.1 to 0.5 mM). Regression coefficients of >0.99 were obtained. The estimation of the quantities of AAS-ABA and GGS-ABA through an ABA standard curve was accomplished by assuming that the fluorescence emitted by 1 mol of ABA is equivalent to that emitted by 1 mol of the derivatized protein carbonyls. Results were expressed as the sum of AAS and GGS in nanomoles of protein carbonyl per gram of protein.
Schiff bases measurements
The emission of fluorescence by SB was assessed by using a fluorescence spectrometer (LS 55 Perkin-Elmer, MA, USA) according to the method described by Estévez et al. (2008). Charqui samples were ground and homogenized according to the process described by Utrera et al. (2012). A 1 mL aliquot of the homogenates was redissolved in 20 mL of the 20 mM sodium phosphate buffer and then dispensed in a 4 mL quartz spectrofluorometer cell. Emission spectra of SB were recorded from 400 to 500 nm with the excitation wavelength set at 350 nm. Excitation and emission slit widths were set at 10 nm and data were collected at 500 nm per minute. Results were expressed as fluorescence intensity units emitted at 460 nm. These values were corrected according to the moisture content of each sample.
Free thiol and disulphide determination
Quantification of free thiol groups and disulphide bond formation in chicken charqui samples was performed according to Rysman et al. (2014) method. Free and total thiols were determined spectrophotometrically after derivatization by 4,4′-dithiodipyridine (4-DPS) in non-reduced and reduced filtrates, respectively. The absorbance was measured at 324 nm before the addition of 4-DPS (Apre) and after exactly 30 min of reaction with 4-DPS in the dark at room temperature (Apost). A 6.0 M Guanidide hydrochloride (GuHCl) solution was used as a blank sample (Ablank). The absorbance corresponding to the thiol content was calculated by subtracting Apre and Ablank from Apost and the thiol concentration was determined using a l-cysteine standard curve ranging from 2.5 to 500 µM. The thiol content was expressed as nanomoles of thiol per milligram of protein. The disulphide content was calculated as half of the difference between total and free thiols.
Instrumental color measurements
Instrumental color parameters were performed by measuring lightness (L), redness (a*), and yellowness (b*). Five measurements per sample and processing step were randomly made on the surface of the breast and thigh chicken charqui by a digital colorimeter (KONICA MINOLTA, Chroma Meter CR-400, Osaka, Japan) using illuminant source C at a 0° standard observer.
Texture measurements
Texture profile analysis was carried out at 15 °C with a Texture Analyser TA-XT2i (Stable Micro Systems, Surrey,UK). Four cylindrical samples (2.5 cm diameter, height 1.0 cm) of each type of charqui were taken and subjected to a two-cycle compression test. Analyses were performed in triplicate on each sample. The samples were compressed to 40 % of their original height with a cylindrical probe of 5 cm diameter and a cross-head speed of 5 mm s−1. The following parameters were determined: hardness (g), adhesiveness (g × s), chewiness (g), springiness (dimensionless), cohesiveness (dimensionless), and resilience (dimensionless). Although all the above mentioned parameters were determined, only results of hardness are shown since the main differences between samples were driven by this attribute.
Statistical analysis
A one-way analysis of variance (ANOVA) was applied to data to assess the effect of the cooking method on the assessed parameters. When significant differences were found (p < 0.05), post hoc analyses (Tukey’s test) were carried out.
Results and discussion
Physicochemical properties of raw, desalted and cooked chicken charqui
Results for physicochemical analysis of chicken charqui are shown in Table 1. Physicochemical characterization of chicken charqui samples was in accordance with Brazilian legislation limits for intermediate moisture meat products (IMMP) such as Jerked beef and Charqui meat (Brazil 1997). As expected, desalting process promoted an increase of moisture (39.1 %) and a decrease of ash (62.2 %) and NaCl (61.0 %) concentration in chicken charqui samples compared to raw treatment. Correia and Biscontini (2003) also reported similar behavior in beef charqui submitted to desalting with distillated water. The authors explained that the water incorporation in charqui pieces during desalting and the high water solubility of NaCl might cause an increase of moisture and a decrease of ash content in salted meat products, respectively. Regarding to cooking methods, no differences were observed between grilled, roasted and fried samples for moisture and ash. However, sous-vide cooked treatment promoted a marked decrease of ash and NaCl. This result was important considering the current concern of health associations in reducing salt level of meat products. Furthermore, application of sous-vide cooking technique in chicken charqui allows for the possibility of developing ready-to-eat meat product. According to Bejerholm and Aaslyng (2004), protein structures of meat are affected by temperature gradient. The formation of Maillard Reaction Products (MRPs) decreased 72.4 % after desalting of chicken charqui as a likely consequence of two events: (1) the water uptake led to a dilution of these compounds in the products and additionally, (2) some of the compounds may have also been lost in the soaking water given the well-known water-linking nature of MRPs. Among cooking methods, as expected, grilled and fried samples showed the highest MRPs values, followed by roasted and sous-vide cooked treatments, which presented no difference (p > 0.05) between them. The rate and extent of Maillard reactions in meat products are influenced by the method of heat transfer, with convection air in oven (roasting) being less effective in promoting the formation of MRPs than conduction through a hot surface (grilling) or a frying oil (Roldán et al. 2015). According to Whitfield (1992), temperatures above 110 °C induce Maillard reactions in meat. On the other hand, cooking methods that use high humidity environment can prevent Maillard reactions in meat products (Bejerholm and Aaslyng 2004), which can explain the low levels of MRPs in desalted and sous-vide cooked samples. Desalting process promoted a relative decrease (52.4 %) in lipid content of chicken charqui probably due the water uptake and this value remained statistically the same compared to grilled, roasted and sous-vide cooked treatments. As expected, fried samples presented the highest level of lipids, which can be explained by the diffusion of olive oil in the samples during cooking. Fatty acid profile of chicken charqui samples was significantly affected by type of cooking treatment. Raw charqui presented high levels of mono-unsaturated fatty acids (MUFA) and low content of poly-unsaturated fatty acids (PUFA). Desalting process did not affected fatty acid profile of chicken charqui. There were no differences in saturated fatty acids (SFA) between raw and cooked samples. Regarding cooking methods, only minor alterations were noted in charqui treatments. The most marked changes were observed in fried samples, which presented a reduction (p < 0.05) of PUFA and saturated fatty acids (SFA). In addition, frying treatment promoted an increase of MUFA, which decreased the ratio between saturated and unsaturated fatty acids (SFA/UFA). This proportional alteration in fatty acid composition of fried samples can be ascribed to the high content of MUFA in olive oils (Santos et al. 2013). Sous-vide cooking technique promoted a decrease of PUFA in chicken charqui samples, which may be associated with the long heat exposure of the charqui meat. During cooking of meat and meat products, several mechanisms can lead to changes in fatty acid composition, such as (1) water loss; (2) lipid oxidation; (3) incorporation of cooking oil in meat pieces and (4) diffusion and exchange of fatty acids between intramuscular fat and cooking oil. It is worth noting that during frying process, a replacement of fatty acids from the food fat with those from the cooking oil takes place, altering lipid profile of fried products (Haak et al. 2007). Moreover, in our experiment, charqui fried in olive oil presented a significant decrease of SFA/UFA ratio, which can promote positive health effects.
Table 1.
Physicochemical characterization of raw, desalted and cooked chicken charqui
| Raw charqui | Desalted | Grilled | Roasted | Fried | Sous-vide | |
|---|---|---|---|---|---|---|
| MoistureA | 45.47d ± 1.19 | 63.27a ± 1.19 | 55.85bc ± 2.81 | 54.43c ± 1.81 | 53.91c ± 2.08 | 57.17b ± 1.63 |
| ProteinA | 32.43b ± 1.85 | 28.21c ± 1.50 | 34.59ab ± 2.99 | 36.37a ± 1.73 | 32.82b ± 0.62 | 35.27a ± 0.72 |
| AshA | 15.96a ± 0.72 | 6.03b ± 0.09 | 6.17b ± 0.46 | 6.84b ± 0.74 | 6.71b ± 0.88 | 4.37c ± 0.24 |
| Sodium ChlorideA | 11.39a ± 0.19 | 4.44d ± 0.85 | 5.82b ± 0.19 | 5.47c ± 0.36 | 5.85b ± 0.48 | 4.30d ± 0.27 |
| MRPsB | 54.24b ± 5.31 | 14.97d ± 1.18 | 62.72a ± 4.90 | 13.47d ± 2.87 | 40.39c ± 8.25 | 14.04d ± 2.86 |
| LipidA | 4.89a ± 0.42 | 2.33b ± 0.28 | 2.49b ± 0.21 | 2.33b ± 0.45 | 4.02a ± 0.78 | 2.84b ± 0.56 |
| SFAC | 37.88ab ± 1.01 | 39.15a ± 1.25 | 39.02a ± 0.72 | 39.96a ± 1.93 | 35.20b ± 2.25 | 39.62a ± 1.73 |
| MUFAC | 46.21b ± 1.60 | 45.79b ± 1.71 | 44.23bc ± 1.15 | 43.21c ± 1.23 | 50.95a ± 2.52 | 45.44b ± 3.66 |
| PUFAC | 15.91ab ± 0.69 | 15.06b ± 0.84 | 16.75a ± 1.79 | 16.85a ± 0.69 | 13.84c ± 0.91 | 14.94bc ± 2.09 |
| SFA/UFA | 0.61a ± 0.03 | 0.64a ± 0.03 | 0.64a ± 0.02 | 0.67a ± 0.05 | 0.54b ± 0.05 | 0.66a ± 0.05 |
Means with different superscripts were significantly different in ANOVA and subsequently grouped by Tukey test (p ≤ 0.05)
AData expressed as g/100 g sample
BData expressed as nmol/g sample
CData expressed as percentage (%)
Protein oxidation in raw, desalted and cooked chicken charqui
Tryptophan and free thiol depletion
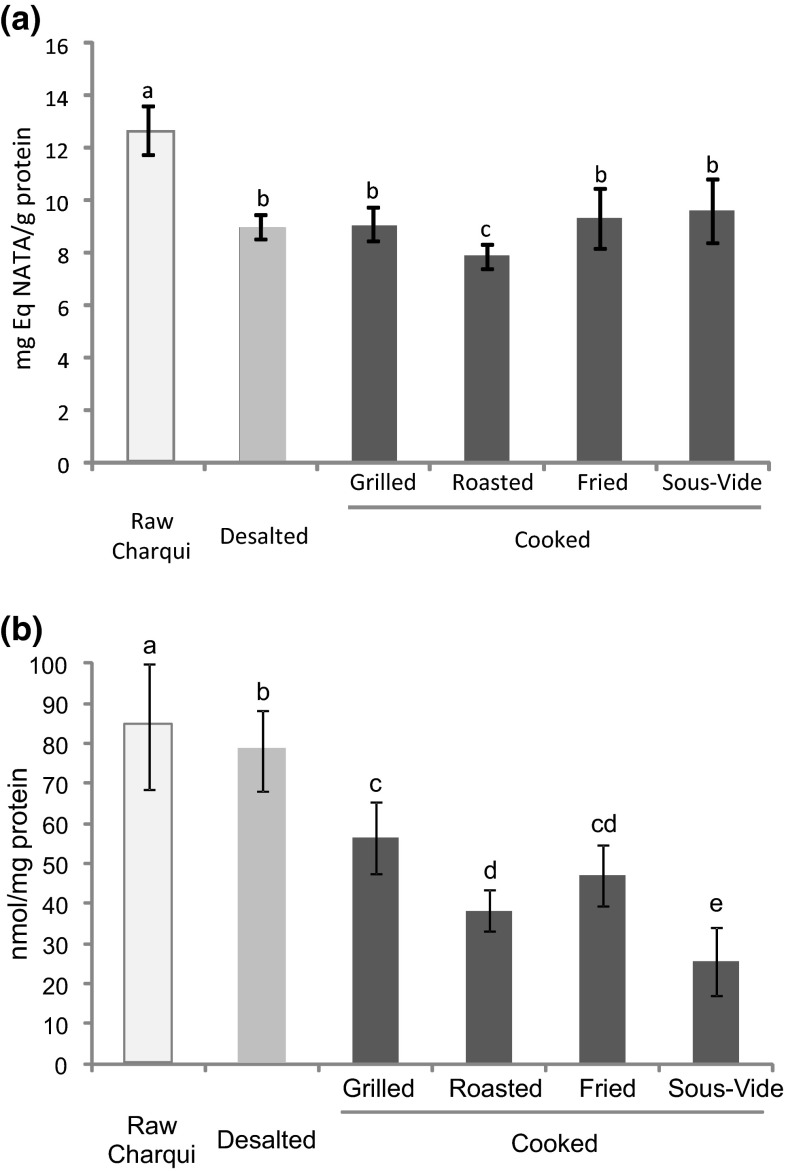
Tryptophan is an essential amino acid very susceptible to oxidative reactions. The degradation of tryptophan leads to the loss of its natural fluorescence and can negatively impact the meat quality, impairing its nutritional value. Figure 1a showed the effect of desalting and type of cooking on the degradation of fluorescence of tryptophan in chicken charqui. There were differences (p < 0.05) in tryptophan fluorescence between raw and cooked samples. After desalting of chicken charqui, a decrease of 30 % in tryptophan was noted. On the other hand, no differences in tryptophan fluorescence were observed between desalted, grilled, fried and sous-vide samples. ANOVA showed an important degradation of tryptophan content in roasted samples, which were submitted to higher cooking temperature (200 °C/15 min) compared to the other cooking treatments. The temperature level and duration of heat treatment displays a marked impact in protein stability of meat and meat products. According to Chan et al. (1992), during heat treatment of fish muscle proteins, there is a decrease in natural fluorescence of aromatic amino acid residues with increasing temperature. Min et al. (2008) also explained that application of high temperatures during cooking associated with a probably enhanced accessibility of different pro-oxidants to tryptophan due to the intense cell disruption promotes the tryptophan oxidation boost.
Fig. 1.
Tryptophan concentration (a) and free thiol content (b) (mean ± standard deviation) in chicken charqui: raw, desalted and subjected to assorted cooking methods. Notes: Different letters on top of bars denote significant differences between treatments in ANOVA (p < 0.05)
Decreases in free thiol group of cysteine have been also associated with protein oxidation development and quality deterioration of meat and meat products (Santé-Lhoutellier et al. 2008). During meat protein oxidation, free thiol groups are converted into oxidation derivatives (i.e. disulphide bonds), promoting protein aggregation and nutritional value losses. Figure 1b shows the effect of desalting and cooking methods in free thiol depletion of chicken charqui. The significant decrease of free thiol groups measured after desalting of charqui samples was low (≈6 %) compared to thermal treatments. Consistently, the type of cooking promoted a remarkable impact in free thiols of chicken charqui. No differences were noted in free thiol between roasted and fried treatments. Sous-vide cooked samples presented the lowest level of free thiol groups, suggesting that sulfur amino acids (i.e. cysteine) of chicken charqui meat are more susceptible to long heating times than aromatic amino acids such as tryptophan. The differences found between tryptophan and free thiol group regarding the type of cooking method could be explained due to (1) the fact of cysteine is greatly affected by oxidation conditions even at low concentration of ROS due to the high susceptibility of sulfur centers, and (2) the necessity of the presence of transition metals to that initiate tryptophan oxidation (Estévez 2011). Santé-Lhoutellier et al. (2008) also reported a decrease of free thiol group content in beef meat after thermal treatment and explained that this phenomenon might have a marked impact on aggregation of meat proteins. It is important to underline that tryptophan depletion during meat processing may involve a relevant nutritional loss given the assorted biological functions covered by this essential amino acid (Soladoye et al. 2015). Similar circumstances apply to sulfur-containing amino acids as besides the loss if nutritional value, the oxidation of methionine and cysteine has been found to lead to the formation of compounds of potential toxicity to humans (Estévez and Luna 2016). On this line, the differences found between cooking methods for the oxidation of these essential amino acids may involve benefits from nutritional and safety points of view.
Protein carbonylation
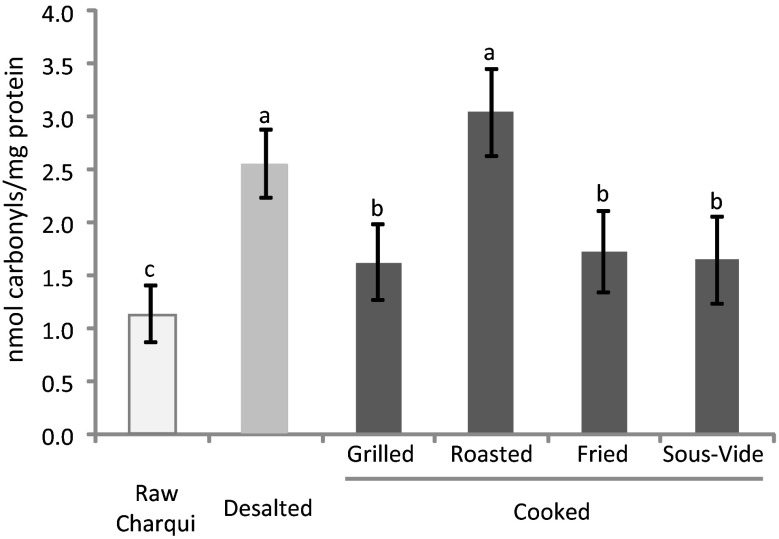
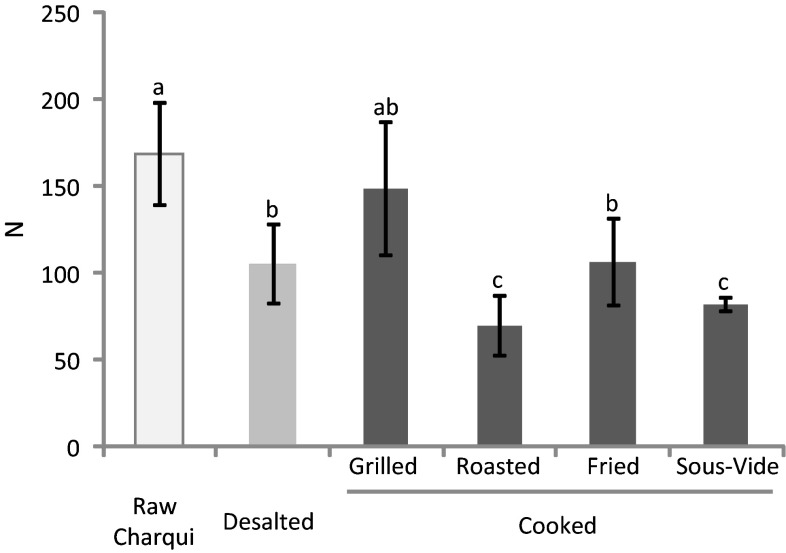
The formation of carbonyl groups from alkaline amino acid side chains has been highlighted as one of the most outstanding modifications in oxidized proteins. This phenomenon is an irreversible and non-enzymatic modification of proteins that promotes a significant decrease in nutritional value of meat in terms of availability of essential amino acids and digestibility of oxidized proteins (Estévez 2011). Thermal processing can negatively affect the quality of meat and meat products by increasing reactive oxygen species (ROS) production. In fact, the application of cooking technologies has been associated with thermal denaturation of protein, which induces structural changes that increase protein carbonylation gain in meat products (Traore et al. 2012; Estévez 2011). The effect of desalting and cooking methods on the concentration of protein carbonyls (AAS + GGS) in chicken charqui is displayed in Fig. 2. Desalting and cooking promoted an increase in protein carbonylation in chicken charqui. Raw charqui samples presented low values of carbonyls. After desalting procedure, an increase of 54 % in carbonyl content was observed in charqui samples. An increase in water activity and the swelling of the myofibrils during water uptake may have facilitated the exposure of meat proteins, the mobility of pro-oxidants and hence, the carbonylation of alkaline amino acids. These mechanisms have been previously proposed by Liu et al. (2009; 2010). Despite the significant differences (p < 0.05) noted among roasted and other cooking methods, no differences were observed between roasted and desalted treatments. Also, grilled, fried and sous-vide cooked samples presented no differences between them. Results from protein carbonylation in raw chicken charqui were slightly higher than that observed by Souza et al. (2013) in raw beef charqui. Estévez (2015) reported that poultry meat is more susceptible to the occurrence of lipid and protein oxidation than meat from other species during the application of processing technologies involving high temperatures. According to Utrera and Estévez (2013), during heat treatment, chicken meat shows a higher susceptibility to lipid oxidation than pork and beef. Furthermore, lipid-derived radicals and hydroperoxides formed during lipid oxidation were found to greatly affect proteins and promote protein carbonylation in meat systems (Estévez 2011). The low content of carbonyl compounds found in grilled and fried samples compared to roasted and desalted ones may be linked to the high content of MRPs, which has been recognized to having antioxidant properties (Silván et al. 2006). Results observed in roasted samples suggest that as previously reported for tryptophan fluorescence, the protein carbonyl formation in chicken charqui seems to be greatly affected by application of high cooking temperatures.
Fig. 2.
Concentration of protein carbonyls (mean ± standard deviation) in chicken charqui: raw, desalted and subjected to assorted cooking methods. Notes: Different letters on top of bars denote significant differences between treatments in ANOVA (p < 0.05)
Schiff base (SB) and disulphide bond formation
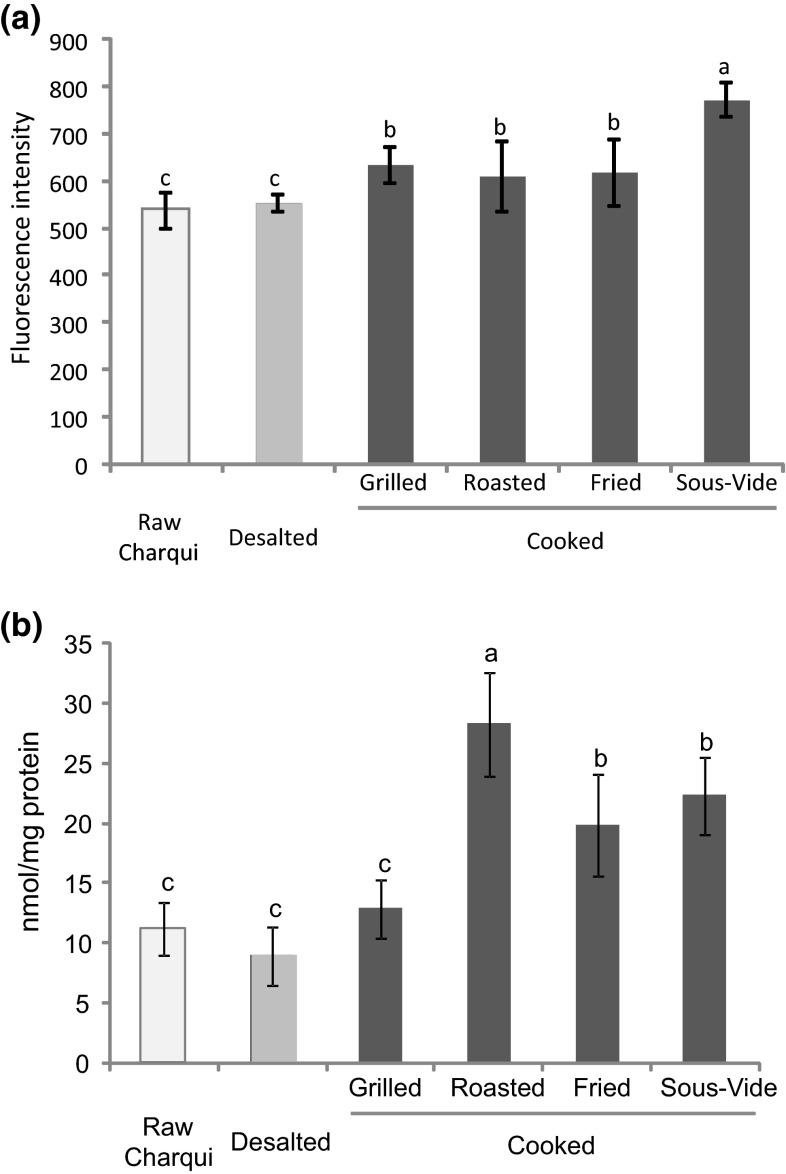
Formation of SB in meat proteins is a result of a crosslinking reaction between aldehyde moiety from protein carbonyls (mainly AAS) and amino groups from alkaline amino acids (mainly lysine) (Estévez 2011). In our experiment, the formation of SB in chicken charqui was significantly affected by the type of cooking (Fig. 3a). Desalting process did not affected SB formation compared to raw charqui and the SB fluorescence increased moderately after heat treatment of charqui samples compared to raw ones, independently of cooking method. Among the thermal treatments, charqui samples submitted to sous-vide cooking had the highest SB increment (28 %) while protein carbonylation decreased (Fig. 2). This SB increment could be attributed to the reaction of AAS with amino groups of proteins (Estévez 2011). The formation of SB has been associated with impaired digestibility and functionality of myofibrillar proteins (Estévez 2011). As previously observed for free thiol groups, SB formation in chicken charqui seems to be associated with long heating times. Traore et al. (2012) also observed a strong increase in SB fluorescence of pig meat after increasing the time of cooking. The high content of SB in grilled and fried samples may also be ascribed to the formation of Maillard fluorescent products, which appears as temperature increases (Silván et al. 2006).
Fig. 3.
Fluorescent Schiff bases (a) and disulphide bond formation (b) (mean ± standard deviation) in chicken charqui: raw, desalted and subjected to assorted cooking methods. Notes: Different letters on top of bars denote significant differences between treatments in ANOVA (p < 0.05)
The formation of disulphide bonds in meat systems is related with the oxidation of two sulfhydryl groups that, as a result, are linked together. This oxidative reaction leads to the formation of cross-linked proteins, resulting in changes of protein functionality and availability (Soladoye et al. 2015). Figure 3b showed the formation of disulphide bonds after desalting and cooking of chicken charqui. Desalting process did not affect disulphide formation in charqui samples compared to raw one. In fact, no differences were noted between raw, desalted and grilled samples. Roasted treatment presented the highest value of disulphide formation in chicken charqui, followed by fried and sous-vide cooked samples. Interestingly, roasted samples also presented the lowest value of tryptophan (Fig. 1a) and the highest content of protein carbonyls (Fig. 2), indicating that protein crosslinking formation and carbonylation of chicken charqui could be associated with the application of high cooking temperature. During heating of meat products, thiol groups are exposed and hydrophobic residues are exteriorized, leading to a breakdown in hydrogen bonds and the formation of disulphide bridges (Traore et al. 2012).
Physical properties of raw, desalted and cooked chicken charqui
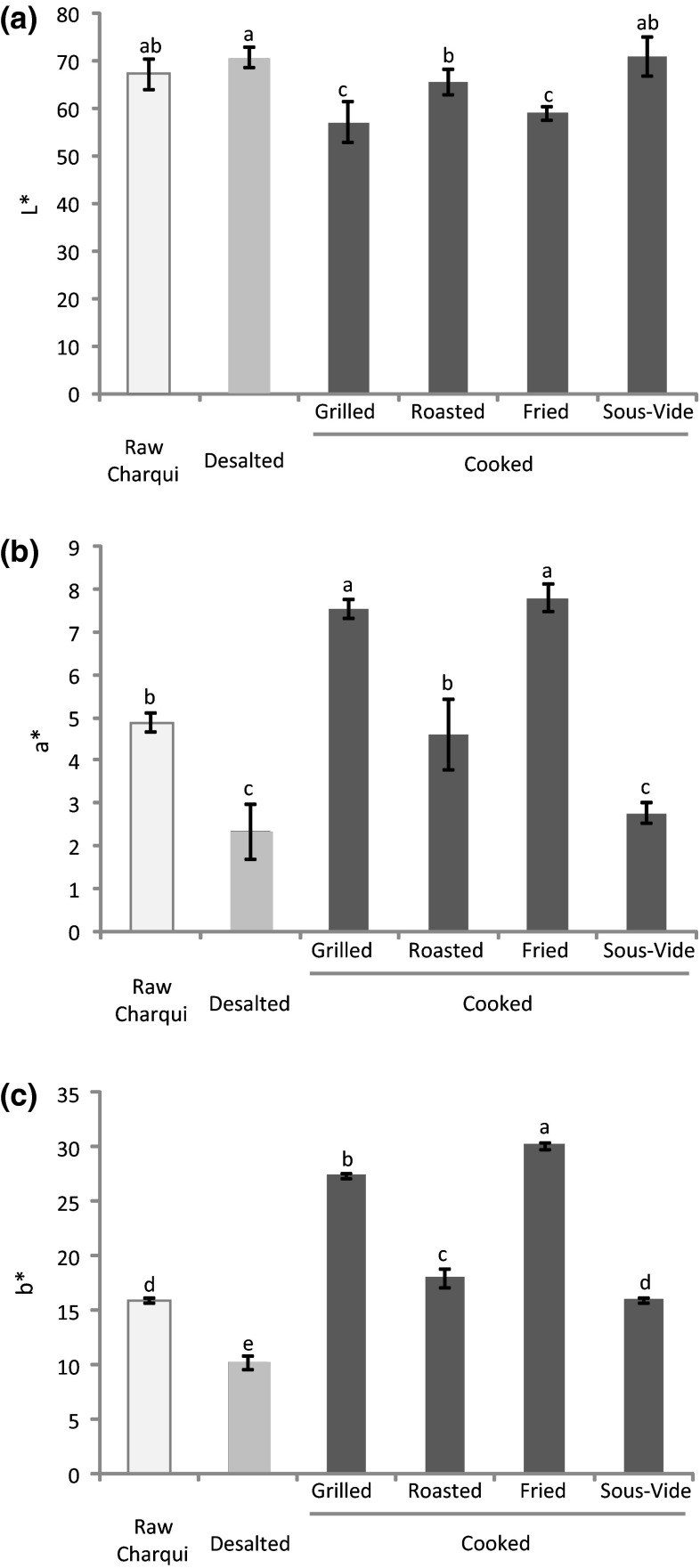
Results for instrumental color (L*, a* and b*) and hardness measurements of chicken charqui samples are shown in Figs. 4 and 5, respectively. No differences in lightness (L*) were noted after desalting of raw charqui, while the type of cooking had a significant impact on this parameter (Fig. 4a). Superficial color was darkest in grilled and fried samples. In agreement with the fact of sous-vide cooked meat presents milder or inexistent browning (Roldán et al. 2015), no significant differences were observed in L* values between sous-vide cooked and desalted charqui. A significant increase of redness (a*) and yellowness (b*) was observed in chicken charqui after grilling and frying compared to the other cooked treatments (Fig. 4b, c, respectively) as a likely result of the presence of colored products formed via Maillard reactions, which are in agreement with MRPs measurements (Table 1). Furthermore, a probable intense dehydration of the grilled and fried charqui samples associated to a heat-denaturation of charqui proteins due to higher endpoint temperatures may also explain the instrumental color results. The increase in b* value after cooking of chicken breast muscle was previously reported by Wattanachant et al. (2005). Final temperatures reached at the meat surface promote a different effect on cooked meat quality (Vittadini et al. 2005). In addition, the formation of red-brownish precipitates after cooking is closely related with color modifications in meat and meat products (Martens et al. 1982). Sous-vide technique did not promoted changes in lightness and yellowness compared to raw product, which may be an advantage to some meat consumers.
Fig. 4.
Lightness (a), redness (b) and yellowness (c) (mean ± standard deviation) in chicken charqui: raw, desalted and subjected to assorted cooking methods. Notes: Different letters on top of bars denote significant differences between treatments in ANOVA (p < 0.05)
Fig. 5.
Instrumental hardness (mean ± standard deviation) in chicken charqui: raw, desalted and subjected to assorted cooking methods. Notes: Different letters on top of bars denote significant differences between treatments in ANOVA (p < 0.05)
The effect of desalting and cooking methods on hardness of chicken charqui is displayed in Fig. 5. Raw charqui presented high values of hardness, which decreased 67 % after desalting processing as a probable result of water uptake. No significant differences in this texture parameter were noted between grilled, fried and desalted samples. However, sous-vide and roasted cooking promoted a marked decrease in hardness of chicken charqui. The low water losses of sous-vide and desalted samples compared to the others cooking treatments may explain this hardening decrease. In addition, this decrement of hardness may be ascribed to a greater collagen solubilization due the long cooking time applied during sous-vide technique, as previously reported by Roldán et al. (2013) in sous-vide cooked lamb loins. During cooking procedures of meat products, heat solubilizes the connective tissue causing meat tenderization. However, cooking temperatures over 65 °C cause denaturation of myofibrillar proteins, which promotes meat toughening (Roldán et al. 2013). According to Baldwin (2012), meat cooked at low temperatures and long times (LTLT), such as those subjected to sous-vide cooking, presents residual collagenolytic activity, which could partially explain the results of texture in our experiment. Despite the close association between the formation of cross-links via protein carbonylation and the toughening of meat products (Estévez 2011), our results seem not to be governed by this mechanism as samples with the highest disulphide bonds formation showed lower hardness.
Conclusion
The oxidative stability and physical traits of chicken charqui are influenced by desalting and the cooking technique. The length of cooking seems to play a major role in free thiol depletion, Schiff base formation and instrumental softening of charqui samples, while the cooking temperature are mainly related to tryptophan degradation, protein carbonylation and disulphide bonds formation. Considering the low water loss, the intense decrease of NaCl content and the improvement of meat texture, sous-vide technique may be indicated to cooking chicken charqui in order to obtain high-quality ready-to-eat chicken products.
Acknowledgments
The authors are thankful to the CNPq and CAPES for the support through the Projects 474300/2011-0 and 401167/2014-3.
References
- AOAC. Association of Official Analytical Chemists . Official methods of analysis. Washington: AOAC; 2000. [Google Scholar]
- Baldwin DE. Sous vide cooking: a review. Int J Gastron Food Sci. 2012;1(1):15–30. [Google Scholar]
- Bejerholm C, Aaslyng MD. The influence of cooking technique and core temperature on results of a sensory analysis of pork—depending on the raw meat quality. Food Qual Prefer. 2004;15(1):19–30. [Google Scholar]
- Brasil (1997) Ministério da Agricultura, Pecuária e Abastecimento. Regulamento de Inspeção Industrial e Sanitária de Produtos de Origem Animal. Brasília: Diário Oficial da União, seção 1
- Chan JK, Gill TA, Paulson AT. The dynamics of thermal denaturation of fish myosins. Food Res Int. 1992;25(2):117–123. [Google Scholar]
- Correia RTP, Biscontini TMB. Desalting and cooking effects on chemical composition and fatty acid profile of charqui and jerked beef. Food Sci Technol (Campinas) 2003;23(1):38–42. [Google Scholar]
- Estévez M. Protein carbonyls in meat systems. Meat Sci. 2011;89(3):259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult Sci. 2015;94(6):1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Estévez M, Luna C. Dietary protein oxidation: a silent threat to human health? Crit Rev Food Sci Nutr. 2016 doi: 10.1080/10408398.2016.1165182. [DOI] [PubMed] [Google Scholar]
- Estévez M, Kylli P, Puolanne E, Kivikari R, Heinonen M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008;80(4):1290–1296. doi: 10.1016/j.meatsci.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(3):497–509. [PubMed] [Google Scholar]
- Gade DW. Llama/Alpaca. In: Kiple KF, Ornelas KC, editors. The Cambridge world history of food. Cambridge: Cambridge University Press; 2000. pp. 555–559. [Google Scholar]
- Gatellier P, Kondjoyan A, Portanguen S, Santé-Lhoutellier V. Effect of cooking on protein oxidation in n-3 polyunsaturated fatty acids enriched beef. Implication on nutritional quality. Meat Sci. 2010;85(4):645–650. doi: 10.1016/j.meatsci.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Haak L, Sioen I, Raes K, Camp JV, Smet SD. Effect of pan-frying in different culinary fats on the fatty acid profile of pork. Food Chem. 2007;102(3):857–864. [Google Scholar]
- Liu G, Xiong YL. Electrophoretic pattern, thermal denaturation, and in vitro digestibility of oxidized myosin. J Agric Food Chem. 2000;48(3):624–630. doi: 10.1021/jf990520h. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xiong YL, Chen J. Identification of restricting factors that inhibit swelling of oxidized myofibrils during brine irrigation. J Agric Food Chem. 2009;57:10999–11007. doi: 10.1021/jf902722j. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xiong YL, Chen J. Protein oxidation enhances hydration but suppresses water-holding capacity in porcine longissimus muscle. J Agric Food Chem. 2010;58:10697–10704. doi: 10.1021/jf102043k. [DOI] [PubMed] [Google Scholar]
- Martens H, Stabursvik E, Martens M. Texture and colour changes in meat during cooking related to thermal denaturation of muscle proteins. J Texture Stud. 1982;13(3):291–309. [Google Scholar]
- Martins SIFS, Van Boekel MAJS. Melanoidins extinction coefficient in the glucose/glycine Maillard reaction. Food Chem. 2003;83(1):135–142. [Google Scholar]
- Min B, Nam KC, Cordray J, Ahn DU. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J Food Sci. 2008;73(6):C439–C446. doi: 10.1111/j.1750-3841.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Rocha Garcia CE, Youssef EY, Souza NE, Matsushita M, Figueiredo E, Shimokomaki M. Preservation of spent leghorn hen meat by a drying and salting process. J Appl Poult Res. 2003;12(3):335–340. [Google Scholar]
- Roldán M, Antequera T, Martin A, Mayoral AI, Ruiz J. Effect of different temperature-time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013;93(3):572–578. doi: 10.1016/j.meatsci.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Roldán M, Antequera T, Armenteros M, Ruiz J. Effect of different temperature-time combinations on lipid and protein oxidation of sous-vide cooked lamb loins. Food Chem. 2014;149(1):129–136. doi: 10.1016/j.foodchem.2013.10.079. [DOI] [PubMed] [Google Scholar]
- Roldán M, Ruiz J, Pulgar JS, Pérez-Palacios T, Antequera T. Volatile compound profile of sous-vide cooked lamb loins at different temperature-time combinations. Meat Sci. 2015;100(1):52–57. doi: 10.1016/j.meatsci.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Rysman T, Jongberg S, Royen GV, Weyenberg SV, Smet SD, Lund MN. Protein thiols undergo reversible and irreversible oxidation during chill storage of ground beef as detected by 4,4′-dithiodipyridine. J Agric Food Chem. 2014;62:12008–12014. doi: 10.1021/jf503408f. [DOI] [PubMed] [Google Scholar]
- Santé-Lhoutellier V, Astruc T, Marinova P, Greve E, Gatellier P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J Agric Food Chem. 2008;56(4):1488–1494. doi: 10.1021/jf072999g. [DOI] [PubMed] [Google Scholar]
- Santos CSP, Cruz R, Cunha SC, Casal S. Effect of cooking on olive oil quality attributes. Food Res Int. 2013;54(2):2016–2024. [Google Scholar]
- Shimokomaki M, Franco BDGM, Biscontini TM, Pinto MF, Terra NN, Zorn TMT. Charqui meats are hurdle technology meat products. Food Rev Int. 1998;14(4):339–349. [Google Scholar]
- Silva FAP, Ferreira VCS, Estévez M, Silva SA, Lemos LTM, Madruga MS (2015) In: Proceedings of 7th Cyta/Cesia. Badajoz, Spain
- Silván JM, Lagemaat VJ, Olano A, Castillo MD. Analysis and biological properties of amino acid derivatives formed by Maillard reaction in foods. J Pharm Biomed Anal. 2006;41(5):1543–1551. doi: 10.1016/j.jpba.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Soladoye OP, Juaréz ML, Aalhus JL, Shand P, Estévez M. Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr Rev Food Sci Food Saf. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Souza MAA, Visentainer JV, Carvalho RH, Garcia F, Ida E, Shimokomaki M. Lipid and protein oxidation in charqui meat and jerked beef. Braz Arch Biol Technol. 2013;56(1):107–112. [Google Scholar]
- Torres EAFS, Shimokomaki M, Franco BDGM, Landgraf M. Parameters determining the quality of charqui, an Intermediate Moisture Meat product. Meat Sci. 1994;38:229–234. doi: 10.1016/0309-1740(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Traore S, Aubry L, Gatellier P, Przybylski W, Jaworska D, Kajak-Siemaszko K, Santé-Lhoutellier V. Effect of heat treatment on protein oxidation in pig meat. Meat Sci. 2012;91(1):14–21. doi: 10.1016/j.meatsci.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Utrera M, Estévez M. Analysis of tryptophan oxidation by fluorescence spectroscopy: effect of metal-catalyzed oxidation and selected phenolic compounds. Food Chem. 2012;135(1):88–93. [Google Scholar]
- Utrera M, Estévez M. Oxidative damage to poultry, pork, and beef during frozen storage through the analysis of novel protein oxidation markers. J Agric Food Chem. 2013;61:7987–7993. doi: 10.1021/jf402220q. [DOI] [PubMed] [Google Scholar]
- Utrera M, Morcuende D, Rodríguez-Carpena J-G, Estévez M. Fluorescent HPLC for the detection of specific protein oxidation carbonyls—σ-aminoadipic (AAS) and γ-glutamic (GGS) semialdehydes—in meat systems. Meat Sci. 2011;89(4):500–506. doi: 10.1016/j.meatsci.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Utrera M, Rodríguez-Carpena J-G, Morcuende D, Estévez M. Formation of lysine-derived oxidation products and loss of tryptophan during processing of porcine patties with added avocado byproducts. J Agric Food Chem. 2012;60(15):3917–3926. doi: 10.1021/jf3001313. [DOI] [PubMed] [Google Scholar]
- Vittadini E, Rinaldi M, Chiavaro E, Barbanti D, Massini R. The effect of different convection cooking methods on the instrumental quality and yield of pork Longissimus dorsi. Meat Sci. 2005;69(4):749–756. doi: 10.1016/j.meatsci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Wattanachant S, Benjakul S, Kedward DA. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005;93(2):337–348. [Google Scholar]
- Whitfield FB. Volatiles from interactions of maillard products and lipids. Crit Rev Food Sci Nutr. 1992;31(1–2):1–58. doi: 10.1080/10408399209527560. [DOI] [PubMed] [Google Scholar]
- Xiao S, Zhang WG, Lee EJ, Ma CW, Ahn DU. Lipid and protein oxidation of chicken breast rolls as affected by dietary oxidation levels and packaging. J Food Sci. 2011;76(4):612–617. doi: 10.1111/j.1750-3841.2011.02137.x. [DOI] [PubMed] [Google Scholar]