Abstract
The effects of transplantation date on milled rice (physicochemical, amino acids composition and protein profiling) of different cultivars and their starch characteristics (granules size distribution, pasting and thermal) were investigated. Head rice yield increased (2.0–4.1 %) and chalky grains (5–10 %) decreased with delaying the paddy transplantation of different cultivars by 20 days. Delayed transplantation of paddy significantly increased asparagine, glutamine, threonine, cysteine, methionine, tryptophan, lysine and proline content in milled rice. Early transplantation of paddy showed higher accumulation of glutelin and prolamines than that in milled rice from delayed transplantation. The change in amino acid composition of milled rice with delay in transplantation was related to variation in accumulation of glutelin and prolamines. Starch from delayed transplanted paddy showed higher peak viscosity and lower breakdown viscosity than those from early transplanted paddy. These differences were due to higher accumulation of amylose in starch from delayed transplanted paddy than that from early transplanted paddy due to exposure of former to lower night air temperature during starch synthesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2293-x) contains supplementary material, which is available to authorized users.
Keywords: Transplanting dates, Protein profiling, Amino acids, Starch, Thermal properties, Granule size
Introduction
Among the different components factors of agronomy packages for rice cultivation, transplanting is one of the important factors as early or late planting rice plants may face different types of abiotic stress (Mahajan et al. 2015). One of the main disadvantages in early transplanted crop is the initiation of sterility induced by high temperature resulting into lower milling quality of rice. Earlier-planted crops have higher water requirements due to high evapotranspiration demand which was generally met through underground water resources. Punjab production of rice was approximately 3738 kg/ha in 2011–2012 and nearly 76 % of which was irrigated by underground water. Early transplanted paddy (ETP) use more water from underground regime which is already depleting at faster rate in this region. Variations in growth temperature by varying the transplantation dates caused variation in productivity and quality of rice. Studies investigating the effects of temperature on kernel development have indicated that higher temperatures during grain-filling stage of plant development result in a decrease in the kernel mass and dimensions, and an increase in the number of chalky kernels (Tashiro and Wardlaw 1991). Variable effects of growth temperatures on amylose content of rice cultivars had been reported (Singh et al. 2014). The changes caused by NTAT (night time air temperature) stress have been attributed to reduced substrate supply to the endosperm, which resulted in slow growth of starch granules and irregular granular organization (Fitzgerald and Resurreccion 2009). The difference in quality attributes of milled rice has been owing to the difference in amylose content, amylopectin structure and protein content (Suwansri and Meullenet 2004). Variations in soil moisture and nutrient availability, ambient temperature, and atmospheric composition also affected starch functionality (Beckles and Thitisaksakul 2014). Elevated temperature led to the most significant changes in cereals and amylose content was reported to be most sensitive parameter under changing environmental conditions. Lanning et al. (2011) demonstrated that higher NTAT resulted in increase in accumulation of lipids and decrease in accumulation of proteins and starch. Starch is the major component of milled rice, and changes in its characteristics caused by NTAT affect the cooking properties of milled rice. The lipids influenced the swelling and viscoelastic properties of the starch by forming inclusion complexes with the helical structures of amylose (Liang et al. 2002). The cultivars exposed to 26 °C showed the reduction in free AAs compared with those grown at lower temperatures.
Changes in properties of rice starch have been reported which depend upon amylose, amylose-lipid complexes, the proportion of intermediate fractions, and short and long chains of amylopectin (Singh et al. 2007). The accumulation of amylose content in rice is regulated genetically and by temperature during grain development. Storage of Wx proteins had been reported to be increased at lower temperatures, resulting in a higher content of amylose in ripened grains of rice (Umemoto et al. 1995). Climate changes resulted in drastic changes in rainfall patterns, with rising temperatures and unfavorable growing conditions. Rice yield and quality are significantly affected by weather conditions. Studies carried out on rice demonstrated the negative impact of drastic changes in rainfall patterns coupled with rising temperatures could be managed by changes in planting dates, transplanting dates, transplant age, and crop spacing (Oteng-Darko et al. 2013). Different developmental stages (panicle initiation, anthesis or flowering, and grain filling) of rice were influenced by TD. The effect of TD on milling quality, AAs composition and starch characteristics of indica rice have not yet been studied. Therefore, the present study was aimed at evaluating the impact of ETP and DTP on physicochemical, milling, AAs composition, protein profiling and starch (pasting, thermal and granule size distribution) characteristics of milled rice.
Materials and methods
Location and experimental detail
Experiment was conducted during kharif season of 2012 on a loamy sand soil at the research farm of rice section, Punjab Agricultural University (PAU), India. The temperature (minimum, maximum and average) and precipitation during May–December is shown in Fig. 1a. The experiment was laid out in a split plot design replicated the rice. The treatments included three dates of transplanting (10, 20 and 30 June) in the main plots and four cultivars (PR114, PR118, PR121, and PR122) in the sub plots resulting in 12 treatment combinations. Irrigation management comprised of continuous flooding for 15 days after transplanting (DAT) followed by the intermittent irrigations at 3 days interval up to 14 days before harvest. Depth of water in irrigation (75 mm) was computed by monitoring water level before and after irrigation using fixed scales installed in each plot. Seedling of 30 days old nursery of each variety was transplanted on a puddled soil with a spacing of 20 cm between rows and 15 cm between hills. Puddling was done by running a cultivator in standing water (75 mm) followed by planking. Forty kg N ha−1 was applied at 5 DAT. Second and third doses of nitrogen (40 kg ha−1 each) were applied at 21 and 42 DAT for each of those 4 dates of transplanting treatment. Herbicide Rifit at 1.5 kg ha−1 (Pretilachlor) was applied 2 days after transplanting to control the weeds. Chlorpyriphos insecticides (2.5 l ha−1) and Tilt 25 EC (250 ml ha−1) were used to control insects and diseases respectively. At harvest, grain yield was measured at 14 % grain moisture content. After harvesting paddy samples were stored for 2 months at room temperature and then milled.
Fig. 1.
a A Different stages of maturation for paddy transplanted on June 10, 20, and 30 June, 2012. B Average precipitation (times) and maximum (open diamond), minimum (open square), and mean (open triangle) temperature of months May–December 2012. b SDS-PAGE analysis of rice seed storage proteins showing granule-bound starch synthase (60 kDa), glutelin acidic subunits (33 and 31 kDa), globulins (24 kDa), glutelin basic subunits (21 and 22 kDa), and prolamins (15, 16 and 18 kDa)
Dehusking and milling
The paddy samples were dehusked on a McGill sample sheller (Rapsco, Brookshire, TX, U.S.A.) and milled in a McGill mill number 2 (Rapsco, Brookshire, TX, U.S.A.) to remove 6 % bran as described earlier (Singh et al. 2000).
Head rice yield
Head rice constituted the grain with length of 3/4 or more of the whole milled grain separated from the 100 g milled rice and head rice yield (HRY) was calculated as percentage of milled rice.
Chalky grains
Chalk is expressed as the proportion of opaque relative to translucent areas in a single layer of the white rice grains. The chalky grains were separated from 100 g of milled rice and calculate as percentage of chalky grains.
Color characteristics
Lightness (L*), redness-greenness (a*), yellowness-blueness (b*) and whiteness of the milled rice from different cultivars were evaluated by method as described earlier (Pal et al. 2016a).
Amino acids (AAs) determination
Amino acids (AAs) determination from milled rice was done as described earlier (Pal et al. 2016b).
Protein profiling
Total seed storage proteins were extracted as described by Kawakatsu et al. (2008). SDS-PAGE analysis of seed storage proteins was carried out according to the modified method of Laemmli (1970). The method was briefly described earlier (Singh et al. 2014).
Starch isolation
Starch was isolated from milled rice of various cultivars by alkali extraction as described earlier (Sodhi and Singh 2003).
Blue value, λmax and amylose content
Iodine absorption spectra and Blue Value (BV) of milled rice and starch were measured according to the method described by Takeda et al. (1983). Amylose content (AC) of starch was determined by the method of Williams et al. (1970).
Pasting properties
Pasting properties of rice starch (3.5 g of milled rice + 24.5 g of distilled water) was evaluated using dynamic rheometer (Anton PaarRheo Plus/32 model MCR-301) as described earlier (Kaur et al. 2013). Pasting temperature (PT), peak viscosity (PV), final viscosity (FV), breakdown viscosity (BD) and setback viscosity (SB) were recorded.
Granule size distribution
Granule size distribution of the starches was measured by using Microtrac S3500 Particle size analyzer (Microtrac Ins. Ltd., USA) as described by Sharma et al. (2015).
Thermal properties
Thermal properties of starches were analyzed using DSC (Mettler Toledo, Greinfense, Switzerland) as described earlier (Singh et al. 2010). Thermal parameters were recorded and analysed using Stare Software (version 8.10). Various thermal parameters measured were onset temperature (To), peak temperature (Tp), conclusion temperature (Tc) and enthalpy of gelatinization (ΔHgel).
Statistical analysis
The data reported in all the tables was average of triplicate observations. The data was subjected to two-way analysis of variance (ANNOVA) using Minitab statistical software (version 14.12.0, Minitab, State College, PA U.S.A.) for determining the effect on different parameters.
Results and discussion
Paddy yield and head rice yield
Paddy yield and HRY was evaluated for ETP and delayed transplanted paddy (DTP). Yield per hectare was 9.82 metric ton (MT), 9.25 MT and 8.85 MT for the paddy transplanted on 10, 20 and 30 June, respectively. The highest average paddy yield of 9.77 MT for PR122 and the lowest of 8.94 MT for PR118 were observed. Milling quality is determined by HRY i.e. defined as the mass percentage of rough rice that remained as head rice after milling. ETP HRY ranged from 57.1 to 58.6 % against 59.3–61.2 % for DTP (Table 1). The cultivar, TD and cultivar × TD showed significant effect on HRY. The cultivar F value for HRY was much larger than TD indicating greater effect of former (Supplementary Table 1). Head rice comprised of milled grains with at least three-fourth length of that of whole milled grains. Rice grain characteristics were influenced by the environmental conditions under which the rice was grown and by their genetic traits. Cultivars with thicker grain size required more milling pressure due to presence of thicker aleurone layer surrounding the endosperm. Furthermore grains with deeper surface grooves also required more milling pressure or longer milling durations to smooth the surface of the grain and to remove an adequate amount of bran to achieve a desired degree of milling. It was thus evident that thickness and morphology of the grains also affected the milling quality of paddy. Earlier studies investigating the effect of temperature on grain development indicated that higher temperatures during the grain-filling stage of plant development resulted in a decrease in the grain weight and dimensions causing an increase in the number of chalky grains (Tashiro and Wardlaw 1991). Environmental temperatures during grain filling had significant effect on starch structure and there by affecting rice quality. High NTAT could possibly have caused an increase in respiration during the non-daylight hours and subsequently the loss of carbohydrate from the grains. To determine the possibilities of significant variation in HRY and other attributes the chalkiness and whiteness of the milled rice was also evaluated.
Table 1.
Effect of transplanting dates on physicochemical properties of milled rice, blue value and λmax of starch from different cultivars
| Cultivars | TD | L*PR | a*PR | b*PR | Whiteness | HRY (%) | Chalky grains (%) | λmax (nm) | BV (%) | AC (%) | BV S (%) | λmax S (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR114 | 10 June | 68.9cd | 1.4d | 13.3d | 66.2e | 58.6c | 33.0e | 575.2c | 0.1058a | 3.1a | 0.1412b | 593.7a |
| PR114 | 20 June | 68.7d | 1.4d | 13.6de | 65.9cd | 59.7d | 30.0ab | 573.4b | 0.1624d | 4.7d | 0.1798d | 597.2b |
| PR114 | 30 June | 67.3b | 1.6e | 13.3d | 64.6a | 60.9e | 28.0a | 587.1f | 0.2812f | 7.5i | 0.1911e | 599.9e |
| PR118 | 10 June | 68.0c | 1.6e | 13.3d | 65.3b | 55.9a | 38.5f | 575.2c | 0.1054a | 4.1c | 0.1666c | 600.8f |
| PR118 | 20 June | 67.9ab | 1.2b | 13.4de | 65.2b | 57.3b | 31.5bc | 573.4b | 0.1067a | 4.7d | 0.1756d | 599.0d |
| PR118 | 30 June | 67.5b | 1.8f | 12.4c | 65.2b | 59.3d | 30.0abc | 590.1g | 0.2119e | 6.1g | 0.2144g | 602.5f |
| PR121 | 10 June | 70.0e | 1.3c | 15.8h | 66.1e | 57.1b | 43.0h | 576.1d | 0.1057a | 5.0e | 0.1310a | 598.1c |
| PR121 | 20 June | 67.8b | 0.7a | 12.0a | 65.6bc | 59.4d | 38.0fg | 576.1d | 0.1068a | 5.7f | 0.1932e | 597.2b |
| PR121 | 30 June | 67.8b | 1.6e | 12.0a | 65.6bc | 61.2f | 33.0e | 587.1f | 0.1436c | 7.1h | 0.2156g | 599.0d |
| PR122 | 10 June | 67.9ab | 1.6e | 13.5f | 65.2b | 58.5c | 40.0fg | 572.5a | 0.1042a | 3.8b | 0.1490b | 599.0d |
| PR122 | 20 June | 68.0c | 0.8a | 13.7g | 65.1b | 59.2d | 32.5bd | 572.5a | 0.1163b | 4.5d | 0.2016f | 598.4d |
| PR122 | 30 June | 66.7a | 1.6e | 11.7b | 64.6a | 60.5e | 30.5bd | 583.1e | 0.1694d | 5.6f | 0.2118g | 599.9e |
TD Transplantation date, PR polished rice, HRY head rice yield, S starch, AC Amylose content, BV blue value
Values with similar superscript do not differ significantly P > 0.05 to P < 0.05
Chalkiness and whiteness
Chalkiness is also an important quality characteristic of rice and occurred most commonly when high temperatures were experienced during grain development. Chalky grains packed loosely leaving some air spaces that scatter light and have opaque appearance. Chalky grains from ETP ranged between 33 and 43 % against 28–33 % from DTP. Chalkiness in milled rice was cultivar dependent and reduced upon delaying transplantation of paddy from 10 to 30 June (Table 1). It was thus evident that DTP resulted in average reduction of 8.25 % chalkiness among different rice cultivars. However, reduction in rice grain chalkiness was 5, 8.5, 10 and 9.5 % for PR114, PR118, PR121 and PR122, respectively with DTP. All the cultivars showed a decrease in chalky grains with DTP. The cultivar, TD and cultivar × TD showed significant effect on the amount of chalky grains (Supplementary Table 1). Furthermore the chalkiness was related to disorder in starch deposition in grains during filling, ripening and maturation that utilize carbon in sucrose during starch deposition. These temperature induced metabolic disorders in starch biosynthesis resulted in generation of the air spaces in the endosperm with cracks that ultimately produced loosely packed granular structures in the grains (Delcour et al. 2010). Milling of chalky grains resulted in the reduction in HRY because they tend to be weaker and more likely to break during milling due to presence of cracks or air spaces than vitreous grains. All the cultivars showed significantly higher HRY upon DTP. Rice containing >2 % of chalky grains are not accepted in most of rice markets. Singh et al. (2014) also demonstrated that increased HRY with DTP may be due to reduction in loose packaging of starch granules resulted in increased grain hardness. Tashiro and Wardlaw (1991) showed that high temperature during specific stages of grain filling and maturation tends to increase the occurrence of chalkiness in rice grains. It was evident that PR114 was least affected by NTAT as compared to PR118, PR121 and PR122. Whiteness is used as an indicator of the degree to which rice was milled. Whiteness of milled rice ranged between 65.2 and 66.2 % in ETP and 64.6 and 65.6 % for DTP (Table 1). All the cultivars showed a decrease in whiteness with DTP. F value indicated significant effect of TD, cultivar and their interaction on whiteness. Whiteness increased with an increase in the degree of milling whereas HRY decreased with same (Saleh and Meullenet 2007). Pre-harvest climate like NTAT during the grain filling stages had deleterious effect on grain formation and resultant quality of milled rice (Singh et al. 2014).
Color characteristics
Delayed transplantation up to 30 June caused a decrease in L* of milled rice of all the cultivars. L* of milled rice from ETP and DTP ranged from 67.9 to 70.0 and 66.7 to 67.8, respectively. The highest and the lowest L* value of 70.0 and 66.7 were observed for PR121 and PR122, respectively. The a* (redness and greeness) of all the cultivars progressively increased with DTP. The a* of milled rice from ETP and DTP ranged from 1.3 to 1.6 and 1.6 to 1.8 respectively. The b* (yellowness and blueness) of milled rice from PR114, PR118 and PR122 increased up to June 20. The b* of milled rice from ETP and DTP ranged from 13.3 to 15.8 and 11.7 to 13.3, respectively. The highest and the lowest b* values of 15.8 and 11.7, respectively were observed for PR121 and PR122 (Table 1). Lower L* indicate darker endosperm of milled rice which may correspond to inherently darker grains in ETP (10 June). The data showed a general trend of lighter color milled rice from DTP. Both cultivar and TD showed significant effect on L*, a* and b* values. F value of L*, a* and b* for cultivars were much larger than those of TD indicating greater difference due to former (Supplementary Table 1).
Amino acids composition
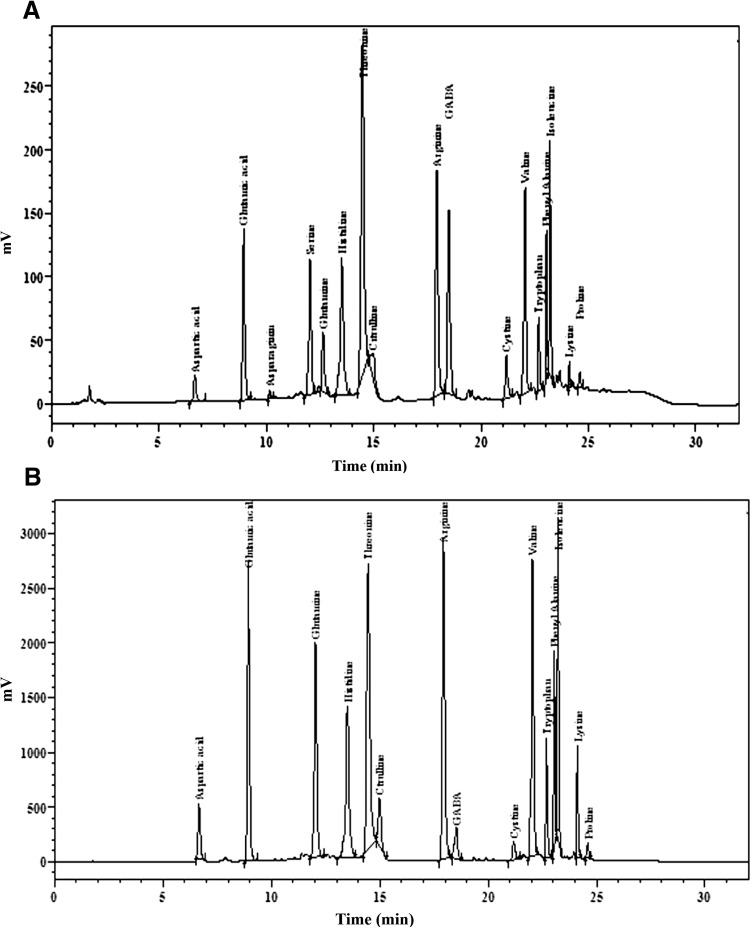
Amino acid composition of ETP and DTP is reported in Table 2. Asparagine, glutamine, threonine, cysteine, methionine, tryptophan, lysine and proline amino acid content increased significantly in the milled rice from DTP while aspartic acid, glutamic acid, serine, histidine, citrulline, arginine, GABA, valine, phenylalanine and isoleucine were decreased as compared with standard of amino acids, Tyrosine was not detected in most of the cultivars (Table 2) (Supplementary Fig. 4). Acidic, basic and aromatic AAs decreased with DTP among all the cultivars. AAs composition as well as aromatic and charged AAs (acidic and basic AAs) of milled rice from different cultivars varied significantly with TD. Figure 2 shows a significant increase in the content of aspartic acid, glutamic acid, glutamine, histidine, citrulline, arginine, valine, tryptophan, iso-lecucine and lysin in DTP as compared to ETP. Crop performance chart showed that vegetative, reproductive and grain maturation stages of ETP was of 46, 34 and 33 days, respectively, against 48, 23, and 35 days, respectively for DTP (Fig. 1a). It was earlier reported that TD significantly influenced different developmental stages of paddy (Oteng-Darko et al. 2013). The key source of assimilated nitrogen (N) in developing grains in the form AAs such as glutamine, asparagine, and serine is from three main sources i.e. roots, stems and leaves were known as source i.e. transport N to the actively developing grains. Shi et al. (2013) also demonstrated that N was stored in the roots and used during active grain filling stage. Accumulation of AAs under abiotic stress condition was frequent process in plants which may occurred due to (1) heat-stable uptake of AAs (2) increased AAs biosynthesis from carbohydrates (3) decreased charging of free AAs to tRNA (4) stress-induced degradation of proteins over protein synthesis and was an adaptive mechanism (Glaubitz et al. 2015). Degenkolbe et al. (2013) also demonstrated that drought stress-induced accumulation of more asparagine in drought-sensitive rice cultivars. Accumulation of glutamic acid, glutamine, glycine, serine and threonine, organic acids such as erythronic acid, galactonic acid and threonic acid was several folds higher in drought sensitive cultivars as compared to the drought tolerant cultivars. Glaubitz et al. (2015) reported that the TCA cycle and AAs metabolism were strongly affected by high night time temperature in sensitive cultivars of rice. Primarily, the protein in rice was reported to be glutelin and 13 kDa prolamines. Prolamines were reported to be rich in glutamine (ranging from 3.4 to 19.2 % with an average 16.2 % of total constituents), leucine (7.7–15.7 % with an average of 12.4 %) and alanine (9.0–12.7 % with an average of 11.6 %) by Yamakawa and Hakata (2010). Whereas glutelin were rich with essential AAs such as lysine, tryptophan and methionine, had high nutritional value and helpful in digestive absorption. SDS-PAGE analysis revealed that accumulation of glutelin and prolamines was higher in the milled rice from ETP whereas storage of these proteins was lower in the milled rice from DTP (Supplementary Figs. 1, 2, 3). Differences in elevated AAs in these varieties were considered to be related to their genetic makeup and TD. F value showed significant effect of cultivar, TD, and cultivar × TD on AAs content, however cultivar showed much larger effect on AAs such as aspartic acid and glutamine while proline > threonine > serine > valine > arginine > glutamic acid > cysteine > phenyl alanine > histidine > methionine > iso-leucine > AAs were highly affected with TD (Supplementary Table 2). Yamakawa and Hakata (2010) demonstrated that the yield was least affected by amount of AAs but the grain quality (palatability and nutritional value) was greatly affected. It was therefore likely that the physico-chemical properties of rice grains may have influenced by date of plantation and NTAT.
Table 2.
Effect of transplanting dates on amino acids composition of milled rice from different cultivars
| Cultivars TD amino acids (%) | PR114 | PR118 | PR121 | PR122 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 June | 20 June | 30 June | 10 June | 20 June | 30 June | 10 June | 20 June | 30 June | 10 June | 20 June | 30 June | |
| Asp | 1.9b | 1.7b | 1.4a | 5.5e | 2.8c | 1.2a | 2.4c | 1.9b | 2.3c | 4.2d | 2.2c | 0.8a |
| Glut | 7.4b | 6.8b | 3.6a | 8.1c | 8.2c | 7.0b | 8.0c | 10.5e | 10.4e | 9.9e | 9.5d | 8.4c |
| Aspr | 0.3a | 0.4ab | 0.6c | 0.5bc | 0.8d | 2.1f | 0 | 0 | 0 | 0.5b | 0.8d | 1.9e |
| Ser | 7.1d | 5.2c | 0 | 9.4g | 9.1fg | 8.0e | 5.3c | 3.5a | 0 | 3.9ab | 5.4c | 3.2a |
| Glu | 2.9bcd | 4.8fg | 5.8h | 3.2de | 4.5f | 6.6i | 1.6b | 4.8g | 9.4j | 1.1a | 1.7b | 2.1bc |
| His | 9.7d | 5.2a | 7.8b | 11.0f | 10.2e | 9.6d | 11.0f | 8.9b | 9.3bc | 10.5ef | 11.0f | 10.0e |
| Thr | 15.1e | 18.8g | 21.6i | 14.5d | 17.8f | 19.6h | 7.0a | 13.9b | 16.9c | 10.3b | 13.6c | 16.6c |
| Cit | 1.6ab | 1.4a | 1.1a | 0 | 0 | 0 | 4.4e | 3.7c | 2.6c | 5.4g | 4.8f | 1.3a |
| Arg | 18.5j | 16.8h | 11.4c | 11.4c | 10.6b | 9.3a | 17.5i | 11.9d | 13.3e | 16.2d | 15.9fg | 15.4f |
| GABA | 9.3hi | 5.0e | 1.6c | 10.1jk | 9.7j | 8.9h | 6.2f | 7.9g | 2.7d | 0.5a | 0.8ab | 0.3a |
| Tyr | 0 | 0 | 0 | 0 | 0 | 0 | 0.9b | 0.7a | 0 | 0 | 0 | 0 |
| Cys | 2.3c | 3.1e | 5.8g | 2.2c | 2.8d | 3.9f | 0 | 0.8a | 1.0a | 0.7a | 0.9a | 1.6b |
| Val | 9.0e | 7.8c | 7.0b | 8.1d | 7.7c | 5.6a | 10.8g | 11.2gh | 10.3f | 15.2j | 12.3i | 10.9g |
| Met | 0.9a | 1.3b | 3.6e | 0 | 0 | 0 | 0.5a | 0 | 0 | 1.3b | 2.3c | 3.1d |
| Try | 1.7a | 1.8a | 2.6c | 2.5b | 3.8e | 4.7f | 3.1d | 2.8c | 3.3d | 2.3b | 3.5e | 7.7g |
| Phe | 4.1d | 3.2c | 3.1a | 3.9b | 2.8b | 2.0a | 5.8g | 4.7e | 4.9e | 7.8i | 6.4h | 5.1f |
| I-Leu | 7.0e | 5.3b | 4.9c | 8.4g | 7.3e | 6.6d | 9.3h | 7.8f | 8.3g | 7.6f | 5.7c | 5.4b |
| Lys | 0.7a | 1.2b | 1.8i | 0.6a | 0.9a | 1.2b | 1.4b | 1.8c | 2.4d | 2.5d | 2.9e | 5.8f |
| Pro | 0.5a | 10.2h | 16.3a | 0.6b | 1.0c | 3.7f | 4.8g | 3.2e | 2.9d | 0.1a | 0.3a | 0.4a |
| Acidic | 9.3c | 8.5b | 5.0a | 13.6h | 11.0e | 8.2b | 10.4d | 12.4g | 12.7h | 14.1i | 11.7f | 9.2c |
| Basic | 28.9e | 23.2c | 21.0a | 23.0c | 21.7b | 20.1a | 29.9f | 22.6c | 25.0d | 29.2d | 29.8f | 31.2g |
| Aromatic | 4.1d | 3.2c | 3.1c | 3.9b | 2.8b | 2.0a | 6.7g | 5.4e | 4.9c | 7.8h | 6.4f | 5.1e |
TD Transplantation date, Asp aspartic acid, Glut glutamic acid, Aspr asparagine, Glu glutamine, His histidine, Thr threonine, Cit citrulline, Arg arginine, GABA λ amino butyric acid, Tyr tyrosine, Cys cystine, Val valine, Met methionine, try Tyrptophan, Phe phenyl-alanine, I-leu isoleucine, Lys lysine, Pro proline, Acidic amino acids Asp and glut, Basic amino acids Lys, Arg and his, Aromatic amino acids Phe and tyr
Values with similar superscript do not differ significantly P > 0.05 to P < 0.05
Fig. 2.
HPLC chromatograms of amino acids from PR121 early (a) and delayed transplanted paddy (b)
Amylose content, blue value and λmax
AC of milled rice from ETP and DTP was ranged from 3.1 to 7.1 % and 5.6 to 7.5 %, respectively (Table 1). Both cultivar and TD showed significant effect on accumulation of AC however, the effect of former was much larger (Supplementary Table 1). AC varied with botanical source and was affected by the climatic and soil conditions during grain development. Furthermore, BV of iodine–starch complexes (monitored at 680 nm) of milled rice from ETP and DTP was ranged from 0.1042 to 0.1058 and 0.1436 to 0.2812, respectively. λmax of milled rice from ETP and DTP ranged from 572.5 to 576.1 and 583.1 to 590.1, respectively. On the contrary BV of starches of milled rice from ETP and DTP was ranged from 0.1310 to 0.1666 and 0.1911 to 0.2156, respectively while λmax ranged from 593.7 to 600.8 nm and 599.0 to 602.5 nm, respectively. All the cultivars showed higher BV and λmax with delayed transplantation due to increased accumulation of amylose (Table 1). Both TD and cultivar showed significant effect on BV and λmax of milled rice and their starch however, the effect of cultivar was much larger as indicated by F value (Supplementary Table 1). BV and λmax provide information on degree of polymerization of amylose and average chain length of amylose and amylopectin. Higher amylose accumulation in the milled rice from DTP may attributed to cool NTAT during active grain filling stage or during the storage of assimilates in the leaf sheath and culms during vegetative phase. DTP of all the cultivars faced nearly identical and favorable agro-climatic conditions during different developmental stages such as panicle initiation, anthesis or flowering and grain filling as compared to ETP (Fig. 1a). The rice plants exposed to cool temperatures for longer periods had higher levels of the GBSS as well as the higher accumulation of amylose. It is thus evident from these studies that the starch biosynthesis was significantly affected by high temperature. The lower accumulation of amylose in the ETP may be associated with impaired accumulation of assimilates associated with starch metabolism during vegetative or flowering phase under heat stress.
SDS-PAGE analysis of milled rice storage proteins
SDS-PAGE analysis of milled rice obtained from different cultivars transplanted at different dates showed presence of 29 major polypeptides ranged from 15.0 to 114.0 kDa (±2.0 kDa) (Fig. 1b). These proteins were classified into granule-bound starch synthase enzyme (GBSS) (60 kDa), glutelin acidic subunits (GAS) (33 and 31 kDa), globulins (24 kDa), glutelin basic subunits (GBS) (24 and 22 kDa) and prolamins (15, 16 and 18 kDa) (Fig. 1b) (Kawakatsu et al. 2008). Densitometry scanning was done to analyze the effect to TD of paddy on accumulation of 60; 33 and 31; 24 and 22, 18, 16, 15 kDa major polypeptides since these polypeptides were showing differential accumulation (Supplementary Figs. 1, 2, 3). Analysis revealed that inter- and intra-cultivar banding pattern of polypeptides was nearly identical (Fig. 1b) but accumulation of the GBSS, GAS, GBS and prolamines was varied in different cultivars and influenced by TD of paddy (Fig. 1b). PR114, PR118, PR121 and PR122 transplanted on 10 June and 20 June showed higher accumulation of GBSS (60 kDa); glutelin acidic subunits (GAS) (33 and 31 kDa), globulins (24 kDa) and glutelin basic subunits (GAB) (21 and 22 kDa) polypeptide followed by a decline in storage of these in the milled rice from DTP. Reduced accumulation of GBSS, GAS, globulins and GBS was observed in DTP may associated to decrease in temperature at the time of delayed transplantation (Fig. 1a, b). Although the accumulation of 60 kDa polypeptide was increased in the milled rice from ETP but AC from the same was significantly lower which revealed that the enzymatic activity of GBSSI may be altered under heat stress. Larkin and Park (1999) revealed that promotor of Wx a allele/GBSSI genes was responsive to cool (18 °C) temperatures and also a mutation at the 5′ splicing site in the first intron of the Wx a allele/GBSSI gene was associated with variation in sensitivity to temperature during seed development.
Storage of prolamines (20, 16; and 15 kDa) was also influenced by TD (Supplementary Fig. 3). Results revealed that accumulation of prolamines was reduced by delayed transplantation however; higher storage of prolamines from milled rice of PR118 as compared to PR114, PR121 and PR122 was observed (Supplementary Fig. 3). Yamakawa and Hakata (2010) demonstrated that the expression of 13 kDa prolamines at transcript and protein levels was significantly reduced under high temperature and heat stress. However, in the present study accumulation of prolamines of 15 kDa was increased in the milled rice from ETP followed by decline in storage of same polypeptide with delayed transplantation. Therefore, the accumulation of some prolamins may be modulated by local temperature and genetic levels. The maximum, minimum and mean temperature for the month of June were 40.6, 27.2 and 34.0 °C, respectively against 35.7, 27.9 and 31.8 °C, respectively, for July 2012 with no rain fall. Mean soil temperature for the depth of 5, 10 and 15 during month of June were 39.4, 37.6 and 36.1 °C, respectively while 36.5, 35.8 and 35.1 °C, respectively for the month of July 2012. Unfavorable climatic conditions at the time of vegetative phase and reproductive phase of paddy significantly affect the grain filling and result in poor grain yield production. Climatic conditions played a crucial role during flowering stage. Paddy transplanted on 10 June flowered in between 20 August to 1 September while 26 August to 6 September for 20 June (Fig. 1a). Whereas, paddy transplanted on 30 June undergoes flowering stage from 9 September to 14 September. Maximum, minimum and average temperature during flowering stage was 33.2, 26.6 and 29.9 °C; 32.8, 23.9 and 28.3 °C, respectively during August and September (Fig. 1a). Average rain fall in month of June was 3.5 mm with three rainy days (10, 18 and 24 June) whereas 10 rainy days were in the month of July (6, 7, 8, 10, 11, 13, 19, 23, 24 and 30 July) with average 76.9 mm rain fall. Paddy transplanted on 30 June completed vegetative and flowering stages in lesser days as compared to paddy transplanted on 10 and 20 June. ETP faced higher temperature regime during both vegetative and flowering stage as indicated in crop performance chart (Fig. 1a) which may be responsible for altered the growth of roots, shoots before heading of paddy. Although all the cultivars faced nearly consistent temperature during grain maturation stages with >75 % humidity. It was therefore likely that effect on yield production, grain size and dry matter may be influenced by significantly higher temperature and lower humidity during early TD (10–20 June). NTAT influenced the rice characteristics and these variations were reported to be cultivar dependent and it was observed that the medium-grain rice cultivars were fairly resistant to elevated NTAT (Cooper et al. 2008). Earlier studies also demonstrated lower accumulation of prolamines and higher values for AC, FV and SB for milled rice from DTP than that of ETP (Singh et al. 2014).
Pasting properties
Pasting properties of starches from ETP and DTP are shown in Table 3. PT of starch from ETP and DTP ranged from 70 to 71.9 °C and 69 to 73 °C, respectively. PV, FV, BD and SB of starch from ETP ranged from 2204 to 3017 cP, 3023 to 5412 cP, 566 to 1458 cP, 1594 to 3193 cP, respectively while DTP ranged from 1995 to 3276 cP, 2411 to 5151, 433 to 1007 cP, 995 to 28543 cP, respectively (Table 3). Starch from ETP showed significant different paste viscosities as compared to starch from DTP. Starch from PR122 showed a significantly higher average PT (75.4 °C) whereas starches from PR114, PR118 and PR121 showed higher PT at the beginning of paddy transplantation except PR122 which showed higher PT at the mid of transplantation of paddy followed by reduced PT with delayed transplantation. PT was strongly correlated with crystalline and amorphous regions in the starch granules and the proportion of short and long chains in amylopectin (Fitzgerald 2004). PV of starch from DTP was higher than that from ETP which attributed to higher AC as compared to former. The formation of starch protein networks is believed to provide mechanical support for starch granules and protect them against rupture and results in increased PV (Saleh and Meullenet 2015). PR118 showed significantly higher average PV as compared to PR114, PR121 and PR122. Starch from DTP showed lower BD than that of ETP because of presence of higher AC. The lower PV and BD of paddy grown at high NTAT compared with rice grown at ambient night temperature had been reported by Song et al. (2013). Therefore, higher BD in starch of ETP may be attributed by higher accumulation of prolamines as compared to delayed transplantation. PR118 showed the higher FV as compared to other cultivars. During cooling increase in paste viscosities may be due to aggregation of amylose molecules. Starch from DTP of PR114 and PR118 showed higher SB than ETP. The cultivar, TD and cultivar × TD effect on pasting viscosities were significant. F values for TD of PT, PV, FV, BD and SB were much larger than cultivar indicated greater effect of TD (Supplementary Table 3).
Table 3.
Effect of transplanting dates on pasting, granule size distribution and thermal properties of starch separated from milled rice from different cultivars
| Cultivars | TD | PT (°C) | PV (cP) | FV (cP) | BD (cP) | SB (cP) | GS (0–5 μm) | GS (5–10 μm) | GS (10–20 μm) | GS (20-60 μm) | To (°C) | Tp (°C) | Tc (°C) | ΔHgel (Jg1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR114 | 10 June | 71.9f | 2961f | 4726f | 1458g | 3167j | 32.76f | 43.37a | 20.71c | 3.16bc | 64.2d | 68.0e | 70.5d | 7.0a |
| PR114 | 20 June | 70.7d | 2966f | 4700f | 1233f | 2967h | 23.92b | 47.27c | 23.79d | 5.02f | 63.6c | 66.4b | 69.2c | 7.9b |
| PR114 | 30 June | 70.7d | 2998e | 4713f | 1007e | 2804g | 25.39c | 49.52d | 21.59c | 3.50d | 62.4a | 66.2b | 69.6c | 8.6c |
| PR118 | 10 June | 70.1c | 2900e | 5412j | 1444g | 3193j | 25.91c | 51.09e | 18.89b | 4.11e | 63.5c | 67.1d | 70.9e | 7.2a |
| PR118 | 20 June | 68.8a | 2998f | 5054h | 986d | 3042i | 22.16a | 44.22b | 27.33f | 6.29g | 63.8d | 66.1b | 68.2a | 10.6e |
| PR118 | 30 June | 69.0b | 3276h | 5151i | 979d | 2854g | 23.63b | 50.59d | 22.48d | 3.30bc | 61.5a | 64.6a | 68.0a | 11.5f |
| PR121 | 10 June | 71.8f | 2985f | 4647e | 989d | 2731f | 23.7b | 49.83d | 22.77d | 3.70d | 63.6c | 67.8d | 71.3e | 8.1c |
| PR121 | 20 June | 70.5d | 2905e | 4865g | 781c | 2661e | 23.45b | 47.81c | 24.41e | 4.33e | 63.0b | 66.3b | 69.5c | 9.1d |
| PR121 | 30 June | 71.2e | 2992d | 4186d | 462a | 1956d | 26.50d | 49.05d | 20.65c | 3.80d | 62.6b | 64.6a | 68.8b | 9.2d |
| PR122 | 10 June | 71.9f | 1704a | 3023c | 566b | 1594c | 25.47c | 53.74f | 17.81b | 2.98b | 63.6c | 66.9c | 70.2d | 7.2a |
| PR122 | 20 June | 81.3h | 1875ab | 2965b | 440a | 1201b | 23.58b | 50.72e | 21.00c | 4.70c | 63.6c | 66.4b | 70.1d | 7.6b |
| PR122 | 30 June | 73.0g | 1995b | 2411a | 433a | 995a | 27.31e | 55.41g | 15.71a | 1.57a | 62.4a | 66.3b | 70.0d | 8.1b |
TD Transplantation date, PT pasting temperature, PV peak viscosity, FV final viscosity, BD breakdown viscosity, SB setback viscosity, GS granule size, T o onset temperature, T p peak temperature, T c conclusion temperature, ΔHgel enthalpy of gelatinization
Values with similar superscript do not differ significantly P > 0.05 to P < 0.05
Granule size distribution
Starch granules size distribution was significantly influenced by cultivars and TD of paddy. The granules of 0–5, 5–10, 10–20 and 20–60 μm size ranged from 23.7 to 32.7, 43.4 to 53.7, 17.8 to 22.8 and 2.9 to 4.1 %, respectively in starch from ETP while ranged from 23.6 to 27.3, 49.5 to 55.4, 15.7 to 21.6 and 1.57 to 3.8 %, respectively was observed in starch from DTP (Table 3). Starch from both the ETP and delayed transplanted rice had highest proportion of granules of 5–10 μm size. The accumulation of starch granules of 20–60 μm had lowest proportion from ETP and DTP. During biosynthesis the proportion of starch granules (0–5 μm) size increased for PR 121 and PR122 while PR114 and PR118 decreased with delayed transplantation than that from ETP. However the accumulation of 5–10 μm was significantly affected by TD except in PR118. F value also showed significant effect on cultivar, TD and cultivar × TD on granules size (Supplementary Table 3). Cultivars granules size varied due to differences in composition and arrangement of the molecules as reported earlier (Wani et al. 2013).
Thermal properties
To, Tp, Tc and ΔHgel of starch isolated from ETP and DTP are summarized in Table 3. To, Tp, Tc and ΔHgel of starch from ETP ranged from 63.6 to 64.2 °C, 66.9 to 68.0 °C, 70.2 to 71.3 °C and 7.0 to 8.1 Jg−1, respectively while 61.5 to 62.6 °C, 64.6 to 66.3 °C, 68.0 to 70.0 °C and 8.1 to 11.5 Jg−1 respectively with delayed transplantation. To, Tp and Tc decreased with delayed transplantation whereas ΔHgel increased. F values of To, Tp, Tc and ΔHgel of the starch was significant for TD and cultivars (Supplementary Table 3). However the effect of cultivar was much larger than TD as indicated by F value. Double helical and crystalline structures of starch was disrupted during gelatinization and the order–disorder phase transition showed melting of crystals (Singh et al. 2007). ΔHgel reflected primarily the loss of molecular (double-helical) order. High transition temperatures had been reported to result from a high degree of crystallinity which provided structural stability and made the granule more resistant to gelatinization. The differences in the gelatinization temperatures among different starches may be due to the presence of crystalline regions within a starch granule composed of small crystallites having slightly different crystal strength. Singh et al. (2003) showed that ΔHgel of starch from different milled rice cultivars varied with AC because amylopectin had been reported to play a major role in the starch crystallinity. The amylose lowered the melting point of crystalline regions and energy for starting the gelatinization process (Flipse et al. 1996). The differences in the gelatinization temperatures may be attributed to the differences in the starch content.
Conclusion
The present study showed changes in grain, protein, AAs and starch characteristics with delay in transplantation of paddy. The higher HRY and lower chalkiness clearly indicated improvement in milling quality of rice. Reduced accumulation of GBSS, GAS, globulins, and GBS was observed in DTP which may be associated due to lowering in temperature at the time of plantation. HRY, whiteness, chalky grains, AC, starch granule size distribution, thermal properties, AAs and pasting viscosities were varied significantly with cultivars and TD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The financial support from the Department of Science and Technology, Ministry of Science and Technology, Government of India, to NS is gratefully acknowledged.
References
- Beckles DM, Thitisaksakul M. Review: how environmental stress affects starch composition and functionality in cereal endosperm. Starch/Starke. 2014;66:58–71. doi: 10.1002/star.201300212. [DOI] [Google Scholar]
- Cooper NTW, Siebenmorgen TJ, Counce PA. Effects of nighttime temperature during kernel development on rice physicochemical properties. Cereal Chem. 2008;85:276–282. doi: 10.1094/CCHEM-85-3-0276. [DOI] [Google Scholar]
- Degenkolbe T, Kopka PT, Zuther J, Hincha EDK, Kohl KI. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS One. 2013;8(5):e63637. doi: 10.1371/journal.pone.0063637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour, Jan A, Hoseney (2010) Structure of cereals. In: Principles of cereal science and technology, 3rd edn. AACC International, pp 1–22. doi:10.1094/9781891127632.001
- Fitzgerald M. Starch. In: Champagne ET, editor. Rice: chemistry and technology. 3. St. Paul: AACC International; 2004. pp. 109–115. [Google Scholar]
- Fitzgerald MA, Resurreccion AP. Maintaining the yield of edible rice in a warming world. Funct Plant Biol. 2009;36:1037–1045. doi: 10.1071/FP09055. [DOI] [PubMed] [Google Scholar]
- Flipse E, Keetels CJAM, Jacobson E, Visser RGF. The dosage effect of the wild type GBSS allele is linear for GBSS activity, but not for amylose content: absence of amylose has a distinct influence on the physico-chemical properties of starch. Theor Appl Genet. 1996;92:21–127. doi: 10.1007/BF00222961. [DOI] [PubMed] [Google Scholar]
- Glaubitz U, Erban A, Kopka J, Hincha DK, Zuther E. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J Exp Bot. 2015 doi: 10.1093/jxb/erv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Kaur P, Singh N, Virdi AS, Singh P, Rana JC. Grains, starch and protein characteristics of rice bean (Vigna umbellata) grown in Indian Himalaya regions. Food Res Int. 2013;54:102–110. doi: 10.1016/j.foodres.2013.05.019. [DOI] [Google Scholar]
- Kawakatsu T, Yamamoto MP, Hirose S, Yano M, Takaiwa F. Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. J Exp Bot. 2008;59:4233–4245. doi: 10.1093/jxb/ern265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:681–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanning SB, Siebenmorgen TJ, Counce PA, Ambardekar AA, Mauromoustakos A. Extreme nighttime air temperatures in 2010 impact rice chalkiness and milling quality. Field Crops Res. 2011;124:132–136. doi: 10.1016/j.fcr.2011.06.012. [DOI] [Google Scholar]
- Larkin PD, Park WD. Transcript accumulation and utilization of alternate and non-consensus splice sites in rice granule-bound starch synthase are temperature-sensitive and controlled by a single nucleotide polymorphism. Plant Mol Biol. 1999;40:719–727. doi: 10.1023/A:1006298608408. [DOI] [PubMed] [Google Scholar]
- Liang X, King JM, Shih FF. Pasting property differences of commercial and isolated rice starch with added lipids and β-cyclodextrin. Cereal Chem. 2002;79:812–818. doi: 10.1094/CCHEM.2002.79.6.812. [DOI] [Google Scholar]
- Mahajan G, Sharma N, Kaur R, Chauhan BS. Comparison of photoperiod-sensitive and photoperiod-insensitivebasmati cultivars for grain yield, water productivity, and quality traits under varied transplanting dates in northwest India. Crop Pasture Sci. 2015;66:793–801. doi: 10.1071/CP14297. [DOI] [Google Scholar]
- Oteng-Darko P, Kyei-Baffour N, Ofori E. Yield of rice as affected by transplanting dates and plant spacing under climate change simulations. J Agric Res. 2013;12:55–63. [Google Scholar]
- Pal P, Kaur P, Singh N, Kaur A, Misra NN, Tiwari BK, Cullen PJ, Virdi AS. Effect of nonthermal plasma on physico-chemical, amino acid composition, pasting and protein characteristics of short and long grain rice flour. Food Res Int. 2016;81:50–57. doi: 10.1016/j.foodres.2015.12.019. [DOI] [Google Scholar]
- Pal P, Singh N, Kaur P, Kaur A, Virdi AS (2016b) A comparison of composition, protein, pasting and phenolic compounds of brown rice and germinated brown rice from different cultivars. doi:10.1094/CCHEM-03-16-0066-R
- Saleh MI, Meullenet JF. Effect of moisture content at harvest and degree of milling (based on surface lipid content) on the texture properties of cooked long-grain rice. Cereal Chem. 2007;84:119–124. doi: 10.1094/CCHEM-84-2-0119. [DOI] [Google Scholar]
- Saleh MI, Meullenet JF. Cooked rice texture and rice flour pasting properties; impacted by rice temperature during milling. J Food Sci Technol. 2015;52:1602–1609. doi: 10.1007/s13197-013-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Singh N, Virdi AS, Rana JC. Himalayan kidney bean germplasm grain flour characteristics, structural-functional properties and invitro digestibility of starches. Food Res Int. 2015;77:498–505. doi: 10.1016/j.foodres.2015.08.030. [DOI] [Google Scholar]
- Shi W, Muthurajan R, Rahman H, Selvam J, Peng S, Zou Y, Jagadish KS. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013;97:825–837. doi: 10.1111/nph.12088. [DOI] [PubMed] [Google Scholar]
- Singh N, Singh H, Kaur K, Bakshi MS. Relationship between the degree of milling, ash distribution pattern and conductivity in bran rice. Food Chem. 2000;69:147–151. doi: 10.1016/S0308-8146(99)00237-X. [DOI] [Google Scholar]
- Singh N, Sodhi NS, Manmeet K, Saxena SK. Physicochemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels. Food Chem. 2003;82:433–439. doi: 10.1016/S0308-8146(03)00007-4. [DOI] [Google Scholar]
- Singh N, Nakaura Y, Inouchi N, Nishinari K. Fine structure, thermal, viscoelastic properties of starch separated from indica rice cultivars. Starch/Stärke. 2007;59:10–20. doi: 10.1002/star.200600527. [DOI] [Google Scholar]
- Singh S, Singh N, Isono N, Noda T. Relationship of granule size distribution and amylopectin structure with pasting, thermal and retrogradation properties in wheat starches. J Agric Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- Singh N, Paul P, Virdi AS, Kaur P, Mahajan G. Influence of early and delayed transplantation of paddy on physicochemical, pasting, cooking, textural and protein characteristics of milled rice. Cereal Chem. 2014;91:389–397. doi: 10.1094/CCHEM-09-13-0193-R. [DOI] [Google Scholar]
- Sodhi NS, Singh N. Morphological, thermal and rheological properties of starches separated from rice cultivars grown in India. Food Chem. 2003;80:99–108. doi: 10.1016/S0308-8146(02)00246-7. [DOI] [Google Scholar]
- Song X, Du Y, Song X, Zhao Q. Effect of high night temperature during grain filling on amyloplast development and grain quality in japonica rice. Cereal Chem. 2013;90:114–119. doi: 10.1094/CCHEM-01-12-0010-R. [DOI] [Google Scholar]
- Suwansri S, Meullenet JF. Physicochemical characterization and consumer acceptance by Asian consumers of aromatic jasmine rice. J Food Sci. 2004;69:250–257. doi: 10.1111/j.1365-2621.2004.tb17883.x. [DOI] [Google Scholar]
- Takeda C, Takeda Y, Hizukuri S. Physicochemical properties of lily starch. Cereal Chem. 1983;60:212–216. [Google Scholar]
- Tashiro T, Wardlaw IF. The effect of high temperature on kernel dimensions and the type and occurrence of kernel damage in rice. Aust J Agric Res. 1991;42:485–496. doi: 10.1071/AR9910485. [DOI] [Google Scholar]
- Umemoto T, Nakamura Y, Ishikura N. Activity of starch synthase and the amylose content in rice endosperm. Phytochemistry. 1995;40:1613–1616. doi: 10.1016/0031-9422(95)00380-P. [DOI] [Google Scholar]
- Wani AA, Singh P, Shah MA, Wani IA, Gotz A, Schott M. Physico-chemical, thermal and rheological properties of starches isolated from newly released rice cultivars grown in Indian temperate climates. Food Sci Technol. 2013;53:76–183. [Google Scholar]
- Williams PC, Kuzina FD, Hlynka I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–420. [Google Scholar]
- Yamakawa H, Hakata M. Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010;51:795–809. doi: 10.1093/pcp/pcq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.