Abstract
Dextran (polyol) was oxidized with 0, 0.5, 1, and 2 % sodium hypochlorite at pH 9.5 and 35 °C to produce polyaldehyde dextran (PD), which was subsequently conjugated with soy peptides (SP) to improve surface activity. SP–PD complexes were formed by heating 1 % SP and 10 % PD at 60 °C and pH 6.5 for 48 h. PD was more reactive than unmodified dextran with SP to produce conjugates based on the Schiff base with absorption at 294 nm. The formation of SP–PD complexes was confirmed by SDS–PAGE with glycoprotein staining. Turbidity and particle size measurements indicated the SP–PD conjugates had significantly improved emulsifying properties compared to non-conjugated SP and the SP/PD mixtures. The results indicate that controlled oxidation of polysaccharides can be a novel technique to efficiently synthesize amphiphilic functional biopolymers.
Keywords: Polysaccharide, Polyaldehyde, Peptide, Maillard conjugation, Oil/water emulsion
Introduction
The foreseeable shortage of the supply of food proteins in the near future underscores the importance of maximizing the functionality of protein ingredients for efficient usage in formulated food products. Most legume proteins in their native structure are of limited functionality (Franzen and Kinsella 1976; Lawal et al. 2007). Accordingly, structure-modifying methods, including thermal, chemical, and enzymatic treatments, are widely employed to enhance the functional behavior for optimal performance (Foegeding and Davis 2011). Reported improvements include solubility, foaming capacity, emulsifying activity, and gelling potential (Jiang et al. 2009; Liu et al. 2012; Morales et al. 2015; Tsumura et al. 2005).
Although generally considered amphiphilic, not all proteins possess satisfactory surface activity due to their poor water solubility. This is particular true for native soy globulins (Johnston et al. 2015). To mitigate the deficiency, there have been numerous attempts aimed at conjugating water-soluble polysaccharides to proteins for improved emulsifying properties (Kato 2002; Oliveira et al. 2016). Essentially, this Maillard-type complexation involves the initial condensation between an aldehyde group in a polysaccharide and an amino group in a protein or peptide to form Schiff base polymers.
There are two ways to form Maillard-type protein–polysaccharide conjugates: dry heating and wet incubation. Because of humidity control requirements (e.g., 79 %) and long preparation time (1–3 weeks) (Diftis and Kiosseoglou 2006; Kato et al. 1992), the dry heating method is not feasible for practical applications. Wet Maillard complexation, on the other hand, is simple, convenient, and easy to control, and can be completed within 2 days (Zhu et al. 2008). Whey protein–dextran conjugates formed by heating aqueous mixtures at 60 °C for 48 h were reported to improve emulsifying activity and emulsion stability (Zhu et al. 2010). However, regardless of the conjugation method, the limited number of free aldehyde groups (usually 1 per mol) naturally present in polysaccharides hinders their efficient cross-linking with proteins. In order to increase free aldehyde groups available for Maillard reaction, a ring-opening oxidative cleavage of the C–C bond that yields free aldehyde groups may be exploited (de Nooy et al. 1995).
Previous studies have shown that oxidation of dextran could generate dialdehydes on glucose monomers as well as low-molecule-weight fragments (Sloan et al. 1954). Maia et al. (2011) reported that low and mild oxidation with periodate could cause C–C bond cleavage in dextran resulting in aldehyde formation. Periodate-oxidized dextran is considered to be a very reactive polyaldehyde compound towards N-nucleophiles, including amines and hydrazines, and synthesized polyaldehyde dextran (PD) has been used in pharmaceuticals (Suvorova et al. 2001).
Sodium hypochlorite (NaOCl), one of the most popular commercial oxidants used in the food industry, has been applied to oxidatively modify starch to produce amylose aldehydes and fragments with improved rheological and baking properties (Kuakpetoon and Wang 2001), as can be demonstrated in the following reaction. It is possible that by taking a similar approach utilizing NaOCl as the oxidizing agent, PD can be formed which would allow efficient Maillard conjugation reaction with proteins to develop functionality-improved protein ingredients.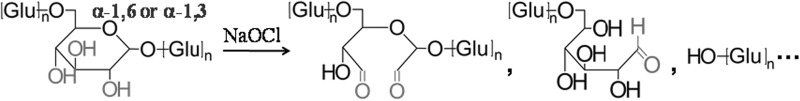
The objectives of this study were to generate PD by means of NaOCl treatment and to assess the efficacy of PD in the formation of functional complexes with soy peptides (SP) through Schiff base condensation under mild heating conditions (60 °C) in a pH 6.5 aqueous solution. This rather mild condition promotes protein–polysaccharide complexation without the generation of Maillard end products, including potentially toxic advanced glycation end products, such as carboxymethyllysine, which typically are formed at extremely high temperatures (Zhu et al. 2008). SP prepared with Flavourzyme® (from Aspergillus oryzae) was used because of the abundance of free amino groups for Maillard reaction and the multi-functional properties, notably antioxidative activity (de Castro and Sato 2014).
Materials and methods
Materials
Soy protein isolate (SPI, Profam 646, 93.5 % protein dry basis) was obtained from Archer Daniels Midland Co. (Decatur, IL, USA). Dextran (MW 40,000) was purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). Chemicals and reagents, all analytical grade unless specified otherwise, were purchased from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich (St. Louis, MO, USA). Nanopure deionized water was used for the preparation of all solutions.
Preparation and analysis of polyaldehyde dextran (PD)
Oxidized dextran was prepared according to a method described previously with minor modifications (Wang and Wang 2003). A 40 % solution of dextran was prepared in the 35 °C water bath and adjusted to pH 9.5 using 2 M NaOH. Oxidation was initiated by adding 6 % NaOCl solution dropwise over a 30-min span with stirring while maintaining the pH 9.5 by adjustment with 2 M H2SO4 as needed until the target concentrations of 0.5, 1.0, and 2.0 g NaOCl per 100 g dextran were achieved. Upon completion of NaOCl addition, the solution was stirred for another 30 min while the pH was still maintained at 9.5 with 1 M NaOH during this time. The oxidized dextran solution was then adjusted to pH 7.0 with 1 M HCl, followed by the addition of 4 vol. of absolute ethanol under constant agitation for 10 min. The mixture was centrifuged at 5000×g for 30 min at room temperature, and the precipitate (oxidized dextran) was dried in a forced air oven at 45 °C for 24 h.
Total aldehyde content in the oxidized dextran samples was measured by the 3,5-dinitrosalicylic acid (DNS) colorimetric method (Miller 1959). An aliquot of 3 mL oxidized dextran solution (5 mg/mL) was mixed with 1.5 mL of DNS reagent, boiled for 5 min, then cooled for 10 min at room temperature. The volume was then brought to 25 mL with H2O, and the absorbance at 540 nm was recorded. Total aldehyde content was calculated from a standard curve prepared with glucose. Transmission Fourier transform infrared spectroscopy (FTIR) was used to measure the aldehyde groups to verify oxidative hydroxyl-to-aldehyde conversion.
Preparation of soy peptides (SP)
A 5 % SPI aqueous suspension (pH 6.5) was hydrolyzed in a 50 °C water bath for 30 min by Flavourzyme® (Novozymes, Bagsvaerd, Denmark) at an enzyme/protein ratio of 5:100 then neutralized to pH 7.0 with 1 M NaOH. The hydrolysate was heated in a 85 °C water bath for 20 min to inactivate the enzyme. After centrifugation at 5000×g for 20 min to remove insoluble particles, the supernatant, which contained mixed peptides referred to as SP, was lyophilized. The dry SP powder was sealed in plastic ziplock bags, and stored in a refrigerator at 4 °C.
Preparation and verification of peptide–dextran complex
Lyophilized SP was dissolved (10 mg/mL) in 10 mM sodium phosphate buffer (pH 6.5) and stirred at room temperature for 2 h. The solution was transferred to a walk-in cooler (approximately 4 ± 1 °C) and pre-chilled PD (100 mg/mL) was added. The mixture was agitated constantly for 12 h in the cooler. Maillard conjugation reaction was carried out by incubation of the SP/PD mixture in a 60 °C water bath for 48 h. The control samples were the same mixture but kept at 4 °C for 48 h. To prevent bacterial growth, sodium azide (0.2 mg/mL) was included in the above solutions. Both the control (SP/PD mixtures) and the conjugated complexes (SP–PD) were centrifuged at 10,000×g for 20 min at room temperature. The supernatants were collected and analyzed immediately for free amines, Schiff base, and advanced Maillard reaction products to verify the SP–PD formation.
Free amines in SP, SP/PD mixture, and SP–PD conjugate samples were quantified using the 2,4,6–trinitrobenzene sulfonic acid (TNBS) method with modifications (Snyder and Sobocinski 1975). Aliquots (200 µL) of diluted sample solutions were mixed with 2.0 mL of 1 mg/mL SDS in 200 mM phosphate buffer (pH 8.0) and 1.0 mL of 0.1 mg/mL TNBS then incubated in a 50 °C water bath for 30 min. The reaction was terminated by adding 2.0 mL of 100 mM sodium sulfite. After cooling to room temperature, the absorbance at 420 nm was read, and the free amine content was calculated from a standard curve generated with 0–10 mM leucine.
Dilute SP, SP/PD mixture, and SP–PD conjugate samples were subjected to UV spectroscopy at 294 nm to verify Schiff base formation and at 420 nm to evaluate advanced Maillard reaction products (Dong et al. 2012). Because binding of polysaccharides to a protein can result in conformation changes, intrinsic tryptophan (Trp) fluorescence of control and conjugated samples were measured. This was done by using a 280 nm excitation wavelength and 300–400 nm emission wavelengths using a fluorescence spectrophotometer (FluoroMax 3, SPEX Industries, Edison, NJ, USA).
Electrophoresis
SDS–PAGE was run according to Laemmli (1970) under non-reducing condition using mini-PROTEAN® TGX™ precast gradient gel (4–20 % polyacrylamide) purchased from Bio-Rad (Hercules, CA, USA). After electrophoresis, one gel was stained for protein with Coomassie blue while another was stained for glycoprotein with the Pierce™ Glycoprotein Staining Kit of the periodic acid–Schiff method (Pierce Biotechnology, Rockford, IL, USA).
Emulsion preparation and analyses
Emulsion preparation
Emulsions were prepared first by homogenization of a mixture of 5 % (v/v) soybean oil and 95 % (v/v) SP, SP/PD, or SP–PD conjugate (1 % protein) in 10 mM sodium phosphate solution (pH 6.5) using a Model PT10-35 Polytron homogenizer equipped with a low-foam probe (Kinematica Inc., Bohemia, NY, USA) at a speed setting 3 for 2 min. Fine emulsions were obtained by passing the crude emulsions once through Nano DeBee high-pressure homogenizer at 34 MPa (BEE International Inc., MA, USA). To control the temperature, the emulsion pipeline was insulated with a cold sleeve. The emulsions were immediately subjected to physical property tests as described below.
Emulsifying activity
The method reported by Pearce and Kinsella (1978) was adopted to measure emulsifying activity. Aliquots of 10 mL freshly-prepared emulsions were immediately transferred to flat-surface glass vials (1.5 cm dia.) and placed in a 4 °C refrigerator. Emulsion samples (25 µL each) were taken by pipetting from the bottom of the vials at 0, 20, 40, 60, and 120 min during storage and diluted with 5 mL of 1 mg/mL SDS. The turbidity (absorbance value) was measured at 500 nm. The absorbance of time 0 samples was used to indicate the emulsifying activity. Since flocculation and eventual coalescence of O/W emulsions are inevitable during storage resulting in the rise of oil droplets, the measured turbidity of the emulsions sampled from the bottom of the vials during storage (up to 120 min) was used to indicate the emulsion stability.
Emulsion morphology
Emulsion droplets were observed by phase contrast microscopy using a 100× oil-immersion objective lens. Images were taken with a MICROPHOT-FXA Nikon photomicroscope equipped with a built-in digital camera (Nikon Inc., Garden City, NY, USA).
Emulsion droplet size
The diameter (Z-average, cumulant size) of emulsion droplets was measured using dynamic light scattering on a Zetasizer Nano S90 (Malvern Instruments Ltd., Malvern, UK). Emulsion samples were dispersed in 10 mM phosphate buffer (pH 6.5) to an appropriate particle density range (generally 200× dilution) before measurement. For emulsion stability evaluation, emulsion samples from various storage times (up to 120 min) were tested.
Statistical analysis
All experiments were repeated 2 times and each measurement was carried out in triplicate. Data was analyzed for variance using the general linear model procedures of Statistix 9.0 software (Analytical Software Inc., St. Paul, MN, USA). A one-way analysis of variance (ANOVA) was performed to determine treatment effects. When a treatment effect was found significant (P < 0.05), Turkey HSD all-pairwise multiple comparisons were performed to identify significant differences between individual means.
Results and discussion
Characteristics of polyaldehyde dextran
Aldehyde content
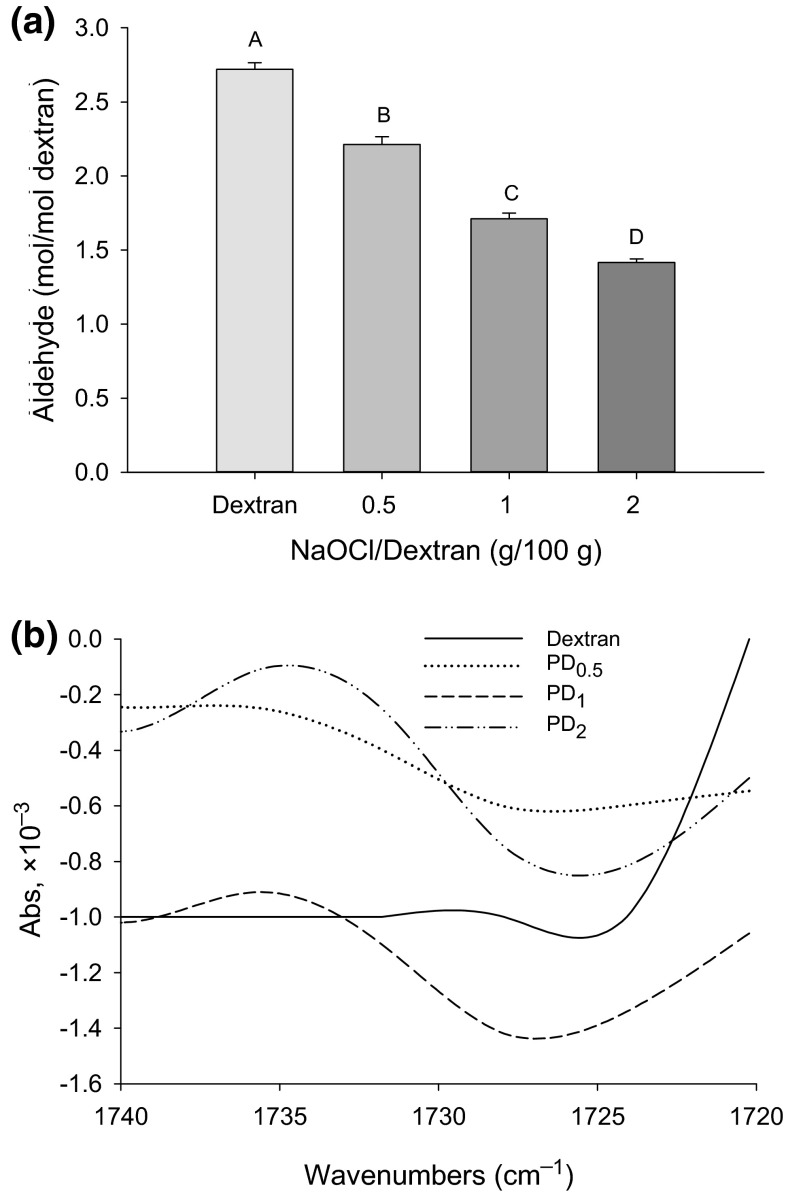
The aldehyde content, detected with the DNS method, shows an unexpected initial (non-oxidized) amount of aldehyde and a surprisingly steady decline at increasing oxidant (NaOCl) concentrations (Fig. 1a). Alternative detection methods from preliminary trials, including the hydroxylamine–HCl titration method (Wang and Wang 2003) and the Cu(I)-reduction or Nelson–Somogyi method (Gusakov et al. 2011), were unsatisfactory because they produced similar trends of underestimated carbonyl production by oxidation. This was due to the oxidative destruction of previously formed aldehydes under strong alkaline conditions, the reagents (e.g., Cu) used during the assay, or the low reactivity of carbonyls with amine reagent. Because the DNS method requires a strong alkaline condition (1 % NaOH) and high temperature (100 °C), the DNSacid-reactive carbonyl groups often include those generated during the assay rather than indigenous aldehydes, hence, the inflation of the carbonyl content (Vettori et al. 2011). This explains why the control (non-oxidized) dextran sample displayed a detected high aldehyde content (2.72 rather than 1 mol/mol).
Fig. 1.
Detectable aldehyde content of dextran (non-oxidized) and polyaldehyde dextran (PD) prepared with different concentrations of NaOCl (a); means (n = 3) with different letters (A–D) differ significantly (P < 0.05). FTIR spectra of dextran and PD (b); PD 0.5, PD 1, and PD 2 denote PD prepared with 0.5, 1, and 2 g NaOCl per 100 g dextran, respectively
On the other hand, Vettori et al. (2011), when comparing different methods for the determination of reducing groups in sugars and polysaccharides, demonstrated that with the DNS method, the higher the molecular weight the greater the degree of over-oxidation of polysaccharides. Since oxidation of dextran is usually accompanied by extensive cleavage of the chain (Manju and Sreenivasan 2011), oxidative conversion by higher concentrations of NaOCl would lead to a decreased size of PD due to fragmentation, hence, less over-oxidation. It must also be noted that in the presence of a strong oxidizing agent, some newly formed aldehyde groups can be further oxidized into carboxyls, which has been observed in oxidatively modified starch (Wang and Wang 2003). This would also contribute to the under-estimation of the aldehyde content in NaOCl-oxidized dextran samples.
FTIR
To confirm the presence of aldehyde groups in PD, FTIR was run and the spectra are shown in Fig. 1b. A small absorbance peak located at 1735 cm−1, typical of an aldehyde functional group according to IR spectra libraries (Bogdanov and Palamareva 2004), indicates significant differences between the control (dextran) and oxidized samples. While the control sample was devoid of the peak, PD1 and PD2 exhibited remarkable absorbance at 1735 cm−1, suggesting the presence of aldehydes in significant quantity. However, no noticeable aldehyde peak was detected in PD0.5. By confirming the oxidative production of aldehydes, the FTIR results indicated the unreliability of the chemical DNS detection method.
Characteristics of Maillard complex
Free amino group content
Because the Maillard-type polysaccharide–protein condensation reaction consumes primary amino groups, the content of free amines after the completion of the conjugation reaction was quantified (Fig. 2a). As expected, the mixture of SP and dextran stored at 4 °C, regardless of oxidation, showed no NH2 reduction (P > 0.05) due to the low temperature. Surprisingly, at 60 °C, where Maillard reaction would occur favoring the Schiff base production (Zhu et al. 2008), a significant increase instead of decrease of the NH2 content was observed in the SP–non-oxidized dextran conjugate as well as in most of the SP–oxidized dextran samples. The increase of the apparent NH2 content may be explained because binding of polyol to a protein may cause protein unfolding leading to greater exposure of the buried amino groups (Samanta et al. 2014). Hence, the amount of detectable amines would reflect the net effect of the two opposing actions of dextran: decreasing the amine content by the formation of carbonyl–amine Schiff base and increasing the accessible (free) amine groups due to protein conformational change.
Fig. 2.
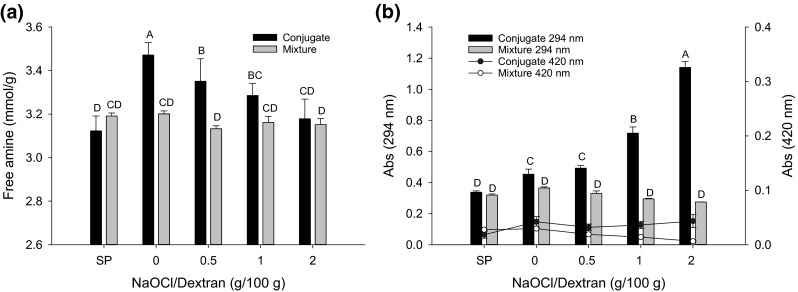
Free amine (a) and UV (294 nm)/VIS (420 nm) absorbance (b) of SP–PD conjugates (60 °C, 48 h) and SP/PD mixtures (4 °C, 48 h). Polyaldehyde dextran (PD) was prepared with different concentrations of NaOCl. Means (n = 3) with different letters (A–D) differ significantly (P < 0.05)
The free amine content in SP–oxidized dextran conjugation samples decreased steadily (P < 0.05) with increasing NaOCl concentrations when compared with the SP–non-oxidized dextran control (Fig. 2a). This is because with an increased amount of aldehyde groups (Fig. 1b), PD prepared with higher NaOCl concentrations is more reactive and consumes more amino groups than non-oxidized dextran at the initial stage of the Maillard reaction. However, for samples incubated at 4 °C, there was no obvious difference between oxidized and non-oxidized dextran samples, indicating that temperature is a critical factor eliciting an impact by oxidative modification.
Schiff base UV absorbance
The formation of Schiff base polymers at the beginning of the Maillard reaction was indicated by the increase in the absorption intensity at 294 nm (Fig. 2b). The absorbance at 420 nm, attributed to brown pigments formed during the advanced stages of the Maillard reaction (Zhu et al., 2008), was also measured to assess the extent of by-product formation in the conjugation process. A remarkable NaOCl dose-dependent increase (P < 0.05) in the Schiff base content (Abs294) can be observed in the conjugated complex; however, there was no significant difference between non-conjugated SP/PD mixture samples. The results are strong evidence that oxidatively-produced PD greatly contributed to the formation of Schiff base polymers in SP–PD1 and SP–PD2 conjugates, and the NaOCl concentration dependency was in accordance with the observed changes of the aldehyde absorbance as revealed by FTIR (Fig. 1b). All SP samples, with or without native and oxidized dextran, exhibited a rather low absorbance at 420 nm, indicating a marginal level of brown pigment production. Even though the SP conjugates with both non-oxidized and oxidized (PD) dextran samples displayed a slightly higher browning intensity (Abs420) than SP alone, the degree of oxidation did not enhance the color development (P > 0.05). Therefore, the processing condition employed to conjugate SP and PD (60 °C, pH 6.5, 48 h) promoted the synthesis and stability of the Schiff base, thereby limiting the generation of advanced Maillard reaction products.
Trp fluorescence
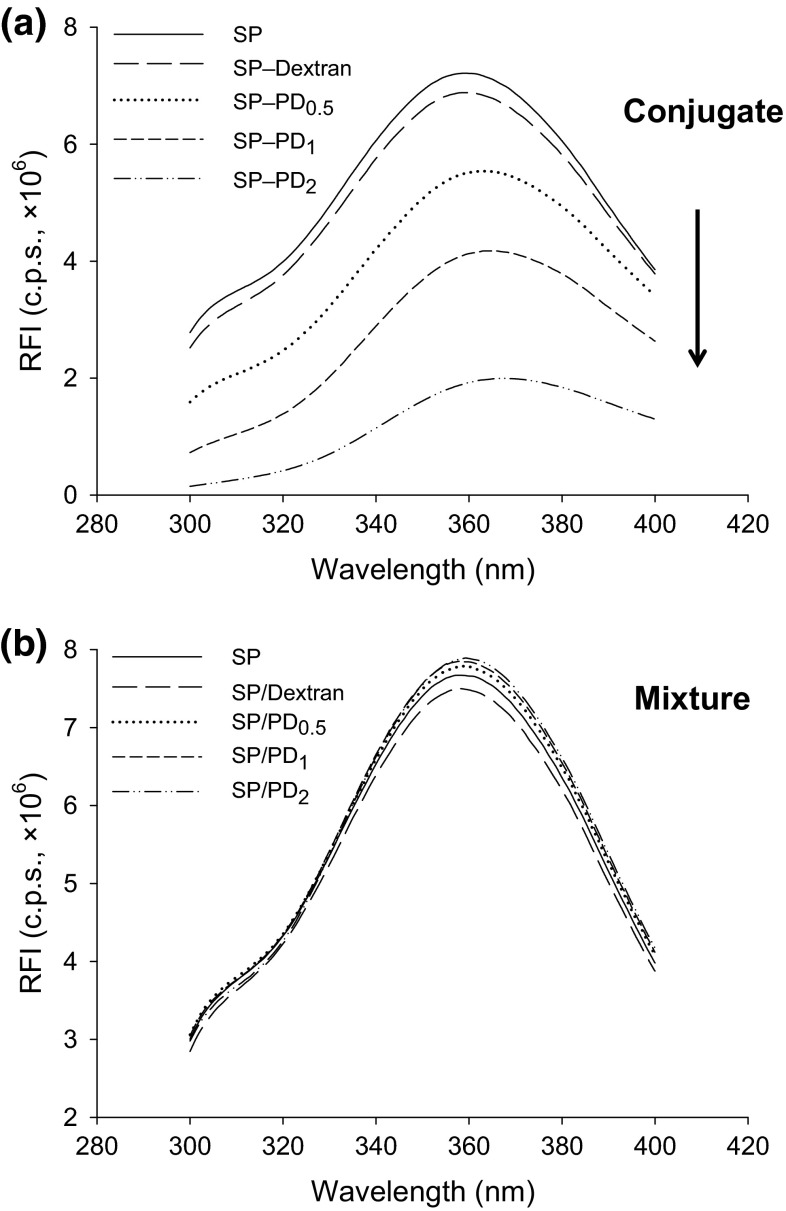
Intrinsic Trp fluorescence was measured to further confirm the Maillard conjugation between SP and PD, because the covalently-bound protein–polysaccharide complex becomes more hydrophilic than unbound protein, therefore, will have reduced electron excitation due to quenching. The fluorescence spectra of conjugated and non-conjugated SP and PD are depicted in Fig. 3. The PD-induced changes in the relative fluorescence intensity (RFI) and the maximal emission wavelength were negligible for SP/PD mixtures (Fig. 3b); however, both changes were remarkable for the SP–PD conjugates (Fig. 3a). While SP and its mixture with PD fluoresced maximally at 358 ± 1 nm, Trp fluorescence of PD-conjugated SP had a major red shift to 360–368 nm, each being accompanied by a markedly reduced RFI in a NaOCl-dependent manner.
Fig. 3.
Relative intensity of intrinsic fluorescence (RFI) of SP–PD conjugates (60 °C, 48 h) (a) and SP/PD mixtures (4 °C, 48 h) (b). PD 0.5, PD 1, and PD 2 denote PD prepared with 0.5, 1, and 2 g NaOCl per 100 g dextran, respectively
The emission spectra at 300–400 nm is mainly due to the excitation of Trp at 280 nm. The intensity and wavelength corresponding to the maximum fluorescence emission of Trp is solvent-dependent, which can be used to monitor protein folding due to the sensitivity of its spectral properties to the environment (Vivian and Callis 2001). A plausible explanation for the observed 5–9 nm red shifting of the SP–PD conjugates could be that, in the SP–PD complex, protein (peptide) structural changes caused by binding with dextran predisposes Try residues to a polar microenvironment, hence, reducing energy of the excited electrons. On the other hand, the loss of the RFI from solvent relaxation quenching may be attributed to the less hydrophobic contacts, hence, a lower energy level of excited Trp (Rizzo et al. 1986).
SDS–PAGE
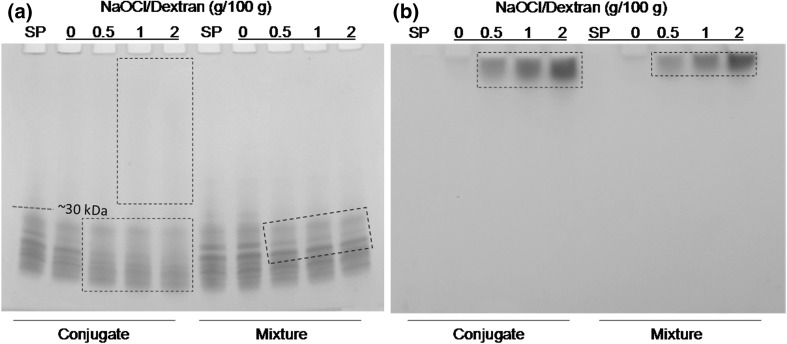
The covalent attachment of SP–PD was verified by SDS–PAGE with protein as well as carbohydrate staining in Fig. 4. The electrophoretic patterns of SP–PD conjugates with peptide-staining Coomassie blue reagent (Fig. 4a) showed a faint, continuing smear that ran through the entire upper region (>30 kDa) of the lane in the SP–PD1 and SP–PD2 samples, differing sharply from the SP control (without dextran) that consisted of only the fragments of soy proteins (<30 kDa). The occurrence of the smear suggests the formation of heterogeneous polymers with each being of a small quantity. It is also noticeable that as the concentration of NaOCl increased, the smear in the conjugate samples became more visible while the original peptide fragments attenuated, suggesting that the smear originated from small peptides.
Fig. 4.
SDS–PAGE of SP–PD conjugates (60 °C, 48 h) and SP/PD mixtures (4 °C, 48 h). a Coomassie blue staining, b glycoprotein staining
Non-oxidized dextran contains a single free aldehyde group per mol, but PD, due to the hydroxyl oxidation by NaOCl, possesses multiple aldehyde groups. Therefore, more covalent bonds in SP–PD conjugates may form Schiff base between aldehyde and ε-amino groups through Maillard reaction, producing an array of heterogeneous polymers. This explains the emergence of the smear above the location of the original SP bands. It is noteworthy that in SP samples mixed with non-oxidized dextran, there were some detectable losses of SP. This was more conspicuous during incubation at 60 °C than 4 °C, evincing heating promoted SP conjugated with dextran to form polymers by Maillard reaction. Similarly, several peptide bands in the SP/PD mixtures became fainter during incubation at 4 °C, suggesting that PD was still somewhat reactive at low temperatures.
To more directly establish the participation of PD in Schiff base formation, another SDS–PAGE gel was stained with the glycoprotein staining kit (Fig. 4b). Broad, high MW bands (on the top of the gel) are evident for both SP–PD conjugates and SP/PD mixtures, and more intense at higher NaOCl concentrations. No clear bands are detected in non-oxidized dextran samples, suggesting that oxidized dextran was an integral part of the polymers formed. By virtue of supplying multiple reactive carbonyl groups, PD functioned as a cross-linking agent to connect different peptides leading to covalently bound agglomerates and aggregates. The polymers detected by glycoprotein staining in the SP/PD mixtures, albeit conspicuously less abundant compared with the SP–PD samples, are somewhat surprising but indicate an enhanced reactivity of dextran once it became a polyaldehyde derivative. Ahmad et al. (2006) also reported that dextran oxidized by periodate could conjugate with the α-amino group of 5-aminosalicylic acid at room temperature for 24 h. The SDS-PAGE results, in corroboration with free amine content and Schiff base quantity measurement (Fig. 2), further proved the efficacy of oxidatively modified dextran in producing non-enzymatic Schiff base peptide polymers.
Emulsifying properties
Emulsifying activity
The ability to disperse oil by conjugated SP and the microscopic images of the O/W emulsions is presented in Fig. 5. The disruption of the compact quaternary structure and exposures of the buried hydrophobic groups have been shown to improve the surface activity of soy proteins (Feng and Xiong 2003). Furthermore, naturally occurring free NH2 groups and those generated from peptide bond cleavages could react with the aldehyde groups present in PD, forming surface active Maillard-type hybridized polymers. Due to increased steric hindrance, these conjugates would provide the emulsions with a greater stability than native proteins. Conjugation with PD would also overcome the general instability of the interfacial membrane formed by short peptides that occur due to charge repulsion.
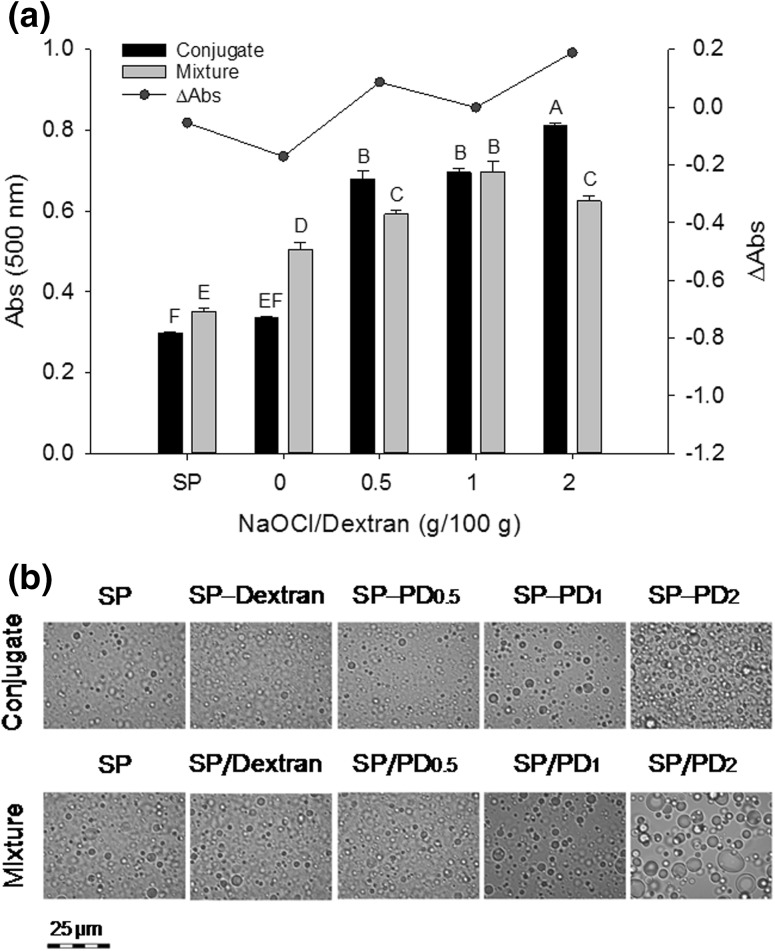
Fig. 5.
Turbidity (a) and phase contrast microscopy (b), measured immediately after emulsification, of SP–PD conjugates (60 °C, 48 h) and SP/PD mixtures (4 °C, 48 h) for soybean oil O/W emulsions. ΔAbs = (Abs of conjugate − Abs of mixture) at 500 nm. Means (n = 3) with different letters (A–D) differ significantly (P < 0.05). PD 0.5, PD 1, and PD 2 denote PD prepared with 0.5, 1, and 2 g NaOCl per 100 g dextran, respectively
Indeed, emulsions with all the SP–PD conjugates showed a higher turbidity (Abs500) in comparison with SP, and the positive ∆Abs values suggest the SP–PD conjugates were more surface active than their non-conjugated SP/PD mixtures (Fig. 5a). The increased turbidity of SP–PD emulsions associated with higher concentrations of NaOCl can be explained because a higher yield of surface active Schiff base polymers can be obtained as more aldehyde groups become available in PD. The improved surface activity of the SP–PD conjugates is due to a newly acquired balance between hydrophobic (in SP) and hydrophilic (in PD) groups in the polymers. It is conceivable that, owing to the multitude of aldehyde groups present, each PD molecule could potentially cross-link with several peptides, thereby increasing the opportunity to adsorb on the oil droplet surface. In several similar studies, reconstructed peptide polymers produced by Maillard conjugation or microbial transglutaminase cross-linking were found to have superior emulsifying properties over free peptides (Babiker 2000; Li et al. 2013). In corroboration, the SP–PD2 emulsion consisted of visibly smaller oil droplets when compared with the SP/PD2 mixture emulsion that displayed mostly inhomogeneous large particles (Fig. 5b). Because of limited conjugation, the SP–non-oxidized dextran sample showed no difference from SP (P > 0.05). However, both had slightly lower emulsifying activity than their respective counterparts, i.e., the mixtures stored at 4 °C, suggesting that long-term incubated SP at the conjugation temperature (60 °C) is less adsorbable onto oil droplets.
Emulsion stability
To test the stability, the evolution of turbidity and growth of the emulsion particle size was monitored during storage (Fig. 6). Throughout 120 min, the turbidity of the SP–PD2 emulsion show no change, and both the SP–PD1 and SP–PD2 emulsions maintained higher turbidity values (P < 0.05) than SP–PD0.5 and control emulsion samples, indicating a relatively high stability. Moreover, conjugated (SP–PD) peptide emulsions were generally more stable than non-conjugated (SP/PD) peptide emulsions, and the most remarkable improvement is demonstrated by the SP–PD2 sample, which is indicated by the increased ∆Abs values (see table). Since 0.1 % SDS, which enables the separation of agglomerates and aggregates of protein-coated oil droplets (Pearce and Kinsella 1978), was used to disperse the emulsion samples prior to the turbidity measurement, the turbidity value would not reveal any physical contacts of emulsion droplets occurring during storage. Therefore, dynamic light scattering was subsequently applied to monitor changes in the apparent emulsion droplet size, and the result is shown in Fig. 6b. In the emulsion prepared with the SP/PD2 mixture, the initial average droplet size was 1.5 µm; it grew to over 2 µm after 120 min. In comparison, the emulsion stabilized by the SP–PD2 conjugate had an initial particle size of 0.5 µm which rose to 1 µm after 120 min. Except for the anomaly recorded at 40 min, all emulsion samples prepared with conjugated PD maintained smaller oil droplets than samples prepared with non-conjugated PD throughout storage, which is indicated by the negative ∆Z-average values (see table).
Fig. 6.
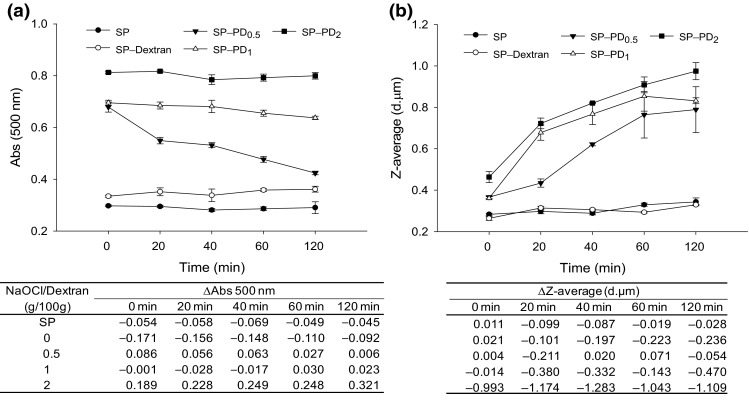
Stability of soybean oil O/W emulsions prepared with SP–PD conjugates, expressed as changes in turbidity (a) and particle size (b) during storage at 4 °C. For succinctness, emulsion stability data of SP/PD mixtures are not plotted, but the differences between SP–PD emulsions and SP/PD emulsions (ΔAbs or ΔZ-average: conjugate minus mixture) are displayed in the underneath tables. PD 0.5, PD 1, and PD 2 denote PD prepared with 0.5, 1, and 2 g NaOCl per 100 g dextran, respectively
It must be noted that while the turbidity measurement indicated no or little change for SP–PD1 and SP–PD2 emulsions during storage, the ∆Z-average values showed otherwise. Considering the principles inherent to the respective testing methods, the larger particle size and higher turbidity of conjugated emulsions suggested the presence of flocculation rather than coalescence. This is because aggregated particles due to flocculation can be readily dispersed by 0.1 % SDS during turbidity testing, allowing the complete recovery of the initial higher absorbance. In spite of the propensity to reversibly associate with each other, emulsions stabilized by protein–polysaccharide complexes may be difficult to merge. This can be explained because the bulky polymeric layer formed by protein–polysaccharide polymers at the interface imparts strong steric hindrances to prevent droplet coalescence (Akhtar and Dickinson 2003). However, as the hydrophilic polysaccharide and their hydrated radii become larger, their increased volume may prevent the droplet surface from becoming saturated with hydrophobic protein. Hence, aggregation, flocculation, and coalescence will occur eventually. Factors that affect solution properties, such as molecular size, degree of branching (α1–3 and α1–6), and conformation or flexibility of oxidized dextran, would have an impact on emulsion stability as well. Therefore, the SP–PD conjugates improved the emulsion stability probably also through increasing the solution viscosity due to the formation of inter-chain hemiacetals in oxidized dextran, as the aldehyde groups would react with nearby hydroxyl groups (Maia et al. 2011).
Conclusions
Oxidative conversion of hydroxyl groups to aldehyde derivatives by NaOCl prove to be an effective technique to produce reactive PD, allowing the high-yield synthesis of SP–dextran polymers at 60 °C in aqueous solutions. The covalent Schiff base SP–PD conjugates are far more surface-active and capable of stabilizing O/W emulsions than the SP/PD mixture or the SP alone. Structural changes in soy polypeptides leading to an enhanced amphiphilicity are implicated in the surface activity of the SP–PD conjugates. The superior emulsifying activity of heterogeneous SP–PD conjugates indicates the adaptability of controlled oxidative modification as a potential, novel processing means to produce functional polypeptides as natural emulsifiers for liquid and semi-solid food products. Certainly, an in situ investigation with actual food systems is needed for validation purposes. Although the dextran–SP conjugate in principle is no different from most other Schiff base Maillard complex food additives, chemical safety and nutrition studies are desirable to assure negligible health risk and uncompromised nutritional status of the developed product.
Acknowledgments
This study was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (Hatch Project 1005724), and an Oversea Study Fellowship from the China Scholarship Council (to X.W.). We thank Ms. Alma True for helpful discussion. Approved for publication as Journal Article Number 15-07-055 by the Director of the Kentucky Agricultural Experiment Station.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interests.
References
- Ahmad S, Tester RF, Corbett A, Karkalas J. Dextran and 5-aminosalicylic acid (5-ASA) conjugates: synthesis, characterisation and enzymic hydrolysis. Carbohydr Res. 2006;341:2694–2701. doi: 10.1016/j.carres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Dickinson E. Emulsifying properties of whey protein–dextran conjugates at low pH and different salt concentrations. Colloids Surf B. 2003;31:125–132. doi: 10.1016/S0927-7765(03)00049-3. [DOI] [Google Scholar]
- Babiker EE. Effect of transglutaminase treatment on the functional properties of native and chymotrypsin-digested soy protein. Food Chem. 2000;70:139–145. doi: 10.1016/S0308-8146(99)00231-9. [DOI] [Google Scholar]
- Bogdanov MG, Palamareva MD. cis/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron. 2004;60:2525–2530. doi: 10.1016/j.tet.2004.01.040. [DOI] [Google Scholar]
- de Castro RJS, Sato HH. Antioxidant activities and functional properties of soy protein isolate hydrolysates obtained using microbial proteases. Int J Food Sci Technol. 2014;49:317–328. doi: 10.1111/ijfs.12285. [DOI] [Google Scholar]
- de Nooy AEJ, Besemer AC, van Bekkum H. Selective oxidation of primary alcohols mediated by nitroxyl radical in aqueous solution. Kinetics and mechanism. Tetrahedron. 1995;51:8023–8032. doi: 10.1016/0040-4020(95)00417-7. [DOI] [Google Scholar]
- Diftis N, Kiosseoglou V. Physicochemical properties of dry-heated soy protein isolate–dextran mixtures. Food Chem. 2006;96:228–233. doi: 10.1016/j.foodchem.2005.02.036. [DOI] [Google Scholar]
- Dong S, Panya A, Zeng M, Chen B, McClements DJ, Decker EA. Characteristics and antioxidant activity of hydrolyzed β-lactoglobulin–glucose Maillard reaction products. Food Res Int. 2012;46:55–61. doi: 10.1016/j.foodres.2011.11.022. [DOI] [Google Scholar]
- Feng J, Xiong Y. Interaction and functionality of mixed myofibrillar and enzyme-hydrolyzed soy proteins. J Food Sci. 2003;68:803–809. doi: 10.1111/j.1365-2621.2003.tb08246.x. [DOI] [Google Scholar]
- Foegeding EA, Davis JP. Food protein functionality: a comprehensive approach. Food Hydrocoll. 2011;25:1853–1864. doi: 10.1016/j.foodhyd.2011.05.008. [DOI] [Google Scholar]
- Franzen KL, Kinsella JE. Functional properties of succinylated and acetylated soy protein. J Agric Food Chem. 1976;24:788–795. doi: 10.1021/jf60206a036. [DOI] [Google Scholar]
- Gusakov AV, Kondratyeva EG, Sinitsyn AP. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem. 2011;2011:1–4. doi: 10.1155/2011/283658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Chen J, Xiong YL. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline ph-shifting processes. J Agric Food Chem. 2009;57:7576–7583. doi: 10.1021/jf901585n. [DOI] [PubMed] [Google Scholar]
- Johnston S, Nickerson M, Low N. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J Food Sci Technol. 2015;52:4135–4145. doi: 10.1007/s13197-014-1523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A. Industrial applications of Maillard-type protein–polysaccharide conjugates. Food Sci Technol Res. 2002;8:193–199. doi: 10.3136/fstr.8.193. [DOI] [Google Scholar]
- Kato A, Mifuru R, Matsudomi N, Kobayashi K. Functional casein–poly saccharide conjugates prepared by controlled dry heating. Biosci Biotechnol Biochem. 1992;56:567–571. doi: 10.1271/bbb.56.567. [DOI] [PubMed] [Google Scholar]
- Kuakpetoon D, Wang Y-J. Characterization of different starches oxidized by hypochlorite. Starch Stärke. 2001;53:211–218. doi: 10.1002/1521-379X(200105)53:5<211::AID-STAR211>3.0.CO;2-M. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawal O, Adebowale K, Adebowale Y. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res Int. 2007;40:1003–1011. doi: 10.1016/j.foodres.2007.05.011. [DOI] [Google Scholar]
- Li Y, Zhong F, Ji W, Yokoyama W, Shoemaker CF, Zhu S, Xia W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo-and poly-saccharides. Food Hydrocoll. 2013;30:53–60. doi: 10.1016/j.foodhyd.2012.04.013. [DOI] [Google Scholar]
- Liu Y, Zhao G, Zhao M, Ren J, Yang B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012;131:901–906. doi: 10.1016/j.foodchem.2011.09.074. [DOI] [Google Scholar]
- Maia J, Carvalho RA, Coelho JFJ, Simões PN, Gil MH. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer. 2011;52:258–265. doi: 10.1016/j.polymer.2010.11.058. [DOI] [Google Scholar]
- Manju S, Sreenivasan K. Detection of glucose in synthetic tear fluid using dually functionalized gold nanoparticles. Talanta. 2011;85:2643–2649. doi: 10.1016/j.talanta.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Morales R, Martínez KD, Pizones Ruiz-Henestrosa VM, Pilosof AMR. Modification of foaming properties of soy protein isolate by high ultrasound intensity: particle size effect. Ultrason Sonochem. 2015;26:48–55. doi: 10.1016/j.ultsonch.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Oliveira FCd, Coimbra JSdR, de Oliveira EB, Zuñiga ADG, Rojas EEG. Food protein–polysaccharide conjugates obtained via the Maillard reaction: a review. Crit Rev Food Sci Nutr. 2016;56:1108–1125. doi: 10.1080/10408398.2012.755669. [DOI] [PubMed] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Rizzo TR, Park YD, Levy DH. Dispersed fluorescence of jet-cooled tryptophan: excited state conformers and intramolecular exciplex formation. J Chem Phys. 1986;85:6945–6951. doi: 10.1063/1.451381. [DOI] [Google Scholar]
- Samanta N, Mahanta DD, Hazra S, Kumar GS, Mitra RK. Short chain polyethylene glycols unusually assist thermal unfolding of human serum albumin. Biochimie. 2014;104:81–89. doi: 10.1016/j.biochi.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Alexander B, Lohmar R, Wolff I, Rist C. Determination of dextran structure by periodate oxidation techniques. J Am Chem Soc. 1954;76:4429–4434. doi: 10.1021/ja01646a045. [DOI] [Google Scholar]
- Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64:284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- Suvorova O, Iozep A, Passet B. Reactivity of polysaccharide aldehydes toward N-nucleophiles. Russ J Appl Chem. 2001;74:1016–1020. doi: 10.1023/A:1013011911022. [DOI] [Google Scholar]
- Tsumura K, Saito T, Tsuge K, Ashida H, Kugimiya W, Inouye K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci Technol. 2005;38:255–261. doi: 10.1016/j.lwt.2004.06.007. [DOI] [Google Scholar]
- Vettori MHPB, Mukerjea R, Robyt JF. Comparative study of the efficacies of nine assay methods for the dextransucrase synthesis of dextran. Carbohydr Res. 2011;346:1077–1082. doi: 10.1016/j.carres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Vivian JT, Callis PR. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J. 2001;80:2093–2109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-J, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2003;52:207–217. doi: 10.1016/S0144-8617(02)00304-1. [DOI] [Google Scholar]
- Zhu D, Damodaran S, Lucey JA. Formation of whey protein isolate (WPI)–dextran conjugates in aqueous solutions. J Agric Food Chem. 2008;56:7113–7118. doi: 10.1021/jf800909w. [DOI] [PubMed] [Google Scholar]
- Zhu D, Damodaran S, Lucey JA. Physicochemical and emulsifying properties of whey protein isolate (WPI)–dextran conjugates produced in aqueous solution. J Agric Food Chem. 2010;58:2988–2994. doi: 10.1021/jf903643p. [DOI] [PubMed] [Google Scholar]