Abstract
Seven edible plants including three unexplored species of high altitude (Ladakh) region were screened for antioxidant activity by bioassay guided fractionation method. The objective of the study was to dereplicate the complex phytochemical matrix of a plant in reference to antioxidant activity in vitro. The screening result showed that ethylacetate fraction of Nepeta longibracteata possesses maximum antioxidant activity, comparable to that of green tea. It also exhibited significant protecting effect against oxidative stress induced by t-BHP in human RBCs. Phytochemical profiling of the most active fraction by nontargeted RP-HPLC–MS and MS/MS technique showed that rosmarinic acid and methylrosmarinate constituted nearly 51 % of the total metabolites apart from twelve other major chemotypes.
Keywords: Nepata longibracteata, Antioxidant activity, Bioassay-guided fractionation, LC-ESI-QTOFMS, Rosmarinic acid, Methylrosmarinate
Introduction
In the recent decades, wild edible and medicinal plants used by ethnic communities of the developing nations have attracted wide response from many scientists across the globe. A number of studies on traditionally used food and medicinal plants have led to the discovery of many compounds including antioxidants and nutraceuticals (Tag et al. 2014). The Indian trans-Himalayan cold desert region of Ladakh represents a valuable source of large number of natural bio-resources beneficial for the armed forces as well as civilian population (Kumar et al. 2015). The adverse climatic conditions of this region leading to sustained energy deficit, malnutrition, vitamin and mineral deficiency and metabolic disorders in human population. However, the diverse flora and fauna growing in this region has the potential of providing additional physiological benefits and basic nutritional requirements to promote health benefits. High altitude stress generates oxidative/reductive stress with the generation of reactive oxygen and nitrogen species (RONS) in humans along with oxidative damage of lipids, proteins and DNA. As a part of our ongoing research to identify bioactive compounds from high altitude plants of Ladakh, we reported two flavonoid compounds from Tanacetum gracile possessing chemopreventive and antioxidant properties (Sinha et al. 2015). In the present study, we selected seven wild edible plants of Ladakh and Kashmir valley. The collection time and location of each plant is provided in Table 1. The literature search showed that Perovskia abrotanoides possesses anti-inflammatory, antimicrobial, antifungal and cytotoxic activity (Ashraf et al. 2014) while, Echinacea purpurea is used for common cold and flu (Yale and Liu 2004). Rhodiola imbricata is widely used as a nutraceutical supplement in the trans-Himalayan region and exhibits antihaemolytic potential by preventing radiation-induced membrane degeneration of human erythrocytes (Arora et al. 2008). Lepidium latifolium (perennial pepperweed) is one of the preferred phytofoods among locals. The leaves, shoots and fruits of the plants are consumed as food (Kaur et al. 2013). Beside their traditional uses, very little scientific information was available for the rest three plants including, Nepeta longibracteata, Waldheimia tomentosa (Rinchen and Pant 2014) and Delphinium brunonianum ( Gupta et al. 2013).
Table 1.
Accession details of plants and their origin
| S. no. | Species | Family | Location | Altitude (m) | Voucher specimen no. | Time of collection |
|---|---|---|---|---|---|---|
| 1 | Nepeta longibracteata Bentham | Lamiaceae | South Polu | 4500 m | 22,081 | July–August |
| 2 | Waldheimia tomentosa Regel | Asteraceae | Khardungla | 4450 m | 21,731 | July–August |
| 3 | Rhodiola imbricata Edgew | Crassulaceae | Khardungla | 5200 m | 22,950 | July–August |
| 4 | Delphinium brunonianum Royle | Ranunculaceae | Khardungla | 4000 m | 21,727 | July–August |
| 5 | Lepidium latifolium L. | Brassicaceae | Leh | 3500 m | 22,107 | July–August |
| 6 | Echinacea purpurea (L.) Moench. | Asteraceae | IIIM—Jammu | 300 m | 21,907 | July–August |
| 7 | Perovskia abrotanoides Karel | Lamiaceae | Khardung—Nubra | 3025 m | 57,323 | July–August |
In the present study, air dried material of each plant (whole plant) was extracted and fractionated with organic and aqueous solvents of increasing polarity. The fractions and extracts, after removal of solvent were screened for antioxidant activity by DPPH based free radical scavenging assay and the most active fraction was identified. The total phenolic and flavonoid content was also determined for each fraction. During exposure to high altitude environment, a wide range of reactive nitrogen and oxygen species (RNOS) generating systems are activated in our body, which weakens the enzymatic and non-enzymatic antioxidant systems. Erythrocytes or red blood cells (RBCs) have widely been used as a model in studying oxidative stress. Oxidative damage in RBCs is induced by tertiarybutyl-hydrogen peroxide (t-BHP) exposure. It causes increased level of haemolysis and damage of poly unsaturated fatty acids, present in cell membrane (lipid peroxidation). Generally, these two parameters are studied to measure oxidative damage caused by an external/internal agent. In order to mimic RBC damage in vivo, the RBCs were treated with t-BHP and the most active fraction was examined to obtain percentage decrease in haemolysis and lipid peroxidation. Finally, the chemical profiling of the most active fraction was performed by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOFMS).
Materials and methods
Chemicals and reagents
Analytical grade solvents and chemicals were used for extraction and fractionation. HPLC grade acetonitrile and methanol (Merck, Darmstadt, Germany) and was used for HPLC and LC–MS analysis. 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tri(2-pyridyl)-S-triazine (TPTZ) and 2-thiobarbituric acid (TBA) were purchased from Sigma-Aldrich. Analytical grade quercetin and ascorbic acid (positive control) were purchased from Hi-Media Laboratories, Mumbai, India, while, luperox TBH70X (t-BHP) was purchased from Sigma-Aldrich. Bruker 500 MHz spectrometer was used for1H NMR and 13C NMR spectra and chemical shift values were expressed in ppm (δ) units. HR-ESI–MS was performed on Bruker mass spectrometer coupled with Agilent 1100 LC.
Plant material
The plant materials were collected and authenticated by Dr. S. Kitchlu, Department of Botany, Indian Institute of Integrative Medicine (IIIM) and the voucher specimen is deposited at the herbarium of IIIM, Jammu.
Extraction and fractionation and phytochemical analysis
Air dried whole plant (100 g) material was crushed and macerated in methanol–water (9:1; 100 mL) for overnight followed by sonication in an ultrasonic bath at 35 °C for 30 min, twice and filtered. The residue was extracted with minimum volume of water. The concentrated organic fraction was fractionated with n-hexane, dichloromethane and ethylacetate successively. The solvent was removed in a rotary evaporator and dry weight was recorded. Each fraction was screened for antioxidant activity (Table 2).
Table 2.
Net weight of the fractions, after complete removal of solvent
| Plant | Fraction | Yield (%) |
|---|---|---|
| Nepeta longibracteata | n-hexane | 0.95 |
| Dichloromethane | 0.42 | |
| Ethylacetate | 0.32 | |
| Aqueous residue | 0.63 | |
| Waldheimia tomentosa | n-hexane | 0.37 |
| Dichloromethane | 0.62 | |
| Ethylacetate | 0.32 | |
| Aqueous residue | 0.51 | |
| Rhodiola imbricata | n-hexane | 0.32 |
| Dichloromethane | 0.45 | |
| Ethylacetate | 0.28 | |
| Aqueous residue | 1.23 | |
| Delphinium brunonianum | n-hexane | 1.12 |
| Dichloromethane | 0.87 | |
| Ethylacetate | 0.23 | |
| Aqueous residue | 1.34 | |
| Lepidium latifolium | n-hexane | 1.11 |
| Dichloromethane | 0.34 | |
| Ethylacetate | 0.67 | |
| Aqueous residue | 1.78 | |
| Echinacea purpurea | n-hexane | 0.82 |
| Dichloromethane | 0.76 | |
| Ethylacetate | 0.53 | |
| Aqueous residue | 1.82 | |
| Perovskia abrotanoides | n-hexane | 0.66 |
| Dichloromethane | 0.23 | |
| Ethylacetate | 0.5 | |
| Aqueous residue | 0.66 |
The concentrated organic extracts were successively fractionated with solvents of increasing polarity viz., n-hexane, dichloromethane and ethylacetate
Total phenolic content (TPC) was determined spectrophotometrically at 760 nm by modified Folin–Ciocalteau method as described by Celiktas et al. (2007) and was expressed as gallic acid equivalent (GAE) in mg per g (mg/g).
The total flavonoid content (TFC) was measured by spectrophotometric assay based on aluminium complex formation as described by Adnan and Fartosy (2011). Briefly, a solution containing NaNO2, AlCl3, NaOH along with plant extract was mixed well and the absorbance was measured at 510 nm. The TFC was calculated in terms of quercetin equivalent (mg/g) by following the calibration curve.
Analysis of antioxidant activities
DPPH free radical scavenging assay
The DPPH free radical scavenging assay was performed according to Celiktas et al. (2007). Briefly, an aliquot of each sample was mixed with 1 mM DPPH solution followed by incubation for 30 min in dark. The absorbance of each sample was measured at 517 nm. The antioxidant activity was expressed in the concentration required to inhibit 50 % methanolic DPPH radical formation (IC50 in µg/mL) with ascorbic acid and quercetin as positive control.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was performed according to modified protocol of Benzie and Strain as adapted by Krishnaraju et al. (2009). The assay was based on the ability of a sample to reduce ferric to ferrous ions in the presence of 2,4,6-tri-(2-pyridyl)-S-triazine (TPTZ), forming an intense blue ferrous–TPTZ complex with absorption maxima at 593 nm. Effective concentration was defined as the concentration of the antioxidant sample having a ferric reducing ability, equivalent to that of 1 mM ferrous salt (EC1).
Inhibition of haemolysis in RBCs
Venous blood samples were collected from healthy donors. The samples were processed by protocol as described by Battistelli et al. (2005). After the removal of plasma and buffy coat, the RBCs were washed thrice and resuspended in phosphate buffer saline (PBS) and was used for subsequent analysis. For haemolysis, modified protocol as described by Okoko and Ere (2012) was followed. The reaction mixture containing 200 µL of RBC suspension and 10 µL of the test solution was incubated for 30 min at 37 °C. Haemolysis was induced by addition of 100 µL of 100 µM of t-BHP followed by incubation at 37 °C for 3 h. Thereafter, 200 µL of the supernatant was diluted with 1.4 mL of PBS and were centrifuged and the absorbance was measured at 540 nm. The experiment without the test sample was considered for 100 % haemolysis and the results were expressed in percentage haemolysis inhibition.
Inhibition of lipid peroxidation
Lipid peroxidation against RBCs was measured by thiobarbituric acid reactive substances (TBARS) method (Maurya and Rizvi 2009). In brief, the processed RBCs were incubated with sample solutions for 3 h. Thereafter, the proteins were precipitated by the addition of 10 % Trichloro-acetic acid (TCA) followed by centrifugation at 3000 rpm for 5 min. To 1 mL of the supernatant, 0.67 % of TBA reagent was added and the reaction mixture was boiled for 20 min. A red colored adduct was formed which was quantified spectrophotometrically at 532 nm. The results were expressed in terms of percentage inhibition of lipid peroxidation.
Cell cytotoxicity assay
The cell cytotoxicity was performed as per the method described by Kakad and Dhembare (2014). Fibroblast cells obtained from chick embryo were cultured in DMEM medium supplemented with Fetal Bovine Serum (FBS) and gentamicin. The cells suspension (2 mL) was treated with sample solution at IC50 concentration and twice the concentration of IC50 (DPPH assay). The microtitre plate was incubated aseptically in CO2 incubator for 24 h at 37 °C. After incubation, cells were dis-aggregated using trypsin (0.25 %). Percent viability was calculated using MTT based assay.
Sample preparation, HPLC and LC/MS analyses
Ethylacetate fraction of N. longibracteata (NlE) was accurately weighed to 5 mg and dissolved in methanol. After centrifugation, the supernatant was concentrated and the residue was reconstituted with methanol.
HPLC analysis
The HPLC experiments were performed on Shimadzu LC system equipped with manual injector and SPD-M20A PDA detector. Solvent systems constituting water with 0.1 % formic acid (v/v) (buffer A) and methanol (buffer B) were used as mobile phase. An injection of 20 µL of aliquot at 1 mg/mL concentration was made on a Phenomenex Luna C-18 analytical column (5 µm, 4.6 × 250 mm) and gradient elution (10–100 % methanol, 25 min, 1 mL/min) was followed to produce a well resolved chromatogram. The retention time of two most intense peaks appearing at 9.15 min and 10.57 min were noted. Further chromatography on a semi-prep C-18 column (10 × 250 mm) was performed with the crude sample in isocratic mode with methanol–water (60:40) for 25 min with a flow rate of 5 mL/min to isolate the pure compounds (retention time 5.72 min and 6.03 min) for characterization.
Characterization of secondary metabolites
Rosmarinic acid (compound 2): C18H16O8; ESIMS (negative ion) m/z 359.07597 [M−H]− (calculated for 359.07656); 1H NMR (CD3OD, 500 MHz): δ 7.36 (1H, d, J = 16 Hz, H-7′), 7.08 (1H, d, J = 8.0 Hz, H-5), 6.95 (br s, H-2), 6.88 (br d, J = 8.0 Hz, H-6), 6.87 (d, J = 8.0 Hz, H-5′), 6.78 (br s, H-2′), 6.75 (br d, J = 8.0 Hz, H-6′), 6.14 (d, J = 16.0 Hz, H-8′), 5.28 (dd, J = 4.3 Hz, and J = 8.6 Hz, H-8), 3.18 (dd, J = 14 Hz and 8.6 Hz, H-7) and 3.07 (dd, J = 14 Hz and 4.3 Hz, H-7); 13C NMR (CD3OD, 125 MHz): δc172.3 (C-9), 167.2 (C-9′), 148.3 (C-3 and C-5′), 146.5 (C-4′), 145.4 (C-7′), 144.7 (C-4), 127.9 (C-7), 126.2 (C-1′), 121.9 (C-6), 120.5 (C-2′), 115.2 (C-5 and C-3′), 115.0 (C-8′), 112.9 (C-6′), 73.3 (C-8) and 37.5 (C-7).
Methylrosmarinate (compound 4): C19H18O8; ESIMS (negative ion): m/z 373.33339 [M−H]− (calculated for 373.33348); 1H-NMR (CD3OD):δ7.52 (1H, d, J = 15.5 Hz, H-7′), 7.04 (1H, d, J = 2.0 Hz, H-2′), 6.91 (H, dd, J = 8.5, 2.0 Hz, H-6′), 6.75 (1H, d, J = 8.5 Hz, H-5′), 6.71 (1H, d, J = 2.0 Hz, H-2), 6.64 (1H, d, J = 8.0 Hz, H-5), 6.61 (1H, dd, J = 8.0, 2.0 Hz, H-6), 6.27 (1H, d, J = 15.5 Hz, H-8′), 5.11 (1H, dd, J = 7.5, 5.0 Hz, H-8), 3.72 (3H, s, OCH3), 3.06 (1H, dd, J = 14.5, 5.5 Hz, H-7a), 3.00 (1H, dd, J = 14.5, 5.5 Hz, H-7b); 13C NMR (CD3COCD3, 125 MHz): δc 170.2 (C-9), 166.3 (C-9′), 148.2 (C-3 and C-3′), 146.2 (C-4′), 145.5 (C-7′), 144.6 (C-4), 143.6 (C-1), 129.2 (C-1′), 122.2 (C-6), 120.9 (C-6′), 116.8 (C-5 and C-5′), 115.6 (C-8′), 114.8 (C-2), 113.7 (C-2′), 73.3 (C-8), 51.6 (C-10), 31.2 (C-7).
LC/MS analysis
LC/MS analysis was performed with Kinetex 1.7u C-18 (100 × 2.1 mm) column on a ultimate 3000–Dionex HPLC system coupled with an electrospray mass spectrometer (QTOF, AB Sciex, Foster City, CA, USA). The LC effluent was introduced into the mass spectrometer in post-column splitting ratio of 5:1. High purity nitrogen (N2) was used as a nebulising gas and ultra-high purity helium (He) as the collision gas. The ion source was operated in both positive and negative ion mode. The sample was scanned over a mass range of 50–1000 m/z with a voltage floating (ISVF) = 5500 V, curtain gas (CUR) = 25, ion source gas 1 (GS1) = 15, ion source gas 2 (GS2) = 25, interface heater temperature (IHT) = 120, column temperature = 40 and declustering potential (DP) = 80 V. Mass spectrometer was operated in a data dependent mode and top 15 m/z peaks were subjected to MS/MS fragmentation in each duty cycle. Peaks with +1 to +5 charge were selected for fragmentation with a threshold value of 120 cps (Basak et al. 2015).
Quantitative data analysis
The LC/MS system was controlled by Analyst TF 1.6 software. The TIC normalization was done using total sum area based module in an excel spread sheet using Marker View (ABSciex).
Identification of secondary metabolites
Each peak was searched against METLIN and MassBank database. Metabolite identification was performed by matching masses with a mass accuracy window of 30 ppm and RT window of ±2 min for METLIN database library. Few metabolites were also identified by comparing their m/z values in the total ion count (TIC) profile with the reported MS/MS spectra. The robustness of the identification was confirmed by matching the masses of the fragments from the MS–MS spectra for each of the metabolite.
All experiments were carried out in triplicates and the results were expressed as mean ± standard deviation (SD) values wherever applicable.
Results and discussions
Initial screening of the plantextracts/fractions for antioxidant activity was evaluated by DPPH radical scavenging and FRAP assay. The antioxidant potential of natural antioxidant, green tea (Camellia sisensis) evaluated by DPPH and FRAP assay exhibited IC50 value of 61.43 and 19.91 µg/mL respectively (Taheri et al. 2011). Also, NlE exhibited significantly higher radical scavenging activity compared to a phytococktail (Dhar et al. 2013), constituting sea buckthorn, apricot and roseroot, commercially used as natural antioxidant. For selection of potential antioxidant fractions amongst experimental plants, green tea extract (GTE) was considered as reference. Amongst seven plants, four plants including N. longibracteata, P. abrotinoides, E. purpurea and D. brunonianum were identified as potential antioxidants. In particular, ethylacetate fraction of N. longibracteata (NlE), P. abrotinoides and E. purpurea along with dichloromethane fraction of D. brunonianum showed maximum potency (Table 3). Fractionation of plant extracts with solvents of increasing polarity could effectively localize activity in a particular fraction. The following method has widely been used to divide complex phytochemical matrix of a plant extract and to confine biological activity in a particular fraction. Amongst these active fractions, NlE exhibiting IC50 value of 64.3 µg/mL, showed comparable activity to that of C. sisensis (Green tea, GTE). Further, the observation was established more emphatically by FRAP assay. At cellular level, antioxidant potential was measured by the degree of inhibition of t-BHP induced haemolysis and lipid peroxidation in reference to an established standard. The standard antioxidant compounds namely ascorbic acid and quercetin, respectively, exhibited 80.8 and 95.8 % inhibition while, NlE showed 78.3 % inhibition against haemolysis. Similar trend was also observed against t-BHP induced lipid peroxidation (Table 4). To correlate antioxidant activity of the fractions with chemical constituents present therein, total phenolic and flavonoid content was determined for each fraction separately. The total phenolic (116.7 GAE/g) and total flavonoid (223.4 QE/g) content was similar to GTE.
Table 3.
(a) Estimation of total phenolic and flavonoid content, (b) measurement of antioxidant activity of the fractions by DPPH and FRAP assay
| Plant name | Fraction | Total phenolic and flavonoid content | Antioxidant activity | ||
|---|---|---|---|---|---|
| TPCa | TFCb | DPPH ICc50 | FRAPd | ||
| N. longibracteata | n-hexane | 33.34 ± 0.11 | 83.42 ± 0.22 | 139.33 ± 0.35 | 93.32 ± 0.03 |
| Dichloromethane | 35.30 ± 0.23 | 39.96 ± 0.17 | 138.72 ± 0.09 | 56.14 ± 0.19 | |
| Ethylacetate | 116.67 ± 0.25 | 223.36 ± 0.08 | 64.31 ± 0.08 | 19.92 ± 0.11 | |
| Aquous residue | 66.69 ± 0.06 | 76.66 ± 0.02 | 151.36 ± 0.016 | 49.17 ± 0.16 | |
| W. tomentosa | n-hexane | 33.33 ± 0.04 | 99.71 ± 0.58 | 130.75 ± 0.03 | 49.87 ± 0.21 |
| Dichloromethane | 55.01 ± 0.02 | 66.69 ± 0.02 | 142.03 ± 0.08 | 101.24 ± 0.24 | |
| Ethylacetate | 40.02 ± 0.01 | 19.99 ± 0.02 | 153.75 ± 0.02 | 106.34 ± 0.25 | |
| Aquous residue | 48.33 ± 0.02 | 46.66 ± 0.01 | >200 | 97.98 ± 0.25 | |
| R. imbricata | n-hexane | 70.1 ± 0.01 | 54.47 ± 0.02 | 178.37 ± 0.21 | 121.53 ± 0.26 |
| Dichloromethane | 133.27 ± 0.07 | 43.34 ± 0.04 | 133.41 ± 0.04 | 132.73 ± 0.19 | |
| Ethylacetate | 56.67 ± 0.03 | 67.34 ± 0.04 | 141.33 ± 0.04 | 92.13 ± 0.14 | |
| Aquous residue | 181.20 ± 0.01 | 22.63 ± 0.01 | 167.36 ± 0.06 | 92.18 ± 0.28 | |
| D. brunonianum | n-hexane | 55.01 ± 0.01 | 19.97 ± 0.04 | 178.43 ± 0.23 | 55.12 ± 0.19 |
| Dichloromethane | 66.65 ± 0.03 | 66.69 ± 0.02 | 123.4 ± 0.01 | 51.23 ± 0.16 | |
| Ethylacetate | 22.27 ± 0.13 | 46.66 ± 0.03 | 185.6 ± 0.30 | 97.12 ± 0.20 | |
| Aquous residue | 133.35 ± 0.03 | 22.65 ± 0.04 | 134.32 ± 0.02 | 91.18 ± 0.23 | |
| L. latifolia | n-hexane | 42.33 ± 0.17 | 22.63 ± 0.17 | 148.56 ± 0.18 | 95.14 ± 0.14 |
| Dichloromethane | 50.00 ± 0.04 | 83.33 ± 0.04 | 139.32 ± 0.03 | 89.85 ± 0.21 | |
| Ethylacetate | 68.34 ± 0.03 | 87.32 ± 0.02 | 132.66 ± 0.02 | 45.3 ± 0.18 | |
| Aquous residue | 81.67 ± 0.01 | 93.35 ± 0.02 | 136.58 ± 0.03 | 51.23 ± 0.19 | |
| E. purpurea | n-hexane | 102.66 ± 0.03 | 22.64 ± 0.03 | 134.33 ± 0.02 | 91.28 ± 0.21 |
| Dichloromethane | 67.32 ± 0.03 | 52.66 ± 0.02 | 146.34 ± 0.03 | 101.27 ± 0.28 | |
| Ethylacetate | 112.67 ± 0.15 | 178.82 ± 0.03 | 97.39 ± 0.03 | 28.71 ± 0.14 | |
| Aquous residue | 78.45 ± 0.18 | 76.78 ± 0.21 | 142.56 ± 0.17 | 78.35 ± 0.15 | |
| P. abrotanoides | n-hexane | 46.67 ± 0.06 | 84.77 ± 0.06 | 134.69 ± 0.02 | 101.38 v 0.17 |
| Dichloromethane | 42.78 ± 0.02 | 38.3 ± 0.01 | 148.79 ± 0.03 | 139.23 ± 0.24 | |
| Ethylacetate | 123.33 ± 0.02 | 226.69 ± 0.02 | 68.47 ± 0.51 | 21.03 ± 0.11 | |
| Aquous residue | 67.34 ± 0.25 | 78.37 ± 0.16 | 156.12 ± 0.16 | 105.26 ± 0.23 | |
| Camellia sinensis (L.) Kuntze | Ethylacetate | 60.47 ± 0.50 | 240.27 ± 0.74 | 61.43 ± 1.16 | 19.91 ± 0.12 |
| Ascorbic acid | 37.31 ± 0.14 | 18.91 ± 0.09 | |||
| Quercetin | 28.41 ± 0.12 | 15.67 ± 0.08 | |||
aTotal phenol content(TPC) expressed as mg GAE/g dry wt. of extract
bTotal flavonoid content(TFC) expressed as mg QE/g dry wt. of extract
cDPPH radical scavenging activity expressed as IC50 in µg/mL extract required to scavenge 50 % of free radicals
dFRAP value expressed as EC1 in µg/mL of extract having a ferric reducing ability equivalent to that of 1 mM Fe2+ salt
Table 4.
Effect of the most active fraction in reducing haemolysis and lipid peroxidation against t-BHP induced RBCs
| Fractions with antioxidant activity | Inhibition of t-BHP induced erythrocyte haemolysis (%) | Inhibition of t-BHP induced erythrocyte lipid peroxidation (%) |
|---|---|---|
| NlEa | 78.32 ± 0.10 | 95.25 ± 0.19 |
| PaEb | 77.9 ± 0.08 | 94.3 ± 0.07 |
| EpEc | 73.4 ± 0.12 | 87.2 ± 0.09 |
| DbDd | 36.3 ± 0.07 | 51.4 ± 0.07 |
| Camellia sinensis (L.) Kuntze | 81.33 ± 0.21 | 95.73 ± 0.03 |
| Ascorbic acid | 80.85 ± 0.13 | 86.3 ± 0.11 |
| Quercetin | 95.8 ± 0.12 | 92.4 ± 0.15 |
% haemolysis/lipid peroxidation calculated by taking hemolysis/peroxidation caused by 100 µM t-BHP as 100 %
% of inhibition at 10 µg of extract/fraction/compound in a total volume of 1900 µL
aEthylacetate fraction of N. longibracteata
bEthylacetate fraction of P. abrotanoides
cEthylacetate fraction of E. purpurea
dDichloromethane fraction of D. brunonianum
An extensive review on pharmacological property and chemical diversity of secondary metabolites of the genus Nepeta has been presented by Formisano et al. 2011. Therein, a large number of secondary metabolites including nepetalactones, monoterpenes (iridoids and their glucosides), diterpenes, triterpenes, phenolics and flavonoids have been reported. Mostly, flavonoids and phenolic compounds of Nepeta species were reported to have antioxidant activity. Additionally nepetalactones, a major constituent of Nepeta oil was reported to have both antioxidant and antibacterial activity.
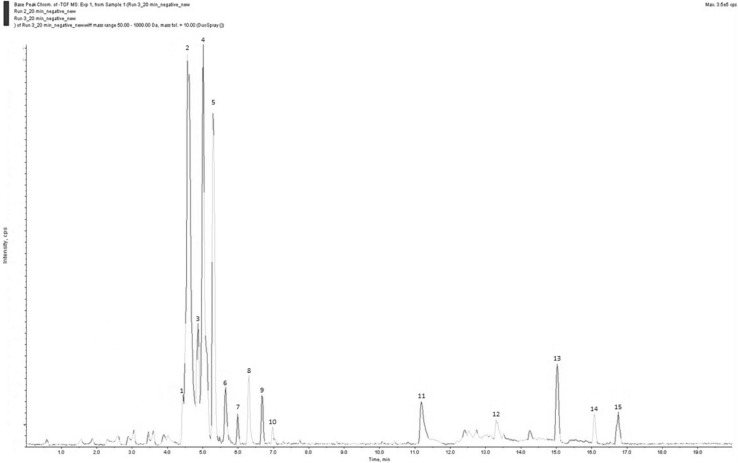
Our investigation of the most active fraction NlE by LC–MS and MS/MS analysis identified 14 major compounds (Fig. 1) by comparing [M−H]− deprotonated molecule and its fragmentation in MS/MS spectra with available standards (Table 5). Best resolution was achieved in negative ion mode, hence, negative ion mode data was considered for analysis. Common fragmentation pathways based on CO2 loss were observed for all phenolic acids with a characteristic deprotonated [M−H]− molecule and [(M−H) − 44]− fragment. The ESI–MS spectra of a major compound (compound 2) appearing at 4.57 min constituting 32.6 % of the total metabolites, showed deprotonated molecules at m/z 359.08. The MS/MS spectra produced ions at m/z 719.16 corresponding to [2M − 1]−, m/z 557.16 for [M+C9H9O5 (dhanshenshu)]−, m/z 395.05 for [M+Cl]− and m/z 197.06 for dhanshenshu [C9H9O5]−, exhibiting close resemblance with the fragmentation pattern of rosmarinic acid (RA) (Altintas et al. 2011). The appearance of higher molecular weight adducts is a common feature in LC–MS spectra of crude extracts. Another major metabolite (compound 4), occupying 18.4 % of the total area, appeared at 5.01 min. It showed deprotonated molecule at m/z 373.08 and a fragment at m/z 359.07 in MS/MS spectra indicating a loss of m/z 14 (methyl moiety), characteristic of the deprotonated molecule of RA. The other significant fragments appeared at m/z 343.08, with a loss of m/z 30 (HCHO) and higher molecular adducts at m/z 747.19 [2M − 1]−, m/z 733.17 [2M − 14]− and m/z 717.17 [2M − 30]−. The mass difference of m/z 14 between deprotonated molecules of 4 and 2 indicated that compound 4 could be the methyl ester derivative of RA. As, both the compounds were present in excess, they were isolated by semi-prep HPLC and were characterized by comparing 1H and 13C NMR data with the reported literature data and were unambiguously identified as rosmarinic acid and methylrosmarinate (Pereira et al. 2013).
Fig. 1.
LC-ESI–MS spectrum of ethylaceate fraction of N. longibracteata in negative ion mode
Table 5.
Identification of chemical constituents of ethylacetate fraction of N. longibracteata by LC-ESI-QTOFMS/MS in −ve ion mode
| Peak | Retention time (min) | % Area | Identificationa | ESI-MS m/z [M−H]−b | (−) ESI-MS2 m/z | Formula |
|---|---|---|---|---|---|---|
| 1 | 4.42 (ill resolved) | 5.29 | Gallic acid | 169.08 | 112.98 [TFA−H]− | C7H6O5 |
| 2 | 4.57 | 32.58 | Rosmarinic acid | 359.08 | 719.16 [2M−H]− 557.16 [M+C9H9O5 (Dhanshenshu)]− 395.04 [M+Cl−]− 197.06 [C9H9O5]− | C18H16O8 |
| 3 | 4.87 | ill resolved | Isopropyl-O-pyrocatecheic acid | 199.1 | 187.1 [M − 12]− 153.09 [M−(HCOOH)]− | C10H15O4 |
| 4 | 5.01 | 18.36 | Methylrosmarinate | 373.08 | 747.19 [2M − 1]− 733.17 [2M−CH2]− 717.17 [2M−2 CH3]− 343.08 [M−CH3]− | C19H18O8 |
| 5 | 5.29 | 12.48 | Isomer of compound 3 | 199.09 | 155.11 [M−CO2]− | C10H16O4 |
| 6 | 5.64 | 2.17 | Kaempferol | 285.03 | 571.08 [2M − 1]− 169.00 [C7H6O5]− | C15H10O6 |
| 7 | 5.99 | 0.74 | Gallic acid derivative | 479.14 | 525.14 [M+(HCOOH)]− | C23H27O11 |
| 8 | 6.3 | 2.53 | Apigenin | 269.04 | 539.10 [2M−1]− 329.06 [M + 59]− 112.98 [TFA−H]− | C15H10O5 |
| 9 | 6.69 | 1.45 | Adenosine | 266.14 | 334.13 | C10H13N5O4 |
| 10 | 6.97 | 0.54 | Derivative of phenylpropionic acid glucoside | 327.22 | 313.07 [M−CH2]− | C19H20O5 |
| 11 | 11.18 | 3.57 | Derivative of phenyl glucoside | 265.15 | 283.26 [M−H+H2O]− 168.99 [M − 96]− | C14H18O5 |
| 12 | 13.3 | 2.59 | Deprotonated TFA | 325.18 | 112.98 [TFA−H]− | C16H21O7 |
| 13 | 15.03 | 3.38 | Phenolic compound | 375.27 | 376.27 [M]− | C19H20O8 |
| 14 | 16.08 | 1.04 | 4-(3,4-Dimethoxyphenyl benzoate), pinocamberine | 255.23 | 323.22 [M + 68]− | C15H12O4 |
| 15 | 16.74 | 1.38 | 4-[2,4-Dicarboxy-3-(4-carboxylatophenyl)phenyl]benzoate | 403.31 | 723.59 | C22H12O8 |
aIdentified using free chemical database, Chem Spider
bExact mass of the parent ion
The first minor compound (compound 1) showed characteristic deprotonated [M−H]− molecule at m/z 169.08, corresponding to gallic acid (C7H6O5). Compound 3, appeared at 4.86 min exhibiting deprotonated molecule at m/z 199.1. The MS/MS fragment at m/z 153.09 [(M−H)− − (CO + H2O)]− could be due to the loss of a carboxyl group present at a terminal position. The molecular formula of C10H15O4 corresponding to camalexin (4-isopropyl-O-pyrocatechuic acid) was suggested for the compound. Compound 5, appearing at 5.29 min showed deprotonated moleculeat m/z 199.01 and a significant MS/MS fragment corresponding to the loss of CO2 (m/z 44), indicating it to be an isomer of compound 3 (Müller et al. 2009). The sixth compound showed deprotonated molecule at m/z 285.04 and higher molecular adduct at m/z 571.08 corresponding to [2M − 1]−. The MS/MS spectra of compound 6 exhibited fragments at m/z 213.0 and m/z 169.0 corresponding to the loss of m/z of 72 and m/z 116 (72 + 44) characteristics of kaempferol (Mišić et al. 2015) with molecular formula C15H10O6. The next deprotonated molecule at m/z 479.14 and the higher molecular adduct at m/z 525.14 [M+HCOOH]−, tentatively indicated the compound could be a gallic acid derivative. On the basis of the higher molecular adduct and deprotonated molecule, the eighth compound was identified as apigenin with molecular formula C15H10O5 (Lin and Harnly 2010). The MS/MS fragments of the deprotonated molecule of compounds 9, 10, 12, 14 and 15 could not be matched directly with the available standard compounds. However, tentative molecular formulae were suggested on the basis of deprotonated molecules as shown in Table 5. Rest two compounds, appearing at 11.18 and 15.03 min exhibited deprotonated molecule at m/z 265.15 and m/z 375.27 corresponding to the molecular formula C14H18O5 and C19H20O8 respectively (Liu et al. 2016). The majority of the compounds as per LC–MS analysis showed that they belong to phenolic or flavonoid class.
Cell cytotoxicity was measured with the fractions against fibroblast cells which were well within permissible limit.
Conclusion
In summary, fourteen compounds of the active antioxidant fraction were identified. Two major compounds constituted almost 51 % of the total chemical constituents and were established as rosmarinic acid and methylrosmarinate. Cell cytotoxicity measured at IC50 (DPPH assay) and twice the concentration of IC50 showed that cell cytotoxicity was well within the permissible limit i.e., 7.5 %. The results of lipid peroxidation suggested that the fraction scan be used as natural antioxidant for the treatment and prevention of diseases mediated by lipid peroxidation. Further, the bioassay guided fractionation method was successful to divide the complex matrix of the plant extract and to concentrate antioxidant compounds in one particular fraction. LC–MS profiling of the antioxidant fraction reveals a distinct pattern, which could be used to differentiate N. longibracteata from related species. The fraction can be used as an alternative source of natural antioxidants with consequential health promoting effects in the oxidative stress conditions.
Acknowledgments
Authors acknowledge Department of Science and Technology, Government of India for financial support, Dr. Ajay Kumar, JNU, New Delhifor NMR support; Ms. Swati, JRF and Dr. Shantanu Sengupta, Scientist from IGIB, New Delhi, for LC–MS support and Dr. A.K. Chauhan, Founder President, Amity University, for hiscontinuous motivation and encouragement.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Adnan DA, AL-Fartosy F. Antioxidant properties of methanolic extract from Inula graveolens L. Turk J Agric. 2011;35:591–596. [Google Scholar]
- Altintas DA, Göger F, Duymuş HG, Kırımer N, Başer KHC. Quantitative analysis of rosmarinic acid in Rosmarinus officinalis growing in Turkey by LC-MS/MS. Planta Med. 2011;77:1394. doi: 10.1055/s-0031-1282737. [DOI] [Google Scholar]
- Arora R, Singh S, Sagar RK, Chawla R, Kumar R, Puri SC, Surender S, Prasad J, Gupta ML, Krishna B, Siddiqui MS, Sharma AK, Tripathi RP, Qazi GN, Sharma RK. Radiomodulatory and free-radical scavenging activity of the fractionated aquo-alcoholic extract of the adaptogenic nutraceutical (Rhodiola imbricata)—a comparative in vitro assessment with ascorbate. J Diet Suppl. 2008;5(2):147–163. doi: 10.1080/19390210802332695. [DOI] [PubMed] [Google Scholar]
- Ashraf SN, Zubair M, Rizwan K, Tareen RB, Rasool N, Zia-Ul-Haq M, Ercisli S. Compositional studies and Biological activities of Perovskia abrotanoides Kar.oils. Biol Res. 2014;47:12–21. doi: 10.1186/0717-6287-47-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak T, Varshney S, Hamid Z, Ghosh S, Seth S, Sengupta S. Identification of metabolic markers in coronary artery disease using an untargeted LC-MS based metabolomic approach. J Proteom. 2015;129(A):169–177. doi: 10.1016/j.jprot.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Battistelli M, De Sanctis R, De Bellis R, Cucchiarini L, Dachà M, Gobbi P. Rhodiola rosea as antioxidant in red blood cells: ultrastructural and haemolytic behaviour. Eur J Histochem. 2005;49(3):243–254. [PubMed] [Google Scholar]
- Celiktas OY, De Girgin G, De Orhan H, Wichers HJ, Bedir E, Vardar-Sukan F. Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times. Eur Food Res Tech. 2007;224(4):443–451. doi: 10.1007/s00217-006-0306-0. [DOI] [Google Scholar]
- Dhar P, Bajpai PK, Tayade AB, Chaurasia OP, Srivastava RB, Singh SB. Chemical composition and antioxidant capacities of phytococktail extracts from trans-Himalayan cold desert. BMC Complement Altern Med. 2013;13:259. doi: 10.1186/1472-6882-13-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano C, Rigano D, Senatore F. Chemical constituents and biological activities of Nepeta species. Chem Biodivers. 2011;8:1783–1818. doi: 10.1002/cbdv.201000191. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Sharma OP, Raina NS, Sehgal S. Ethno-botanical study of medicinal plants of Paddar valley of Jammu and Kashmir, India. Afr J Tradit Complement Altern Med. 2013;10(4):59–65. doi: 10.4314/ajtcam.v10i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakad SB, Dhembare AJ. The cytotoxicity of different plant extract on chick embryo fibroblast cell line. Arch Appl Sci Res. 2014;6(4):139–142. [Google Scholar]
- Kaur T, Hussain K, Koul S, Vishwakarma R, Vyas D. Evaluation of nutritional and antioxidant status of Lepidium latifolium Linn: a novel phytofood from Ladakh. PLoS ONE. 2013;8(8):e69112. doi: 10.1371/journal.pone.0069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraju AV, Rao CV, Rao TVN, Reddy KN, Trimurtulu G. In vitro and in vivo antioxidant activity of Aphanamixis polystachya bark. Am J of Infect Dis. 2009;5(2):60–67. doi: 10.3844/ajidsp.2009.60.67. [DOI] [Google Scholar]
- Kumar J, Dhar P, Tayade AB, Gupta D, Chaurasia OP, Upreti DK, Toppo K, Arora R, Suseela MR, Srivastava RB. Chemical composition and biological activities of Trans-Himalayan alga Spirogyra porticalis (Muell.) Cleve. PLoS ONE. 2015;10(2):e0118255. doi: 10.1371/journal.pone.0118255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LZ, Harnly JM. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat) Food Chem. 2010;120:319–326. doi: 10.1016/j.foodchem.2009.09.083. [DOI] [Google Scholar]
- Liu Y, Li L, Xiao YQ, Yao JQ, Li PY, Yu DR, Ma YL. Global metabolite profiling and diagnostic ion filtering strategy by LC–QTOF MS for rapid identification of raw and processed species of Rheum palmatum L. Food Chem. 2016;192:531–540. doi: 10.1016/j.foodchem.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Rizvi SI. Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat Prod Res. 2009;23(12):1072–1079. doi: 10.1080/14786410802267643. [DOI] [PubMed] [Google Scholar]
- Mišić M, Šiler B, Gašić U, Avramov S, ŽivkovićS ŽivkovićJN, ŽivoslavTešić MM. Simultaneous UHPLC/DAD/(±) HRESI–MS/MS analysis of phenolic acids and nepetalactones in methanol extracts of Nepeta species: a possible application in chemotaxonomic studies. Phytochem Anal. 2015;26:72–85. doi: 10.1002/pca.2538. [DOI] [PubMed] [Google Scholar]
- Müller L, Reinnig MC, Hayen H, Hoffmann T. Characterization of oligomeric compounds in secondary organic aerosol using liquid chromatography coupled to electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:971–979. doi: 10.1002/rcm.3957. [DOI] [PubMed] [Google Scholar]
- Okoko T, Ere D. Reduction of hydrogen peroxide-induced erythrocyte damage by Carica Papaya leaf extract. Asian Pac J Trop Biomed. 2012;2(6):449–453. doi: 10.1016/S2221-1691(12)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira OR, Peres AM, Silva AMS, Domingues MRM, Cardoso SM. Simultaneous characterization and quantification of phenolic compounds in Thymus × citriodorus using a validated HPLC–UV and ESI–MS combined method. Food Res Int. 2013;54:1773–1780. doi: 10.1016/j.foodres.2013.09.016. [DOI] [Google Scholar]
- Rinchen T, Pant S. Ethnopharmacological uses of plants among inhabitants surrounding Suru and Zanskar valleys of cold desert, Ladakh. Int J Pharm Biol Sci. 2014;5(1):486–494. [Google Scholar]
- Sinha S, Amin H, NayakD BhatnagarM, Kacker P, Chakraborty S, Kitchlu S, Vishwakarma R, Goswami A, Ghosal S. Assessment of microtubule depolymerization property of flavonoids isolated from Tanacetum gracile in breast cancer cells by biochemical and molecular docking approach. Chem Biol Interact. 2015;239:1–11. doi: 10.1016/j.cbi.2015.06.034. [DOI] [PubMed] [Google Scholar]
- Tag H, Tsering J, Hui PK, Gogoi BJ, Veer V. Nutritional potential and traditional uses of high altitude wild edible plants in eastern Himalayas. Int J Biol Biomol Agric Food Biotechnol Eng. 2014;8(3):238–243. [Google Scholar]
- Taheri M, Giahi M, Shahmohamadi R, Ghafoori H, Aghamaali MR, Sariri R. Screening antioxidant activity of extracts from different tea samples. Pharmacologyonline. 2011;3:442–448. [Google Scholar]
- Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2004;164(11):1237–1241. doi: 10.1001/archinte.164.11.1237. [DOI] [PubMed] [Google Scholar]