Abstract
Shellac-based surface coating was used in combination with passive modified atmosphere (MA) packaging for shelf life extension of fresh green chillies. The green chillies were coated with shellac coating, packed in anti-fog film and kept at 8 ± 1 °C for storage along with uncoated control. The coated and MA packed chillies showed significantly lower respiration rates as compared to control. The physico-chemical characteristics showed significantly lesser variations in terms of physiological loss in weight, firmness, colour, pigments, ascorbic acid and antioxidant activity during storage. A shelf life extension of 48 days was observed for coated and MA packed chillies against uncoated and MA packed (28 days) and control (15 days) ones. Shellac coated chillies showed a shelf life of 30 days at 8 ± 1 °C. Shellac coating along with the passive MA packaging resulted in restriction of metabolic activities (respiration) and delayed senescence and was found most effective in maintaining the postharvest quality of green chillies during low temperature storage.
Keywords: Green chillies, Shellac, MAP, Postharvest quality
Introduction
Fresh green chillies (Capsicum annuum L.) which represent a culinary and cultural symbol undergo high postharvest losses due to poor postharvest handling during transportation and storage (Singh et al. 2009). They are characterized by high moisture content and active metabolism as a consequence, significant losses resulting in senescence, desiccation, physiological disorders, mechanical injuries and microbial spoilages occur at any point from harvest through utilization (Edusei et al. 2012). Application of semi-permeable coatings has been shown to improve the storage life of fruits and vegetables (Xing et al. 2011; Chandrahas and Laxmi 2016; Babak et al. 2013; Hossein et al. 2015). They improve the mechanical handling properties or structural integrity of fresh commodities and provide an effective barrier to respiratory gases and water vapour (Ojagh et al. 2010). Shellac (an exudate of insect Lacciferlacca) is non-toxic and physiologically harmless and therefore, listed as GRAS (generally recognized as safe) substance by FDA (Okamoto and Ibanez 1986). Modified atmospheric packaging (MAP) is being used for preserving fruits and vegetables by reducing their quality changes and quantity losses during storage (Zhaojun et al. 2014). MAP results in the accumulation of CO2 and depletion of O2 around the commodity which increases their storage life (Kader et al. 1989). Additionally, the desired atmosphere can reduce the respiration rate, ethylene production and physiological changes (Kader 1992). MAP can inhibit chemical, enzymatic and microbiological mechanisms associated with the decay of fresh products (Kader et al. 1989; Mangaraj et al. 2009). However, literature is as such scarce with respect to postharvest shelf life extension of green chillies using combination techniques by application of coating along with MAP. Therefore, the present work was undertaken to develop a protocol for shelf-life extension of green chillies (Capsicum annuum L.) using shellac-based surface coating in combination with modified atmosphere packaging.
Materials and methods
Sample preparation, shellac coating and modified atmosphere packaging
Matured fresh green chillies (C. annuum L.) of uniform size were procured from the local market of Mysore, India. The chillies were sorted for absence of defects, washed with tap water followed by washing with sodium hypo-chlorite solution (100 mg/kg) for 10 min to reduce the microbial load and then surface dried at room temperature. The surface sanitized chillies were divided into four blocks, the first block is control (uncoated and unpacked), second block is shellac coated. The shellac coatings were prepared by dissolving dewaxed and bleached shellac (5 %) in alkaline aqueous medium (0.6 % ammonia) at 95 °C. To this, PVA (binding and coating agent) (3 %) previously dissolved in hot water was added along with triethanolamine (surfactant) (1 %) and 0.1 % oleic acid (lubricant, binder and defoaming agent). The composite was mixed thoroughly and slowly brought down to room temperature followed by the addition of 0.5 % tween 80 (emulsifier). Sodium alginate (0.5 %) was incorporated and the emulsion was homogenized (Virtis homogenizer, Gardiner, New York) at a high-speed of 3000 rpm for 5 min. The coatings were applied by dipping the chillies in the coating solution for 5 min and then surface dried in the stream of dehumidified air.
The third block of chillies were packed using passive modified atmosphere packaging (MAP) (anti-fog RD 45 film) (Cryovac, Sealed Air Corporation, Duncan, South Carolina). The fourth block of chillies are coated with shellac and MA packed (100 ± 2 g pack size). The O2 (OTR) and CO2 (CTR) transmission rate of the film was 10,800–13,800 and 32,000–36,000 cm3 m−2 day atm at 22.7 °C, respectively. The water vapour transmission rate of the film was 2.2 g H2O m−2 day at 22.7 °C and 100 % RH. The samples were stored at 8 ± 1 °C along with control and were analyzed for an interval of every 7 days for physico-chemical and visual sensory (marketability) attributes during storage.
Physico-chemical analysis
Headspace gaseous composition and Respiration rate
Head space gases (O2 and CO2) were measured every day with a headspace gas analyser (Checkmate 9900, PBI Dansensor Co., Denmark) by drawing up 2 ml of air samples. Sampling took place with a hypodermic needle through a septum pasted on the packaging film. The CO2 and O2 production rate of unpackaged control chillies was measured by placing the chillies in an air tight container (PET jar) and the changes in the head space were measured using a PBI Dansensor CO2/O2analyzer (Manolopoulou et al. 2012). For determining the respiration rate, concentration of CO2 inside the packets was checked at every 1 min intervals over a period of 2 h and respiration rate was calculated from the regression slope of CO2 concentration versus time data and evaluated as mg CO2 kg−1 h−1 (Maftoonazad and Ramaswamy 2005).
Physiological loss in weight
The green chillies were weighed initially and at regular intervals during storage. The results were expressed as the percentage loss of initial weight.
Firmness
Firmness of the fruits were measured using a texture analyzer (TAHDi, Stable Microsystems, Godalming, UK) loaded with Texture Expert software (Version 1.22; Stable Microsystems, Godalming, UK) and 25 kg load cell. The firmness was measured by cutting the chillies exactly at the geometric centre with a Warner–Bratzler Blade at a test speed of 0.5 mm s−1 with automatic return. The pre-test and post-test speeds were set at 2 mm s−1.
Colour values
The CIE colour values (L*, a*, b*) were measured using D-65 illuminant and 10º observer. The equipment (Mini Scan XE Plus, Model No. 45/0-S, Hunter Associates Laboratory, Inc., Reston, VA, USA) was calibrated using white and black standard ceramic tiles and the readings were recorded with inbuilt software (Easy Match QC, Hunter Associates Laboratory, Inc., Reston, VA, USA).
Chlorophyll
Chlorophyll content in chillies was measured following the method reported by (Ranganna 1999). Ten grams sample was taken and extracted with acetone in a blender. The extraction was repeated with acetone till the residue was colourless. The filtered contents were taken in a separating funnel along with 50 ml of diethyl ether. Water was added 5–10 times to remove the acetone layer until the ether layer is free of acetone. The ether extract was transferred to a 100 ml volumetric flask and made up the volume with ether. AnhydrousNa2SO4 was added to remove the traces of moisture. The OD was measured using spectrophotometer (Shimadzu 1609, Tokyo, Japan) at 660 and 642.5 nm. The total chlorophyll was calculated using following formula:
Carotenoids
Total carotenoids (TC) were determined according to the procedure described by Kuti (2004). A 5 g of sample was extracted with a solvent mixture containing 40 ml acetone and 60 ml hexane until the residue became colourless. The homogenate was filtered through Whatman No. 4 filter paper and transferred to a separating funnel. Fifty millilitres of hexane was added, and acetone was separated from the extract by repeated washings with distilled water and 5 % NaCl solution. The upper hexane layer with extracted pigment was shifted to a 100 ml volumetric flask, and the volume is made up with hexane. The absorbance was measured using spectrophotometer (Shimadzu 1609, Tokyo, Japan) at 450 nm with hexane as blank. The total carotenoids were calculated using the following formula:
Ascorbic acid
Ascorbic acid was measured as per the method described by Ranganna (1999). Ten grams of sample was macerated using 3 % meta-phosphoric acid and volume was made up to100 ml with meta-phosphoric acid. An aliquot of 10 ml of the extract was taken and titrated with the standard dye (2,6-dichlorophenol indophenol) till pale pink endpoint was observed which persisted for 15–20 s.
Total phenolics
Ten grams of macerated sample extract was diluted to 100 ml with water and 1 ml of Folin–Ciocalteu reagent was added to 5 ml of this aliquot. After 6 min, 10 ml of Na2CO3 (7 %) was added and the volume was made up to 25 ml with distilled water followed by incubation at room temperature for 90 min. The absorbance was measured at 750 nm by spectrophotometer (Shimadzu 1609, Tokyo, Japan) using gallic acid as standard and distilled water as blank (Singleton and Rosi 1965).
Total flavonoids
The sample extract of 15 g was dissolved in water and the volume was made up to 100 ml with distilled water. 5 ml of this aliquot was taken and 0.3 ml of NaNO2 (5 %) was added, kept for 5 min followed by addition of 0.3 ml of AlCl3 (10 %), again kept for 6 min followed by addition of 2 ml sodium hydroxide (1N) solution. Volume was made up to 10 ml with distilled water and absorbance was measured at 510 nm by spectrophotometer using catechin as standard and distilled water as blank (Zhishen et al. 1999).
Antioxidant activity
The antioxidant activity was determined by DPPH method described by Blois (1958). One gram of sample was taken along with 29 ml methanol, shaken vigorously, and centrifuged at 5000 rpm for 15 min at 4 °C. To that filtrate 0.5 ml DPPH (Diphenylpicrylic hydrazine) reagent was added and incubated for 45 min in dark. Methanol was taken as blank and 3 ml methanol with 0.5 ml DPPH reagent was taken as control. The absorbance was measured at517 nm. The antioxidant activity was calculated using the following formula:
Capsaicin content
The capsaicin content of chillies was measured by using spectrophotometric method reported by Sadasivam and Manickam (1997) with slight modifications. Two grams of green chilli paste was taken in 100 ml ethyl acetate and allowed it to stand for 24 h, from that an extract 1 ml was taken and made the volume to 5 ml using ethyl acetate, to that 0.5 ml of vanadium oxy-chloride (Sigma Aldrich, India) solution (vanadium oxy chloride 0.5 % in ethyl acetate) was added just before taking the absorbance of the sample. Pure capsaicin (0.01 %) in ethyl acetate (10 mg in 100 ml) was taken as standard. The absorbance of the blank, standard and sample was read at 720 nm. A colour change was observed after the addition of vanadium oxy chloride, the extract changes from green to yellow colour. The readings were taken when the extract was in green colour.
Keeping quality and shelf life
The keeping quality and shelf-life of chillies were evaluated on the basis of sensory attributes (% Marketability) in terms of visual sensory score and physiological loss in weight.
Statistical analysis
All measurements were performed in triplicates for different physico-chemical parameters and the data obtained were analyzed statistically for analysis of variance (ANOVA) using completely randomized design (CRD) and least significant difference (LSD) at p < 0.05 using Statistica 7 software (StatSoft, Tulsa, OK, USA).
Results and discussions
Coating properties and thickness of the film
Sodium alginate which has the potential to form a biopolymer film, has improved the overall properties of the composite without effecting pH (at 30 °C) (7.4 ± 0.1) and drying time (5 ± 1 min). The viscosity (20 °C) of the coating was (17.7 cP ± 0.2). The thickness of the film (MAP) (anti-fog RD 45 film) was 18.1 µm; surface area of the film exposed to the sample was 0.064 m2.
Headspace gaseous composition and Respiration rate
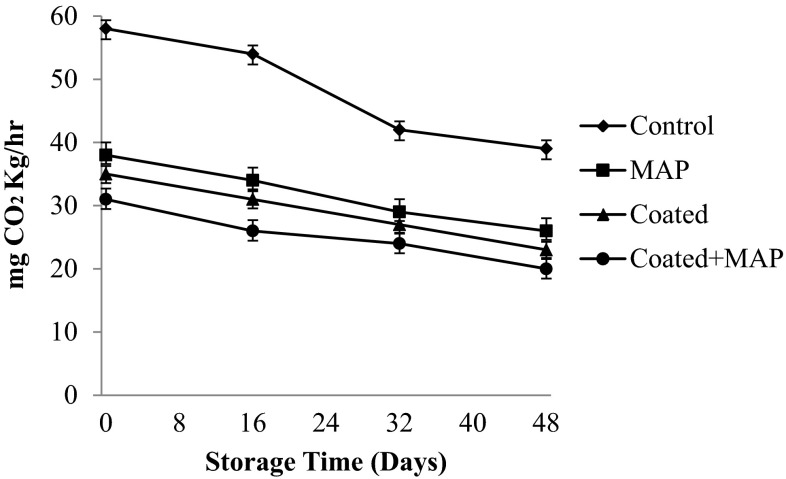
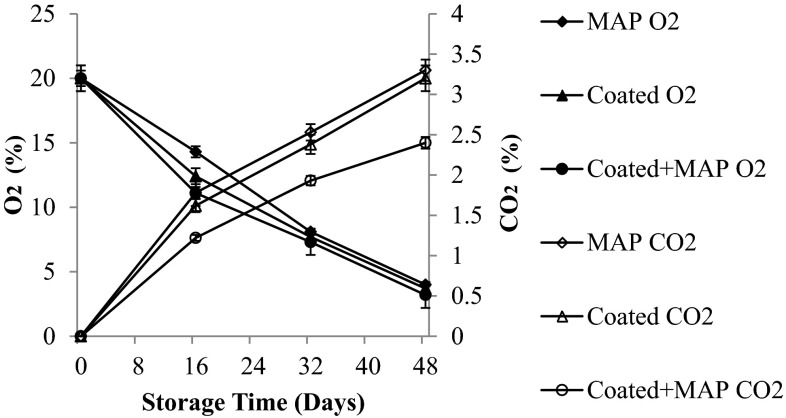
The rate of respiration is an important consideration in extending the postharvest life of chillies and optimizing their postharvest quality. The respiration rate decreased significantly (p < 0.05) with increase in storage period among all the samples indicating non-climacteric pattern (Fig. 1). The coated and packed chillies showed a significant suppression of respiratory activity compared to that of control chillies (unpacked and uncoated). This could be attributed to the barrier properties of the coating and the RD45 anti-fog film towards respiratory gases and water vapour, causing generation of modified atmosphere within the tissues as well as within the packets (Chitravathi et al. 2014, 2015). The coated and MA packed chillies showed an optimum oxygen transmission rate (OTR) and (CTR) CO2 transmission rate which generated optimum atmosphere in the tissues lowering the respiration rate of chillies. The atmosphere in the packages was evaluated for O2 and CO2 concentrations. The change in O2 and CO2 concentrations in the headspace package of fresh green chillies is shown in Fig. 2. The green chillies showed a decrease in O2 concentration, and increase in CO2 concentration during storage. The patterns of change in O2 and CO2 concentration were similar in treated chillies. The MAP packed chillies showed slightly faster gaseous transmission rates than the coated and coated + MAP packed chillies. The headspace gaseous composition for coated + MAP packed chillies showed 3.2 % O2, 2.4 % CO2 followed by coated and MA packed chillies (3.7 % O2, 3.2 % CO2; 4 % O2, 3.3 % CO2) after 48 days of storage at 8 ± 1 °C. For green pepper, the recommended limit for O2 concentration should be not less than 3 %, and not greater than 10 % for CO2 (Ohta et al. 2002). Although, the O2 and CO2 concentrations are strongly affected by film permeability, temperature, respiration of sample, surface area of film, void volume, and weight of sample. The results in this study indicate that O2 and CO2 concentrations in the treated chillies were suitably maintained with acceptable limits. Shellac + sodium alginate coating in combination with MAP decreased water vapour and oxygen transmission rates, thus diminishing respiration rate, retarded ripening, delayed senescence and increased shelf-life of chillies. Similar trend of respiration pattern was observed in capsicum (Manolopoulou et al. 2012).
Fig. 1.
Respiration rate of shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
Fig. 2.
In package headspace gas composition changes (O2 and CO2) of shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
Physiological loss in weight
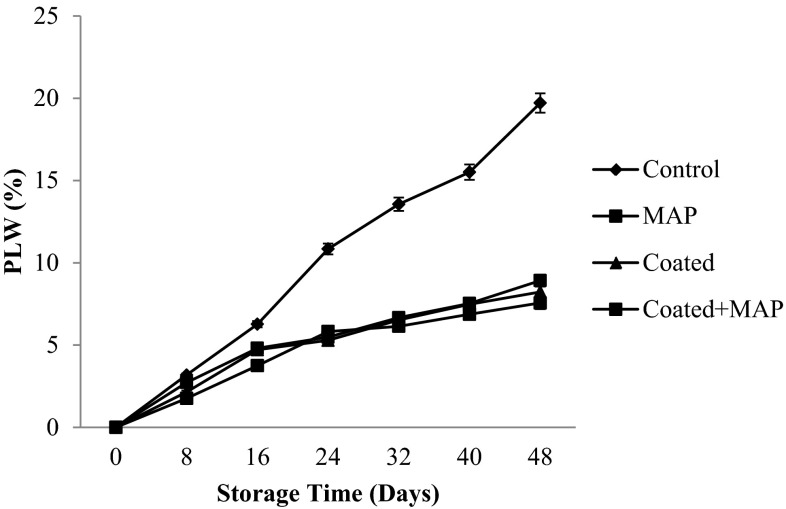
Fresh produce generally shows symptoms of freshness loss with 3–10 % weight loss (Ben-Yehoshua and Rodov 2002). The weight loss of the produce is due to the loss of moisture which in turn shrinks the commodity. Shellac coatings, MAP (anti-fog RD45) films and other biodegradable materials such as alginates have been reported to retard weight loss in fresh produce (Chauhan et al. 2013; Chitravathi et al. 2014, 2015; Koide and Shi 2007; Tay and Perera 2004). The coated + MAP green chillies showed minimal weight loss and were able to maintain a fresh appearance after 48 days at 8 ± 1 °C (Fig. 3). A significant (p < 0.05) difference in weight loss was observed between control and treated samples. The control chilli samples showed the highest loss of about 19.71 % compared to the treated chillies, where the weight loss was in the range of 7.56–8.92 %. The increase in weight loss may be due to the effect of trans-evaporation and respiration (water and heat production). Respiration causes a weight reduction because a carbon atom is lost from the fruit in each cycle (Kablan et al. 2008). Similar results were observed by Xing et al. (2011) in the case of bell peppers.
Fig. 3.
Physiological loss in weight of shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
Firmness
One of the main factors used to determine fruit quality and postharvest shelf-life is the loss of firmness during storage. Firmness is related to gas composition. Decreased softness in fresh produce has been reported when exposed to high levels of CO2 (Tanada-Palmu and Grosso 2005). Firmness is sometimes correlated to weight loss and the degree of injury due to decay or microbial growth. In this study, the firmness value measured dropped slightly as storage time increased, but very little changes were observed among the treated samples compared to the control chillies during storage. The radicals (superoxide and nitric oxide) generated by aerobic respiration loosen the cell wall organization and render the wall pectins accessible to the pectinases, causing loss of firmness during postharvest life of the commodity (Chitravathi et al. 2015). The coated + MAP treated samples exhibited a less reduction in flesh softening and cell wall disassembly during low temperature storage (8 ± 1 °C) (Table 1). Similar results were obtained for MAP treated green peppers (Edusei et al. 2012) and soy protein–cysteine based edible coating and modified atmosphere packaging treated fresh-cut eggplant (Ghidelli et al. 2014). Thus, the combination techniques can adequately be used to preserve the firmness of green chillies during postharvest storage.
Table 1.
Physico-chemical attributes of Shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
| Parameters | Storage time (days) | Control | MAP | Coated | Coated + MAP |
|---|---|---|---|---|---|
| Firmness (N) | 0 | 6.62 ± 0.17aw | 6.70 ± 0.35aw | 6.63 ± 0.28aw | 6.65 ± 0.21aw |
| 16 | 3.98 ± 0.32ax | 4.12 ± 0.25ax | 4.69 ± 0.19bx | 4.85 ± 0.27bx | |
| 32 | 2.06 ± 0.12ay | 2.99 ± 0.34by | 3.76 ± 0.23cy | 3.89 ± 0.44cy | |
| 48 | 1.24 ± 0.17az | 2.09 ± 0.41by | 2.12 ± 0.38bz | 2.24 ± 0.18bz | |
| Total phenolics (mg gallic acid Eq./100 g) | 0 | 144.95 ± 1.05aw | 145.87 ± 1.23aw | 145.05 ± 1.26aw | 145.17 ± 1.65aw |
| 16 | 148.25 ± 1.31ax | 147.01 ± 1.54aw | 146.98 ± 1.39bx | 146.36 ± 1.29bw | |
| 32 | 152.16 ± 1.82ay | 149.57 ± 1.91bx | 148.92 ± 1.47by | 148.41 ± 1.53bx | |
| 48 | 159.85 ± 1.64az | 153.74 ± 1.87by | 152.81 ± 1.15bz | 152.03 ± 1.67by | |
| Total flavonoids (mg catechin Eq./100 g) | 0 | 27.38 ± 1.74aw | 27.09 ± 1.49aw | 26.98 ± 1.28aw | 27.14 ± 1.87aw |
| 16 | 23.89 ± 1.89ax | 25.61 ± 1.73ax | 24.62 ± 1.32ax | 25.07 ± 1.63ax | |
| 32 | 20.74 ± 1.56ay | 23.12 ± 1.65by | 23.47 ± 1.46bx | 22.96 ± 1.71by | |
| 48 | 18.59 ± 1.64az | 22.17 ± 1.58by | 22.03 ± 1.55bx | 21.34 ± 1.59bz | |
| Ascorbic acid (mg/100 g) | 0 | 49.17 ± 0.95aw | 48.26 ± 0.83aw | 48.35 ± 0.74aw | 48.69 ± 0.69aw |
| 16 | 38.56 ± 0.88ax | 34.72 ± 0.63bx | 33.48 ± 0.81cx | 35.14 ± 0.78bx | |
| 32 | 19.89 ± 0.91ay | 28.68 ± 0.94by | 29.72 ± 0.76by | 28.02 ± 0.84cy | |
| 48 | 12.54 ± 0.77az | 20.72 ± 0.78bz | 21.18 ± 0.89cz | 21.96 ± 0.89cz |
First superscript letter (a–d) shows the significant difference (p < 0.05) among a particular row, second superscript letter (w–z) shows the significant difference (p < 0.05) among a particular column for a specific attribute. Control (uncoated and unpacked); MAP (Anti-fog-RD 45 film); Coated (Shellac + sodium alginate) and Coated + MAP (Shellac + sodium alginate and MAP (Anti-fog -RD 45 film))
CIE colour values, chlorophyll and carotenoids
As the fruits mature physiological changes occur. One of the features of fruit ripening is the change in colour. During the ripening of chilli fruits, de novo synthesis of carotenoid pigments occurs, (capsanthin and capsorubin exclusive to this genus) (Minguez-Mosquera and Hornero-Mendez 1994). This process is accompanied by a sharp decrease in chlorophyll as a consequence of the degeneration of chloroplast into chromoplast. A significant (p < 0.05) difference in chlorophyll content was observed among the control and treated samples (Table 2). Respiration of the chillies causes fruit ripening. As the treated chillies had a low/delayed respiration rate, it in turn increased shelf-life and delayed senescence. The combination treatment (shellac + sodium alginate and MAP) partly inhibited chlorophyll degradation during storage, retaining green colour (lower a* values) in the chillies (Fig. 4). The L* and b* values increased in both treated and control samples with progress in senescence during storage. The carotenoid content varied significantly (p < 0.05) among the chilli samples. There was a sharp increase in the carotenoid content in the control samples showing faster senescence (Table 2). Furthermore, the chlorophyll and carotenoids contents of chillies can vary in composition and concentration owing to differences in genetics and maturation (Howard et al. 2000). Similar results were observed in the case of peppers and bell peppers by Deepa et al. (2007) and Xing et al. (2011) respectively.
Table 2.
Physico-chemical attributes of Shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
| Parameters | Storage time (days) | Control | MAP | Coated | Coated + MAP |
|---|---|---|---|---|---|
| Total chlorophyll (mg/100 g) | 0 | 43.15 ± 0.95aw | 43.59 ± 0.84aw | 42.98 ± 0.92aw | 43.21 ± 0.92aw |
| 16 | 33.91 ± 0.87ax | 35.87 ± 0.56bx | 36.12 ± 0.63bx | 37.46 ± 0.59cx | |
| 32 | 21.42 ± 0.72ay | 28.96 ± 0.68by | 28.17 ± 0.54by | 29.32 ± 0.65by | |
| 48 | 15.86 ± 0.83az | 21.71 ± 0.71bz | 22.16 ± 0.75bz | 23.23 ± 0.51cz | |
| Total carotenoids (mg/100 g) | 0 | 5.48 ± 0.52aw | 5.21 ± 0.59aw | 5.15 ± 0.92aw | 5.39 ± 0.41aw |
| 16 | 17.25 ± 0.63ax | 7.92 ± 0.72bx | 7.21 ± 0.87bx | 6.54 ± 0.65cx | |
| 32 | 26.41 ± 0.27ay | 10.88 ± 0.83by | 10.03 ± 0.59cy | 9.67 ± 0.53dy | |
| 48 | 31.48 ± 0.48az | 13.87 ± 0.94bz | 12.13 ± 0.46bz | 11.01 ± 0.84cz | |
| Capsaicin content (%) | 0 | 0.270 ± 0.08aw | 0.271 ± 0.07aw | 0.268 ± 0.04aw | 0.268 ± 0.03aw |
| 16 | 0.265 ± 0.09aw | 0.268 ± 0.08aw | 0.266 ± 0.02aw | 0.267 ± 0.02aw | |
| 32 | 0.259 ± 0.07aw | 0.266 ± 0.06aw | 0.265 ± 0.01aw | 0.265 ± 0.08aw | |
| 48 | 0.252 ± 0.04aw | 0.265 ± 0.01aw | 0.263 ± 0.08aw | 0.264 ± 0.04aw | |
| Antioxidant activity (%) | 0 | 40.12 ± 1.56aw | 39.78 ± 1.83aw | 40.23 ± 1.72aw | 40.28 ± 1.84aw |
| 16 | 35.25 ± 1.38ax | 36.29 ± 1.42ax | 37.12 ± 1.64ax | 38.54 ± 1.52bx | |
| 32 | 29.87 ± 1.42ay | 33.81 ± 1.26by | 32.43 ± 1.59by | 34.36 ± 1.37cy | |
| 48 | 23.67 ± 1.71az | 29.95 ± 1.31bz | 29.87 ± 1.48bz | 30.98 ± 1.29bz |
First superscript letter (a–d) shows the significant difference (p < 0.05) among a particular row, second superscript letter (w–z) shows the significant difference (p < 0.05) among a particular column for a specific attribute. Control (uncoated and unpacked); MAP (Anti-fog-RD 45 film); Coated (Shellac + sodium alginate) and Coated + MAP (Shellac + sodium alginate and MAP (Anti-fog -RD 45 film))
Fig. 4.
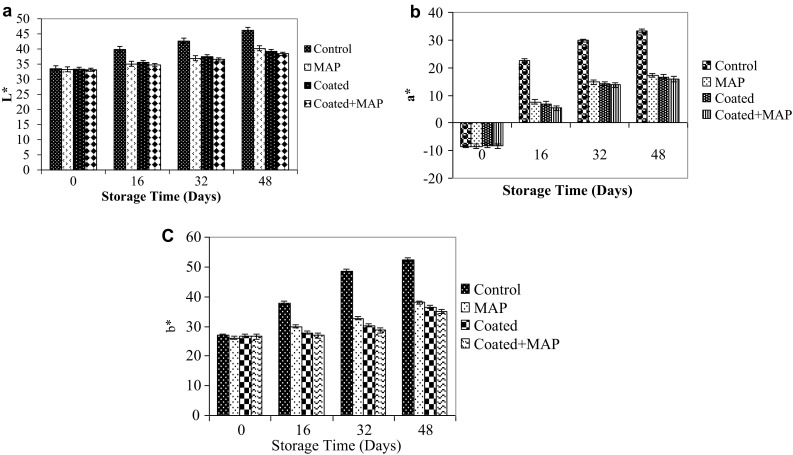
The CIE L*, a*, b* values of shellac coated and passive MAP of green chillies at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
Ascorbic acid
Ascorbic acid is a predominant vitamin in green chillies and tends to decrease during ripening/senescence of the fruit. The ascorbic acid had shown a decreasing trend in all the samples during storage at (8 ± 1 °C). A significant (p < 0.05) higher reduction was noted in the ascorbic acid content of control samples (uncoated and unpacked) during storage. The coated and MA packed chillies retained ascorbic acid content compared to the control and individually treated samples (Table 1). It is reported that in storage atmosphere with low levels of O2 preserves the vitamin C levels (Arvanitoyannis et al. 2005). Ascorbic acid is usually degraded by oxidative process, which is stimulated in the presence of light, heat, oxygen, peroxides and enzymes (ascorbate oxidase or peroxidase) (Plaza et al. 2006). The levels of ascorbic acid vary with genotypic differences, pre-harvest climatic conditions, maturity and postharvest handling procedures. The results are in good agreement with those obtained by Deepa et al. (2007) and Ghasemnazhad et al. (2011) in the case of bell peppers.
Total phenolics and flavonoids
Maturity is one of the major factors that determine the content of phenolics in fruits and vegetables. The control samples had the highest phenolics content due to the early ripening of chilli fruit compared to the treated samples. Phenolics increased significantly (p < 0.05) in all the chilli samples during storage (Table 1). The increase in phenolics is due to the conversion of flavonoids to secondary phenolic compounds (Barz and Hoesel 1977). The results were found to be in conformity with those of Ghasemnazhad et al. (2011). A decreased trend of flavonoid content was observed among all the samples (Table 1). During pepper fruit development, flavonoid synthesis may compete with casaicinoid synthesis in the phenylpropanoid pathway. The onset of capsaicinoid accumulation in chilli pepper fruit was paralleled by the disappearance of flavonoids (Sukrasano and Yeoman 1993). Cultivars which had low flavonoid content exhibited antioxidant activity (Lee et al. 1995). The bioactive compounds in peppers vary due to genotype and maturation/ripening (Ghasemnazhad et al. 2011). The degradation of flavonoids can be attributed to the enzyme action (peroxidase). Various factors such as cultivars, seasons and pre and postharvest conditions also affect the chemical composition of plant foods (Howard et al. 2000). The results are in good agreement with Howard et al. (2000), Ghasemnazhad et al. (2011) and Edusei et al. (2012) in case of bell peppers and green chillies, respectively.
Antioxidant activity and capsaicin content
A significant (p < 0.05) difference in antioxidant activity was found among the control and treated samples. A decrease in antioxidant activity was observed in all samples with progress in senescence during storage. The control samples exhibited a decrease in antioxidant activity at the 48th day of storage, due to early colour break (green to red) (Table 2). The results are in good agreement with those reported by Shaha et al. (2013). Capsaicin is the most abundant pungent principle in chillies. Capsaicinoids are synthesized by the condensation of vanillylamine with a short-chain branched fatty acid. There was no significant difference in the capsaicin content in treated and control samples. The coated samples in combination with MAP retained capsaicin content to a better extent as compared to the individually treated (coated, MAP) and control samples during storage (Table 2). Many studies have reported the accumulation of capsaicinoids in chillies in relation to the fruit age, size and stage of development (Estrada et al. 2002). Other studies on capsaicinoids have demonstrated that capsaicinoid content is genetically controlled but also subjected to the environmental variables such as temperature, light, soil moisture and fertilization level (Estrada et al. 2002). Capsicum peroxidase oxidizes capsaicin. Due to the vacuolar localization of alkaloid-oxidizing peroxidases and capsaicin (Bernal et al. 1993). Capsaicin concentration gradually tends to degrade into secondary compounds due to peroxidase action (Reyes-Escogido et al. 2011). The results are in good agreement with those reported by Margarita and Yahia (1998), where the decrease in capsaicinoid concentration coincided with a high or increased peroxidase activity.
Keeping quality and shelf life
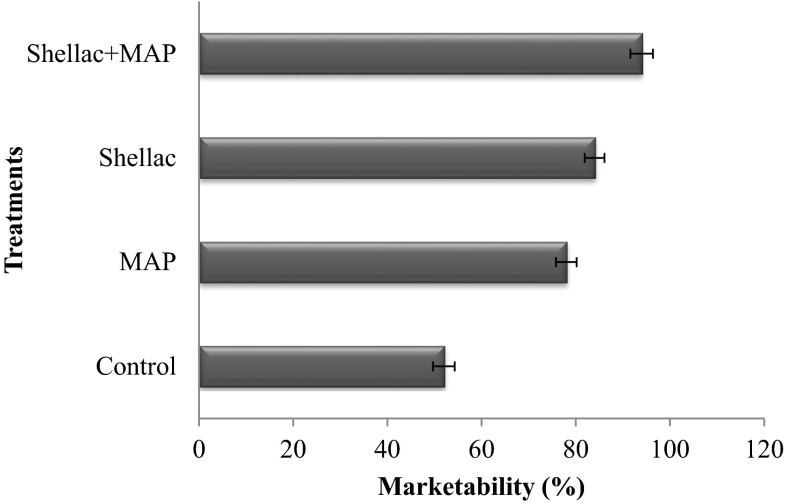
The effect of combined treatments of shellac + sodium alginate in combination with passive modified atmospheric packaging of fresh chillies had resulted in an increased shelf-life and retention of bio-active compounds, firmness, decreased tissue respiration with a restricted weight loss during storage. Coated + MAP treated chillies showed highest (94 %) marketability by the end of 48 days of storage period, followed by 84, 78 and 52 % for coated, MAP and control samples respectively (Fig. 5). The percentage of marketability of control samples decreased with storage time due to loss of firmness and colour deterioration. Delayed senescence due to reduced respiration rate and retention of firmness and chlorophyll pigments maintained the marketability of shellac + sodium alginate and MA packed chillies. The combination treatment found promising and extended the shelf-life up to 48 days, compared to MA packed (28 days), shellac + sodium alginate coated (30 days) and control (15 days) at low temperature (8 ± 1 °C) storage.
Fig. 5.
Marketability (%) of green chillies at the end of storage period at low temperature (8 ± 1 °C) storage, (mean ± SD; n = 3)
Conclusion
The postharvest treatments studied showed significant extension in the shelf-life of green chillies at low temperatures (8 ± 1 °C) up to a period of 48 days compared to the control samples (15 days). Application of shellac based surface coating along with modified atmosphere packaging not only reduced the metabolic activities but also maintained the quality attributes of the chillies during storage. Further research work is needed with regards to other pre-treatments in combination with surface coating and MAP for maximum benefits.
References
- Arvanitoyannis IS, Khah EM, Christakou EC, Bletsos FA. Effect of grafting and modified atmosphere packaging on eggplant quality parameters during storage. Int J Food Sci Techol. 2005;40(3):311–322. doi: 10.1111/j.1365-2621.2004.00919.x. [DOI] [Google Scholar]
- Babak GN, Chaudhari ML, RamanaRao TV. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J Food Sci. 2013 [Google Scholar]
- Barz W, Hoesel W. Metabolism and degradation of phenolic compounds in plants. Phytochemistry. 1977;12:339–369. [Google Scholar]
- Ben-Yehoshua S, Rodov V (2002) Transpiration and water stress. In: Barz J, Brecht JK (eds) Postharvest physiology and pathology of vegetables. Marcel Dekker, Inc., New York, USA, pp 111–159
- Bernal MA, Calderon AA, Pedreno MA, Munoz R, Barcelo RA, Caceres FM. Dihydrocapsaicin oxidation by Capsicum annuum (var. annuum) peroxidase. J Food Sci. 1993;58(3):611–613. doi: 10.1111/j.1365-2621.1993.tb04337.x. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chandrahas V, Laxmi A. Postharvest shelf-life extension of pink guavas (Psidium guajava L.) using HPMC-based edible surface coatings. J Food Sci Techol. 2016;53(4):1966–1974. doi: 10.1007/s13197-015-2164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan OP, Nanjappa C, Ashok N, Ravi N, Roopa N, Raju PS. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J Food Sci Tech. 2013 doi: 10.1007/s13197-013-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitravathi K, Chauhan OP, Raju PS. Postharvest shelf-life extension of green chillies (Capsicum annuum L.) using shellac-based edible surface coatings. Postharvest Biol Technol. 2014;92:146–148. doi: 10.1016/j.postharvbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitravathi K, Chauhan OP, Raju PS. Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.) Food Packag Shelf-life. 2015;4:1–9. doi: 10.1016/j.fpsl.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa N, Kaur C, George B, Singh B, Kapoor HC. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) LWT Food Sci Technol. 2007;40:212–219. doi: 10.1016/j.lwt.2005.09.016. [DOI] [Google Scholar]
- Edusei VO, Ofosu-Anim J, Johnson PNT, Cornelius EW. Extending postharvest life of green chilli pepper fruits with modified atmosphere packaging. Ghana J Hortic. 2012;10:131–140. [Google Scholar]
- Estrada B, Bernal MA, Diaz J, Pomar F, Merino F. Capsaicinoids in vegetative organs of (Capsicum annuum L.) in relation to fruiting. J Agric Food Chem. 2002;50:1188–1191. doi: 10.1021/jf011270j. [DOI] [PubMed] [Google Scholar]
- Ghasemnazhad M, Sherafati M, Payvast GA. Variation in phenolics compounds, ascorbic acid and antioxidant activity of five coloured bell peppers (Capsicum annuum) fruits at two different harvest times. J Funct Foods. 2011;3(1):44–49. doi: 10.1016/j.jff.2011.02.002. [DOI] [Google Scholar]
- Ghidelli C, Mateos M, Rojas-Argudo C, Pérez-Gago MB. Extending the shelf life of fresh-cut eggplant with a soy protein–cysteine based edible coating and modified atmosphere packaging. Postharvest Biol Technol. 2014;95:81–87. doi: 10.1016/j.postharvbio.2014.04.007. [DOI] [Google Scholar]
- Hossein M, Mahmood G, Davood B. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J Food Sci Techol. 2015;52(7):4507–4514. doi: 10.1007/s13197-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum Species) as influenced by maturity. J Agric Food Chem. 2000;48:1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- Kablan T, Koffi NR, Marina K, Oule MK. The effects of different storage temperatures on the quality of bell pepper (Capsicum annuum L.) Agric J. 2008;3(2):157–162. [Google Scholar]
- Kader AA. Postharvest biology and technology: an overview. In: Kader AA, editor. Postharvest technology of horticultural crops. Oakland: University of California Publishing; 1992. pp. 15–20. [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmospheric packaging of fruits and vegetables. Crit Rev Food Sci Nutr. 1989;28(1):1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Koide S, Shi J. Microbial and quality evaluation of green peppers stored in biodegradable film packaging. Food Control. 2007;18:1121–1125. doi: 10.1016/j.foodcont.2006.07.013. [DOI] [Google Scholar]
- Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004;85:527–533. doi: 10.1016/S0308-8146(03)00184-5. [DOI] [Google Scholar]
- Lee Y, Howard LR, Villalon B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. J Food Sci. 1995;60(3):473–476. doi: 10.1111/j.1365-2621.1995.tb09806.x. [DOI] [Google Scholar]
- Maftoonazad N, Ramaswamy HS. Postharvest shelf-life extension of avocados using methyl cellulose based coating. LWT Food Sci Technol. 2005;38:617–624. doi: 10.1016/j.lwt.2004.08.007. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK, Mahajan PV. Applications of plastic films for modified atmosphere packaging of fruits and vegetables: a review. Food Eng Rev. 2009;1(2):133–158. doi: 10.1007/s12393-009-9007-3. [DOI] [Google Scholar]
- Manolopoulou H, Lambrinos G, Xanthopoulos G. Active modified atmosphere packaging of fresh-cut bell peppers: effect on quality indices. J Food Res. 2012;1(3):148–158. doi: 10.5539/jfr.v1n3p148. [DOI] [Google Scholar]
- Margarita C-P, Elhadi YM. Changes in capsaicinoids during development, maturation and senescence of chile peppers and relation with peroxidase activity. J Agric Food Chem. 1998;46(6):2075–2079. doi: 10.1021/jf970972z. [DOI] [Google Scholar]
- Minguez-Mosquera MI, Hornero-Mendez D. Comparative study of the effect of paprika processing on the carotenoids in peppers (Capsicum annuum) of the Bola and Agridulce varieties. J Agric Food Chem. 1994;42(7):1555–1560. doi: 10.1021/jf00043a031. [DOI] [Google Scholar]
- Ohta H, Shiina T, Sasaki K. Dictionary of freshness and shelf-life of food. Tokyo: Science Forum Co., Ltd; 2002. [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Okamoto MY, Ibanez PS. Final report on the safety assessment of shellac. J Am Coll Toxicol. 1986;5:309–327. [Google Scholar]
- Plaza L, Sanchez-Moreno C, Elez-Martinez P, De-Ancos B, Marin-Belloso O, Cano MP. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur Food Res Technol. 2006;223(4):487–493. doi: 10.1007/s00217-005-0228-2. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality for fruit and vegetable products. 2. New Delhi: Tata McGraw-Hill Publishing Company Limited; 1999. pp. 1–29:163, 164, 578–582. [Google Scholar]
- Reyes-Escogido ML, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules. 2011;16(2):1253–1270. doi: 10.3390/molecules16021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. New Delhi: New Age International Ltd; 1997. p. 413. [Google Scholar]
- Shaha RK, Shafiqur R, Afandi A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann Biol Res. 2013;4(8):27–34. [Google Scholar]
- Singh Y, Sharma M, Sharma A. Genetic variation, association of characters and their direct and indirect contributions for improvement in chilli peppers. Int J Veg Sci. 2009;15(4):340–368. doi: 10.1080/19315260903004158. [DOI] [Google Scholar]
- Singleton VL, Rosi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sukrasano N, Yeoman MM. Phenylpropanoid metabolism during growth and development of Capsicum frutescens fruits. Phytochemistry. 1993;32:839–844. doi: 10.1016/0031-9422(93)85217-F. [DOI] [Google Scholar]
- Tanada-Palmu PS, Grosso CRF. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol Technol. 2005;36(2):199–208. doi: 10.1016/j.postharvbio.2004.12.003. [DOI] [Google Scholar]
- Tay SL, Perera CO. Effect of 1-methylcyclopropene treatment and edible coatings on the quality of minimally processed lettuce. J Food Sci. 2004;69(2):131–135. [Google Scholar]
- Xing Y, Xihong L, Qinglian X, Juan Y, Yaqing L, Yao T. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.) Food Chem. 2011;124:1443–1450. doi: 10.1016/j.foodchem.2010.07.105. [DOI] [Google Scholar]
- Zhaojun B, Li L, Junfeng G, Jianhua F, Maoyu W, Xinming X, Jiang L. Modified atmosphere packaging (MAP) and coating for improving preservation of whole and sliced Agaricus bisporus. J Food Sci Techol. 2014;51(12):3894–3901. doi: 10.1007/s13197-013-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoids content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;54:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]