Abstract
In red winemaking de-stemming is crucial since the stems contain polymeric phenolic compounds responsible for the astringency of wine. Wine such as Primitivo has low phenolic constituents and tannins and stems affect aroma, taste body and olfactory characteristics. The aim of the study was to evaluate the effects of presence of stems during fermentation on polyphenolic, volatile compounds and sensory characteristics of wine. Primitivo grapes vinified in presence of different percentage of stems: 100 % de-stemmed (D100), 75 % de-stemmed (D75) and 50 % de-stemmed (D50). Results confirmed that the wines vinified in presence of stems were higher in tannins, flavans, to vanillin and proanthocyanidins, colour intensity with lower anthocyanins. The presence of stems during fermentation conferred more structure and flavour to wines. They facilitated must aeration thus promoting synthesis of higher alcohols and ethyl esters by yeast. In particular, a higher content of hexan-1-ol, hex-3-en-1-ol and 2-phenyl ethanol in D50 and D75 gave the wines that suggest green grass, herb and floral. Wine from D75 seemed to be better than D50 in terms of volatile compounds as well as fruity, floral and balsamic components preserved, without any unpleasant taste of long chain fatty acids found in D50.
Keywords: Primitivo wine, Stem contact fermentation, Phenolic and volatile compounds, Sensorial analysis
Introduction
Most of the compounds of technological interest present in grape berries such as aromas, aromatic precursors and polyphenols are localized in the solid parts of the grape: skin, pulp and seeds (Gambacorta et al. 2011). Stems, as part of a grape cluster, contain many polymeric phenolic compounds and flavans monomers. These compounds are extracted from grape clusters during red grape maceration and fermentation and contribute to the astringency of a red wine. To avoid this sensorial characteristic in red grape winemaking grapes are usually de-stemmed. Stems need to be removed as they can add unwanted characters and flavours to the wine. If the de-stemming process is not delicate enough, the berries can burst and stems can be bruised, broken or damaged, leaving juice negative substances from the stems end up in the wine. (Lombard 2011). The practice of de-stemming process, sometimes overlooked, assumes a strategic importance in contributing character to a red wine. Red wines made by skin-contact fermentation in presence of stems contain higher total and polymeric phenols compared to wines made without stems, and this seems to be proportional to the duration of pomace contact with must (Kantz and Singleton 1991), on the contrary increasing maceration time decreases both total and some individual anthocyanins (Spranger et al. 2004). Beside this, healthy properties of polyphenols content in red wine are well documented (Dell’Agli et al. 2004).
Primitivo is one of the typical non aromatic red grape varieties cultivated in Apulia Region (Southern Italy) and due to its importance it has been chemically characterized, concerning the phenolics and aroma precursors (Tamborra and Esti 2010), which can represent a tool for the authenticity and traceability of derived wines. Primitivo grapes, in addition to Southern-Italy, is also grown in the United States as the second most widely cultivated black wine grape in California, behind Cabernet Sauvignon, under the name of Zinfandel (Maletic et al. 2004; Fidelibus et al. 2005). Primitivo wines, respect to other varieties like Merlot or Cabernet Sauvignon, normally exhibit higher alcohol content and titratable acidity but give rise to unstructured, “short-lived” and with not very stable colour over, with low polymerization rate of anthocyanic pigments, which does not allow a wine to age (Lovino et al. 2006; Moio 2015).
During ageing of wines changes occur mainly for acetaldehyde-mediated condensation, co-pigmentation and self-association reactions (Boulton 2001). Under oxidative conditions condensed tannins, highly present in grape seeds and skins, undergo polymerization phenomena Tannins–Anthocyanines (T–A) or Tannins–Tannins (T–T) and precipitate, allowing a wine to soften and mature preventing oxidation phenomena. Some studies focused on the influence of the amount of stems on chemical composition of wine (Gambacorta et al. 2011), even an in depth research involving correlations between polyphenolic and volatile compounds and sensory analysis on Primitivo wine grapes is lacking.
The aim of this work was to evaluate the chemical profile in terms of major phenolic and volatile compounds, and a sensorial analyses, of Primitivo wines in relation to three different de-stemming levels (1) 100 % de-stemmed grapes; (2) 75 % de-stemmed grapes; (3) 50 % de-stemmed grapes.
Materials and methods
Experimental design and winemaking
The research was conducted in 2012 on Primitivo grapes harvested in a vineyard located in the district of Sava (Taranto province, Apulia region, Italy). Wines were grown under organic farming conditions with a Guyot pruning system. Grapes were harvested when showed 34.40 Brix, pH 3.95 and total acidity 3.84 g/L. Three different percentages of stems with grape bunches during the winemaking were performed. The wine was made with no stem-contact fermentation by de-stemming a 100 % of grapes (D100). Grapes were pressed-destemmed with an Electric Press-Destemmer, Zambelli Enotech srl, Comisano Vic. De-stemmed and crushed grapes were transferred to a 100 kg capacity stainless steel tank, thereafter 60 mg/L of SO2was added. Total acidity was corrected to 5.80 g/L by adding tartaric acid to achieve a more efficient fermentation. Must was inoculated with Lalvin V1116 yeast (Lallemand) at 20 g/100 kg.
Skin maceration and fermentation lasted for 10 days at an average temperature of 25 °C, pumping over the fermentation cap twice a day. Racking was performed after pressing in a vertical hydro-press at 2.5 bar with a Hydro80 Press—Zambelli Enotech srl, Comisano Vic. Wines were prepared by de-stemming and pressing 75 and 50 % in weight of grapes to obtain D75 and D50, while the complement to 100 kg of each was achieved by adding grapes pressed without de-stemming. Both these were further processed as described above. All the treatments were performed in three replicates in 100 kg capacity stainless steel tanks. During the fermentative pomace contact period, temperature and must density were recorded. After racking, wines were stored at room temperature (18 °C) and racked one month later, sulphur dioxide was added without any malolactic fermentation. The wines were cold stabilised (−5 °C) for 1 month, then bottled and stored at room temperature (18 °C). All the analyses were made in triplicate at racking, while sensory analyses were performed after 4 months of bottling.
Chemicals and reference compounds
Malvidin-3-O-glucoside, (+)-catechin, (−)-epicatechin, procyanidin B2, procyanidin B3, procyanidin B4, (+)-epigallocatechin and epigallocatechingallate were supplied by Extrasynthese. Quercetin, myricetin, kaempferol, caffeoyl tartaric acid and coumaroyl tartaric acid were purchased from Sigma AldrichInc. (MI, USA). Standards purities were above 95 %. All the solvents (methanol, acetonitrile, ethyl acetate, diethyl ether) were supplied by Carlo Erba (Milan, Italy) and were HPLC grade. All the solutions were obtained with distilled deionised water.
Wine composition
Total acidity, volatile acidity, reducing sugar, total SO2, alcohol and total dry extract were all determined on wines at racking according to EEC regulation 2676/90 (1990).
Spectrophotometric analysis
Phenolic compounds were determined by spectrophotometric methods (Di Stefano et al. 1997) using a UV/VIS Mod Lambda 25 double beam Spectrophotometer (Perkin Elmer SpA). Colour intensity and hue were estimated by measuring absorbance at 420, 520 and 620 nm according to EEC regulation 2676/90 (1990).
HPLC analysis
The wines were separated into two fractions containing individual catechins and oligomeric proanthocyanidins respectively using C18 Sep-Pak cartridge for the determination of flavan-3-ols as described by Sun et al. (1999). About 5 mL of wine were adjusted to pH 7 and then passed through the Sep-Pak cartridges preconditioned with H2O adjusted to pH 7.0. Elution was carried with 10 mL of H2O to eliminate phenolic acids. After drying the cartridges with N2, elution was carried out with 15 mL ethyl acetate to elute catechins and oligomeric proanthocyanidins (F I + F II). Each fraction was evaporated and dissolved in methanol, followed by HPLC analysis. An HPLC 1100 series Agilent technologies with binary pump and diode array detector (DAD) was used with a Thermo ODS RP-C18 Hypersil 200 × 2.1 (5 μm) column with a guard ODS Hypersil 20 × 2.1 mm (5 μm) was used for flavans analysis. Two millilitres of each extracted fraction was filtered through a 0.45 μm nylon membrane filter and immediately injected according to Squadrito’s et al. method (2007). Peak identification was performed comparing the retention times and absorption spectra of the following pure compounds (+)-catechin, (−)-epicatechin, procyanidin B2, procyanidin B3, procyanidin B4, (+) epigallocatechin and epigallocatechingallate (supplied by Extrasynthese) and were found analogues to those reported in literature (Ricardo-da-Silva et al. 1991; Sun et al. 1998; Garcia-Beneytez et al. 2003). A Phenomenex Synergi 4u Hydro-RP 80Å (250 × 4.60 mm, 4 micron) with guard column was used for organic acid quantification. The analysis was performed with an isocratic elution using H3PO4 10−3 M at 25 °C, 0.7 mL/min flow rate and the detector was set at 210 nm according to the method proposed by Cane (1990).
Anthocyanins were isolated directly from the wine by passing 3 mL of wine through C 18 Sep-pak cartridge previously conditioned by 2 mL methanol and 2 mL fosforic acid 0.01 N. Anthocyanins were eluted with 3 mL methanol, was evaporated to dryness and redissolved in a mixture of 1 mL methanol: H3PO4 10−3 M (40:60) before injection into chromatographic system according to Squadrito’s et al. method (2007). The samples, previously filtered on 0.45-μm nylon membrane, were injected into a Thermo ODS RP-C18 Hypersil 100 × 2.1 (5 μm) column with a guard ODS Hypersil 20 × 2.1 mm (5 μm). Separation was carried out at 30 °C, the flow rate was 0.25 mL/min and the injection volume 10 μL. The detection was at 520 nm, using solvent: A formic acid 10 %; B formic 10 % and methanol 50 %. Linear gradients from 72 to 55 % A in 15 min; from 55 to 30 % A in 20 min; from 30 to 10 % A in 10 min; from 10 to 1 % A in 5 min; from 1 to 72 % A in 5 min; equilibration time 5 min. Anthocyanins were identified according to the retention time and the UV–Vis spectral features described in the literature (Kelebek et al. 2007; López et al. 2009). Detected compounds were quantified by integration of the peak area and the results were expressed in terms of malvidin-3-O-glucoside (mg/L).
GC–MS analysis
Volatile compounds were quantified using a 6890 gas chromatograph interfaced with a 5973 mass selective detector (Agilent, Palo Alto, CA, USA). The identification of compounds was performed using a NIST 75 library (using a percent matching higher than 95 % as the threshold value for acceptance) and comparing the linear retention index and the electron impact (EI) mass spectra with data from reference compounds. The concentration was calculated as µg/L of 1-heptanol (internal standard). Twenty-three fermentation-derived volatile compounds, including acetate esters, ethyl fatty acid esters, higher alcohols and fatty acids, were detected and quantified in each wine using a C18 solid phase extraction/gas chromatography/mass spectrometry (SPE/GC/MS) technique described by Giannotti and Di Stefano (1991). Twenty mL of wine were added with 200 µL of internal standard 1-heptanol (676 µg/L), and diluted with 40 mL of H2O prior to reverse solid phase extraction with C18 cartridges (1 g) and 6 mL of CH2Cl2 as eluent; dehydration of the extract, concentration under N2 flow, and storing at −25 °C until analysis.
Sensory evaluation
The tasters panel was composed by 20 trained judges of the Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria (CREA), Experimental Winery of Barletta (Apulia region, Italy), who participated, at least twice a week, in wine sensory sessions. The judges were requested not to smoke or eat for 1 h prior to the sensory sessions. The wines were evaluated for colour, aroma and flavour attributes.
Prior to the evaluation, five experienced wine judges selected twelve sensorial descriptors to characterize the wines relatively to colour attributes: red colour intensity, violaceous tastes; olfactory perceptions: spicy (licorice, cloves, cinnamon), red berries (raspberry, currant, blackberries), cherry–black cherry, prune-jam, green grass odour (herb, green olive, tea), taste perceptions: acidity, astringency (relative to tannins or procyanidins), pleasantness, body (mouthcoat and/or ethanol), overall judgement. Judges participated in a training session to practice the tasting and rinsing (with water) protocol, which required judges to swirl and sniff the glass for the aroma assessment, then sip the wine for the flavour. All aroma and flavour of wine evaluations were conducted in individual tasting booths, with 30 mL wine at 20 °C in 250 mL ISO wine glasses, labelled with three-digit random numbers and covered with plastic film. Judges scored each attribute on a 100 mm unstructured line scale, anchored at 1 and 100 mm with low and high (or light and dark for colour), respectively.
Statistical analysis
Chemical analyses were repeated three times for each sample. The one way analysis of variance (ANOVA), and Duncan multiple comparison test to measure variation between treatments at a probability level of p < 0.05 was applied.
The odour activity value (OAV) of volatile compounds detected was calculated to determine their influence onto the perception of the aromas. OAV was defined as the concentration of a single compound divided by the odour threshold (i.e. the lowest concentration that can be detected by human nose) (Gómez-Míguez et al. 2007; Jiang and Zhang 2010). Perceptive odour thresholds were referred to Jiang and Zhang (2010), Tao and Zhang (2010), Francis (2013).
A nonparametric Kruskall–Wallis test was applied to compare each of the twelve selected sensorial descriptors scored among the three experimental theses and considering each judge as a replicate. Volatile compounds resulted statistically different at p < 0.01 among the investigated theses by the ANOVA or exhibiting OAVs > 1 were selected to perform a principal component analysis (PCA). All statistical analyses were performed with Statgraphics Centurion XV ver. 15.1.02 was employed.
Results and discussion
Wine chemical composition
Grapes were harvested on 20th August 2012, earlier if compared to the previous year (29th August 2011), due to the dry climatic conditions and the high day temperatures occurred during the ripening of the grapes. It must be noticed that the favourable climate brought forward the ripening of the grapes employed in this study and strongly enhanced the parameters related to the technological and phenolic maturity. As often it happens with Primitivo grapes, its high sugar content developed a high alcohol content in the wines obtained. The high alcohol content can contrast the harshness brought by tannins present in the wines for the presence of a greater mass of stems in contact with must during the fermentation, as in the case of D50 and D75. Table 1 lists the physical and chemical characteristics of the three different types of wines. D50 showed significant the highest alcohol %, the lowest total SO2and malic acid content, anyway these parameters seemed to be not strictly linked to the amount of stems present in the must during fermentation, since they do not behave in a graduate scale as the amount of stems grows passing from to the other. On the contrary, there were not significant differences between D50 and D75 and the completely de-stemmed grapesD100 in terms of reducing sugars, volatile acidity, pH and titratable acidity.
Table 1.
Chemical-composition of three different Primitivo wines vinified without (D100) and with 25 % (D75) and 50 % (D50) of stems
| D100 | D75 | D50 | ANOVA | |
|---|---|---|---|---|
| Alcohol % | 19.67 ± 0.05b | 19.38 ± 0.06c | 20.05 ± 0.06a | *** |
| Reducing sugars (g/L) | 3.2 ± 0.70 | 2.8 ± 0.90 | 3.4 ± 0.70 | n.s. |
| Total acidity (g/L) | 6.15 ± 0.28 | 6.38 ± 0.25 | 6.3 ± 0.28 | n.s. |
| Volatile acidity (g/L) | 0.75 ± 0.03 | 0.75 ± 0.03 | 0.66 ± 0.30 | n.s. |
| pH | 3.91 ± 0.10 | 3.84 ± 0.08 | 3.90 ± 0.09 | n.s. |
| Total SO2 (mg/L) | 96 ± 3.3a | 77 ± 3.1b | 64 ± 3.1c | *** |
| Free SO2 (mg/L) | 24 ± 0.18a | 9 ± 0.10c | 10 ± 0.12b | *** |
| Tartaric acid (g/L) | 1.6 ± 0.15 | 1.53 ± 0.14 | 1.69 ± 0.15 | n.s. |
| Malic acid (g/L) | 1.73 ± 0.12b | 2.4 ± 0.14a | 1.7 ± 0.13b | *** |
| Lactic acid (g/L) | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | n.s. |
n.s. not significant, * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001. Different letters in the same row indicate significant differences (p ≤ 0.05) Mean ± standard deviation
Phenolic composition
Table 2 lists the main phenolic compounds found in the three different wines at racking. All phenolic compounds showed higher concentration in D50 compared to D75 and D100. In general, as the percentage of stems included during the maceration/fermentation processes increases, an increase in (+)-catechin and (−)-epicatechin, procyanidins dimers and a slight reduction of anthocyanins occurred. The wines obtained from the three different winemaking treatments were characterized by the prevalence of epigallocatechin with respect to all flavan-3-ol monomers, followed by (+)-catechin and epicatechingallate while gallocatechins were present in the lowest amounts. The procyanidins dimers B2 and B2 gallate were present in the highest concentrations. As expected, both the stem-contact wines D50 and D75 had a higher concentration of catechin monomers, dimers and gallates, compared to D100 wines. This was consistent with the reports reported by Spranger et al.(2004), who stated that the presence of flavan-3-olspresent in grape stems were transferred into the must during the fermentation. The results also indicated that the transfer of both monomeric catechins and oligomeric procyanidins from stems to must during maceration was not selective but affected the whole series of flavan-3-ols detected. This was confirmed that stems represented a natural source of tannins capable to enrich wine, providing more structure and complexity.
Table 2.
Phenolic compounds (mg/L) and colour characteristics of three different Primitivo wines vinified without (D100) and with 25 % (D75) and 50 % (D50) of stems
| D100 | D75 | D50 | ANOVA | |
|---|---|---|---|---|
| Non-flavonoids—hydroxycinnamate derivatives | ||||
| Caftaric acid | 264.80 ± 4.84b | 260.42 ± 5.04b | 283.23 ± 6.36a | ** |
| Coutaric acid | 75.4 ± 3.11 | 73.2 ± 2.85 | 77.42 ± 2.79 | n.s. |
| Flavonoids | ||||
| Flavan-3-olmonomers | ||||
| (+)-Catechin | 17.78 ± 0.74c | 21.20 ± 0.83b | 28.30 ± 0.88a | *** |
| (−)-Epicatechin | 12.40 ± 0.21c | 17.61 ± 0.31b | 19.62 ± 0.29a | *** |
| Gallocatechin | 6.20 ± 0.25a | 3.30 ± 0.10c | 4.02 ± 0.03b | *** |
| Epicatechingallate | 19.30 ± 0.75b | 19.60 ± 0.67ab | 21.40 ± 0.98a | * |
| Epigallocatechin | 23.80 ± 0.38c | 25.81 ± 0.41b | 30.30 ± 1.04a | *** |
| Dimers | ||||
| Procyanidin B2 | 45.87 ± 1.24c | 62.30 ± 1.88b | 68.31 ± 1.93a | *** |
| Procyanidin B1 | 25.21 ± 0.44c | 33.40 ± 0.53b | 39.40 ± 0.67a | *** |
| Procyanidin B4 | 18.89 ± 0.37c | 28.40 ± 0.46a | 26.33 ± 0.63b | *** |
| Procyanidin B2 gallate | 48.30 ± 1.24c | 61.30 ± 1.08b | 76.24 ± 2.34a | *** |
| Flavonols | ||||
| Myricetin gr. + gs. | 12.45 ± 0.44a | 9.60 ± 0.49c | 11.20 ± 0.37b | *** |
| Quercetin gr. + gs. | 25.8 ± 0.69b | 25.3 ± 0.73b | 27.90 ± 0.37a | ** |
| Kaempferol gr. + gs. | 20.3 ± 0.61b | 19.9 ± 0.54b | 23.54 ± 0.52a | *** |
| Mono-glycoside anthocyanins | ||||
| Delphinidin-3-G† | 5.67 ± 0.15a | 4.42 ± 0.12b | 4.3 ± 0.15b | *** |
| Cyanidin-3-G† | 0.77 ± 0.01a | 0.66 ± 0.01b | 0.54 ± 0.01c | *** |
| Petunidin-3-G† | 15.31 ± 0.56a | 12.6 ± 0.68b | 12.25 ± 0.52b | ** |
| Peonidin-3-G† | 8.72 ± 0.45a | 7.51 ± 0.44b | 7.31 ± 0.39c | * |
| Malvidin-3-G† | 181.37 ± 5.22a | 150.50 ± 4.75b | 149.21 ± 6.80b | *** |
| Total Acetylated forms† | 19.6 | 21.65 | 18.27 | |
| Total Coumaroylated forms† | 26.44 | 22.98 | 22.36 | |
| Sum anthocyanins | 257.88 | 220.32 | 214.24 | |
| Col.Int. 420 + 520 + 620 | 11.97 | 15.9 | 15.2 | |
| Tint 420/520 | 0.72 | 0.67 | 0.7 | |
| Flavans, vanillin | 1078 ± 16c | 1333 ± 18b | 1587 ± 16a | *** |
| Sum proanthocyanidins | 1744 ± 36c | 2180 ± 43b | 2275 ± 44a | ** |
†Expressed as mg/L malvidin-3-O-glucoside. n.s. not significant, * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001. Different letters in the same row indicate significant differences (p ≤ 0.05) Mean ± standard deviation
Primitivo wines have higher levels of hydroxycinnamate derivatives (caftaric and coutaric acid), compared to other varieties (Gómez-Plaza et al. 2001; Suriano et al. 2012). Hydroxycinnamyl tartaric acids (HCTA), in particular the T-caftaric acid, are important because they are substrates of polyphenol oxidase (PPO) and are thus responsible for the processes of musts browning. Among HCTA, caftaric acid ester was present in greater concentrations as the percentage of stems augmented in the wines, while minor and not significant differences occurred for coutaric acid concentrations in wines among the three treatments.
Among the three compounds of the class of flavonols (myricetin, quercetin and kaempferol), present as glucuronide and glucoside, it was observed that quercetin was the most abundant, with the highest amount detected in D50 wines. Kaempferol proved to be the highest in D50 wines, while myricetin was most abundant in D100 wines. In this case too, they seem not to follow the gradient of stem content of the investigated wines.
The overall anthocyanins profiles of wines showed a slight prevalence of coumarylated anthocyanins compared to the acetylated forms. The most abundant glucosylated anthocyanins encountered were the malvidin-3-O-glucoside, followed by petunidin-3-O-glucoside, peonidin-3-O-glucoside, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside. D50 and D75 had lower concentrations of individual anthocyanins, compared to the totally de-stemmed thesis, in line with Spranger et al. (2004). This could be due to the fact that anthocyanins were absorbed by stems during the maceration/fermentation process or to degradation reactions and condensations with other compounds. In general, the colour intensity of red wine is positively correlated with the concentration of anthocyanins, thus the increase of anthocyanins, achievable with a total de-stemming process, usually entails an increase of colour intensity (Gómez-Plaza et al. 2001). D50 and D75 had higher colour intensity with respect to D100 (Tab. 2), probably due to an easier oxygenation of musts favoured by a better aeration of wines for the presence of stems during the treatments performed. The colour enhancement in wines has been found to be between two and ten times that expected from the pigment alone (Mazza and Brouillard 1990; Boulton 2001) and tannins act as co-factors in co-pigmentation phenomena. Indeed, D75 and D50 showed a significant higher content of flavans reacting to vanilline and total pronthocyanidins (high molecular tannins) than D100.
Oxygen plays an important role in several reactions during winemaking, mainly involving phenolic compounds, encouraging their polymerisation and condensation reactions, and leading to the formation of stabilised compounds (Cejudo-Bastante et al. 2011). Moreover, the presence of greater amounts of metal cations (Cu++, Mg++) might have contributed with the anthocyanins to the formation of complexes responsible for abathochromic effect, causing a shift toward an intense blue colour (Boulton 2001), already favoured in part by the significant concentration of the malvidin-3-O-glucoside found among the glucosylated anthocyanins.
Volatile compounds
The concentration of the volatile compounds, identified by GC–MS in D50, D75 and D100 Primitivo wines is shown in Table 3. Approximately 50 volatile compounds were identified and the most representatives of these (23) belong to different classes including alcohols, acids and esters. Considering the components of the volatile fraction of wines grouped by chemical class, the treatments used in the winemaking did not show significant differences. Indeed, all the wines were characterized by a good content of higher alcohols, on average the 87 % of the volatile fraction, followed by esters of 9 % and at lower levels by acids of 3.2 %, reported by Moreno-Pérez et al. (2013). Higher alcohols, in wines of D50 differed significantly inhigher1-butanol, isobutanol, hexan-1-ol, hex-3-en-1-ol and 2-phenylethanol content when compared to D75 and D100 wines, confirming effects reported by other authors (Spranger et al. 2004), who stated that pomace contact time during winemaking can improve the formation of this compounds, also may be due to a better aeration of wines due to the presence of stems. The 1-butanol, hex-3-en-1-ol and hexan-1-ol, which give the wine an alcoholic, green grass and herb odour, did not reach the threshold of perception, while the 2-phenyl-ethanol, responsible for flowery, pollen, and perfume nuances was 2–4 times higher than the threshold and were potentially perceptible. A higher concentration of benzyl alcohol, responsible for citrusy and sweet hints in wine (Gómez-Míguez et al. 2007) was found for both D50 and D75.
Table 3.
Perceived thresholds, odour activity value (OAV) and Mean ± standard deviation and Duncan test (n = 3) on the concentrations of volatile compounds (μg/L) of three different Primitivo wines vinified (D100) and with 25 % (D75) and 50 % (D50) of stems
| Threshold (μg/L) | Aroma | D100 | D75 | D50 | ANOVA | D100 OAV | D75 OAV | D50OAV | |
|---|---|---|---|---|---|---|---|---|---|
| Higher alcohols | |||||||||
| Propan-1-ol | 306,000 | Fresh, alcohol | 1179 ± 88 | 1059 ± 201 | 1108 ± 219 | n.s. | 0.004 | 0.003 | 0.004 |
| Isobutanol | 40,000 | Fusel, alcohol | 10,657 ± 480b | 11,801 ± 500ab | 12,137 ± 550a | * | 0.266 | 0.295 | 0.303 |
| 1-Butanol | 15,000 | Medicinal, alcohol | 602 ± 20b | 622 ± 34ab | 691 ± 35a | * | 0.040 | 0.041 | 0.046 |
| 1-Pentanol | 80 | Fruity, balsamic | 93 ± 10b | 139 ± 5a | 110 ± 18ab | * | 1.163 | 1.738 | 1.375 |
| Hexan-1-ol | 8000 | Green, grass | 696 ± 20b | 948 ± 18a | 954 ± 21a | *** | 0.087 | 0.119 | 0.119 |
| Hex-3-en-1-ol | 400 | Green, floral | 615 ± 32c | 840 ± 28b | 920 ± 35a | *** | 1.538 | 2.100 | 2.300 |
| 3-Ethoxy-propan-1-ol | 100† | Blackcurrant | 182 ± 10 | 193 ± 21 | 171 ± 12 | n.s. | 1.820 | 1.930 | 1.710 |
| Benzyl alcohol | 200,000 | Citrusy, sweet | 178 ± 31b | 210 ± 48ab | 309 ± 52a | * | 0.001 | 0.001 | 0.002 |
| 2-Phenylethanol | 14,000 | Flowery, pollen perfumed | 36,560 ± 368c | 40,439 ± 396b | 41,612 ± 453a | *** | 2.611 | 2.889 | 2.972 |
| Total higher alcohols | 50,384 | 55,411 | 57,092 | ||||||
| Ethyl esters | |||||||||
| Ethyl isobutyrate | 15‡ | Fruity | 148 ± 21 | 124 ± 25 | 153 ± 24 | n.s. | 9.867 | 8.267 | 10.200 |
| Ethyl isopentanoate | 3‡ | Fruity | 31 ± 5 | 21 ± 4 | 28 ± 6 | n.s. | 10.333 | 7.000 | 9.333 |
| Ethyl hexanoate | 5 | Fruity, anise | 173 ± 14 | 182 ± 23 | 201 ± 30 | n.s. | 34.600 | 36.400 | 40.200 |
| Ethyl lactate | 154,636 | Lactic, raspberry | 4460 ± 309 | 4541 ± 240 | 4366 ± 421 | n.s. | 0.029 | 0.029 | 0.028 |
| Ethyl octanoate | 2 | Pineapple, pear, floral | 80 ± 1b | 73 ± 2c | 87 ± 4a | ** | 40.000 | 36.500 | 43.500 |
| Ethyl 3-hydroxybutyrate | 20† | Fruity | 315 ± 30b | 320 ± 29b | 399 ± 35a | * | 15.750 | 16.000 | 19.950 |
| Ethyl decanoate | 200 | Fruity, fatty, pleasant | 90 ± 5a | 70 ± 9b | 69 ± 5b | * | 0.450 | 0.350 | 0.345 |
| Ethyl dodecanoate | 1500 | Flowery, fruity | 1 ± 0 | 2 ± 1 | 2 ± 0 | n.s. | 0.001 | 0.001 | 0.001 |
| Total ethyl esters | 5298 | 5333 | 5305 | ||||||
| Acetate esters | |||||||||
| Isoamyl acetate | 30 | Banana | 291 ± 48 | 312 ± 26 | 307 ± 47 | n.s. | 9.700 | 10.400 | 10.233 |
| Ethyl acetate | 7500 | Fruity, sweet | 34 ± 2b | 42 ± 4a | 44 ± 2a | * | 0.005 | 0.006 | 0.006 |
| Hexyl acetate | 670 | Pleasant, fruity, pear | 15 ± 1b | 18 ± 1ab | 20 ± 2a | * | 0.022 | 0.027 | 0.030 |
| Total acetate esters | 340 | 372 | 371 | ||||||
| Acids | |||||||||
| Butanoic acid | 173‡ | Rancid, cheese, vomit | 501 ± 35b | 587 ± 30a | 594 ± 25a | * | 0.003 | 0.003 | 0.003 |
| Hexanoic acid | 3000 | Cheese, rancid, fatty | 677 ± 72b | 698 ± 70ab | 870 ± 80a | * | 0.226 | 0.233 | 0.290 |
| Octanoic acid | 500 | Rancid, harsh, cheese, fatty acid | 465 ± 38b | 549 ± 44ab | 629 ± 62a | * | 0.930 | 1.098 | 1.258 |
| Decanoic acid | 15,000 | Fatty, unpleasant | 36 ± 5c | 115 ± 12b | 161 ± 14a | *** | 0.002 | 0.008 | 0.011 |
| Sum acids | 1679 | 1949 | 2254 | ||||||
The presence of stems during maceration/fermentation had no or very low impact on ethyl esters among theses. Ethyl esters, with respect to the other volatile compounds investigated, exhibited the highest OAVs, thus proving to be very peculiar of the aromatic profile of Primitivo red wines. Ethyl lactate was the most representative among all ethyl esters, in line with Jiang and Zhang (2010), responsible for lactic and raspberry aroma (Tominaga et al. 1998). Its concentration, ranging from 4366 μg/L in D50 wines to 4541 μg/L in D75 wines, was below the perceived threshold levels (Guth 1997). Fractions like hexanoate, octanoate, hydroxybutyrate, decanoate and dodecanoate, contributed to the fruity aromas, i.e. apple, pineapple and tropical fruit (Takeoka et al. 1989; Guth 1997), had different behaviours. Among these octanoate and hydroxybutyrate seemed to be promoted by high stem percentage during fermentations (D50), while less differences occurred between D75 and D100. Both compounds showed the highest OAVs among the volatile compounds investigated in all the theses, presumably too high to be sensorially discriminated. Less important appeared decanoate, much higher in D100 with respect to non de-stemmed theses, which did not reach the perceived odour threshold in any of the wines. It resulted in a very low OAV and therefore may had little contribution to perceived aromas of Primitivo wines.
Total acetate esters were almost similar among theses, little higher in non de-stemmed theses D75 and D50. Ethyl and Hexil-acetate represented a very small part of acetate esters and remained below the perceived threshold, with small differences in favour of stemmed wines. Conversely, isoamyl acetate, responsible for flavour of banana and melon, mainly contributed to total acetate esters. It exhibited a very high OAV, but no differences among theses were found.
Total fatty acids were most abundant in D50 and D75 compared to D100. This behaviour was similar when considering the single fatty acids found in the samples. Four fatty acids were present in all the wines with significant quantitative differences. Hexanoic and octanoic acids, responsible for aroma defects like rancid, cheese, harshy, were the most present in all the treatments. An increase in these compounds was observed with an increase in the quantity of stems present during the fermentation. The butanoic and decanoic acids showed the same trend, the last one in particular was found 4–5 times higher in D75 and D50. Although the presence of C6–C10 fatty acids are usually related to the occurrence of negative odours, in our analyses they were present in quantities far below or just around the threshold of perception and could be very important for aromatic equilibrium in wines because they oppose the hydrolysis of the corresponding esters (Edwards et al. 1990).
Sensory evaluation
Figure 1 shows the differences in sensory attributes of wines for the three different treatments based on Kruskal–Wallis test. Colour attributes did not show statistical difference among theses. D75 showed the best red colour intensity and violaceous scores. D100 and D75 substantially exhibited the red colour intensity, higher than D50, on the contrary D100 lost part of violaceous scores similar to D50. The olfactory attributes were much more discriminant among the wines. D75 revealed significant higher (p < 0.01) of spices, red berries and cherry with respect to D50 and D100 which resulted almost similar each other with exception for red berries notes, much present in D50. Mature fruit and jam were substantially similar among theses.
Fig. 1.
Sensory profile of three different Primitivo wines vinified (D100) and with 25 % (D75) and 50 % (D50) of stems
Finally, taste descriptors revealed differences for astringency (p < 0.05), resulted higher in D50, and substantially similar in D100 and D75, the last one still higher in value. The analytical results showed that monomeric catechins and procyanidins dimers were the highest among the flavanols. The wines fermented in the presence of the stems were expected to be higher in potassium, calcium and other cations. This would have resulted in the salification of organic acids and thus changed the acidity of the wines. Instead, both chemical and sensory analyses did not reveal differences in this regard. All the wines had sufficient concentrations of acidity conferring the wines a good structure. The tasters noticed higher herbaceous in D75 and D50 respect to D100, even not statistically supported. Interestingly, however, the increase of grassy/vegetative sensations increased with the percentage of stems present during fermentation.
The structure (body) of a wine was determined by a combination of flavours such as bitterness, sweetness, sourness together with tactile sensations such as astringency. D50 scored the highest value (p < 0.01) for body descriptor, followed by D75 and D100, the last one the less bodied. D50 wines seemed to be the less pleasant, while D75 and D100 scored almost similar values. The overall judgment partially reflected this trend, for which D75 resulted the preferred by tasters followed by D100 and D50.
No unpleasant odour or taste was reported by tasters, confirming that some molecules responsible for negative sensorial notes like some alcohols or fatty acids occurred in the wines did not reach the perceived threshold, thus not compromising the quality of the final product.
PCA analysis
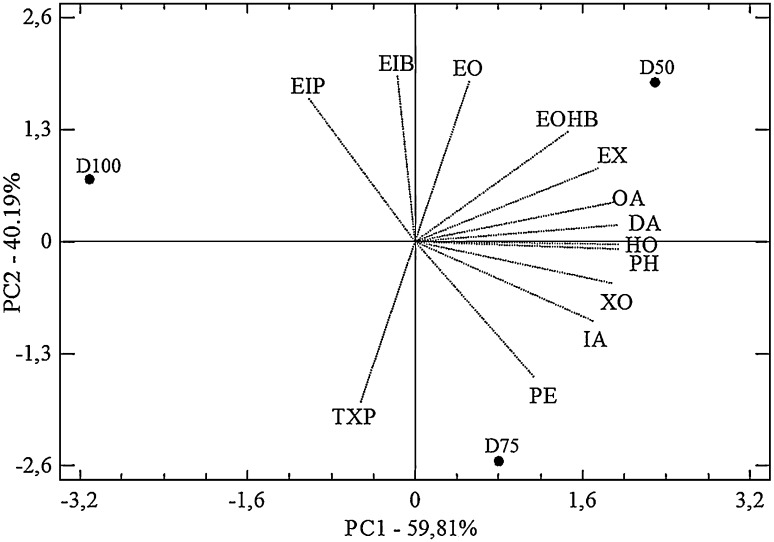
Prior to perform PCA on volatile compounds, the odour activity value (OAV) of the compounds detected was calculated to determine their influence onto the perception of the aromas (Tab. 3). Volatile compounds exhibiting an OAV > 1 and those resulted significantly different for the previous ANOVA at least at p < 0.01 were considered in the PCA analysis (Fig. 2). In total, 13 volatile compounds were subject to PCA: 5 alcohols, 5 ethyl esters, 1 acetate ester and 2 acids, which were able to distinguish the wines each other, with fruity and green-floral notes increasingly evident as the amount of stems present in the must during fermentation increases, in line with sensorial panel. In details, PC1 and PC2 were able to describe 100 % of variability. In particular, the wines differentiated each other along PC1 for positive loadings principally exhibited by hex-3-en-1-ol (HO), which confers green floral aroma, 2-phenylethanol (PH) responsible for flowery, pollen and perfumed hints, and decanoid acid (DA), which in contrast develops fatty and unpleasant sensations, for a total of 59.8 % of variance explained. PH, in particular, seemed to be influenced by the presence of stems during fermentation. PH synthesis in wine was highly influenced by many variables of the media composition. Among these, the right amount of the precursor l-phenylalanine, approximately 3 g/L, was fundamental for maximum efficiency of 2-phenyletanol synthesis (Garavaglia et al. 2007). It can be hypothesized that stems contributed to nitrogen content in must that was used by yeasts to synthesize aminoacids and among these l-phenylalanine, successively converted to 2-phenylethanol.
Fig. 2.
Principal component analysis based on volatile compounds selected on the base of their OAVs and on their significant statistical differences among three different Primitivo wines vinified without stems (D100) and in presence of 25 % (D75) and 50 % (D50) of stems, respectively. PE1, pentanol; XO, hexan-1-ol; HO, hex-3-en-1-ol; TXP, 3-ethoxy-propan-1-ol; PH, 2-phenylethanol; EIB, ethyl isobutyrate; EIP, ethyl isopentanoate; EX, ethyl hexanoate; EO, ethyl octanoate; EOHB, ethyl 3-hydroxybutyrate; IA, isoamyl acetate; OA, octanoic acid; DA, decanoic acid
PC2 described 40.2 % variability and the wines differentiated mainly for Ethyl isobutyrate (EIB) and ethyl octanoate (EO) with positive loadings, both responsible for fruity, pineapple, pear and floral aroma, and 3-ethoxy-propan-1-ol (TXP) with negative loading, responsible for blackcurrant notes. The trend shown by PCAs reflected the stem content of the experimental theses, revealing D50 to exhibit the most green and floral aromas for both PC1 and PC2, with traces of negative notes inferred by long chain fatty acids like octanoic (OA) and DA. OA showed OAV > 1 for all the theses and then was little discriminant, while DA, considered in the PCA for its statistically significant difference recorded among the wines, had OAV far below 1 and therefore resulted not perceived by the tasters. D75 was positioned in an intermediate position in the plot along PC1 and was characterized by balsamic and blackcurrant notes, while D100 projected on the negative side of PC1.
Conclusion
The aim of the study was to evaluate the effects of the presence of stems during the fermentation on the polyphenolic composition and the volatile compounds of Primitivo wines and their reflexes on sensorial analysis.
The stems of grape bunches, rich in polymeric phenolic compounds, are able to affect aroma and taste improving on one side structure, body and olfactory characteristics, conferring on the other side astringency. Molecules conferring green aromas were higher in presence of stems, even their concentration was below or around the perceived threshold. This resulted in no difference perceived by tasters on vegetal notes among the wines. Therefore, the ‘‘right’’amount of stems to be used in winemaking is fundamental to assure balance to sensorial and organoleptic characteristics to a wine. As expected the analyses confirmed that the wines which underwent maceration in presence of stems were richer in tannins, higher colour intensity and lower concentrations of anthocyanins. The extraction of tannins from the stems favoured the polymerization processes between anthocyanins-tannins and acetaldehyde, thereby enhancing the colour component.
The analysis on the volatile compounds showed differences mainly concerning higher alcohols and fatty acids, which gave the wines D50 and D75 hints of green grass, herb and floral.
The statistical analysis (PCA) performed showed a correspondence between features described by the panel of tasters and the actual composition of wine especially in terms of volatile compounds. On the base of our results D75 seemed represented the recommended percentage of stems to be used in winemaking since it preserved the fruity and balsamic components, with moderate astringency and no unpleasant notes. These results encourage a rational use of stems in a red winemaking as a natural source of tannins, which confer structure aromatic complexity to a wine.
Acknowledgments
The authors thank Apulia Region for the financial support in the Regional Development Program 2007/2013, Axis I Improvement of competitiveness in agricultural and forestry sectors, Integrated Projects of the Production Chain—Measure 124. Authors also thank Mr. GianFranco Fino for grapes and for support in the winemaking.
References
- Boulton R. The Copigmentation of anthocyanins and its role in the color of red wine: a critical review. Am J Enol Vitic. 2001;52(2):67–87. [Google Scholar]
- Cane P. Il controllo della qualità dei vini mediante HPLC: determinazione degli acidi organici. L’Enotecnico. 1990;26(1/2):69–72. [Google Scholar]
- Cejudo-Bastante MJ, Hermosín-Gutiérrez I, Pérez-Coello MS. Micro-oxygenation and oak chip treatments of red wines: effects on colour-related phenolics, volatile composition and sensory characteristics. Part II: Merlot wines. Food Chem. 2011;124(3):738–748. doi: 10.1016/j.foodchem.2010.07.064. [DOI] [Google Scholar]
- Dell’Agli M, Buscialà A, Bosisio E. Vascular effects of wine polyphenols. Cardiovasc Res. 2004;63:593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Di Stefano R, Ummarino I, Gentilini N (1997). Alcuni aspetti del controllo di qualità nel campo enologico. Lo stato di combinazione degli antociani. Annali Istituto Sperimentale. Enologia di Asti, pp 105–121
- Edwards CG, Beelman RB, Bartley CE, McConnell AL. Production of decanoic acid and other volatile compounds and the growth of yeast and malolactic bacteria during vinification. Am J Enol Vitic. 1990;41(1):48–56. [Google Scholar]
- EEC (1990) Commission regulation No 2676/90 on community analysis methods to use in wine sector. Official Journal of the European Communities No. L272/3.10.90
- Fidelibus MV, Christensen LP, Katayama DG, Verdenal PT. Performance of Zinfandel and Primitivo grapevine selections in the central San Joaquin Valley, California. Am J Enol Vitic. 2005;56(3):254–286. [Google Scholar]
- Francis L (2013) Fermentation derived aroma compounds and grape-derived monoterpenes. In: Proceedings of the 15th Australian wine industry technical conference Sydney New South Wales 13–18 July. http://www.awri.com.au/wp-content/uploads/2013/08/francis-W07-AWITC15.pdf Accessed 5 Nov 2015
- Gambacorta G, Antonacci D, Patì S, La Gatta M, Faccia M, Coletta A, La Notte E. Influence of winemaking technologies on phenolic composition of Italian red wines. Eur Food Res Technol. 2011;233(6):1057–1066. doi: 10.1007/s00217-011-1613-7. [DOI] [Google Scholar]
- Garavaglia J, Hickmann Flôres S, Pizzolato TM, Peralba MdC, Zachia Ayub MA. Bioconversion of l-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J Microbiol Biotechnol. 2007;23(9):1273–1279. doi: 10.1007/s11274-007-9361-3. [DOI] [Google Scholar]
- Garcia-Beneytez E, Cabello F, Revilla E. Analysis of grape and wine anthocyanins by HPLC-MS. J Agric Food Chem. 2003;51(19):5622–5629. doi: 10.1021/jf0302207. [DOI] [PubMed] [Google Scholar]
- Giannotti S, Di Stefano R. Metodo per la determinazione dei composti volatili di fermentazione. L’Enotecnico. 1991;10:61–64. [Google Scholar]
- Gómez-Míguez MJ, Cacho JF, Ferreira V, Vicario IM, Heredia FJ. Volatile components of Zalema white wines. Food Chem. 2007;100:1464–1473. doi: 10.1016/j.foodchem.2005.11.045. [DOI] [Google Scholar]
- Gómez-Plaza E, Gil-Muñoz R, López-Roca JM, Martínez-Cutillas JM, Fernández JI. Phenolic compounds and colour stability of red wines: effect of skin maceration time. Am J Enol Vitic. 2001;53(3):266–270. [Google Scholar]
- Guth H. Quantitation and sensory studies of character impact odorants of different white varieties. J Agric Food Chem. 1997;45(8):3027–3032. doi: 10.1021/jf970280a. [DOI] [Google Scholar]
- Jiang B, Zhang Z. Volatile compounds of young wines from Cabernet Sauvignon Cabernet Gernischet and Chardonnay varieties grown in the Loess Plateau Region of China. Molecules. 2010;15:9184–9196. doi: 10.3390/molecules15129184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantz K, Singleton VL. Isolation and determination of polymeric polyphenols in wines using Sephadex LH-20. Am J Enol Vitic. 1991;42(4):309–316. [Google Scholar]
- Kelebek H, Canbas A, Cabaroglu T, Selli S. Improvement of anthocyanin content in the cv. Öküzgözü wines by using pectolytic enzymes. Food Chem. 2007;105:334–339. doi: 10.1016/j.foodchem.2006.11.068. [DOI] [Google Scholar]
- Lombard SG (2011) Experimental and numerical investigation into the destemming of grapes. Degree Master of Science in Mechanical Engineering at the University of Stellenbosch. http://scholar.sun.ac.za. Accessed 8 Nov 2015
- López N, Puértolas E, Hernández-Orte P, Álvarez I, Raso J. Effect of a pulsed electric field treatment on the anthocyanins composition and other quality parameters of Cabernet Sauvignon freshly fermented model wines obtained after different maceration times. LWT Food Sci Technol. 2009;42(7):1225–1231. doi: 10.1016/j.lwt.2009.03.009. [DOI] [Google Scholar]
- Lovino R, Baiano A, Patì S, Faccia M, Gambacorta G. Phenolic composition of red grapes grown in southern Italy. Ital J Food Sci. 2006;18:177–186. [Google Scholar]
- Maletic E, Pejic I, Karoglan Kontic J, Piljac J, Dangl GS, Vokurka A, Lacombe T, Mirosevic N, Meredith CP. Zinfandel, Dobricic, and Plavac mali: the genetic relationship among three cultivars of the Dalmation coast of Croatia. Am J Enol Vitic. 2004;55:174–180. [Google Scholar]
- Mazza G, Brouillard R. The mechanism of copigmentation of anthocyanins in acqueous solution. Phytochemistry. 1990;29(4):1097–1102. doi: 10.1016/0031-9422(90)85411-8. [DOI] [Google Scholar]
- Moio L (2015) Colori, odori ed enologia del primitivo. In: Proceedings of 70th Congresso nazionale Assoenologi Castellaneta marina (TA-ITA), 30th May–2nd June 2015. http://www.assoenologi.it/main/images/pics/relazione_luigi_moio_70congresso.pdf Accessed 11 May 2016
- Moreno-Pérez A, Vila-López R, Fernández-Fernández JI, Martínez-Cutillas A, Gil-Muñoz R. Influence of cold pre-fermentation treatments on the major volatile compounds of three wine varieties. Food Chem. 2013;139:770–776. doi: 10.1016/j.foodchem.2013.01.052. [DOI] [PubMed] [Google Scholar]
- Ricardo-da-Silva JM, Bourzeix M, Cheynier V, Moutounet M. Procyanidin composition of chardonnay Mauzac and Grenache blanc grapes. Vitis. 1991;30:245–252. [Google Scholar]
- Spranger MI, Clìmaco MC, Sun B, Nilza E, Fortunato C, Nunes A, Leandro MC, Avelar ML, Belchior AP. Differentiation of red winemaking technologies by phenolic and volatile composition. Anal Chim Acta. 2004;513:151–161. doi: 10.1016/j.aca.2004.01.023. [DOI] [Google Scholar]
- Squadrito M, Corona O, Ansaldi G, Di Stefano R. Relazioni fra i percorsi biosintetici degli HTCA, dei flavonoli e degli antociani nell’acino d’uva. Riv Vitic Enol. 2007;60(3):59–70. [Google Scholar]
- Sun B, Leandro C, Ricardo-da-Silva JM, Spranger I. Separation of grape and wine proanthocyanidins according to their degree of polymerization. J Agric Food Chem. 1998;46(4):1390–1396. doi: 10.1021/jf970753d. [DOI] [Google Scholar]
- Sun B, Pinto T, Leandro MC, Ricardo-da-Silva JM, Spranger MI. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am J Enol Vitic. 1999;50(2):179–184. [Google Scholar]
- Suriano S, Ceci G, Tamborra P. Impact of different winemaking techniques on polyphenols compounds of Nero di Troia wines. Ital Food Beverage Technol. 2012;70:5–15. [Google Scholar]
- Takeoka G, Buttery RG, Flath RA, Teranishi R, Wheeler EL, Wieczorek RL, Guentert M. Volatile constituents of pineapple (Ananas Comosus [L] Merr.) In: Teranishi R, Buttery RG, Shahidi F, editors. Flavor chemistry: trends and developments. ACS Symposium Series 388. Washington, DC: American Chemical Society; 1989. pp. 223–237. [Google Scholar]
- Tamborra P, Esti M. Authenticity markers in Aglianico Uva di Troia Negramaro and Primitivo grapes. Anal Chim Acta. 2010;660:221–226. doi: 10.1016/j.aca.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Tao Y, Zhang L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China) LWT Food Sci Technol. 2010;43:1550–1556. doi: 10.1016/j.lwt.2010.06.003. [DOI] [Google Scholar]
- Tominaga T, Murat ML, Dubourdieu D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv Sauvignon Blanc. J Agric Food Chem. 1998;46(3):1044–1048. doi: 10.1021/jf970782o. [DOI] [Google Scholar]