Abstract
The physicochemical properties, colour characteristics and dynamic viscosity of fresh rapeseed honey (control, CON) and those stored for 18 months at room temperature (RT) or in freezer (FRO) were compared. The significant decrease of pH after storage was found. Honey stored at RT showed an increase of 5-hydroxymethylfurfural (HMF) (+543.0 %) and decrease of diastase activity (−24.4 %), while in FRO samples the decreasing course (−50.5 and −7.3 %, p > 0.05) were found in comparison with CON samples (3.07 and 28.37 mg kg−1). The negative correlation between HMF content and diastase activity was found. The temperature of storage significantly influenced colour parameters, however, RT differentiated colour of honey to a greater extent (ΔE = 20.37) than FRO (ΔE = 8.07). The lightness (L*), hue (h°), whiteness index (WI) and colour intensity (ABS450) of CON honey samples were similar to honey from FRO. The dynamic viscosity was significantly higher in honey stored in FRO in comparison to CON honey and honey stored at RT. However, in comparison with CON honey, higher (almost twice) viscosity for honey stored at RT was observed.
Keywords: Honey, Storage conditions, Viscosity, Colour, Hydroxymethylfurfural, Diastase activity
Introduction
Rape (Brassica napus L.) is largely cultivated in Central and Eastern Europe for oil production from seeds. This plant represents one of the most important spring sources to bees both for nectar and pollen, giving rise to large amounts of very pure unifloral honey (Persano Oddo et al. 2004). Due to high concentration of glucose on average 40.5 %, the ratio of glucose to fructose of about 1.1, and reducing sugars content equal to approx. 80 %, the rape honey undergoes granulation relatively fast (often in crystallized form with very small crystals) (Devillers et al. 2004). The granulation of honey consists of two processes: the formation of crystals and their gradual growth (in shape and size), which depend on composition of honey and temperature of storage (Lupano 1997). Despite the fact that the crystallisation is natural process and does not reduce the quality of honey, Polish consumers consistently prefer fresh liquid honey (‘patoka’) (Kędzierska-Matysek et al. 2016).
After collecting, honey passes subsequent stages that include different processing operations and periods of storage until consumption. The room temperature of honey storage in domestic conditions is differentiated by the specific geographical features of some regions, seasons and household equipment. Besides, hermetically sealed honey can be stored safely in the freezer. Low temperatures slow down (or stop almost completely) the crystallization, prevent fermentation and reduce the viscosity (Horn 2008). Moreover, with decreasing temperatures other chemical processes come to pass more slowly, especially at temperatures below 0 °C. Nevertheless, the freezing process does not occur in honey, and what is more, lowering the temperature of honey down to 0 °C does not have an overall negative effect on its quality (Bakier 2011). As a consequence, storage of honey in the freezer is the best and most gentle way to maintain honey quality in the long term (Horn 2008). Even lowering the temperature of honey down to 233 K (−40.15 °C) will not result in its change of state in spite of the phenomenon of the so called glass transition. This clearly proves that water in honey can be found only in a strongly bound form (Bakier 2006).
The aim of the present investigation was to study the effect of storage temperature for 18 months (room vs. freezing temperature) on the physicochemical properties, colour and rheological behaviour of rapeseed honey.
Materials and methods
Honey samples
The present study was carried out using seven samples of raw (unprocessed) rapeseed honey procured directly from beekeepers. Apiaries were located in Lublin region. Honey samples were collected in May/June 2014. Three samples of the same fresh honey were poured into glass jars with a volume of one litre and sealed. After a few (3–5) days of storage at 20 °C in the dark, honey showed the complete and homogeneous granulation close to that of a solid substance of plastic consistency. The control honey samples (CON) were analyzed until 15th July, 2014. The experimental groups were stored for 18 months, the first batch in the freezer at −20 °C (FRO), and the second one kept in darkness at room temperature (RT), i.e. between 20 and 26 °C. All analyses for both experimental groups were performed until 30th December, 2015. CON honey and honey stored at RT were analyzed at 20 °C in duplicate and the average values were reported. The samples stored in the freezer were warmed in a water bath (LaboPlay, W615, Poland) to 20 °C and after achieving the desired temperature, the glass jar was held for a while in an insulated box to obtain a homogeneous temperature and analysed.
Measurements and analysis
Descriptive analysis
The moisture content was determined based on refractometric method using an Abbe Carl Zeiss refractometer (Jena, Germany). The refractive indices of honey samples were measured at ambient temperature, and the readings were corrected for a standard temperature of 20 °C (Bogdanov et al. 2009).
The pH and electrical conductivity were determined as described earlier (Bogdanov et al. 2009) using a pIONneer 65 Meter (Radiometer Analytical, Villeurbanne, CEDEX-France) with a combined pH electrode (E16M340) and a 4-pole conductivity cell (CDC 30T) with a built-in temperature sensor. Free acidity was determined by potentiometric titration with 0.1 N sodium hydroxide solution (NaOH) and expressed as the milliequivalents of acid per kg of honey (Bogdanov et al. 2009).
Analytical determinations
The 5-HMF (expressed as mg per kg of honey) was determined by White (1979) method using a Varian Cary 300 Bio spectrophotometer (Varian Australia PTY, Ltd.). The diastase activity, expressed as the diastase number in Schade units, was measured with the Phadebas tablets (Honey Diastase Test, Magle AB, Lund, Sweden) according to Bogdanov et al. (2009) using a Varian Cary 300 Bio spectrophotometer (Varian Australia PTY, Ltd.) at 620 nm.
Colour measurements
The colour of the honey samples was measured by a Minolta CR-310 Chroma Meter (Minolta Camera Co. Ltd., Osaka, Japan) using D65 as the standard light source. Honey samples were poured into small disposable petri dishes (60 mm in diameter, height layer of honey 10 mm), and then put onto a white standard plate. The measuring head (50 mm diameter of aperture) was inserted directly into the sample, and the reflectance of honey surface was measured. The CIE colour parameters were L* (lightness), a* (redness/greenness), b* (yellowness/blueness), C* (chroma), and h° (hue angle) (CIE 2004). The colour difference (ΔE) between control samples of honey and after storage denotes the square root of (ΔL2 + Δa2 + Δb2). The whiteness index was calculated using following formula
| 1 |
Colour intensity
The honey samples for colour intensity were diluted to 50 % (w/v) with warm (45–50 °C) deionised water and the solution was filtered through a 0.45 μm filter before measuring the optical density. The absorbance was measured using a Varian Cary 300 Bio spectrophotometer (Varian Australia PTY, Ltd.) at 450 and 720 nm and the difference in absorbance was expressed as mAU (Beretta et al. 2005).
Viscosity measurements
Viscosity was measured using a Zwick/Roell universal testing machine Proline BDO-FB0.5TS (Zwick GmbH and Co, Ulm, Germany) with the back extrusion rig. The measuring system consists of a back extrusion cell (a diameter 50 mm, a length of 60 mm), and a plunger (45 mm diameter). The dynamic viscosity η (Pa s) was evaluated from the measured force difference and the flow rate of the honey sample in the annular gap between the piston and back extrusion cell. The mean value was calculated based on 4 cycles (50, 100, 200 and 400 mm/min) using the testXpert II program especially developed for viscosity testing.
Statistical analysis
The analyses were performed using STATISTICA ver. 6.0 software (StatSoft Inc. 2003). One-way analysis of variance (ANOVA) followed by Tukey’s (HSD) test was used to compare means of physicochemical properties, colour parameters, and dynamic viscosity of different storage conditions. The data were expressed as means ± standard deviations (s.d.). Differences between means at the 95 and 99 % (p < 0.05 and p < 0.01, respectively) confidence levels were considered statistically significant.
Results and discussion
Physicochemical parameters
The results of physicochemical parameters are presented in Table 1. The moisture content did not differ significantly before and after storage, however, a slight decrease for RT and FRO groups were observed. However, irrespective of temperatures the significant (p < 0.05) decrease of pH after storage was found. Concomitantly, free acidity of honey from RT and FRO groups were raised (p > 0.05), while the electrical conductivity of honey remained stable regardless of storage conditions. Results obtained for physicochemical properties of rapeseed honey were in close agreement with values reported by other authors (Persano Oddo et al. 2004; Szczęsna et al. 2011; Wilczyńska 2012). Furthermore, physicochemical properties of raw rapeseed honey before (CON) and after storage at different temperatures (RT and FRO) were consistent with the Polish criteria for consumer honey. According to Regulation (MARD 2004), the acceptable moisture content, free acids and conductivity must not exceed 200 g kg−1, 50 mEq kg−1 and 0.8 mS cm−1, respectively. Singh et al. (2006) reported the average pH values for ripened and unripened Brassica juncea honey of 4.1 and 5.0, respectively. Electrical conductivity is a parameter correlated with the mineral content, which increased in stored honeys with respect to fresh honeys, whereas moisture content and pH did not show a significant variation among fresh and stored honeys (Gulati and Kumari 2005).
Table 1.
Physicochemical properties of raw and stored rapeseed honey
| Specification | CON (n = 7) | RT (n = 7) | FRO (n = 7) |
|---|---|---|---|
| Moisture (%) | 18.53 ± 0.74 | 17.80 ± 0.78 | 18.06 ± 0.89 |
| Free acidity (mEq kg−1) | 18.71 ± 4.16 | 22.21 ± 4.62 | 21.71 ± 3.09 |
| pH | 4.20b ± 0.16 | 3.94a ± 0.18 | 3.93a ± 0.16 |
| Electrical conductivity (mS cm−1) | 0.25 ± 0.06 | 0.25 ± 0.05 | 0.25 ± 0.06 |
| 5-HMF (mg kg−1 of honey) | 3.07A ± 1.77 | 19.74B ± 2.84 | 1.52A ± 0.70 |
| Diastase activity | 28.37b ± 6.53 | 21.44a ± 4.37 | 26.30ab ± 5.79 |
Mean with different letters in the same column are significantly different: a–b –p < 0.05; A–B –p < 0.01
CON fresh honey, RT honey stored at room temperature (20–26 °C) for 18 months, FRO honey stored in freezer (at −20 °C) for 18 months
HMF content and diastase activity
Content of 5-HMF in the control rapeseed honey was much lower (approx. ten times) than the acceptable limit (not exceed 40 mg kg−1) permitted by the Polish regulation (MARD 2004) (Table 1). Wilczyńska (2012) reported similar 5-HMF content (between 0.6 and 4 mg kg−1) for Polish honeys. In fresh honeys there was practically no HMF, and subsequent level of HMF depends on the chemical properties and floral origin of honey (Singh and Bath 1997, Fallico et al. 2008), temperature and time of heating (Singh and Bath 1998), and storage conditions (Sancho et al. 1992). Thus, obtained results indicated that honey samples were fresh and unheated earlier. Honey stored at RT showed significant (p < 0.01) increase of 5-HMF (approximately +543.0 %). However, the opposite (approximately −50.5 %) was observed for frozen samples; but this difference was insignificant (p > 0.05). Previous observations of Yilmaz and Küfrevioğlu (2001) and Castro-Vazquez et al. (2008) indicated increase in HMF during 12 months of storage of Turkish floral honey at constant temperature 25 °C (from 3.3 to 19.1 mg kg−1) and in citrus honey at 20 °C (from 10.2 to 30.4 mg kg−1). Also Ramírez Cervantes et al. (2000) revealed that the HMF rises up to concentrations around 30 mg kg−1 (900 %) and 16 mg kg−1 (433 %) of Tahonal and the Dzidzilché honeys stored at 26 ± 2 °C for 23 weeks. Contrary, Fallico et al. (2008) reported the decrease of HMF averaged approximately 45.0, 61.0 and 60.0 %, respectively for citrus, chestnut and multifloral honeys stored at constant temperature 25 °C for 75 days. On the other hand, Piekut and Baranowska (2001) investigated the influence of storage conditions for 12 months on honey quality of six different varieties. The highest increase of HMF (approximately 2.89 mg kg−1) was observed for honey stored at room temperature (18–20 °C), while the lowest (about 1.76 mg kg−1) for honey stored at –15 °C. Nevertheless, all honey samples contained less than 10 mg kg−1 of HMF. According to Śliwińska et al. (2012), the decrease of HMF content in some multifloral honeys could be related to intensification of complex processes favoring the HMF decomposition and concomitant slowing down the creating processes of HMF precursors.
The results of diastase activity in fresh honey samples and those stored for 18 months at different temperatures are presented in Table 1. The significant (p < 0.01) decrease of diastase activity in honey stored at RT (−24.4 %) compared to CON was observed. FRO samples also showed an insignificant decrease in diastase activity (−7.3 %). The values obtained for fresh rapeseed honeys in the present study for diastase activity were in range reported by Polish authors (Szczęsna et al. 2011; Wilczyńska 2012; Semkiw et al. 2010). Furthermore, in spite of a decrease in diastase activity the lowest level obtained for honey stored at RT (21.44) was much higher than the regulatory value (not less than 8) set in Polish regulations (MARD 2004). Other authors also reported depletion in diastase activity after storage for three and half months at 26 °C (Ramírez Cervantes et al. 2000), 1 year at 20 (±5 °C) (Yilmaz and Küfrevioğlu 2001), and for 2 years at 20 °C (Sancho et al. 1992). However, Castro-Vazquez et al. (2008) stated that diastase activity for citrus honey stored for 12 months at 40 °C exceeded permissible limit.
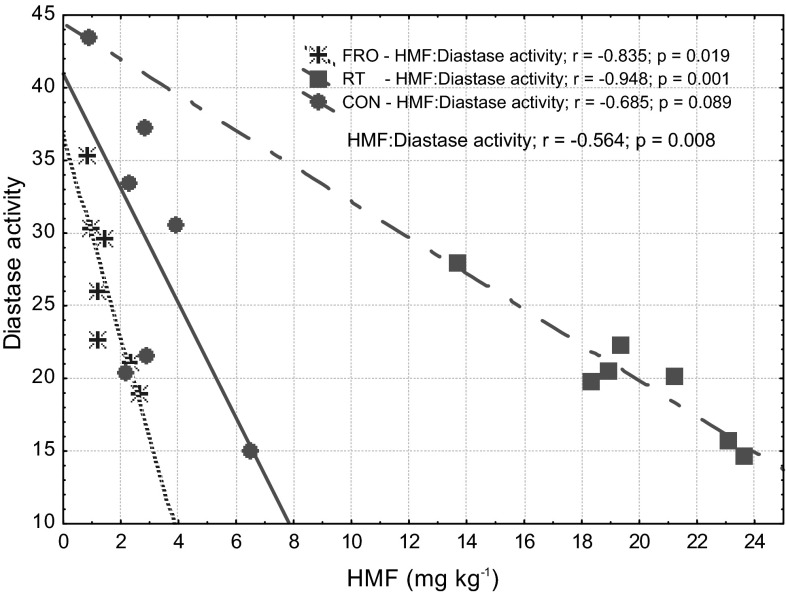
Both HMF and diastase activity are the international parameters used to control the freshness, thermal treatment and storage history of honey (Sancho et al. 1992). The data presented in Fig. 1 show an inverse relationship between the diastase activity and HMF content within each honey group (−0.685 < r < −0.948). Furthermore, the significant (p < 0.01) correlation between these two parameters (r = −0.564) was confirmed irrespective of the storage conditions. Chua and Adnan (2014) also reported the strong negative correlation (r = −0.605) between HMF content and diastase activity.
Fig. 1.
HMF and diastase activity relationship of raw and stored rapeseed honey. CON fresh honey, RT honey stored at room temperature (20–26 °C) for 18 months, FRO honey stored in freezer (at −20 °C) for 18 months
Colour characteristics
The results of colour analysis of investigated honey are reported in Table 2. The temperature of storage significantly (p < 0.01) influenced colour parameters, however, the room temperature differentiated colour to a greater extent (ΔE = 20.37) than freezing (ΔE = 8.07). In present study, a significant (p < 0.01) reduction in lightness (L*), hue (h°) and whiteness index (WI) and increase in redness (a*) and colour intensity (ABS450), as well as insignificant rise of yellowness (a*) and chroma (C*) values were observed for CON and RT honey samples. Honey after freezing showed a significant (p < 0.01) decrease for a*, b* and C* values. However, the lightness (L*), hue (h°), whiteness index (WI) and colour intensity (ABS450) of honey from control samples were similar to those stored in the freezer. Obtained results indicate that all samples of honey can be classified as light honeys (with L* > 50) (González-Miret et al. 2005). Kuś et al. (2014) obtained average L* value of 81.8, and b* of 28.4 for rapeseed honey collected in Poland in May. Contrary to our findings, the value of a* was slightly below zero (green component). Darkening of honey could be related with non-enzymatic browning due to Maillard reaction incorporating the sugars and free amino acids or fructose caramelisation. These reactions lead to formation of a variety of brown pigments, and simultaneously the formation of intermediate products as HMF. Other factors that would influence the kinetics of Maillard browning could be the type and thermal stability of amino acids and reducing sugars which participate in the reaction (Turkmen et al. 2006). Visquert et al. (2014) reported that citrus and rosemary honeys stored for 104 days at 25 °C had total colour difference (ΔE) of 3.89 and 8.30, respectively while at 30 °C approximately 16.01 and 9.51. Additionally, the decrease of a*, b* and C* values in frozen samples, could be linked to changes in the morphology of crystalline structure of analysed honeys. Such changes (more finer crystals) were reported by Bakier (2008), may perhaps modified the optical properties, and consequently caused the lightening of frozen samples of honey (higher L* and WI values) compared to samples stored at RT (Table 2). Some authors pointed the possibility of using WI as an indicator of the presence of crystals in honey. Costa et al. (2015) reported that samples stored at 15 °C for 34 days contained more small crystals than those stored at 25 °C. Furthermore, the WI of samples stored at 15 °C were higher than those stored at 25 °C. However, Kuroishi et al. (2012) found a trend for the WI to increase in samples stored for 3 weeks at 11 and 21 °C, as a result the formation of crystals in the honey. Contrary, Visquert et al. (2014) stated the decrease of WI for citrus and rosemary honeys stored at 25 °C for 104 days from 48.3 to 45.1 and from 33.7 to 30.2. As well, the decrease of WI after storage of honey at RT was observed in the present study.
Table 2.
Colour parameters and ABS450 of raw and stored rapeseed honey
| Specification | CON (n = 7) | RT (n = 7) | FRO (n = 7) |
|---|---|---|---|
| CIE | |||
| L* | 84.32B ± 5.55 | 64.34A ± 2.94 | 79.78B ± 2.38 |
| a* | 7.52B ± 0.85 | 10.12C ± 0.73 | 5.82A ± 0.68 |
| b* | 23.37B ± 2.44 | 25.80B ± 1.25 | 18.60A ± 1.72 |
| C* | 24.56B ± 2.51 | 27.72B ± 1.27 | 19.48A ± 1.81 |
| h° | 72.2B ± 1.33 | 68.7A ± 1.47 | 72.7B ± 1.13 |
| ΔE | – | 20.37B ± 8.19 | 8.07A ± 3.05 |
| WI | 70.7B ± 5.10 | 54.8A ± 2.32 | 71.8B ± 1.81 |
| ABS450 (mAU) | 416.88A ± 93.37 | 661.74B ± 77.39 | 475.97A ± 96.04 |
Mean with different letters in the same column are significantly different: A–C –p < 0.01
CON fresh honey, RT honey stored at room temperature (20–26 °C) for 18 months, FRO honey stored in freezer (at −20 °C) for 18 months mean value ± SD
Honey viscosity
Figure 2 provides the temperature-dependent characteristics of dynamic viscosity for rapeseed honey samples. As expected, viscosity significantly (p < 0.01) increased for FRO honey in comparison to CON. Such changes as granulation effect were also observed to be insignificant for honey samples stored at RT. According to Bakier (2006) the rapeseed honey had Newtonian fluid behaviour and its rheological properties can be characterised individually by dynamic viscosity. The rapeseed honey samples (with 17.8–18.3 % of moisture content) and measured between 293 and 295 K (19.85 and 21.85 °C) showed the dynamic viscosity from 11.4 to 16.9 Pa s (Bakier 2006). This range was less than half that average value found in present study for control samples (33.6 Pa s). However, Bakier (2006) for rapeseed honey samples contained 17.8–18.3 % of moisture content and viscosity at 275 K (1.85 °C) between 261.6 and 317.7 Pa s. In present study the average value of dynamic viscosity for samples stored in freezer (280.5 Pa s) was similar to this range. The honey granulation was accelerated between 13 and 15.5 °C, whereas this process was retarded at freezer temperatures (White 1974). In addition, Conforti et al. (2006) emphasize that factors which determine honey crystallization at room temperature showed different influence than freezing temperature. Lupano (1997) revealed that honey stored at −20 °C showed lower values of crystal melting enthalpies than honey stored at 20 °C. This suggests that different values of enthalpy should correspond to the presence of diverse crystals. Honey stored at 20 °C formed coarse crystals with melting temperatures (Tm) between 45 and 65 °C, whereas honey stored at −20 °C granulated as a finely-grained, fondant-like honey, with Tm between 25 and 45 °C. Therefore, the frozen honey showed a higher viscosity as a result of a crystallized structure formed by fine crystals.
Fig. 2.
Dynamic viscosity of raw and stored rapeseed honey. CON fresh honey, RT honey stored at room temperature (20–26 °C) for 18 months, FRO honey stored in freezer (at −20 °C) for 18 months
Conclusion
The moisture content, free acidity and electrical conductivity of raw rape honey are not impacted by storage temperatures. Freezing temperature maintained freshness of raw honey (expressed as HMF content and diastase activity) and colour characteristics (the lightness, hue, whiteness index and colour intensity), but significantly increased viscosity. The storage of honey at room temperature differentiated colour to a greater extent than freezing, and significantly increased of HMF content and decreased of diastase activity.
Compliance with ethical standards
Conflict of interest
None.
References
- Bakier S. Characteristics of water state in some chosen types of honey found in Poland. Acta Agrophys. 2006;7:7–15. [Google Scholar]
- Bakier S. Investigations of the rheological properties of honey in crystallized states. Treatises and monographs. Warsaw: Univ Life Sci Press; 2008. [Google Scholar]
- Bakier S. Quality changes of honey during collecting and processing. In: Nowak D, editor. Quality and safety of food—impact of technological processing on nutritive quality. Warszawa: Wydaw SGGW; 2011. pp. 129–144. [Google Scholar]
- Beretta G, Granata P, Ferrero M, Orioli M, Maffei Facino R. Standarization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 2005;533:185–191. doi: 10.1016/j.aca.2004.11.010. [DOI] [Google Scholar]
- Bogdanov S, Martin P, Lüllmann C (2009) Harmonised methods of the European Honey Commission, Apidologie extra issue: 1–59, http://www.ihc-platform.net/ihcmethods2009.pdf. Accessed 15 June 2014
- Castro-Vazquez L, Diaz-Maroto MC, Gonzalez-Vinas MA, De La Fuente E, Perez-Coello MS. Influence of storage conditions on chemical composition and sensory properties of citrus honey. J Agric Food Chem. 2008;56:1999–2006. doi: 10.1021/jf072227k. [DOI] [PubMed] [Google Scholar]
- Chua LS, Adnan NA. Biochemical and nutritional components of selected honey samples. Acta Sci Pol Technol Aliment. 2014;13(2):169–179. doi: 10.17306/J.AFS.2014.2.6. [DOI] [PubMed] [Google Scholar]
- CIE . Colorimetry. 3. Vienne: Commission International de l’Eclairage.; 2004. pp. 16–20. [Google Scholar]
- Conforti PA, Lupano CE, Malacalza NH, Arias V, Castells CB. Crystallization of honey at −20 °C. Int J Food Prop. 2006;9:99–107. doi: 10.1080/10942910500473962. [DOI] [Google Scholar]
- Costa LCV, Kaspchak E, Queiroz MB, de Almeida MM, Quast E, Quast LB. Influence of temperature and homogenization on honey crystallization. Braz J Food Technol. 2015;18:155–161. doi: 10.1590/1981-6723.7314. [DOI] [Google Scholar]
- Devillers J, Morlot M, Pham-Delegue MH, Dore JC. Classification on monofloral honeys based on their quality control data. Food Chem. 2004;86:305–312. doi: 10.1016/j.foodchem.2003.09.029. [DOI] [Google Scholar]
- Fallico B, Arena E, Zappala M. Degradation of 5-hydroxymethylfurfural in honey. J Food Sci. 2008;73:C625–C631. doi: 10.1111/j.1750-3841.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- González-Miret ML, Terrab A, Hernanez D, Fernández-Recamales MA, Hereida FJ. Multivariate correlation between colour and mineral composition of honeys and by their botanical origin. J Agric Food Chem. 2005;53:2574–2580. doi: 10.1021/jf048207p. [DOI] [PubMed] [Google Scholar]
- Gulati R, Kumari B. Chemical composition of unifloral, stored and commercial Apis mellifera L. honeys. J Food Sci Technol. 2005;42:492–495. [Google Scholar]
- Horn H. Darf man Honig in der Kühltruhe lagern? Die Biene. 2008;9:20. [Google Scholar]
- Kędzierska-Matysek M, Florek M, Wolanciuk A, Skałecki P, Litwińczuk A. Characterisation of viscosity, colour, 5-hydroxymethylfurfural content and diastase activity in raw rape honey (Brassica napus) at different temperatures. J Food Sci Technol. 2016;53:2092–2098. doi: 10.1007/s13197-016-2194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroishi AM, Queiroz MB, Almeida MM, Quast LB. Avaliação da cristalização de mel utilizando parâmetros de cor e atividade de água. Braz J Food Technol. 2012;15:84–91. doi: 10.1590/S1981-67232012000100009. [DOI] [Google Scholar]
- Kuś PM, Congiu F, Teper D, Sroka Z, Jerković I, Tuberoso CIG. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT Food Sci Technol. 2014;55:124–130. doi: 10.1016/j.lwt.2013.09.016. [DOI] [Google Scholar]
- Lupano CE. DSC study of honey granulation stored at various temperatures. Food Res Int. 1997;30:683–688. doi: 10.1016/S0963-9969(98)00030-1. [DOI] [Google Scholar]
- MARD (2004) Regulation of The Polish Ministry of Agriculture and Rural Development of 18 February 2004 amending regulation on detailed requirements regarding merchantable quality of honey (The Official Journal, 40, Pos. 370, 12.03.2004)
- Persano Oddo L, Piro R, et al. Main European unifloral honeys: descriptive sheets. Apidologie. 2004;35(Suppl. 1):S38–S81. doi: 10.1051/apido:2004049. [DOI] [Google Scholar]
- Piekut J, Baranowska E. Storage of natural bee honeys. Pszczelarstwo. 2001;12:7–9. [Google Scholar]
- Ramírez Cervantes MA, González Novelo SA, Sauri Duch E. Effect of the temporary thermic treatment of honey on variation of the quality of the same during storage. Apiacta. 2000;4:162–170. [Google Scholar]
- Sancho MT, Muniategui S, Huidobro JF, Lozano S. Aging of honey. J Agric Food Chem. 1992;4:134–138. doi: 10.1021/jf00013a026. [DOI] [Google Scholar]
- Semkiw P, Skowronek W, Skubida P, Rybak-Chmielewska H, Szczęsna T. Changes occurring in honey during ripening under controlled conditions based on α-amylase activity, acidity and 5-hydroxymethylfurfural content. J Apic Sci. 2010;54:55–64. [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian honey. Food Chem. 1997;58:129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Singh N, Bath PK. Relationship between heating and hydroxymethylfurfural formation in different honey types. J Food Sci Technol. 1998;35:154–156. [Google Scholar]
- Singh B, Sarkar BC, Sharma HK, Singh C. Comparative studies on ripened and unripened honey of unifloral Brassica juncea. J Food Sci Technol. 2006;43:535–537. [Google Scholar]
- StatSoft Inc. (2003) STATISTICA. Data analysis software system, version 6. www.statsoft.com
- Śliwińska A, Przybylska A, Bazylak G. Effect of changes in storing temperature on the content of 5-hydroxmethylfurfural in some unifloral and multifloral honeys. Bromat Chem Toksykol. 2012;XLV:271–279. [Google Scholar]
- Szczęsna T, Rybak-Chmielewska H, Waś E, Kachaniuk K, Teper D. Characteristics of Polish unifloral honeys. I. Rape Honey (Brassica napus L. var oleifera metzger) J Api Sci. 2011;55:111–119. [Google Scholar]
- Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006;95:653–657. doi: 10.1016/j.foodchem.2005.02.004. [DOI] [Google Scholar]
- Visquert M, Vargas M, Escriche I. Effect of postharvest storage conditions on the colour and freshness parameters of raw honey. Int J Food Sci Technol. 2014;49:181–187. doi: 10.1111/ijfs.12296. [DOI] [Google Scholar]
- White JW. Beekeeping: honey and honey products. In: Johnson AH, Peterson MS, editors. Encyclopedia of food technology. Westport: Avi Publishing Company; 1974. pp. 103–108. [Google Scholar]
- White J. Spectrophotometric method for hydroxymethylfurfural in honey. J Assoc Off Anal Chem. 1979;62:509–514. [PubMed] [Google Scholar]
- Wilczyńska A. Evaluation of honey’s quality in aspect of factors affecting its antioxidant properties. Polska: Prace Nauk, Wydaw Akad Morskiej w Gdyni; 2012. [Google Scholar]
- Yilmaz H, Küfrevioğlu I. Composition of honeys collected from Eastern and South-Eastern Anatolia and effect of storage on hydroxymethylfurfural content and diastase activity. Turk J Agric For. 2001;25:347–349. [Google Scholar]