Abstract
Platensimycin (PTM) and platencin (PTN), isolated from several strains of Streptomyces platensis, are potent antibiotics against multi-drug resistant bacteria. PTM was also shown to have antidiabetic and antisteatotic activities in mouse models. Through a novel genome-mining method, we have recently identified six PTM and PTN dual-producing strains, and generated several mutants with improved production of PTM or PTN by inactivating the pathway-specific transcriptional repressor gene ptmR1. Among them, S. platensis SB12026 gave the highest titer of 310 mg/L for PTM. In this study, we now report titer improvement by medium and fermentation optimization and pilot-scale production and isolation of PTM from SB12026. The fermentation medium optimization was achieved by manipulating the carbon and nitrogen sources, as well as the inorganic salts. The highest titer of 1560 mg/L PTM was obtained in 15-L fermentors, using a formulated medium mainly containing soluble starch, soybean flour, morpholinepropanesulfonic acid sodium salt and CaCO3. In addition, a polyamide chromatographic step was applied to facilitate the purification and 45.14 g of PTM was successfully obtained from a 60 L scale fermentation. These results would speed up the future development of PTM as human medicine.
Keywords: Platensimycin, fermentation medium optimization, polyamide chromatography, Streptomyces platensis SB12026
Introduction
Bacterial infection is a public health crisis worldwide, mainly due to the increase of drug resistance and the lack of new classes of antibiotics (Spizek et al. 2010; Walsh and Wencewicz 2014). Platensimycin (PTM) and platencin (PTN) were discovered by Merck Research Laboratories from Streptomyces platensis MA7327 or MA7339, isolated from soil samples in South Africa or Spain respectively (Wang et al. 2006; Singh et al. 2006; Wang et al. 2007; Jayasuriya et al. 2007). Both PTM and PTN consist of a substituted phenolic acid and a terpene-derived cage unit (Supporting information, Fig. S1). By blocking the bacterial fatty acid synthesis, they have shown strong antibacterial activity against Gram-positive pathogens, especially strains of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. PTM has also been found to inhibit the biosynthesis of mycolic acids and is an excellent lead compound against Mycobacterium tuberculosis (Brown et al. 2009). Subsequently, PTM has been shown to be a potent and selective inhibitor of mammalian fatty acid synthase and could potentially be used to treat diabetes and other metabolic diseases (Wu et al. 2011). Based on the statistics from International Diabetes Federation in 2015, diabetes affects a huge world population, with an estimate of 109.6 million adults with diabetes in China alone (International Diabetes Federation, 2015). Therefore, PTM and PTN are promising drug leads for human medicine. However, their poor in vivo pharmacokinetics has significantly limited the future drug development and none of their analogs prepared so far showed superior activities (Martens and Demain, 2011). In addition, the low titer of PTM or PTN from the wild-type strains limited their production, thereby preventing cost-effective preparation of analogues via semi-synthesis methods (Herath et al. 2008; Shen et al. 2009).
Medium development is critical to improve the production of PTM or to study its biosynthesis. Singh and his colleagues obtained a PTM titer of 52 mg/L from S. platensis MA7327 in a 50 L CLA production medium (mainly consisting of yellow corn meal, lactose and ambrerex) (Zhang et al. 2011). Demain and his colleagues developed several semi-defined or chemically-defined media for S. platensis MA7327, all of which supported PTM production (Aluotto et al. 2013; Falzone et al. 2013).
Using an alternative approach, we generated several mutant strains, which over-produced PTM or PTN to the highest titer of 323 mg/L or 255 mg/L, by inactivating the pathway-specific transcriptional repressor gene ptmR1 in S. platensis MA7327 or ptnR1 in S. platensis MA7339 (Smanski et al. 2009; Yu et al. 2010). Through a novel genome-mining method, we have recently identified six PTM and PTN dual-producing S. platensis strains, all isolated from soil samples collected from various places in China. The similar ptmR1 mutants were generated and the strain S. platensis SB12026 was not only shown to have the highest PTM titer of 310 mg/L and the PTN titer of 170 mg/L, but it sporulated better than the previous S. platensis MA7327ΔptmR1 mutant or S. platensis MA7339ΔptnR1 mutant (Hindra et al. 2014). This strain was also amenable to further genetic manipulation, as demonstrated in the generation of the ptmR1 in-frame deletion mutant (Rudolf et al. 2015).
Since PTM has superior biological activity and was produced in higher amount than PTN in S. platensis SB12026, the first systematic effort to further increase the yield of PTM is warranted through the medium and fermentation optimization. In this study, we report PTM titer improvement up to 1560 mg/L by medium and fermentation optimization, as well as pilot-scale production (6 L in a 15-L fermentor or 60 L in a 150-L fermentor, respectively) and isolation of PTM, featuring a polyamide chromatography step, from S. platensis SB12026, affording 45.14 g of PTM.
Materials and methods
Strain, media and chemicals
The spore suspension of S. platensis SB12026 (deposited at China Center for Type Culture Collection under accession No. CCTCC M 2014286) in 20% glycerol was stored at −80°C. All inorganic salts, carbon and nitrogen sources used in this study were from common commercial sources, unless otherwise specified. The ISM-3 seed medium contained 20 g dextrose, 15 g yeast extract (Difco), 10 g malt extract, 0.244 g MgSO4·7H2O and 0.3 g FeCl3·6H2O in 1 L deionized water (pH 7.0). The initial production medium contained 40 g dextrin (Sigma), 40 g α-lactose, 5 g yeast extract and 5 g morpholinepropanesulfonic (MOPS) acid sodium salt in 1 L deionized water (pH 7.2).
Cultivation of S. platensis SB12026 and medium optimization in flasks
About 1.0 – 3.0 × 105 fresh S. platensis SB12026 spores from a spore stock solution of 107 spores/mL were inoculated into 50 mL ISM-3 seed medium in 250-mL flasks, and incubated at 30°C and 220 rpm in a rotary shaker for about 60 hours. Then 2 mL of such seed culture was inoculated into 50 mL initial production medium and incubated at 30°C and 220 rpm for 7 days. The effects of inorganic salts, carbon and nitrogen sources on the production medium were investigated. An orthogonal test was used to examine the desired ratio of the selected carbon and nitrogen sources. Three different concentrations of soluble starch (Sangon Biotech, Shanghai) (50, 60, and 70 g/L) and four different concentrations of soybean flour (Kerui Biotech, Qingdao) (7.5, 10, 12.5 and 15 g/L) were tested.
Optimization of fermentation conditions in fermentors
S. platensis SB12026 was grown in 15 L fermentors with a working volume of 6.0 L using PTM production medium (70 g/L starch soluble, 15 g/L soybean flour, 5 g/L MOPS acid sodium salt, 5 g/L CaCO3, 15 mg/L MnCl2·4H2O, 30 mg/L (NH4)6Mo7O24·4H2O and 1 mL/L anti-foaming agent) at 30°C, air flow rate 200 L/h and air pressure 0.05 MPa for 7 days. The agitation speed was at 350 rpm unless otherwise specified. To determine the preferred volume of inoculum, different amount of seed culture (4, 10, 13 and 17% (v/v)) was used. To determine the optimal agitation speed, 4% inoculum was used for testing different agitation speeds (200, 270, 350 and 450 rpm). To determine the optimal dissolved oxygen (DO) level, 4% inoculum was used and the agitation speed was correlated with the DO level.
The fermentation of S. platensis SB12026 was further scaled up in a 150-L fermentor using a 3-stage process. Briefly, 250 mL SB12026 seed culture was prepared and inoculated into a 3 L seed medium in a 15-L fermentor. After 24-hour growth, the 3 L seeds were pumped into the 150-L fermentor containing 60 L PTM production medium. The fermentation ran for 7 days at 30°C. During the initial trial to keep the DO level above 40%, the air flow rate was 2000 L/h and the air pressure was at 0.05 MPa when the agitation speed was set between 270 – 350 rpm. Afterwards the air flow rate was set at 1200 L/h, the air pressure was at 0.02 MPa and the agitation speed was set between 145 – 305 rpm.
HPLC analysis of PTM
To analyze the samples from shaking flasks, about 50 mL S. platensis SB12026 culture broth was centrifuged at 4000 rpm for 15 minutes, and 1.5 g of XAD-16 resin was used to absorb PTM from the supernatant. The resin was dried and extracted thoroughly with 15 volume (m/v) of absolute ethanol, and the extract was used for HPLC analysis. For PTM analysis from the fermentors, about 1 mL fermentation broth was centrifuged at 14000 rpm for 5 minutes, and 10 μl supernatant was directly subjected to HPLC analysis. All samples were analyzed on a Waters e2695 HPLC system equipped with a PDA detector and a Waters Sunfire C18 column (150 mm × 4.6 mm). The mobile phase consisted of buffer A (ultrapure H2O containing 0.1% HCOOH and 0.1% CH3CN) and buffer B (chromatographic grade CH3CN containing 0.1% HCOOH) was applied at a flow rate of 1 mL·min−1. A linear gradient program (70% buffer A and 30% buffer B to 5% buffer A and 95% buffer B for 8 mins, followed by 5% buffer A and 95% buffer B for 2 mins) was applied to detect PTM at 240 nm.
PTM purification from the 150-L fermentor
The fermentation broth from 150-L fermentor was centrifuged to give about 60 L supernatant. Two batches of highly porous XAD-16 resin (2000 g for 5 hours and 200 g for 1.5 hours) were sequentially added into the supernatant and mixed by a pitch blade impeller at 100 rpm. The adsorbed resins were charged to a chromatography column (19 cm × 100 cm) and washed with 3.0 L deionized water, then eluted with 12.5 L 80% ethanol (pH 10) at a flow rate of 4.8 mL·min−1. After most ethanol was removed by rotary evaporation, the PTM crude extract was basified by 200 mL 2 M NaOH and centrifuged to remove insolubles. The resulting 3.75 L basic supernatant was extracted three times with the same volume of CH2Cl2 to remove impurities. The residual aqueous layer was then acidified by 250 mL 2 M HCl and extracted again with CH2Cl2 (3.8 L × 3). The latter CH2Cl2 fractions were combined with 150 g polyamide powder (100 – 200 mesh), and evaporated to remove CH2Cl2. The resulting dried polyamide mixture was charged directly onto a deionized water-equilibrated polyamide column (3 L).
The PTM-loaded polyamide column was washed with 3 L deionized water and 10 L 30% ethanol, then eluted with 6 L 80% ethanol at a flow rate of 6.4 mL·min−1. The collected elute was concentrated under reduced pressure to remove ethanol. The remaining oily material was redissolved in 400 mL 50 mM NaOH and transferred to a 1-L beaker. Then pH of this basic solution was adjusted to pH 1.0 using 2 M HCl, resulting in a large amount of precipitate. The precipitate was harvested and lyophilized to afford 45.14 g purified PTM as amorphorous powder, which was further validated by 1H NMR analysis in CDCl3 using tetramethylsilane as an internal standard with a Bruker Advance III 500 MHz Spectrometer.
Results
Initial medium selection
In our previous work, the seed culture of S. platensis SB12026 was prepared sequentially in R2YE and ISM-3 media, and then fermented in the production medium containing 40 g/L dextrin, 40 g/L α-lactose, 5 g/L yeast extract, and 20 g/L MOPS acid sodium salt, supplemented with 5 mL trace element solution (Hindra et al. 2014). We firstly inoculated SB12026 directly into ISM-3 medium to prepare the seed culture and then simplified the production medium by only using 5 g/L MOPS acid sodium salt and excluding the trace element solution. With these modifications, the PTM titer in shaking flasks reached to 463 mg/L, which was higher than the previously reported titer of 310 mg/L (Table 1). Thus, this medium was selected as the starting point for medium optimization in 250-mL shaking flasks.
Table 1.
Effect of inorganic salts and vitamin B12 on production of platensimycin in S. platensis SB12026.
| Inorganic salts and vitamin | Concentration (mg/L) | PTM (mg/L)a |
|---|---|---|
| – | 0 | 463 ± 6 |
| (NH4)6Mo7O24·4H2O | 30 | 519 ± 2 |
| (NH4)2SO4 | 2000 | 199 ± 8 |
| CoCl2 | 0.5 | 436 ± 96 |
| CuSO4·5H2O | 1 | 432 ± 8 |
| FeSO4·7H2O | 30 | 477 ± 13 |
| MnCl2·4H2O | 15 | 512 ± 10 |
| MgSO4·7H2O | 1000 | 179 ± 10 |
| Vitamin B12 | 0.1 | 419 ± 56 |
| ZnSO4·7H2O | 20 | 254 ± 41 |
The values are averages of at least two independent trials.
Effect of inorganic salts and vitamin on the production of PTM
Inorganic salts and vitamins are crucial factors for the survival and growth of Streptomyces and could increase secondary metabolite production (Kieser et al. 2000; Hara et al. 1989). In our study, various inorganic salts, including (NH4)6Mo7O24·4H2O, (NH4)2SO4, CuSO4·5H2O, CoCl2, FeSO4·7H2O, MnCl2·4H2O, MgSO4·7H2O, ZnSO4·7H2O as well as vitamin B12 were individually evaluated (Table 1). Although the addition of CuSO4·5H2O, CoCl2, FeSO4·7H2O and vitamin B12 had negligible effects on PTM production, supplement of (NH4)6Mo7O24·4H2O or MnCl2·4H2O could improve the PTM production up to 519 mg/L or 511 mg/L respectively, while the addition of ZnSO4·7H2O, (NH4)2SO4 or MgSO4·7H2O exerted negative effects on PTM production.
Effect of different carbon sources on the production of PTM
Carbon sources, such as carbohydrates, lipids and carbonic acids, play an essential role in the process of bacterial cytoskeleton formation and secondary metabolites biosynthesis. In our study, 10 different carbon sources, including D-fructose, D-galactose, D-maltose, D-xylose, glycerol, glucose, α-lactose, mannitol, soluble starch and sucrose, were evaluated at a final concentration of 40 g/L, respectively. The fermentation results showed that soluble starch was the preferred carbon source to support the PTM production of 635 mg/L, which is about 37 % higher than the original mixed carbon sources including dextrin and α-lactose (Fig. 1A). All other tested carbon sources supported the production of PTM, albeit at much lower levels (less than 200 mg/L). Subsequent concentration variation of soluble starch from 10 to 100 g/L indicated that PTM was produced with the titer of 697 mg/L when soluble starch was used at 60 g/L (Fig. 1B).
Figure 1.
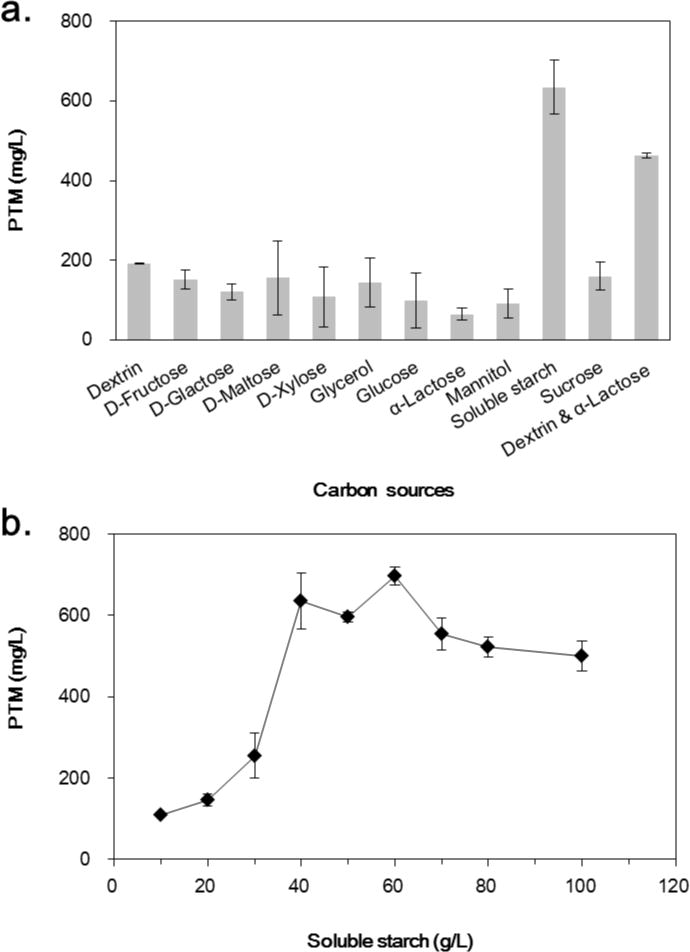
Effect of carbon sources on the production of platensimycin (a) and platensimycin production titers as a function of soluble starch concentration in S. platensis SB12026 (b). There were at least three independent trials for the data bars.
Effect of different nitrogen sources on the production of PTM
Organic nitrogen is the indispensable resource for various primary and secondary metabolic pathways, such as amino acids and nucleic acids. In this study, 11 different nitrogen sources, including beef extract, corn flour, corn gluten meal, corn steep liquor, cotton seed meal, fish meal, malt extract, peanut peptone, peptone, soya peptone and soybean flour, were compared to the previous yeast extract at a concentration of 5 g/L (Fig. 2A). The carbon source was the original dextrin/α-lactose combination. The comparison indicated that only soybean flour and peanut peptone were superior than yeast extract on the production of PTM, and soybean flour could produce highest amount of PTM at a titer of 573 mg/L. Then various concentrations of soybean flour ranging from 1 – 25 g/L were then investigated. To our delight, the titer of PTM reached to 1278 mg/L when 12.5 g/L soybean flour was used (Fig. 2B).
Figure 2.
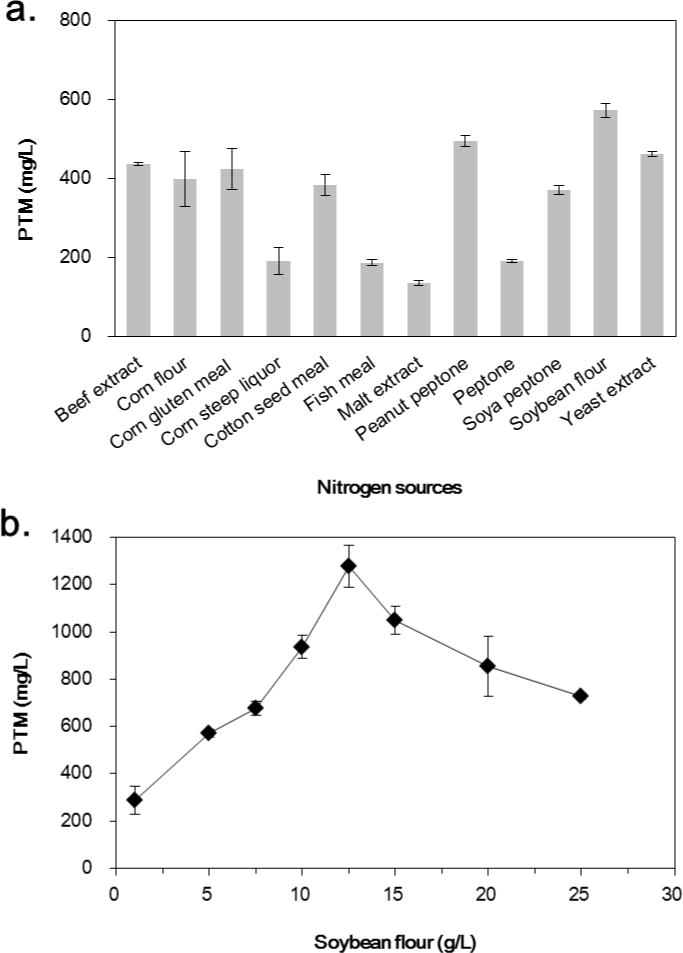
Effect of nitrogen sources on the production of platensimycin (a) and platensimycin production titers as a function of soybean flour concentration in S. platensis SB12026 (b). There were at least three independent trials for the data bars.
The formulation of optimal medium for PTM production
Based on the above single-factor optimization experiments, the PTM production medium was then determined to consist of 60 g/L starch soluble, 12.5 g/L soybean flour, 5 g/L MOPS, 15 mg/L MnCl2·4H2O and 30 mg/L (NH4)6Mo7O24·4H2O. An orthogonal test, using three soluble starch concentrations and four soybean flour concentrations, was further carried out to determine the appropriate combination of carbon and nitrogen sources (Fig. 3). Higher amount of soluble starch and soybean flour was beneficial to the production of PTM, because 70 g/L soluble starch and 15 g/L soybean flour supported the production of PTM to 1420 mg/L, while the combination of 60 g/L starch soluble and 12.5 g/L soybean flour in the production medium only produced 1166 mg/L PTM.
Figure 3.
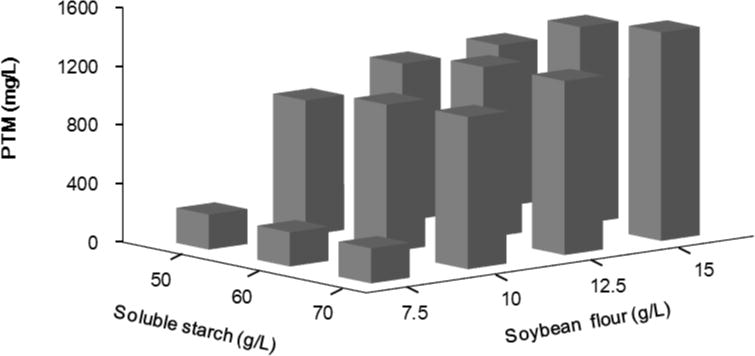
Platensimycin production titers as a function of soluble starch and soybean flour concentration in S. platensis SB12026.
Batch fermentation of S. platensis SB12026 in 15-L fermentors
The fermentation production of PTM was first scaled up in 15-L fermentors using the production medium from the shaking flask experiments. It was observed that reduction of foaming could be achieved by the addition of 1 mL/L anti-foaming agent and using 6 L production volume in the fermentors. However, the PTM titer could only reach to about 600 mg/L after 7-day fermentation, much lower than those of the previous shaking flask experiments (Fig. 4A). We quickly found out that the addition of CaCO3 (5 g/L) would help to increase PTM production to more than 1000 mg/L, probably because of the decreased viscosity (data not shown) and the increased dissolved oxygen (DO) concentration (Fig. 4B). Therefore the optimized production medium used in fermentors was finally determined to consist of 70 g/L starch soluble, 15 g/L soybean flour, 5 g/L MOPS acid sodium salt, 5 g/L CaCO3, 15 mg/L MnCl2·4H2O, 30 mg/L (NH4)6Mo7O24·4H2O and 1 mL/L anti-foaming agent, which was used for the remaining experiments.
Figure 4.
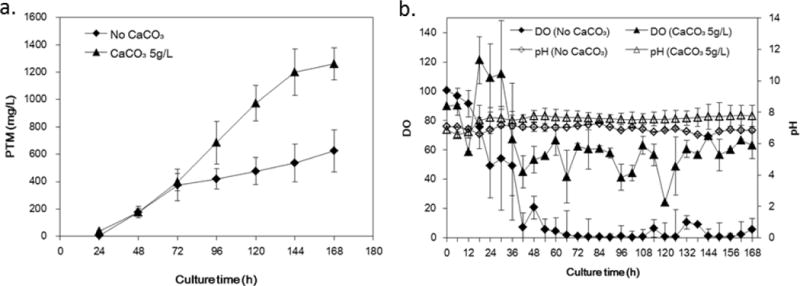
The time course and effects of CaCO3 on platensimycin production in S. platensis SB12026 in 15-L fermentors. (a) platensimycin titers as a function of fermentation time; (b) The dissolved oxygen and the medium pH as a function of fermentation time. There were at least two independent trials for the data bars.
As important fermentation parameters, the effects of inoculum volume, agitation speed and DO on PTM production were investigated (Fig. 5 and Supporting information, Fig. S3). The volume of the seed culture (4%, 10%, 13% and 17%) was first tested. 4% and 10% inoculum produced similar amount of PTM with the titer around 1200 mg/L, while the higher inoculation volume had detrimental effect on PTM production (Fig. 5A). The agitation speed (200 rpm, 270 rpm, 350 rpm and 450 rpm) was next tested and they had strong effects towards PTM production. The PTM titer could only reach to 174 mg/L when the agitation speed was 200 rpm, and it was also low when the speed was 270 rpm or 450 rpm. On the contrary, the PTM titer could reach to 1259 mg/L when the agitation speed was 350 rpm, only slightly lower than those from shaking flask experiments (Figure 5B). Finally, three different DO levels of 20% – 40%, 40% – 60% and 60% – 80% were tested. Satisfactorily, the PTM titer was able to reach to 1513 mg/L, 1560 mg/L and 1558 mg/L respectively (Fig. 5C).
Figure 5.
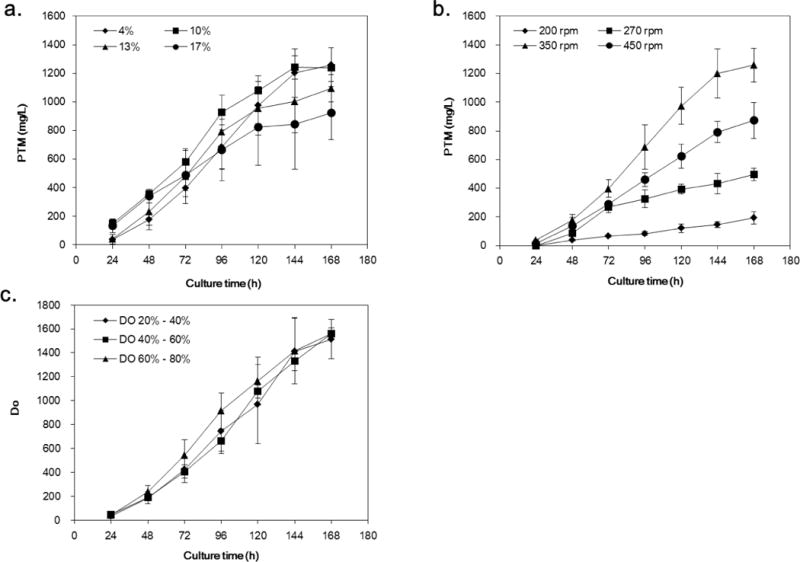
Platensimycin production titers as a function of inoculation volume (a), agitation speed (b), and dissolved oxygen level (c) in S. platensis SB12026 in 15-L fermentors. There were at least two independent trials for the data bars.
PTM scaled-up fermentation in a 150-L fermentor and purification of gram-scale PTM
We finally evaluated the production of PTM from S. platensis SB12026 in a 150-L fermentor (Fig. 6A). 3 L seed culture was prepared and inoculated into a 60 L production medium for PTM production during 7-day fermentation, using the optimal parameters from 15-L batch fermentation. Under these conditions, the PTM yield in the 150-L fermentor was only 1155 mg/L after 7-days fermentation when the agitation speed was set between 270 – 400 rpm. The higher DO suggested that there was very low oxygen uptake by SB12026 in the 150-L fermentor, in comparison to those in 15-L fermentators. The air flow rate was therefore lowered from 2000 L/h to 1200 L/h, and the agitation speed was set between 145 – 305 rpm, resulting in a higher PTM titer of 1250 mg/L after 6-day fermentation. However, there was a slightly decrease of the PTM titer at the end of the fermentation.
Figure 6.
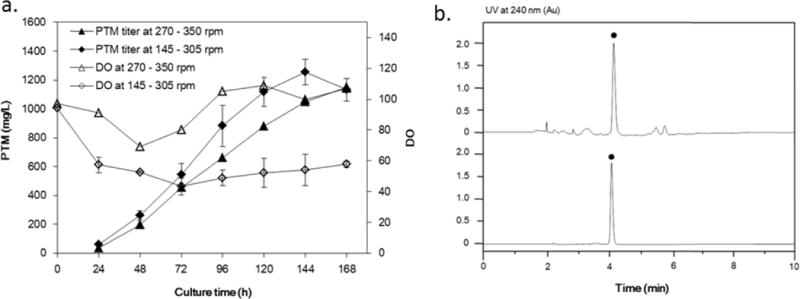
(a) Platensimycin production in S. platensis SB12026 in a 150-L fermentor. The PTM production at agitation speed of 270 – 350 rpm (a single fermentation trial) or at agitation speed of 145 – 305 rpm (The values reported are averages of three independent trials and are reported with standard deviations). (b) The purification of platensimycin from a 60 L fermentation. i, crude S. platensis SB12026 fermentation extract; ii, purified platensimycin. ●, platensimycin.
For purification of PTM from the above fermentation, highly porous XAD-16 resin was applied to adsorb PTM from about 60 L fermentation supernatant. Then the resin was eluted by 80% (pH 10) ethanol, and the resulted elutes were basified by NaOH and extracted by CH2Cl2 to remove hydrophobic impurities. The obtained aqueous layer was subsequently acidified by HCl and extracted by CH2Cl2 again to acquire crude PTM. The crude PTM extract was further purified through the polyamide chromatography and the final 80% ethanol PTM elute was acidified by HCl to precipitate out PTM. After lypholization, 45.14 g of purified PTM was finally obtained, resulting in an isolation yield of 65.1% (Figure 6B and Supporting information, Fig. S4).
Discussion
Natural products remain very important sources of new drugs, especially as anti-infective or anti-cancer therapeutics (Newman and Cragg 2012). However, drug discovery and development from natural products were often hampered by their limited availability in nature, and the access to large quantity of them through other methods, such as total synthesis, remained a formidable task due to their rich stereochemistry and complex structure (Nicolaou et al. 2007; Cragg et al. 2012). PTM is a promising drug lead to treat bacterial infection and diabetes, thus the acquisition of large quantity of PTM is critical for its future application.
In this study, we achieved the highest PTM titer of 1560 mg/L in S. platensis SB12026 through the traditional medium and fermentation optimization approach, which was significantly higher than previous reported PTM titers (Smanski et al. 2009; Zhang et al. 2011; Hindra et al. 2014). In our optimized fermentation medium, soybean flour was used to replace yeast extract as nitrogen source, and soluble starch was used to replace dextrin and α-lactose as carbon source. Interestingly, soluble starch was also observed to stimulate PTM production in S. platensis MA7327 (Falzone et al. 2013). However, the concentration of soybean flour seemed more critical than the soluble starch because the production media containing 7.5 g/L soybean flour could only produced PTM with a titer less than 400 mg/L, while the media with higher soybean flour concentration (> 10 g/L) minimally produced 1000 mg/L PTM, regardless of the soluble starch concentration used (Fig. 3). Therefore the current nitrogen and carbon sources for PTM production were significantly cheaper and more sustainable, which would reduce the cost to produce PTM on an industrial scale. In addition, our finding suggested that the biosynthesis of PTM in S. platensis SB12026 was very sensitive to some inorganic salts, especially ZnSO4·7H2O or MgSO4·7H2O, since the addition of 20 mg/L ZnSO4·7H2O or 1000 mg/L MgSO4·7H2O could dramatically reduce the yield of PTM.
During the scaled-up fermentation of S. platensis SB12026, we identified some important factors for the production of PTM. For example, the addition of CaCO3 (5 g/L) in the production medium seemed indispensible for PTM production in fermentors, while it was not necessary in shaking flask experiments. It seemed that CaCO3 only slightly increases the pH in the batch fermentation, because there was 0.5% MOPS salt in the production medium (Fig 4B). Instead, the presence of CaCO3 in the production medium might affect the strain morphology, which therefore resulted in the drastic increase of the DO level during the batch fermentation. Therefore PTM titer was typically very low when DO levels reached and were kept below 10% for certain period of time during batch fermentation (Supporting information, Fig. S3A and Fig. S3B), while moderate DO level (20% – 80%) was beneficial to PTM production (Fig. 5 and Fig. 6A).
It was noted that the PTM titer in 150-L fermentor was comparable to those in 15-L fermentors in their first 6-day fermentation, while the PTM production slowed down dramatically in 150-L fermentors despite the maintenance of DO level above 20% and the utilization of different DO levels (Fig. 5C and Fig. 6A). Therefore the PTM titer was lower in 150-L fermentor (~1200 mg/L) comparing to those from 15-L fermentors or shaking flasks (both about 1500 mg/L). These distinct behaviors during large scale and small scale fermentations suggested that the changing microenvironment under different fermentation conditions played an important role for PTM production. Therefore the established fermentation would be helpful for optimization of larger scale fermentation in the future.
We obtained 45 g purified PTM using a polyamine chromatography procedure, by taking the advantage of the presence of a phenolic acid moiety in PTM structure, and confirmed its structure by NMR analysis. Taken together, our studies notably enhanced the PTM titer in a formulated cost-effective medium and showcased a very rapid approach to access gram-scale of PTM, which should facilitate the future development of this important natural product. Furthermore, since S. platensis SB12026 is a dual producer of PTM and PTN, we envision that the similar approach might also be applicable to increase the titer of PTN in the future.
Supplementary Material
Acknowledgments
This work is supported in part by a startup fund from Central South University (CSU) 165000001 (to Y.D. and Y.H.), the Chinese Ministry of Education 111 Project B0803420 (to Y.D.), National High Technology Joint Research Program of China grant 2011ZX09401-001 (to Y.D.), and U. S. National Institutes of Health Grants GM086184 and AI079070 (to B.S.). We are grateful to Dr. Lin Qiu and the Center for Advanced Research in CSU in assisting to acquire the 1H NMR of platensimycin.
Footnotes
Conflict of interest
All authors have declared that they have no conflict of interest.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Aluotto S, Tynan H, Maggio C, Falzone M, Mukherjee A, Gullo V, Demain AL. Development of a semi-defined medium supporting production of platensimycin and platencin by Streptomyces platensis. J Antibiot. 2013;66:51–54. doi: 10.1038/ja.2012.97. [DOI] [PubMed] [Google Scholar]
- Brown AK, Taylor RC, Bhatt A, Futterer K, Besra GS. Platensimycin activity against mycobacterial β-ketoacyl-ACP synthases. PLoS One. 2009;4:e6306. doi: 10.1371/journal.pone.0006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ. New horizons for old drugs and drug leads. J Nat Prod. 2014;77:703–723. doi: 10.1021/np5000796. [DOI] [PubMed] [Google Scholar]
- Falzone M, Martens E, Tynan H, Maggio C, Golden S, Nayda V, Crespo E, Inamine G, Gelber M, Lemence R, Chiappini N, Friedman E, Shen B, Gullo V, Demain AL. Development of a chemically defined medium for the production of the antibiotic platensimycin by Streptomyces platensis. Appl Microbiol Biotechnol. 2013;97:9535–9539. doi: 10.1007/s00253-013-5201-6. [DOI] [PubMed] [Google Scholar]
- Hara M, Asano K, Kawamoto I, Takiguchi T, Katsumata S, Takahashi K, Nakano H. Leinamycin, a new antitumor antibiotic from Streptomyces: producing organism, fermentation and isolation. J Antibiot. 1989;42:1768–1774. doi: 10.7164/antibiotics.42.1768. [DOI] [PubMed] [Google Scholar]
- Herath KB, Zhang C, Jayasuriya H, Ondeyka JG, Zink DL, Burgess B, Wang J, Singh SB. Structure and semisynthesis of platensimide A, produced by Streptomyces platensis. Org Lett. 2008;10:1699–1702. doi: 10.1021/ol800251v. [DOI] [PubMed] [Google Scholar]
- Hindra, Huang T, Yang D, Rudolf JD, Xie P, Xie G, Teng Q, Lohman JR, Zhu X, Huang Y, Zhao LX, Jiang Y, Duan Y, Shen B. Strain prioritization for natural product discovery by a high-throughput real-time PCR method. J Nat Prod. 2014;77:2296–2303. doi: 10.1021/np5006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation IDF Diabetes. 7. Brussels, Belgium: International Diabetes Federation; 2015. http://www.diabetesatlas.org. [Google Scholar]
- Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Isolation and structure of platencin: a FabH and FabF dual inhibitor with potent broad-spectrum antibiotic activity. Angew Chem Int Ed Engl. 2007;46:4684–4688. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces genetics. The John Innes Foundation; Norwich: 2000. [Google Scholar]
- Martens E, Demain AL. Platensimycin and platencin: promising antibiotics for future application in human medicine. J Antibiot. 2011;64:705–710. doi: 10.1038/ja.2011.80. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou KC, Edmonds DJ, Li A, Tria GS. Asymmetric total syntheses of platensimycin. Angew Chem Int Ed Engl. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- Rudolf JD, Dong LB, Huang T, Shen B. A genetically amenable platensimycin- and platencin-overproducer as a platform for biosynthetic explorations: a showcase of PtmO4, a long-chain acyl-CoA dehydrogenase. Mol Biosyst. 2015;11:2717–2726. doi: 10.1039/c5mb00303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Ding FX, Singh SB, Parthasarathy G, Soisson SM, Ha SN, Chen X, Kodali S, Wang J, Dorso K, Tata JR, Hammond ML, Maccoss M, Colletti SL. Synthesis and biological evaluation of platensimycin analogs. Bioorg Med Chem Lett. 2009;19:1623–1627. doi: 10.1016/j.bmcl.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. Isolation, structure, and absolute stereochemistry of platensimycin, a broad spectrum antibiotic discovered using an antisense differential sensitivity strategy. J Am Chem Soc. 2006;128:11916–11920. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- Smanski MJ, Peterson RM, Rajski SR, Shen B. Engineered Streptomyces platensis strains that overproduce antibiotics platensimycin and platencin. Antimicrob Agents Chem. 2009;53:1299–1304. doi: 10.1128/AAC.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizek J, Novotna J, Rezanka T, Demain AL. Do we need new antibiotics? The search for new targets and new compounds. J Ind Microbiol Biotechnol. 2010;37:1241–1248. doi: 10.1007/s10295-010-0849-8. [DOI] [PubMed] [Google Scholar]
- Walsh CT, Wencewicz TA. Prospects for new antibiotics: a molecule-centered perspective. J Antibiot. 2014;67:7–22. doi: 10.1038/ja.2013.49. [DOI] [PubMed] [Google Scholar]
- Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci U S A. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, Li C. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A. 2011;108:5378–5383. doi: 10.1073/pnas.1002588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Smanski MJ, Peterson RM, Marchillo K, Andes D, Rajski SR, Shen B. Engineering of Streptomyces platensis MA7339 for overproduction of platencin and congeners. Org Lett. 2010;12:1744–1747. doi: 10.1021/ol100342m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ondeyka J, Herath K, Jayasuriya H, Guan Z, Zink DL, Dietrich L, Burgess B, Ha SN, Wang J, Singh SB. Platensimycin and platencin congeners from Streptomyces platensis. J Nat Prod. 2011;74:329–340. doi: 10.1021/np100635f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


