Abstract
There is a strong need to better understand the neurobiology of juvenile sociability (tendency to seek social interaction), a phenotype of central relevance to autism spectrum disorders (ASD). Although numerous genetic mouse models of ASD showing reduced sociability have been reported, and certain brain regions, such as the amygdala, have been implicated in sociability, there has been little emphasis on delineating brain structures and circuits activated during social interactions in the critical juvenile period of the mouse strain that serves as the most common genetic background for these models—the highly sociable C57BL/6J (B6) strain. We measured expression of the immediate early genes Fos and Egr-1 to map activation of brain regions following the Social Approach Test (SAT) in juvenile male B6 mice. We hypothesized that juvenile B6 mice would show activation of the amygdala during social interactions. The basoloateral amygdala (BLA) was activated by social exposure in highly sociable, 4-week-old B6 mice. In light of these data, and the many lines of evidence indicating alteration of amygdala circuits in human ASD, future studies are warranted to assess structural and functional alterations in the BLA, particularly at BLA synapses, in various mouse models of ASD.
Keywords: social, behavior, amygdala, autism, Fos, mouse model
Introduction
Social interactions enable animals to communicate with and learn from one another, and can increase the likelihood of survival and reproduction. Markedly reduced sociability, defined as a decreased tendency to seek out and engage in social interactions, is associated with a number of neurodevelopmental and psychiatric disorders in humans, and is highly disabling. Reduced sociability is one of the core symptoms of autism spectrum disorder (ASD), and impairs social cognition and social skills during the sensitive developmental period of childhood (Dawson et al., 2002; Jones et al., 2008; Chevallier et al., 2012). The experimental control afforded by mouse models is useful for elucidating the brain regions and circuits that mediate social approach behaviors, which can lead to better mechanistic understanding of sociability development and, ultimately, better treatment of ASD. Various mouse models relevant to ASD have been developed, many on a C57BL/6J inbred strain genetic background (Jamain et al., 2008; Bozdagi et al., 2010; Peñagarikano and Geschwind, 2012; Won et al., 2012; Yang et al., 2012). However, few studies have attempted to delineate the brain structures and circuits activated during juvenile social interactions, which are particularly relevant to ASD, a childhood-onset disorder. Here, we investigate the brain regions and cell types activated during a widely used assay of mouse social approach behavior in juvenile C57BL/6J males.
We investigated brain region expression of the immediate early genes (IEG), c-Fos and Egr-1, in juvenile (4-week-old) B6 mice following exposure to a modified version of the Social Approach Test (SAT), an assay that has been widely used to assess sociability in mice, including mouse models relevant to ASD (Brodkin et al., 2004; Moy et al., 2004; Tabuchi et al., 2007; Fairless et al., 2012; Peñagarikano and Geschwind, 2012; Won et al., 2012). Based on previous studies of adult rodent social behaviors (Ferguson et al., 2001; Petrulis, 2009; Samuelsen and Meredith, 2011; Trezza et al., 2012), as well as human social behaviors, we hypothesized that juvenile mice would show increased IEG expression in olfactory and limbic areas of the brain, particularly the amygdala, following exposure to a social stimulus mouse. We hypothesized that both glutamatergic and GABAergic neurons in these regions would show increased c-Fos expression.
Experimental Procedures
Subjects
Male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 3 weeks of age, were allowed to acclimate for 1 week, and were tested for social approach behavior at 4 weeks of age. Gonadectomized A/J mice were also obtained from JAX (gonadectomized at JAX at 3–4 weeks of age) and were used at 60–100 days old as stimulus mice for the Social Approach Test. Mice were housed 4–5 per cage in a temperature and humidity controlled environment (12-hour light-dark cycle) with ad libitum access to food (LabDiet 5010, Purina Mills, Richmond, IN) and water. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. A total of 58 mice completed the modified Social Approach Test after which their brains were processed for immunochemistry, described below. Mice were randomly placed into one of three experimental conditions: home cage (HC), nonsocial exposure (NS), or social exposure (SOC), described below. Thirteen mice were excluded from analysis because of excessive climbing on cylinders during behavioral testing or due to damage to the tissue during processing/ inadequate staining. In total samples sizes were as follows: Cohort 1: HC, n=4; NS, n=5; SOC, n=4; Cohort 2: NS, n=3; SOC, n=4; Cohort 3: NS, n=5; SOC, n=6; Cohort 4: NS, n=7; SOC, n=7.
Behavior
Four-week-old B6 mice underwent a modified version of the Social Approach Test (SAT) (Sankoorikal et al., 2006; Fairless et al., 2008, 2011). Mice were placed in a three-chambered topless and bottomless box (10 × 20.5 × 9 in) made of black acrylic Plexiglass. The apparatus was placed on a clear Plexiglass stand covered with a fresh piece of bench mat paper and was illuminated from beneath with infrared light panels. Testing was conducted under low light (= 5 lux) and filmed from overhead using a Sony Hard Disk Drive Handycam DCR-SR85 camcorder set to an infrared-sensing “nightshot” mode. The two end chambers contained identical clear Plexiglass cylinders which had breathing holes that allowed for air circulation and olfactory exploration. The test mouse was able to move freely between the chambers.
The modified SAT consisted of two ten-minute phases. During Phase 1 (habituation), both cylinders were empty and the test mouse was allowed to freely explore the arena. During Phase 2, one cylinder contained a non-social stimulus--a novel object (OBJ; a plastic paperweight). The other cylinder either remained empty (EMP; nonsocial exposure group (NS)) or contained a social stimulus (SS)--a same-sex gonadectomized adult A/J mouse (social exposure group (SOC)). The test mouse was again able to freely explore the apparatus for ten minutes. A group of mice designated the Home Cage (HC) group was not subjected to the modified SAT but remained in their home cages in the testing room for approximately 30 min. Nothing new was placed in their home cages, and they were sacrificed at the same time as the NS and SOC groups of mice.
Time spent sniffing the cylinders was measured using an automated behavioral analysis system (TopScan, Version 2.00, Clever Sys. Inc., Reston, VA). The primary variable of interest in the SAT was time spent sniffing the social cylinder in Phase 2, because our previous work has indicated that that variable is the most reliable and valid measure of sociability (Fairless et al., 2011).
Immunohistochemistry
One hour after the SAT, mice were trans-cardially perfused with 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO). The brains were post-fixed for 24 h and cryoprotected in a 30% sucrose solution. Coronal cryosections (40 µm) were incubated with a rabbit polyclonal anti-c- Fos (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA; Figure 1A) or anti-Egr-1 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA; Figure 4F) antibody for 48 hours at 4°C and then with secondary biotinylated anti-Rabbit IgG (1:200, Jackson Immunoresearch, West Grove, PA) antibody. The c-Fos and Egr-1 immunoreactivity were visualized using standard ABC method (Vectastain ABC kit, Vector) and diaminobenzidine (DAB)-nickel solution (Sigma Aldrich, St. Louis, MO). For double immunofluorescence experiments, cryosections were incubated with a mixture of rabbit polyclonal anti-c-Fos antibody (1:500, EMD Millipore, Billerica, MA) and either a mouse anti-calcium calmodulin kinase II (CaMKII, 1:250; EMD Millipore) or guinea pig anti-gamma-aminobutyric acid antibody (GABA, 1:200; EMD Millipore). A mixture of fluorescent secondary anti-rabbit and anti-mouse or anti-guinea pig antibodies (Alexa 488 and Alexa 546 or Alexa 647, 1:200; Jackson Immunoresearch) were used to visualize staining.
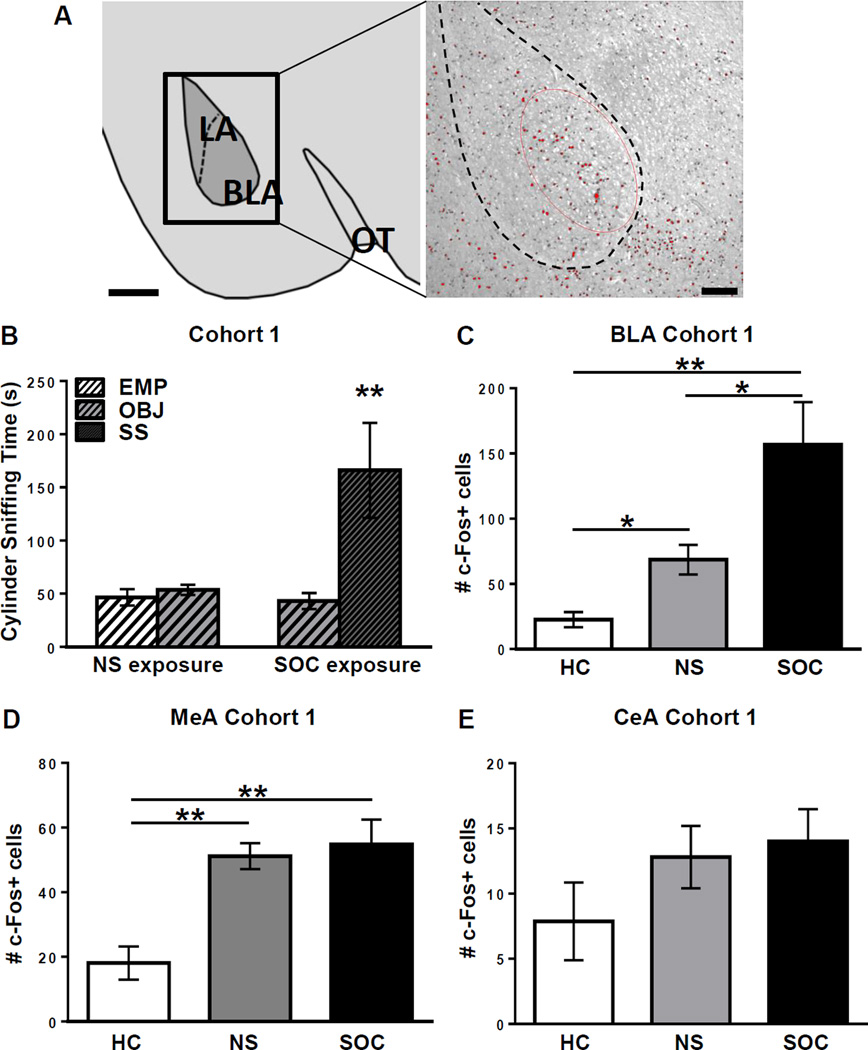
Figure 1.
Cohort 1: B6 mice show high social approach and activation of the BLA during social approach. A: Amygdala region analyzed for c-Fos immunoreactivity. Left panel: Atlas illustration based on Paxinos and Franklin, 2007, of the lateral (LA) and basolateral (BLA) nuclei of the amygdala (−1.34 mm from bregma). OT indicates the optic tract, for reference. Scale bar = 500 µm. Right panel: Representative c-Fos-labeled section containing the BLA. The dotted line indicates the limits of the LA/BLA and the red circle shows the standardized region of interest used to count labeled cells in the BLA. Cells in red are those that were counted and included in analysis after meeting a standardized intensity threshold set in NIH ImageJ. Scale bar = 100 µm. B: Cylinder sniffing times of B6 juvenile males in Cohort 1 exposed to nonsocial (NS, n=5) or social (SOC, n=4) stimuli in Phase 2 of the Social Approach Test (SAT). In the NS condition, mice could sniff an empty (EMP, white striped bar) cylinder or one containing a novel object (OBJ, gray striped bar). In the SOC condition, mice could sniff a cylinder containing either an OBJ or a social stimulus (SS, black striped bar; same-sex gonadectomized A/J mouse). ** = p<0.010 SS cylinder vs. OBJ cylinder sniffing. C–E: Number of c-Fos-positive cells in the basolateral, medial, and central nuclei of the amygdala (BLA, MeA, and CeA, respectively) of mice from Cohort 1 in the NS and SOC groups, as well as a home cage (HC, n=4) group that remained in their home cages in the testing room during the SAT. Mice were sacrificed 60 min after behavioral testing and brains were collected for immunohistochemistry. * = p<0.050, ** = p<0.010. Raw data is presented in the graphs, but a log transformation was required for normality to perform data analysis.
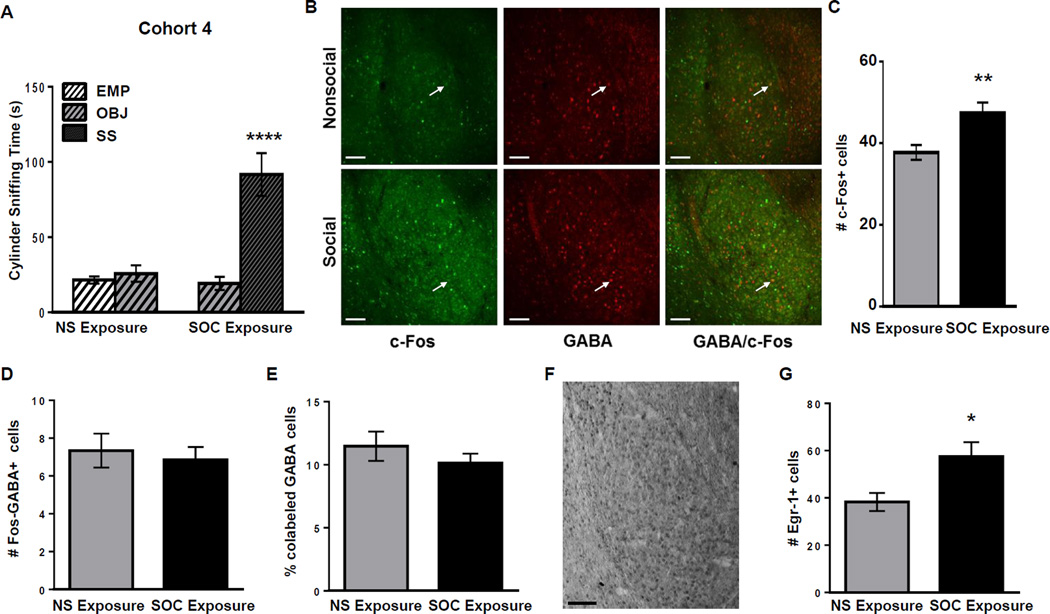
Figure 4.
Cohort 4: B6 mice show high social approach and GABAergic activation of the BLA. A: Cylinder sniffing times for the NS (n=7) and SOC (n=7) B6 mice in Cohort 4 in Phase 2 of the SAT. **** =p<0.0001 compared to OBJ cylinder. B: Representative double immunofluorescent staining in the BLA with c-Fos (green), GABA (red) and both (yellow) in Cohort 4 B6 juvenile males in nonsocial (NS) and social (SOC) exposure groups. White arrows indicate representative co-labeled cells. Scale bars = 100 µm. C: Number of c-Fos+ cells in the BLA of B6 NS or SOC mice. ** = p<0.010. D: Number of BLA cells colabeled with c-Fos and GABA. E: Percent of GABA+ BLA cells that are co-labeled with c-Fos in the NS or SOC exposure groups. F: Representative Egr-1-labeled section containing the BLA. Scale bar = 100 µm. G: Quantification of Egr-1+ cells in the BLA of mice from Cohort 4 in the NS and SOC groups. * = p<0.050. Raw data is presented in the graphs, but a log transformation was required for normality to perform data analysis.
Image Analysis
Images were acquired using an Olympus BX41 light microscope with SPOT basic digital imaging software (Diagnostic Instruments, Sterling Heights, MI), a Leica DM6000 upright widefield microscope, or a Leica TCS SP5 AOBS confocal microscope (Leica, Wetzlar, Germany). Images were processed in NIH ImageJ by an experimenter blind to behavioral group (NS, SOC, or HC) using the binary function to convert to grayscale, after which a threshold for staining intensity was applied. The watershed function was applied to separate clustered cells, and a standardized shape based on the Paxinos and Franklin (2001) mouse brain atlas was placed over each of the 19 brain regions of interest (ROI). Nuclei within each ROI were counted using the analyze particles function. An example of the threshold and region of interest used to quantify c-Fos cells in the basolateral amygdala (BLA) is shown in Figure 1A. For colocalization experiments, the number of c-Fos+, GABA+, and double-labeled nuclei were counted using the RG2B colocalization plugin of NIH ImageJ and the percent of co-labeled cells was determined. CaMKII+ and co-labeled cells were counted by hand due to their abundance and non-nuclear staining.
Table 1 includes the bregma coordinates within which each ROI was analyzed. Numbers of cells were counted bilaterally in 2–4 separate cryosections containing the same brain area and were averaged within each animal to give one value per animal. In order to minimize variability in the tissue analyzed, we used sections within a small range of rostral-caudal spread as defined by bregma coordinates, excluding the most rostral and caudal areas (see Table 1). When a brain area spanned 0.4–0.6 mm, the majority (~80%) of the time, 4 evenly spaced serial sections (every 4th section), were averaged. For smaller brain areas, 2–3 slices were averaged, and in the case of the olfactory bulb and cingulate cortex, only 1 slice was used per animal.
Table 1.
Numbers of c-Fos-positive cells in each of 19 brain areas. A standardized region of interest (ROI) based on a mouse brain atlas (Paxinos and Franklin, 2001) was placed over each brain area and cells within that ROI were counted using NIH ImageJ. Bilateral counts from a small rostral-caudal range (distance from bregma indicated above) of 1–4 coronal sections (40 µm) were averaged and are expressed here as mean ± SEM.
| Brain Region | Bregma (mm) | Home Cage | Nonsocial | Social | ANOVA |
|---|---|---|---|---|---|
| Amygdala-Basolateral | −1.22 to −1.70 | 23 ± 5†^ | 73 ± 12 | 157 ± 33* | p=0.0002 |
| Amygdala-Medial | −1.34 to −1.70 | 18 ± 5†^ | 51 ± 4 | 55 ± 8 | p=0.002 |
| Amygdala-Central | −1.22 to −1.58 | 8 ± 3 | 13 ± 2 | 14 ± 2 | p=0.259 |
| Nucleus Accumbens | 1.42 to 0.86 | 62 ± 9 | 109 ± 17 | 91 ± 14 | p=0.071 |
| Ventral Tegmental Area | −3.28 to −3.08 | 13 ± 1 | 8 ± 1 | 12 ± 4 | p=0.438 |
| Accessory Olfactory Bulb | 2.80 to 2.58 | 36 ± 3†^ | 126 ± 26 | 105 ±21 | p=0.001 |
| Olfactory Bulb | 3.56 | 1443 ± 561 | 3002 ± 439 | 2621 ± 603 | p=0.099 |
| Bed Nucleus Stria Terminalis | 0.26 to 0.14 | 28 ± 6 | 48 ± 11 | 47 ± 12 | p=0.271 |
| Hippocampus-CA1 | −1.50 to −2.06 | 28 ± 5 | 61 ± 10 | 47 ± 11 | p=0.075 |
| Hippocampus-CA3 | −1.50 to −2.06 | 25 ± 5 | 46 ± 6 | 41 ± 2 | p=0.035 |
| Hippocampus-DG | −1.50 to −2.06 | 33 ± 5 | 52 ± 7 | 60 ± 3 | p=0.034 |
| Lateral Septum | 0.74 to 0.38 | 251 ± 78 | 628 ± 114 | 544 ± 144 | p=0.046 |
| Locus Ceruleus | −5.34 to −5.52 | 9 ± 2 | 20 ± 5 | 20 ± 3 | p=0.058 |
| Paraventricular Nucleus Hypothalamus |
−0.34 to −0.82 | 37 ± 13 | 89 ± 12 | 76 ± 4 | p=0.011 |
| Subiculum | −4.16 to −4.24 | 65 ± 22†^ | 468 ± 75 | 329 ± 63 | p=0.0005 |
| Cingulate Cortex | 0.26 to −0.22 | 242 ± 57 | 673 ± 71 | 642 ± 117 | p=0.005 |
| Infralimbic Cortex | 1.94 to 1.34 | 26 ± 6†^ | 77 ± 7 | 62 ± 9 | p=0.0006 |
| Entorhinal Cortex | −4.16 to −4.38 | 75 ± 26†^ | 314 ± 32 | 267 ± 26 | p=0.002 |
| Prelimbic Cortex | 1.94 to 1.34 | 48 ± 4 | 73 ± 5 | 52 ± 10 | p=0.088 |
B6 mice were randomly placed in one of the following groups: social exposure (SOC; n=4, except n=3 for locus ceruleus), nonsocial exposure (NS; n=5, except n=4 for ventral tegmental area and locus ceruleus), and home cage (HC; n=4, except n=3 for ventral tegmental area, olfactory bulb, and locus ceruleus). All mice are from Cohort 1. Bolded p-values indicate significance of p<0.0026 (0.05/19, Bonferroni correction for multiple comparisons) in a one-way ANOVA. ANOVAs were performed on log transformed data due to non-normality of distribution; however, raw count averages are presented here.
The following symbols refer to results of Tukey post hoc tests:
= p<0.05, NS vs. SOC:
= p<0.05, HC vs. NS;
= p<0.05, HC vs. SOC.
Data analysis
Data are presented as mean ± SEM. All graphs presented reflect raw data counts of time, cell number, or percent colabeled cells; however, because the sample sizes were small, we could not assume normal distribution and equal variances in our data. Therefore, all data was log transformed before statistical analysis. Behavioral measures were compared using a two-way ANOVA with Bonferroni posthoc comparisons. Fos counts for cohort 1 were analyzed with a one-way ANOVA. When ANOVA results were p=0.05/19 (0.0026, Bonferroni correction for multiple comparisons), further analysis was done using Tukey posthoc tests. Fos counts and double-labeled cells in cohorts 2–4 were analyzed using a two-tailed Student’s t-test, in which values were considered significant at p=0.05. Statistical tests were conducted using Prism 6.0 statistical software (GraphPad; San Diego, CA).
Results
In the first cohort of 4-week-old C57BL/6J (B6) mice, there was no significant difference in time spent sniffing each of two empty (EMP) cylinders during Phase 1 (habituation period) of the Social Approach Test (SAT). This was true for mice in both the Social exposure group (SOC, n=4) and the Nonsocial exposure group (NS, n=5); a two-way ANOVA revealed no significant main effects of exposure group or cylinder choice, and no significant exposure group×cylinder choice interaction in Phase 1 (p=0.253, p=0.591, p=0.130, respectively; data not shown). In Phase 2 for the nonsocial (NS) exposure group, a novel object (OBJ) was placed in one cylinder, while the second cylinder remained empty (EMP). In Phase 2 for the social exposure condition (SOC), a novel OBJ was placed in one cylinder and a novel mouse (social stimulus; SS) was placed in the other cylinder. Mice in the social exposure group (SOC, n=4) and mice in the nonsocial exposure group (NS, n=5) were allowed 10 minutes to explore the arenas and sniff the cylinders in Phase 2 of the SAT. A two-way ANOVA revealed significant main effects of exposure group (F(1,14)=14.33; p=0.014) and cylinder choice (F(1,14)=5.866; p=0.030), as well as a significant exposure group × cylinder choice interaction (F(1,14)=7.916; p=0.014). Bonferroni post-hoc tests revealed that B6 mice in the SOC exposure group spent significantly more time sniffing the SS cylinder than the OBJ cylinder (p=0.007), while in the B6 mice in the NS group, there was no significant difference in the time spent sniffing the EMP cylinder vs. the OBJ cylinder in Phase 2 (p>0.999; Figure 1B). There was no significant difference in total distance traveled in the arena in the NS and SOC groups in Phases 1 or 2 (p=0.817 and p=0.445, respectively; data not shown). The finding that these juvenile B6 mice showed a high level of approach toward a social stimulus mouse is consistent with previous studies from various laboratories (Brodkin et al., 2004; Sankoorikal et al., 2006; Moy et al., 2007; Panksepp et al., 2007; Fairless et al., 2012).
One hour after the SAT, mice in the SOC (n=4) and NS (n=5) exposure groups, as well as a home cage group (HC, n=4), which remained in their home cages in the testing room during the SAT, were sacrificed and their brains were processed for immunohistochemistry, using a c-Fos antibody as a marker of neuronal activity. For each animal, the number of c-Fos-positive cells was averaged over 2–4 cryosections (40-µm) containing each brain region of interest (ROI). In the majority of mice (~80%), four equally spaced serial cryosections, excluding the most rostral and caudal areas, were averaged when the rostral-caudal extent of the ROI was 0.4–0.6 mm. In the minority of mice (~20%), random damage to particular cryosections led us to use 2–3 cryosections for these ROIs. The incidents of damage to cryosections occurred randomly across mice exposed to different conditions (e.g. social and nonsocial conditions). For smaller brain areas, 1–3 slices were averaged (see Methods and Table 1). Nineteen ROIs were assayed, primarily olfactory and limbic regions (Table 1). Based on human and rodent studies indicating its role in processing social stimuli (Ferguson et al., 2001; Adolphs, 2010), we hypothesized that the amygdala would be activated by social exposure in B6 mice. One-way ANOVAs demonstrated significant differences (after Bonferroni correction for multiple comparison) in numbers of c-Fos+ cells among the three exposure groups in 6 brain regions, including the basolateral amygdala (BLA; F(2,10)=22.000; p<0.001; Table 1, Figure 1C), medial amygdala (MeA; F(2,10)=12.340; p=0.002; Table 1, Figure 1D), accessory olfactory bulb (AO; F(2,10)=14.440; p=0.001; Table 1), subiculum, (S; F(2,10)=17.870; p<0.001; Table 1), infralimbic cortex (IL; F(2,10)=17.280; p<0.001; Table 1), and entorhinal cortex (EC; F(2,10)=12.470; (p=0.002; Table 1). Tukey post-hoc comparisons revealed that all 6 regions showed increased c-Fos activation in the NS group compared to the HC group: BLA (p=0.005; Figure 1C), MeA (p=0.004; Figure 1D), AO (p=0.001), S (p<0.001), IL (p<0.001), and EC (p=0.002) and increased c-Fos labeling in the SOC condition compared to the HC condition: BLA (p<0.001; Figure 1C), MeA (p=0.004; Figure 1D), AO (p=0.005), S (p=0.003), IL (p=0.004), and EC (p=0.006). However, only the BLA had increased c-Fos expression in the SOC versus NS group (p=0.049), which is the comparison that reveals brain region activation specific to exposure to the social stimulus mouse. Thus, while a number of regions were activated by exposure to the SAT testing apparatus and procedure, the only brain region of juvenile B6 mice that we found to be specifically activated in response to a social stimulus was the BLA (Figure 1A and 1C, Table 1). This finding in the BLA was consistent with our hypothesis; however, no significant difference in activation of the medial or central nuclei of the amygdala was found between NS and SOC exposure groups (p>0.999, post hoc for MeA; and p=0.259, main effect for CeA), Figures 1D and 1E, respectively).
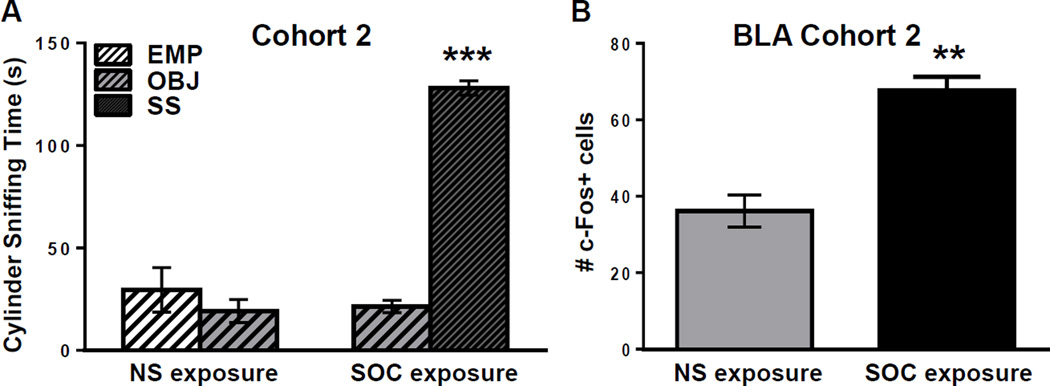
Following the initial set of experiments in Cohort 1 in which many brain areas were analyzed and multiple comparisons were made, we repeated the modified SAT and immunohistochemistry in a second cohort of B6 juvenile males, with a focused hypothesis that c-Fos expression would be significantly increased in the BLA of mice in the SOC exposure group relative to the NS exposure group. By testing a focused hypothesis about the BLA in this second cohort, we diminished the possibility that the BLA finding in the first cohort was a type I statistical error. Figure 2A shows the results of the modified SAT for the NS (n=3) and SOC (n=4) exposure groups in Cohort 2. Again, a two-way ANOVA of Phase 1 (habituation) revealed no significant main effects of exposure group or cylinder choice, and no significant exposure group × cylinder choice interaction (p=0.773, p=0.577, p=0.681, respectively; data not shown). A two-way ANOVA of Phase 2 (Cohort 2) demonstrated significant main effects of exposure group (F(1,10)=12.180; p=0.006) and cylinder choice (F(1,10)=7.363; p=0.022), as well as a significant interaction between the two factors (F(1,10)=16.630; p=0.002). Post-hoc analysis demonstrated that B6 juvenile males in the SOC exposure group spent significantly more time sniffing the social stimulus (SS) than the novel object (OBJ) (p<0.001), while there was no significant difference in the amount of time that B6 juvenile males in the NS exposure group spent sniffing the empty cylinder (EMP) vs. the cylinder that contained the OBJ (p=0.776). There was no significant difference between the NS and SOC groups in locomotor activity (distance traveled in the apparatus) in Phases 1 and 2 (p=0.682 and p=0.749, respectively; data not shown). As in Cohort 1, quantification of Cohort 2 c-Fos expression in the BLA 60 minutes after the SAT confirmed that the mice in the SOC group had significantly more c-Fos labeled cells than did mice in the NS exposure group (t(5)=5.334; p=0.003; Figure 2B). Thus, in a separate cohort of mice (Cohort 2), with a focused hypothesis of BLA activation in response to social exposure, we found a significantly greater level of BLA c-Fos expression in the SOC exposure group relative to the NS exposure group, which replicated the activated BLA finding from Cohort 1.
Figure 2.
Cohort 2: B6 mice show high social approach and activation of the BLA during social approach. A: Cylinder sniffing times of B6 juveniles in Cohort 2 NS (n=3) or SOC (n=4) exposure conditions during Phase 2 of the SAT. *** =p<0.0001 SS cylinder vs. OBJ cylinder sniffing. B: Number of c-Fos-positive cells in the BLA of mice from Cohort 2 in the NS and SOC groups. ** = p<0.010. Raw data is presented in the graphs, but a log transformation was required for normality to perform data analysis.
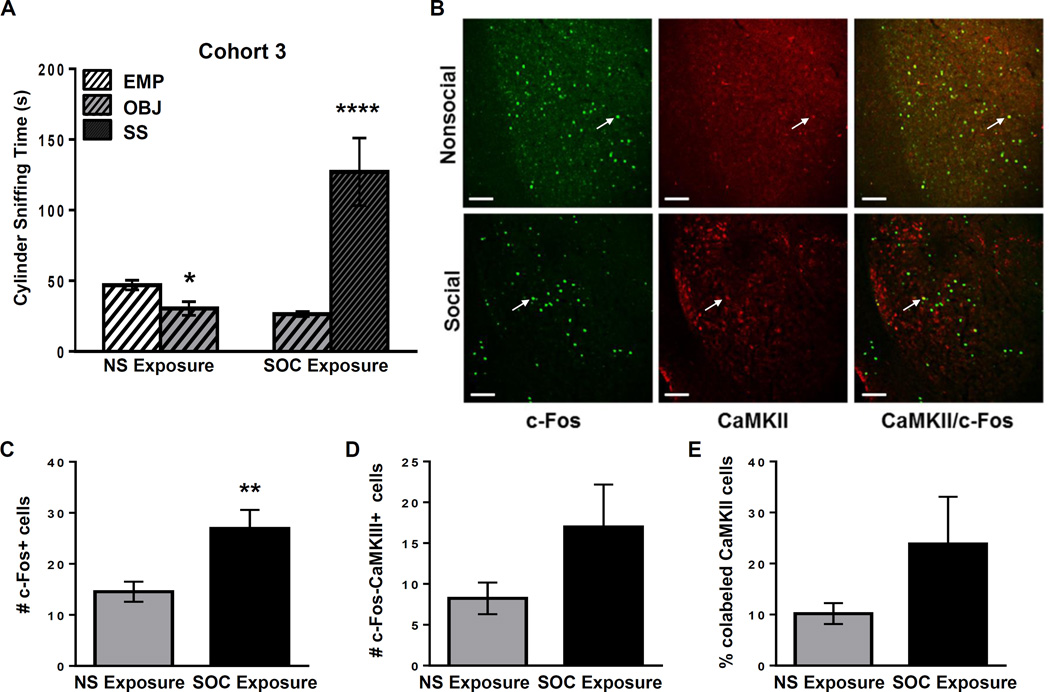
To further understand the population of BLA cells activated in response to social exposure, a third cohort of juvenile B6 mice was tested in the SAT, and 60 min later BLA cells were labeled for c-Fos and calcium calmodulin kinase II (CaMKII) to determine the proportion of c-Fos+ neurons that expressed CaMKII, a protein kinase associated with glutamatergic neurons. This third cohort of B6 juveniles underwent social approach testing, with NS (n=6) and SOC (n=5) exposure groups. A two-way ANOVA revealed no significant main effects of exposure group or cylinder choice, and no significant exposure group × cylinder choice interaction in Phase 1 (habituation; p=0.148, p=0.636, p=0.503, respectively; data not shown). In Phase 2, there were significant main effects of exposure group (F(1,18)=7.982; p=0.011), cylinder choice (F(1,18)=11.290; p=0.004), and a significant exposure group × cylinder choice interaction (F(1,18)=44.590; p<0.001; Figure 3A). Post-hoc analysis demonstrated that SOC exposure group mice spent more time sniffing the SS than the OBJ (p<0.001) while the NS exposure group mice spent slightly but significantly less time sniffing the cylinder that contained the OBJ vs. the empty cylinder (EMP) (p=0.048; Figure 3A). There was no significant difference between the NS and SOC groups in total distance traveled in the arena during Phases 1 and 2 (p=0.743 and p=0.809, respectively; data not shown).
Figure 3.
Cohort 3: B6 mice show high social approach and glutamatergic activation of the BLA. A: Cylinder sniffing times for the NS (n=6) and SOC (n=5) B6 mice in Cohort 3 in Phase 2 of the SAT. **** =p<0.0001 compared to OBJ cylinder. B: Representative double immunofluorescent staining in the BLA with c-Fos (green), CaMKIIα (red) and both (yellow) in Cohort 3 B6 juvenile males in nonsocial (NS) and social (SOC) exposure groups. White arrows indicate representative co-labeled cells. Scale bars = 100 µm. C: Number of c-Fos+ cells in the BLA of B6 NS or SOC mice. ** = p<0.010. D: Number of BLA cells colabeled with c-Fos and CaMKII. E: Percent of CaMKII+ BLA cells that are co-labeled with c-Fos in the NS or SOC exposure groups. Raw data is presented in the graphs, but a log transformation was required for normality to perform data analysis.
Using fluorescent confocal microscopy, we again replicated the result that mice in the SOC group exhibit significantly more c-Fos+ cells in the BLA than those in the NS group (t(9)=3.314; p=0.009; Figure 3B and 3C). In addition, the number of cells colabeled with c-Fos and CaMKII was not different between the NS and SOC groups (t(9)=1.204; p=0.259; Figure 3B and 3D). Similarly, comparison of the percentage of CaMKII+ cells (red) that were co-labeled with c-Fos (green) in the SOC and NS groups revealed no significant difference (t(9)=0.857; p=0.414; Figure 3B and 3E).
Finally, a fourth cohort of animals underwent social approach testing followed by immunohistochemistry labeling for c-Fos and gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the brain. In Phase 1 (habituation) of social approach testing (NS (n=7) and SOC (n=7), a two-way ANOVA revealed no significant main effects of exposure group or cylinder choice, and no significant exposure group × cylinder choice interaction (p=0.981, p=0.702, p=0.509, respectively; data not shown). In Phase 2, there were significant main effects of exposure group (F(1,24)=24.91; p<0.001), cylinder choice (F(1,24)=10.100; p=0.004), and a significant exposure group × cylinder choice interaction (F(1,24)=19.790; p<0.001; Figure 4A). Post-hoc tests determined that SOC exposure group mice spent more time sniffing the SS than the OBJ (p<0.001) while the NS exposure group mice spent similar amounts of time sniffing the empty cylinder (EMP) vs. the cylinder that contained the OBJ (p=0.755; Figure 4A). There was no significant difference between the NS and SOC groups in total distance traveled in the arena during Phases 1 and 2 (p=0.735 and p=0.867, respectively; data not shown). Significantly more c-Fos+ cells were labeled in the BLA of mice in the SOC group than those in the NS group (t(12)=2.553; p=0.025; Figure 4B and 4C). The number of cells co-labeled with c-Fos and GABA was not significantly different between the SOC and NS groups (t(12)=0.823; p=0.426; Figure 4B and 4D). The percentage of GABA+ cells that were also Fos+ was not different between the NS and SOC groups (t(12)=0.262; p=0.798; Figure 4B and 4E).
A second set of sections from this fourth cohort of animals was used to analyze the expression of another immediate early gene, Egr-1, in order to validate the neural activation of the BLA in response to social exposure (Figure 4F). Mice from the SOC group had significantly more Egr-1+ BLA cells than those in the NS group (t(12)=3.308; p=0.006; Figure 4G).
Therefore, BLA neurons were activated upon exposure to a social stimulus, as evidenced by an increase in expression of two immediate early genes, demonstrated by the number of c-Fos+ and Egr-1+ cells, compared to the NS group. The c-Fos+ activated cells included both CaMKII+ and GABA+ cells; however, the percentage of excitatory and inhibitory cells active in the BLA was not affected by exposure to a social stimulus.
Discussion
Using c-Fos immunohistochemistry, we have demonstrated that exposure to a social stimulus in the SAT preferentially activated the BLA of highly sociable, 4-week-old B6 mice. Importantly, we validated the activity of the BLA using markers for two discrete immediate early genes, c-Fos, and Egr-1 (Guzowski et al., 2005; Wheeler et al., 2013). This study is novel in its focus on neural circuits mediating social approach behavior in juvenile mice, a developmental stage of particular relevance to maturing social circuits and to neurodevelopmental disorders such as ASD (Scherf et al., 2013). Our findings have important implications for studies of social approach in mouse models of ASD, as B6 is the strain used as the genetic background for most mouse models of ASD (Kazdoba et al., 2015). Previous studies of brain activation following social behaviors have often utilized adults in testing conditions other than the SAT, or have involved social stimulus mice that are likely to induce sexual and/or aggressive interactions (Ferguson et al., 2001; Duncan et al., 2009; Samuelsen and Meredith, 2009). In adult rodents, social behaviors that may be influenced by sexual or aggressive drive are mediated, in part, by the medial amygdala (Petrulis, 2009; Samuelsen and Meredith, 2009; Maras and Petrulis, 2010). In the present study of juvenile mice that were exposed to gonadectomized, same-sex stimulus mice, the medial amygdala did not exhibit significant activation during social exposure. B6 mice at four weeks of age are pre-pubertal and therefore unlikely to exhibit sexual or aggressive behaviors (Chan et al., 2011) and have different structural and functional amygdala circuitry (Scherf et al., 2013). For example, inputs to and synaptic responses of the rodent amygdala change over the course of adolescence (Pan et al., 2009; Pattwell et al., 2012). In addition, changes in behaviors mediated by the limbic system, including fear conditioning and depression-like behaviors, are observed during this time in both rodents and humans (Hefner and Holmes, 2007; Pattwell et al., 2012). Importantly, reduced social approach behavior is often more severe in the juvenile period than in adulthood in ASD (McGovern and Sigman, 2005).
Social approach behavior in the SAT also may be influenced by olfactory or reward pathways. While we found significant changes in c-Fos activation in only the BLA after exposure to social stimulus, and no increase in other areas of the brain involved in reward, including the NAc, many of these regions have reciprocal interactions with the BLA (Stuber et al., 2011). Likewise, BLA-prefrontal cortex (PFC) connections likely play a role in juvenile social behavior in the SAT. Optogenetic experiments have demonstrated that increasing excitatory activity in the PFC leads to reduced social approach in adult mice, whereas increasing inhibitory activity in the PFC ameliorates this deficit (Yizhar et al., 2011). This is consistent with our data, as the PFC has been shown to inhibit the BLA (Kita and Kitai, 1990). Finally, previous clinical and animal studies demonstrate a role for the amygdala and reciprocally connected cortical circuitry, including prefrontal cortex and cingulate cortex, in mediating both positive and negative social interactions in rodents as well as humans (Everitt et al., 2003; Mahler and Berridge, 2011). Although we did not find evidence of increased c-Fos expression in these other regions, the BLA is a central hub of the circuitry that is reciprocally connected to many other structures. Future studies should aim to analyze the connectomics of social behavior in further depth (Bilker et al., 2004; Wheeler et al., 2013). Because amygdala structure and function are fairly conserved across mammalian evolution, findings in mouse models likely have important relevance to humans (Adolphs, 2013).
The amygdala has been associated with a myriad of social behaviors in rodents, monkeys, and humans (Allsop et al., 2014 for review). For example, lesion studies in various species have resulted in altered social behavior (Rosvold et al., 1954; Emery et al., 2001; Amaral et al., 2003; Machado and Bachevalier, 2006; Machado et al., 2008; Adolphs, 2010; Bliss-Moreau et al., 2011, 2013). Likewise, neuroanatomical, fMRI, and electrophysiological studies have shown that amygdala volume, connectivity, and activity are associated with social functioning or cognition (Leonard et al., 1985; Vuilleumier et al., 2004; Duncan et al., 2009; Davis et al., 2010; Bickart et al., 2011; Troiani et al., 2014). Specifically, the BLA has been identified as an amygdalar nucleus important for social behavior and has been shown to mediate play behaviors in 4–5 week old rats (Trezza et al., 2012). In addition, optogenetic manipulation of projections from the BLA to ventral hippocampus regulates juvenile sociability in the SAT as well as a resident-intruder test (Felix-Ortiz and Tye, 2014). These data are consistent with our findings that the BLA plays an important role in mediating juvenile mouse social interactions in the SAT.
The BLA is a salience detector that integrates sensory information, mediates the emotional and motivational valence of stimuli, emotional memory formation and consolidation, and modulates reward (Cardinal et al., 2002; Everitt et al., 2003; Mahler and Berridge, 2011). Histologically, the majority of the neurons in the rodent BLA are glutamatergic pyramidal cells, which are predominantly projection neurons with outputs to cortical regions and other amygdala nuclei (Spampanato et al., 2011). Approximately 18% of the BLA is composed of GABA-producing interneurons, which are important for inhibiting conditioned fear learning and expression (Spampanato et al., 2011). Enhanced overall activation of the BLA may lead to an excitatory drive on downstream nuclei, including the nucleus accumbens (NAc), hippocampus, and hypothalamus, which modulate reward, aversion, and motivated movement (Kita and Kitai, 1990; Everitt et al., 1999). This circuitry may be involved in social approach tendencies in B6 mice. However, while it appears in the present study that more Fos+ cells were excitatory versus inhibitory, social exposure-induced Fos activation in CaMKII+ and GABAergic cells was not assessed in the same group of animals, making it difficult to determine an exact breakdown of specific cell types. Thus, further studies are needed to determine what proportion of socially responsive neurons are excitatory versus inhibitory. Finally, it seems likely that the increase in BLA activity in response to a social stimulus occurs in other types of cells that were not identified in the particular double-labeling immunohistochemistry experiments reported here. Identifying these cell types will be an important future direction.
Identifying the role of the largely glutamatergic neuronal population in the BLA involved in juvenile social behavior can have important implications for neurodevelopmental disabilities that involve social deficits, namely ASD. Altered amygdala structure and function have been reported in numerous studies of children and adults with ASD. Researchers have demonstrated changes in cell number and size (Kemper and Bauman, 1993; Schumann and Amaral, 2006), volume (Abell et al., 1999; Howard et al., 2000; Sparks et al., 2002; Munson et al., 2006), gray matter volume (Abell et al., 1999; Waiter et al., 2004), and activity during social reward or perception tasks (Schultz, 2005; Baron-Cohen et al., 1999; Wang et al., 2004; Grelotti et al., 2005; Ashwin et al., 2006; Kaiser et al., 2010; Kohls et al., 2013). One study of children with ASD identified an increase in volume of the BLA in particular (Kim et al., 2010). Additionally, the amygdala theory of autism indicates that the sociability deficits of ASD may be attributable, at least in part, to developmental and functional anomalies of the amygdala and limbic circuits that mediate the salience and emotional/motivational significance of social stimuli (Bachevalier, 1996; Baron-Cohen et al., 2000; Schultz et al., 2003; Chevallier et al., 2012). However, progress towards mechanistic understanding has been hampered by a paucity of animal model studies focused on the impact of ASD susceptibility gene mutations on the development and function of the circuits and synapses that specifically mediate juvenile sociability. In this study, we have implicated the BLA as an important hub in the circuitry mediating social affiliative behaviors in juvenile mice. Future studies elucidating the function of BLA circuitry in juvenile social behaviors, particularly in genetic mouse models of ASD, will be important in developing improved understanding and more effective treatments for social behavior deficits in ASD.
Conclusions
The basoloateral amygdala (BLA) was activated specifically by social approach behavior in highly sociable, 4-week-old B6 mice. These data implicate the BLA as an important hub in the circuitry mediating social approach behaviors in juvenile mice. Future studies of genetic mouse models of ASD should address potential alterations in circuitry involving the BLA that may mediate social approach phenotypes in juvenile mice.
Highlights.
Juvenile C57BL/6J male mice exhibit high social approach behavior in a three-chamber apparatus.
Social approach preferentially activates the basolateral amygdala (BLA), indicated by increased c-Fos and Egr-1 expression.
Our data indicate that the BLA is an important hub in the circuitry mediating juvenile social approach behavior.
Future studies of sociability in mouse models of ASD should address potential alterations in circuitry involving the BLA.
Acknowledgments
The authors wish to thank the following funding sources: National Institutes of Health Grants R01MH080718 (E.S.B.), ARRA supplement 3R01MH080718-03S1 (E.S.B.), 1P50MH096891 (Raquel Gur) subproject 6773 (E.S.B.), Training Program in Neurodevelopmental Disabilities T32 NS077413 (S.L.F.), Pennsylvania Department of Health (SAP # 4100042728) (R. Schultz), NARSAD Distinguished Investigator Award (R.G.), Burroughs-Wellcome Fund Career Award in the Biomedical Sciences (E.S.B.). This content is solely the responsibility of the authors and does not represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happé F, Frith C, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The biology of fear. Curr Biol. 2013;23:R79–R93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, Mendoza SP. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: a test of the amygdala theory. Soc Neurosci. 2006;1:349–363. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. Brief report: medial temporal lobe and autism: a putative animal model in primates. J Autism Dev Disord. 1996;26:217–220. doi: 10.1007/BF02172015. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilker WB, Brensinger C, Gur RC. A Two Factor ANOVA-like Test for Correlated Correlations: CORANOVA. Multivariate Behav Res. 2004;39:565–594. doi: 10.1207/s15327906mbr3904_1. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, Amaral DG. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci. 2013;25:2124–2140. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chan T, Kyere K, Davis BR, Shemyakin A, Kabitzke PA, Shair HN, Barr GA, Wiedenmayer CP. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci. 2011;31:4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex. 2010;20:612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH, Moy SS. Neural activation deficits in a mouse genetic model of NMDA receptor hypofunction in tests of social aggression and swim stress. Brain Res. 2009;1265:186–195. doi: 10.1016/j.brainres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, Morton EA, Tan J, Berrettini WH, Li H, Abel T, Brodkin ES. Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res. 2012;228:299–310. doi: 10.1016/j.bbr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–217. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec (Hoboken) 2011;294:1713–1725. doi: 10.1002/ar.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the Medial Amygdala is Essential for Social Recognition in the Mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of Preferential Looking to the Eyes of Approaching Adults Predicts Level of Social Disability in 2-Year-Old Toddlers With Autism Spectrum Disorder. Arch Gen Psychiatry. 2008;65:946. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Deen B, Pitskel NB, Sugrue DR, Voos AC, Saulnier CA, Ventola P, Wolf JM, Klin A, Vander Wyk BC, Pelphrey KA. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdoba TM, Leach PT, Crawley JN. Behavioral Phenotypes of Genetic Mouse Models of Autism. Genes Brain Behav. 2015 doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11:175–187. [PubMed] [Google Scholar]
- Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, Kim DJ, Yoon SJ, Hwang J, Dager SR. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, Müller K, Fink GR, Kamp-Becker I, Herpertz-Dahlmann B, Schultz RT, Konrad K. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. 2013;8:565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res. 1985;15:159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behav Neurosci. 2008;122:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2011;221:407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Lesions that functionally disconnect the anterior and posterodorsal subregions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus) Neuroscience. 2010;165:1052–1062. doi: 10.1016/j.neuroscience.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. J Child Psychol Psychiatry. 2005;46:401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Pan B-X, Ito W, Morozov A. Divergence between thalamic and cortical inputs to lateral amygdala during juvenile-adult transition in mice. Biol Psychiatry. 2009;66:964–971. doi: 10.1016/j.biopsych.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Geschwind DH. What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol Med. 2012;18:156–163. doi: 10.1016/j.molmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neuroscience. 2009;164:1468–1476. doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav. 2013;64:298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Südhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof LWM, Pasterkamp RJ, Zhou Y, Campolongo P, Cuomo V, Di Marzo V, Vanderschuren LJMJ. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Price ET, Schultz RT. Unseen fearful faces promote amygdala guidance of attention. Soc Cogn Affect Neurosci. 2014;9:133–140. doi: 10.1093/scan/nss116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, McIntosh AR, Parkinson J, Frankland PW. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput Biol. 2013;9:e1002853. doi: 10.1371/journal.pcbi.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee H-R, Gee HY, Mah W, Kim J-I, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park S-G, Lee J-S, Lee K, Kim D, Bae YC, Kaang B-K, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]