Abstract
Background
Aberrant expression of cancer-testis antigens (CTA) in breast carcinoma tissue, and its natural expression in the testis, the tissue away from the immune system, makes them good candidates for cancer immunotherapy and vaccines designing.
Objectives
The aim of this study was to assess the expression of a CTA (MAGE-1) in invasive breast cancer and its correlation with prognostic factors.
Methods
Paraffin blocks of breast cancer tissues from 113 patients operated in 2011 - 2013 were stained for MAGE-1expression by immunohistochemistry (IHC). The associations of MAGE-1 expression with known prognostic factors were assessed by statistical analysis using SPSS 16.
Results
MAGE-1 expression was found in cancer cell cytoplasms of 30.1% of patients, with different degrees of intensity, (23.9% moderate and 6.2% strong). Nuclear staining turned positive in 31.8%, stratified from moderate in 26.5%to to strong in 5.3%. There was a significant association between the number of lymph nodes involved and both nuclear (P = 0.042) and cytoplasmic (P = 0.003) MAGE-1 expression. There was also a significant correlation between the nuclear expression of MAGE-1 and tumor size (P = 0.018). Cytoplasmic expression of MAGE-1 increased with increasing pathologic grade of tumors although the association was not statistically significant (P = 0.119).
Conclusions
CTA MAGE-1 has significant association with some prognostic factors in breast cancer and may have the role of a prognostic factor.
Keywords: Breast Cancer, Cancer-Testis Antigens, MAGE-1, Immunohistochemistry
1. Background
Breast cancer is the most prevalent malignancy in women and affects about 1 in 8 women around the world (1). Therefore, investigation of early biomarkers and molecular aspects is valuable for improvement of breast cancer therapy and outcome.
Cancer-testis antigens (CTA) are proteins with physiological expression restricted to adult testicular germ cells. They are down-regulated in somatic adult tissues but may be aberrantly re-expressed in various malignancies. The first CTA was discovered by taking advantage of a newly developed DNA-cloning method to identify targets of T-cell recognition. Cytotoxic T lymphocytes (CTL) recognizing autologous tumor cells were obtained from a patient bearing melanoma with an unusually favorable clinical course. Using the melanoma cell line MZ2- MEL and autologous CTL clones cytolytic to this line, MAGE-1, subsequently was renamed as MAGE-A1, and was identified as the target antigen. This was the first molecularly characterized tumor antigen eliciting autologous CTL responses in a cancer patient. Further analysis of the MAGE-A family revealed 12 closely related genes clustered at Xq28. A growing number of tumor-associated antigens (TAA), with similar characteristics, identified by cellular or serological screening techniques, have been reported since. Although some of them may be expressed in placenta as well, they are collectively referred to as CTA. CTA presently include 44 distinct gene families, some comprising multiple members, such as MAGE-A and GAGE1, as well as splice variants, such as XAGE1a and XAGE1b, for a total of 89 transcripts. CTA can be classified into those that are encoded on the X chromosome (X-CTA) and those that are not (non-X CTAs) (2).
To date, almost 100 genes and gene families encoding CTAs have been identified. CTAs mapping to chromosome X are referred to as X -CTAs and are distinguished from non-X CTAs located on other chromosomes (2-4). X-CTAs expression in breast cancer tissues is associated with a poor outcome and is more prevalent in higher grade and advanced stage tumors (5-9). Due to testis blood barrier and the immune privileged status of germinal cells (10), expression of CTAs in tissues other than testis can trigger an immune response. These antigens are also expected to become new candidates for cancer-specific immunotherapy, but little information is available on the comprehensive expression of CTAs in a large number of samples of gastrointestinal and breast carcinomas (11). Expression of CTAs of the MAGE family has been also reported in human breast carcinomas although only to a limited degree (12). Several clinical trials have assessed their therapeutic potentials in cancer patients. Breast cancers, especially triple-negative cancers, show higher expression of CT genes, which is the prerequisite for any immunotherapeutic approach. CT genes have also gained attention for immunoprevention in high-risk patients (13).
2. Objectives
The purpose of the present study is to assess immunohistochemical expression of CTA MAGE-1 in tissue samples of invasive breast cancer and its correlation with known prognostic factors.
3. Methods
3.1. Patients
A total of 113 patients with invasive breast cancer (112 ductal and one lobular) were included. Age ranged between 27 and 78 years (median: 46 years). All patients were surgically treated at Omid hospital in Mashhad University of Medical Sciences, Iran between 2011 and 2013. Data related to tumor size, grade, stage, estrogen receptor (ER) and progesterone receptor (PR) status, human epithelial growth factor receptor 2 (HER-2/neu), and axillary lymph node status are summarized in Table 1.
Table 1. Frequency and Percentage of Tumor Size, Grade, Stage, ER/PR Status, HER-2 Status, LN Stage.
| Clinicopathological Parameter | No. (%) |
|---|---|
| Tumor Size, cm | |
| ≤ 4 | 51 (45.2) |
| > 4 | 35 (31) |
| Miss | 27 (23.9) |
| Tumor Grade | |
| 1 | 9 (8) |
| 2 | 44 (38.9) |
| 3 | 41 (36.3) |
| Miss | 19 (16.8) |
| Primary Tumor Stage | |
| T1 | 15 (13.3) |
| T2 | 54 (47.8) |
| T3 | 18 (15.9) |
| Miss | 26 (23) |
| Lymph Node Status | |
| N0 | 9 (8) |
| N1 | 28 (24.8) |
| N2 | 19 (16.8) |
| N3 | 8 (7.1) |
| Miss | 49 (43.4) |
| ER Status | |
| Neg | 22 (19.5) |
| Pos | 1 (0.9) |
| Miss | 90 (79.6) |
| PR Status | |
| Neg | 24 (21.2) |
| Pos | 1 (0.9) |
| Miss | 88 (77.9) |
| Her2/Neu | |
| Neg | 23 (20.4) |
| + | 10 (8.8) |
| ++ | 9 (8) |
| +++ | 14 (12.4) |
| Miss | 57 (50.4) |
3.2. Immunohistochemistry
Immunohistochemical staining of MAGE-1 was performed on the invasive breast cancer. For the detection of MAGE-1 protein, we used undiluted NCL-MAGE-1 monoclonal antibody (mAb), staining is described in detail elsewhere (14). Briefly, tissue slides from paraffin embedded breast cancer tumor samples were places on Silane (3 aminopropyltriethoxysilane, A 3648, Sigma, St. Louis MO, USA). After de paraffinization, slides were heated in an 800-W microwave oven at maximum power for 30 minutes, held in 10 mmol/L edta buffer (pH 6.0) for 5 minutes and then rinsed with a tris buffer solution (PBC, pH 7.2). To suppress endogenous peroxidase activity, slides were treated with H2O2. After additional rinsing with PBC, they were incubated for 20 minutes with a 1:10 dilution of normal rabbit serum (DakoX0902, Dako A/S) in a wet chamber at room temperature for 20 minutes to prevent non-specific binding of immunoglobulin. Slides were then treated with undiluted mAbs at room temperature for 90 minutes.
The Envision (Dako) system was used as a secondary detection tool and diaminobenzidine tetrahydrochloride served as a chromogen. Slides were counterstained with hematoxylin prior to evaluation. Sections of normal human testis with intact spermiogenesis were used as positive controls for MAGE-1 mAbs.
3.3. Scoring
MAGE-1 staining results were scored using Allred scoring system (15). This method takes into account percentages of positive cells (scored on a 0 - 3 scale) and the intensity of their staining (scored on a 0 - 3 scale). The percentage of positive cells is then multiplied by the intensity of staining, and the final score ranges from 0 (no staining) to 9 (diffuse and strong staining). The final results were further classified as 0 (no staining), 1 (score 1, 2, 3), 2 (score 4, 5, 6) and 3 (score 7, 8, 9). For statistical analysis, MAGE-1 scores of 0 and 1 were considered negative, whereas scores 2 and 3 were considered positive.
3.4. Statistical Analysis
The association between immunohistochemical data and different clinicopathological parameters were evaluated by chi2 and T-test. A P value < 0.05 was considered significant. For all statistical analyses, the computer program SPSS 16 software (SPSS for Windows, 2007) was used.
4. Results
4.1. MAGE-1 Cytoplasmic Expression
One hundred and thirteen samples were examined for cytoplasmic MAGE-1 expression by IHC. Table 2 summarizes IHC staining results. MAGE-1 expression (score ≥ 2+) was detected in 34/111 (30.1%) of patients (Figure 1).
Table 2. Frequency and Percentage of MAGE-1 Expression.
| Expression Score | No. (%) | |
|---|---|---|
| Cytoplasmic Expression | 0 | 41 (36.3) |
| 1 | 36 (31.9) | |
| 2 | 27 (23.9) | |
| 3 | 7 (6.2) | |
| Miss | 2 (1.8) | |
| Nuclear expression | 0 | 48 (42.5) |
| 1 | 27 (23.9) | |
| 2 | 30 (26.5) | |
| 3 | 6 (5.3) | |
| Miss | 2 (1.8) |
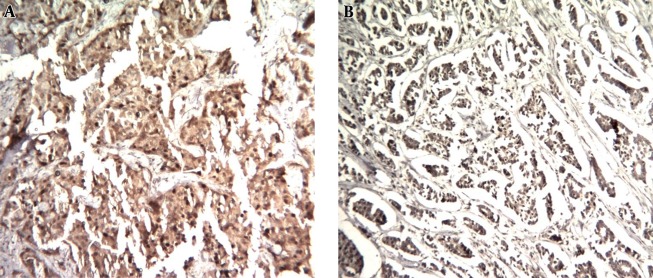
Figure 1. MAGE-1 Nuclear and Cytoplasmic Expression by Immunohistochemistry in Human Breast Cancer.
A, strong nuclear and cytoplasmic staining of most of neoplastic cells (H & E, 100 ×); B, strong nuclear and cytoplasmic staining of most of neoplastic cells (H & E, 100 ×).
4.2. MAGE-1 Nuclear Expression
One hundred and thirteen samples were examined for nuclear MAGE-1 expression by IHC. Table 2 summarizes IHC staining results. MAGE-1 expression (score ≥ 2+) was detected in 36/111 (31.8%) of patients (Figure 1).
4.3. Association Between MAGE-1 Cytoplasmic Expression and Different Clinicopathological Parameters
Table 3 presents associations between MAGE-1expression with clinicopathological variables. Expression of MAGE-1 was significantly associated with lymph node (P = 0.003) breast cancers, but no association was found between MAGE-1 cytoplasmic expression and tumor size, age, HER-2 status, Tumor stage, grade, and ER/ PR status.
Table 3. Association Between MAGE-1 Nuclear and Cytoplasmic Expression and Different Clinicopathological Parametersa.
| Clinicopathological Parameter | Cytoplasmic Expression | Nuclear Expression | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | P Value | Negative | Positive | P Value | |||
| ER status | - | (n = 23) | 15 (65.2) | 8 (34.8) | 0.501 | 16 (69.6) | 7 (30.4) | 0.298 |
| + | (n = 34) | 25 (73.5) | 9 (26.5) | 19 (55.9) | 15 (44.4) | |||
| PR status | - | (n = 23) | 15 (65.2) | 8 (34.8) | 0.912 | 16 (69.6) | 7 (30.4) | 0.337 |
| + | (n = 30) | 20 (66.7) | 10 (33.3) | 17 (56.7) | 13 (43.3) | |||
| HER2/neu | Pos | (n = 32) | 25 (78.1) | 7 (21.9) | 0.087 | 19 (59.4) | 13 (40.6) | 0.66 |
| Neg | (n = 23) | 13 (56.5) | 10 (43.5) | 15 (65.2) | 8 (34.8) | |||
| Tumor stage | T1 | (n = 15) | 10 (66.7) | 5 (33.3) | 0.942 | 10 (66.7) | 5 (33.3) | 0.751 |
| T2 | (n = 53) | 37 (69.8) | 16 (30.2) | 37 (69.8) | 16 (30.2) | |||
| T3 | (n = 18) | 13 (72.2) | 5 (27.8) | 14 (77.8) | 4 (22.2) | |||
| Lymph node status | N0 | (n = 9) | 6 (66.7) | 3 (33.3) | 0.003 | 3 (33.3) | 6 (66.7) | 0.042 |
| N1 | (n = 25) | 22 (78.6) | 6 (21.4) | 21 (75) | 7 (25) | |||
| N2 | (n = 19) | 15 (78.9) | 54 (21.1) | 16 (84.2) | 3 (15.8) | |||
| N3 | (n = 8) | 1 (12.5) | 7 (87.5) | 6 (75) | 2 (25) | |||
| Tumor grade | I | (n = 9) | 8 (88.9) | 1 (11.1) | 0.119 | 6 (66.7) | 3 (33.3) | 0.718 |
| II | (n = 44) | 32 (72.7) | 12 (27.3) | 33 (75) | 11 (25) | |||
| III | (n = 40) | 23 (57.5) | 17 (42.5) | 27 (67.5) | 13 (32.5) | |||
| Patient age, y | ≤ 46 | (n = 52) | 36 (69.2) | 16 (30.8) | 0.312 | 43 (82.7) | 9 (17.3) | 0.418 |
| > 46 | (n = 46) | 36 (78.3) | 10 (21.7) | 35 (76.1) | 11 (23.9) | |||
| Tumor Size, cm | ≤ 4 | (n = 51) | 35 (68.6) | 16 (31.4) | 0.137 | 38 (74.5) | 13 (25.5) | 0.018 |
| > 4 | (n = 35) | 29 (82.9) | 6 (17.1) | 33 (94.2) | 2 (5.7) | |||
aValues are expressed as No. (%).
4.4. Association Between MAGE-1 Nuclear Expression and Different Clinicopathological Parameters
Table 3 presents associations between MAGE-1 nuclear expression with clinicopathological variables. Expression of MAGE-1 was significantly associated with tumor size (P = 0.018) and lymph node (P = 0.042) breast cancers. No association was found between MAGE-1 nuclear expression and age, HER-2 status, Tumor stage, grade, and ER/ PR status.
5. Discussion
Breast cancer is among the leading causes of death in women worldwide (16).
The search for human tumor antigens as potential immunotherapeutic targets represents an appealing therapeutic concept since decades ago. Recent advances in molecular characterization of human tumor-associated antigens have paved the way toward active specific immunotherapy of cancer (17).
CTAs are of particular interest, because they are expressed in a very limited number of healthy tissues typically including HLA class I negative spermatogonia, while they are expressed in a wide range of malignancies (18).
Few studies have examined CTA expression in breast cancer. In present study, we analyzed expression of MAGE-1 antigen on archival paraffin-embedded samples of invasive breast cancer tissue of 113 patients and correlated their expression with other clinicopathological variables. To our knowledge, this is the first report specifically examining expression of MAGE-1, at the protein level, in breast cancer.
MAGE-1 cytoplasmic expression (score ≥ 2+) was detectable in 30.1% and nuclear expression (score ≥ 2+) was detectable in 31.8% of patients.
Data on MAGE-A and NY-ESO-1 expression in literature are highly variable. The frequency of multispecific MAGE-A and NY-ESO-1 positivity in published studies ranges between 17 and 74% and 2% - 40%, respectively (2). Stefan found a 18% positive CTA7 (MAGE-C1), defined as immunoreactivity in more than 50% of tumor cells, in 124 women with invasive breast cancer (16).
In the study of recurrent ductal breast cancer, Bandic found 74% of MAGE-A and 40% of NY-ESO-1-positivity in samples (19).
These discrepancies observed between studies may be due to different antibodies used for CT detection or difference in scoring system. Some authors define positive X-CTA expression according to percentage of cells (19-21), while others combine the extent and intensity of CTA expression using semi-quantitative scoring systems (22, 23).
Studies exploring potential prognostic significance of CTA expression in breast cancer have yielded contradictory findings. Some authors found that expression of CTA is associated with poorly differentiated histological phenotypes (20, 22). Others found no association between their expression and various pathological parameters (19) or only an association between MAGE-A1 and Ki-67 labeling index (23). According to the present study (Table 3), a positive nuclear expression MAGE-1 status correlated significantly with lymph nodes status (P = 0.042), and positive cytoplasmic expression MAGE-1 status correlated significantly with lymph nodes (LN) status (P = 0.003). Positive nuclear Expression MAGE-1 status correlated significantly with tumor size (P = 0.018). However, the expression of MAGE-1 was not associated with other typical adverse clinicopathological features, namely tumor stage, and pathological grade.
In Badovinac Crnjevic et al. study (2) MAGE-A10 expression was significantly associated with ER-negative (P = 0.002), PR-negative (P = 0.002) and HER-2-negative (P = 0.044) tumors. They showed that MAGE-A10 was frequently expressed in the triple negative (TN) subgroup of patients, where the majority (85.7%) of tumors expressed CTA (19). Curigliano showed a significantly higher expression of MAGE-A (26%) in TN breast cancers compared with ER-positive tumors (10%) (P = 0.07) (23).
Although the exact biological function of CTAs is still unknown, future studies will hopefully allow more insights into the activities of CTAs in tumor cells on the molecular level.
In conclusion, due to MAGE-1 expression (score ≥ 2+) in about 30% of our patients, even more frequent than her2 positivity in breast cancer, this marker could be potentially regarded for target therapy in these patients.
Acknowledgments
This work was supported by the Mashhad University of Medical Sciences.
Footnotes
Authors’ Contribution:None declared.
Conflict of Interest:None declared.
Financial Disclosure:None declared.
Funding/Support:None declared.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac Crnjevic T, Spagnoli G, Juretic A, Jakic-Razumovic J, Podolski P, Saric N. High expression of MAGE-A10 cancer-testis antigen in triple-negative breast cancer. Med Oncol. 2012;29(3):1586–91. doi: 10.1007/s12032-011-0120-9. [DOI] [PubMed] [Google Scholar]
- 3.Almeida LG, Sakabe NJ, deOliveira AR, Silva MC, Mundstein AS, Cohen T, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37(Database issue):D816–9. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105(51):20422–7. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–62. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O'Neill D, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade VC, Vettore AL, Felix RS, Almeida MS, Carvalho F, Oliveira JS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 8.Napoletano C, Bellati F, Tarquini E, Tomao F, Taurino F, Spagnoli G, et al. MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic factors and effects of chemotherapy. Am J Obstet Gynecol. 2008;198(1):99 e1–7. doi: 10.1016/j.ajog.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20(1):3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 11.Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85(5):713–20. doi: 10.1054/bjoc.2001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo V, Traversari C, Verrecchia A, Mottolese M, Natali PG, Bordignon C. Expression of the MAGE gene family in primary and metastatic human breast cancer: implications for tumor antigen-specific immunotherapy. Int J Cancer. 1995;64(3):216–21. doi: 10.1002/ijc.2910640313. [DOI] [PubMed] [Google Scholar]
- 13.Ghafouri-Fard S, Shamsi R, Seifi-Alan M, Javaheri M, Tabarestani S. Cancer-testis genes as candidates for immunotherapy in breast cancer. Immunotherapy. 2014;6(2):165–79. doi: 10.2217/imt.13.165. [DOI] [PubMed] [Google Scholar]
- 14.Bolli M, Schultz-Thater E, Zajac P, Guller U, Feder C, Sanguedolce F, et al. NY-ESO-1/LAGE-1 coexpression with MAGE-A cancer/testis antigens: a tissue microarray study. Int J Cancer. 2005;115(6):960–6. doi: 10.1002/ijc.20953. [DOI] [PubMed] [Google Scholar]
- 15.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68. [PubMed] [Google Scholar]
- 16.Kruger S, Ola V, Feller AC, Fischer D, Friedrich M. Expression of cancer-testis antigen CT7 (MAGE-C1) in breast cancer: an immunohistochemical study with emphasis on prognostic utility. Pathol Oncol Res. 2007;13(2):91–6. doi: 10.1007/BF02893483. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 18.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–21. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandic D, Juretic A, Sarcevic B, Separovic V, Kujundzic-Tiljak M, Hudolin T, et al. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat Med J. 2006;47(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Kavalar R, Sarcevic B, Spagnoli GC, Separovic V, Samija M, Terracciano L, et al. Expression of MAGE tumour-associated antigens is inversely correlated with tumour differentiation in invasive ductal breast cancers: an immunohistochemical study. Virchows Arch. 2001;439(2):127–31. doi: 10.1007/s004280100421. [DOI] [PubMed] [Google Scholar]
- 21.Schultz-Thater E, Piscuoglio S, Iezzi G, Le Magnen C, Zajac P, Carafa V, et al. MAGE-A10 is a nuclear protein frequently expressed in high percentages of tumor cells in lung, skin and urothelial malignancies. Int J Cancer. 2011;129(5):1137–48. doi: 10.1002/ijc.25777. [DOI] [PubMed] [Google Scholar]
- 22.Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, et al. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One. 2011;6(3):e4404. doi: 10.1371/journal.pone.0017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curigliano G, Viale G, Ghioni M, Jungbluth AA, Bagnardi V, Spagnoli GC, et al. Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol. 2011;22(1):98–103. doi: 10.1093/annonc/mdq325. [DOI] [PubMed] [Google Scholar]