Significance
Language is viewed as a predominantly perisylvian function typically studied in isolation from memory. We demonstrate that the same neuronal computations used by the hippocampus for memory function also subserve online language usage. Our findings specify that the hippocampal complex contributes to language in an active fashion, relating incoming words to stored semantic knowledge, a necessary process in the generation of sentence meaning. These findings represent a major step in integrating the studies of language and memory, significantly expanding the role of hippocampal theta oscillations and adding hippocampal structures to the neural network supporting language.
Keywords: recollection, relational memory, semantic memory, sentence completion, word production
Abstract
Language is classically thought to be supported by perisylvian cortical regions. Here we provide intracranial evidence linking the hippocampal complex to linguistic processing. We used direct recordings from the hippocampal structures to investigate whether theta oscillations, pivotal in memory function, track the amount of contextual linguistic information provided in sentences. Twelve participants heard sentences that were either constrained (“She locked the door with the”) or unconstrained (“She walked in here with the”) before presentation of the final word (“key”), shown as a picture that participants had to name. Hippocampal theta power increased for constrained relative to unconstrained contexts during sentence processing, preceding picture presentation. Our study implicates hippocampal theta oscillations in a language task using natural language associations that do not require memorization. These findings reveal that the hippocampal complex contributes to language in an active fashion, relating incoming words to stored semantic knowledge, a necessary process in the generation of sentence meaning.
Language is viewed as a predominantly perisylvian function typically studied in isolation from memory. The medial temporal lobe, and in particular the hippocampus, has not been considered as a key brain structure supporting language. For example, the hippocampal complex is still absent from prominent language models (1, 2).
Recently, studies examining patients with hippocampal lesions have found behavioral deficits in online language use (3; but see also refs. 4 and 5). From these findings, it has been proposed that the hippocampus is necessary for the integration and maintenance of memory representations required for the online use of language (3). However, the neurophysiological mechanism by which the hippocampus might support linguistic processing is unknown.
Oscillations play a key role in neuronal communication and provide optimal windows for neural excitability and network interactions (6). In the mammalian hippocampus, theta oscillations are ubiquitous and have been described as an index of neuronal computations by which different sources of information are integrated (7). The tight relationship between hippocampal theta oscillations and behavior is well studied. In particular, increases in oscillatory theta power are observed in relation to various memory processes in rodents (7, 8) and in humans (9, 10), but similar physiological data are lacking for language. We hypothesized that the same neurophysiological mechanism used by the hippocampus for memory function, as measured by theta oscillations, is also used for the online use of language.
One way to probe the integration and maintenance of memory representations for the online use of language is through context association. Context plays a fundamental role in language, facilitating word retrieval (11, 12) and enabling prediction during language comprehension (13). The activation of a concept invariably results in the coactivation of other associated features or concepts (14, 15). Given the critical role of the medial temporal lobe in retrieving learned associations (16), we examined activity recorded directly from the hippocampal complex of participants performing a linguistic-context task.
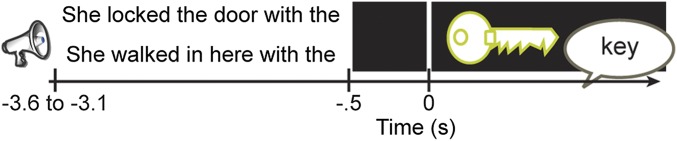
Twelve patients implanted stereotactically with depth electrodes for the localization of epileptic foci participated in the study. Table 1 provides additional information on their handedness, electrode coverage, and electrode analysis. The patients heard lead-in sentences missing the final word; then, they were shown a picture to name that completed the sentence. Half of the sentences had a linguistic context constraining the final word [e.g., “She locked the door with the” (picture: key)], and the other half had an unconstrained context [e.g., “She walked in here with the” (picture: key)] (Table S1). An example of the trial structure is shown in Fig. 1. All of our sentences were well-formed, acceptable English sentences (as in natural language use), with a duration of 2.6–3.1 s. Our task required online language comprehension and production in both conditions. Crucially, in the constrained sentences, the words are more strongly associated with a particular context/concept, constraining the sentence meaning and facilitating retrieval of the picture name (11, 12). Instead of comparing active versus rest time-periods, we directly compared the two conditions. Our effects thus reflect the differential role of stronger associations between the words in the sentence to a particular context. By asking participants to produce the last word of the sentence, we could assess whether they used the stronger associations between the words to enhance their behavior (11, 12).
Table 1.
Patient (P) information, hemisphere included in the analyses, number of bipolar contacts in the hippocampal-complex (used for Bonferroni correction), and number of bipolar contacts showing a significant context effect
| P | Hand | Pathology | Coverage | Hemisphere analyzed | Bipolar contacts | Significant (subfields) |
| 1 | R | Right TLE | Bilateral | Left | 2 in H | 1 in H (#1*: CA3-CA1) |
| 2 | R | Right TLE | Left | Left | 2 in H | 2 in H (#1: CA3-CA1; #2*: CA1) |
| 3 | NA | Left TLE | Bilateral | Right | 3 (2 in PH, 1 in EC) | 2 in PH, 1 in EC (#1–2*: PH; #3: EC) |
| 4 | R | Left TLE | Bilateral | Right | 3 in H | 0 (Decreases: 2) |
| 5 | R | Right frontal encephalomalacia | Right | Right | 5 in H | 1 in H (#1*: CA1) |
| 6 | R | Left TLE | Bilateral | Right | 5 (4 in H, 1 in SB) | 0 |
| 7 | R | Right TLE | Bilateral | Left | 8 in H | 5 in H (#1*-5: CA1) |
| 8 | R | Right TLE | Bilateral | Left | 1 in H-EC | 1 in H-EC (#1*) |
| 9 | R | Right TLE | Bilateral | Left | 2 in H | 1 in H (#1*: CA1) |
| 10 | L | Right TLE | Bilateral | Left | 4 (2 in PC, 2 in PH) | 1 in PH (#1*) |
| 11 | L | Right TLE | Bilateral | Left | 6 (3 in H, 3 in PH) | 2 in H (#1*: CA3; #2: CA1-CA3) |
| 12 | R | Bilateral frontal lesion (no focus) | Bilateral | Bilateral | 5 in H | 2 in H (#1*-2: Left CA1) |
| Total | 46 (34 in H, 7 in PH, 1 in EC, 1 in SB, 2 in PC, 1 in H-EC) | 19 (14 in H, 3 in PH, 1 in EC, 1 in H-EC) |
The location of the contacts are shown: entorhinal cortex (EC), hippocampus proper (H), perirhinal cortex (PC), parahippocampal cortex (PH), and subiculum (SB). For the significant contacts, the subfield in which it is located is given in parentheses, as determined by a neurologist based on a hippocampal subfield atlas (17). When the bipolar contact comprises two different subfields, both are indicated. Significant contacts are numbered (#), which correspond to the order of contacts in Figs. S1 and S2. The contacts shown in Fig. 3 are indicated by the asterisk. L, left; NA, not available; no focus, no epileptic focus identified; R, right; TLE, temporal lobe epilepsy.
Table S1.
Sentence materials
| Constrained sentence | Target | Unconstrained sentence | Target |
| He chopped the wood with an | Axe | The man came here with an | Axe |
| They bought a crib for the | Baby | They bought an egg for the | Baby |
| The hungry monkey eats | Bananas | The happy mother eats | Bananas |
| Joe hit the ball with the | Bat | She did something with the | Bat |
| She put fresh sheets on the | Bed | She found a hair on the | Bed |
| The teacher wrote on the | Board | The woman wrote on the | Board |
| We sailed in a wooden | Boat | He looked at a wooden | Boat |
| Read the chapter in the | Book | They described him in the | Book |
| Put the cat's milk in the | Bowl | Try to fit it in the | Bowl |
| John swept the floor with a | Broom | She was wandering with a | Broom |
| Kids ride to school on the | Bus | They were happy on the | Bus |
| Pat put frosting on the | Cake | Sam put flowers on the | Cake |
| Most rabbits like to eat | Carrots | Some people like to eat | Carrots |
| The mouse is eating the | Cheese | My friends are eating the | Cheese |
| The priest preaches in the | Church | The man spent time in the | Church |
| She was typing on the | Computer | She spilled something on the | Computer |
| He watches TV on the | Couch | Sandy waited on the | Couch |
| The boy played fetch with his | Dog | The man drove home with his | Dog |
| Girls play mommy with a | Doll | They are outside with a | Doll |
| A stranger knocked on the | Door | She placed her hand on the | Door |
| The young bride bought a white | Dress | My uncle bought a white | Dress |
| She put a stamp on the | Envelope | She saw a heart on the | Envelope |
| Phil put some drops in his | Eyes | Jim had something in his | Eyes |
| The brand new shoes hurt his | Feet | A bad sunburn hurt his | Feet |
| He poured the wine in the | Glass | It looks better in the | Glass |
| He can’t see without his | Glasses | He can't play without his | Glasses |
| He shot someone with a | Gun | He was happy with a | Gun |
| He hit the nail with a | Hammer | I saw someone with a | Hammer |
| He locked the door with the | Key | She walked in here with the | Key |
| Pam walked her dog on his | Leash | She couldn't pull on his | Leash |
| He found the road with a | Map | The boy arrived with a | Map |
| Please wipe your feet on the | Mat | The boy sat down on the | Mat |
| She saw herself in the | Mirror | She saw someone in the | Mirror |
| The wolf pack howled at the | Moon | The woman looked at the | Moon |
| The cat is chasing the | Mouse | The boy was chasing the | Mouse |
| He had coffee in his | Mug | He dropped something in his | Mug |
| She signed her name with a | Pen | She came over with a | Pen |
| She boiled the fish in the | Pot | She saw a hole in the | Pot |
| The birthday girl got a | Present | The little girl got a | Present |
| The young shepherd watched his | Sheep | The friendly man watched his | Sheep |
| The knight battled with a | Sword | Richard knelt down with a | Sword |
| She left a note on the | Table | She saw a mark on the | Table |
| The campers slept in the | Tent | The people were in the | Tent |
| She spread butter on her | Toast | She put one thing on her | Toast |
| The nice dentist pulled my | Tooth | His grandfather pulled my | Tooth |
| She dried her hands with a | Towel | He left the room with a | Towel |
| The pirate found all of the | Treasure | The woman brought all of the | Treasure |
| The leaf fell under the | Tree | We found this under the | Tree |
| The winning team raised the | Trophy | The teenager raised the | Trophy |
| Paul took a bath in the | Tub | There were two things in the | Tub |
| She put flowers in the | Vase | There was nothing in the | Vase |
Fig. 1.
Trial structure. An example of a trial with constrained (Upper) and unconstrained (Lower) sentence contexts with auditory sentences and visual pictures. Only one sentence was presented at each trial, but here both sentences are shown at the same time. Sentences lasted between 2.6 and 3.1 s. For picture-locked data, sentences started between 3.1 and 3.6 s before picture presentation.
Results
Sentence Context Facilitates Word Retrieval.
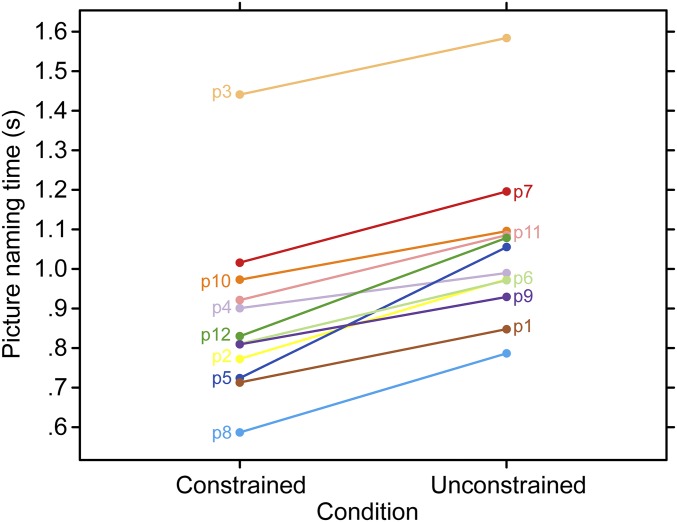
Participants named pictures on average 174-ms faster in the constrained contexts, t(11) = 9.23, P < 0.001 (Fig. 2). These results confirm that participants used the sentence context to guide their picture-naming responses.
Fig. 2.
Picture naming times. Mean picture naming times for correct responses as a function of condition for each participant.
Context Increases theta Power During Sentence Processing.
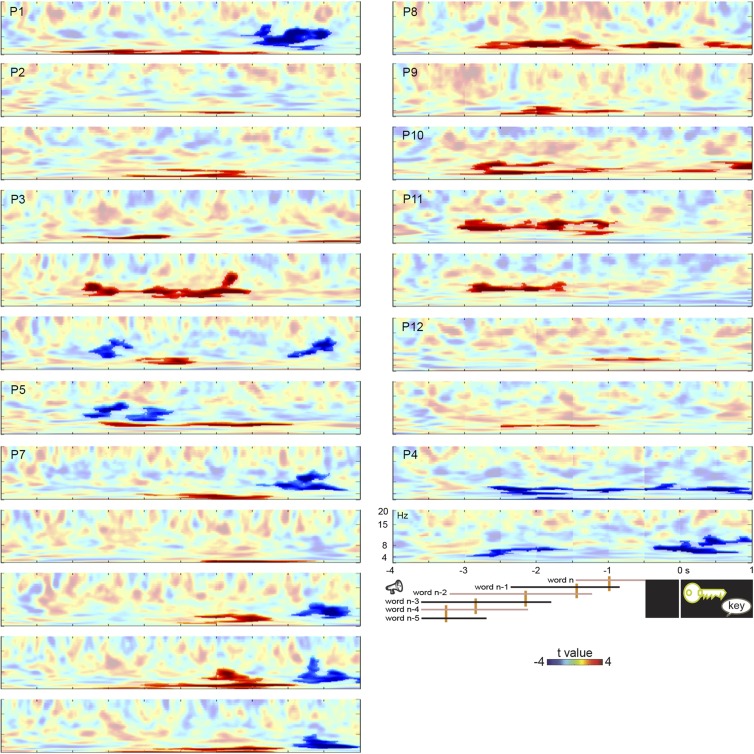
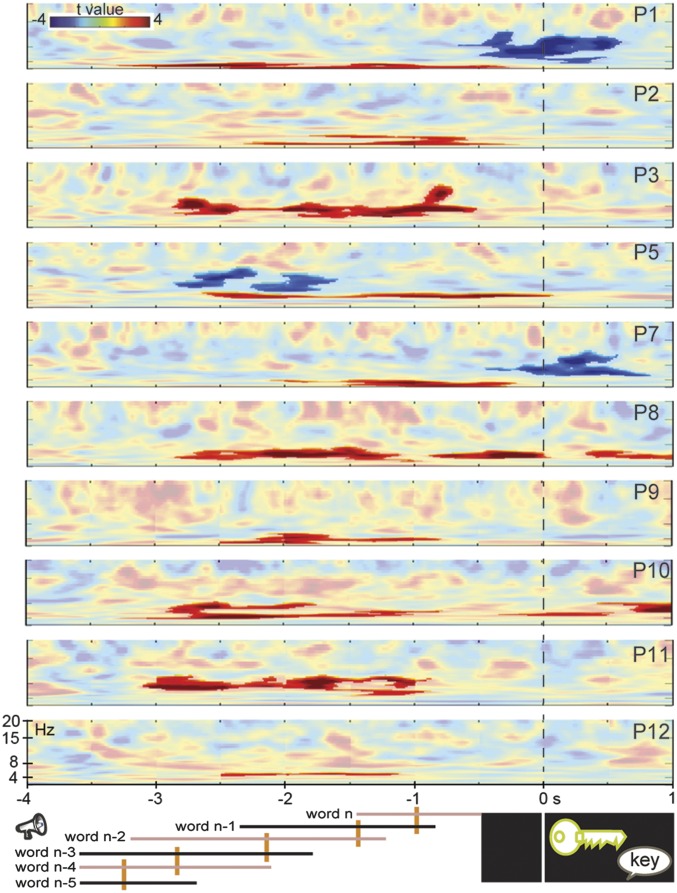
We assessed the neurophysiological mechanism of the context effect by recording from bipolar contacts in the hippocampal complex of the patients’ nonepileptic hemisphere (Table 1). For 10 of 12 patients, hippocampal-complex contacts showed sustained power increases during the lead-in sentences in constrained relative to unconstrained contexts (all cluster-based permutation tests Ps < 0.012, Bonferroni-corrected for the number of contacts tested per patient) (see Table 1 and Figs. S1 and S2 for all spectral data). Fig. 3 shows the time-resolved spectra of the context effect (constrained vs. unconstrained) in one bipolar contact of each patient time-locked to picture onset. On average, 1.6 contacts per patient were significant. The power increases occurred between 2 and 10 Hz (mean centered at 5 Hz) in the human hippocampal theta range (9).
Fig. S1.
Context effect. Time-resolved spectra of the context effect (constrained vs. unconstrained) time-locked to the picture in all 11 patients for bipolar contacts with significant effects. The color scale represents t-values. Significant effects are shown in stronger colors (multiple comparisons-corrected). Trial events are shown at the bottom (from left to right: the words of the sentence, picture presentation, and response). The timing of each word position is indicated by the continuous lines. The left end of each line indicates the earliest possible word onset. The right end indicates the latest possible word offset (and next word onset). Median word onset (and previous word offset) is indicated by the orange vertical bars. Results for P4, who only showed theta-power decreases, are shown in the Bottom Right panels. P6 did not have a significant theta effect.
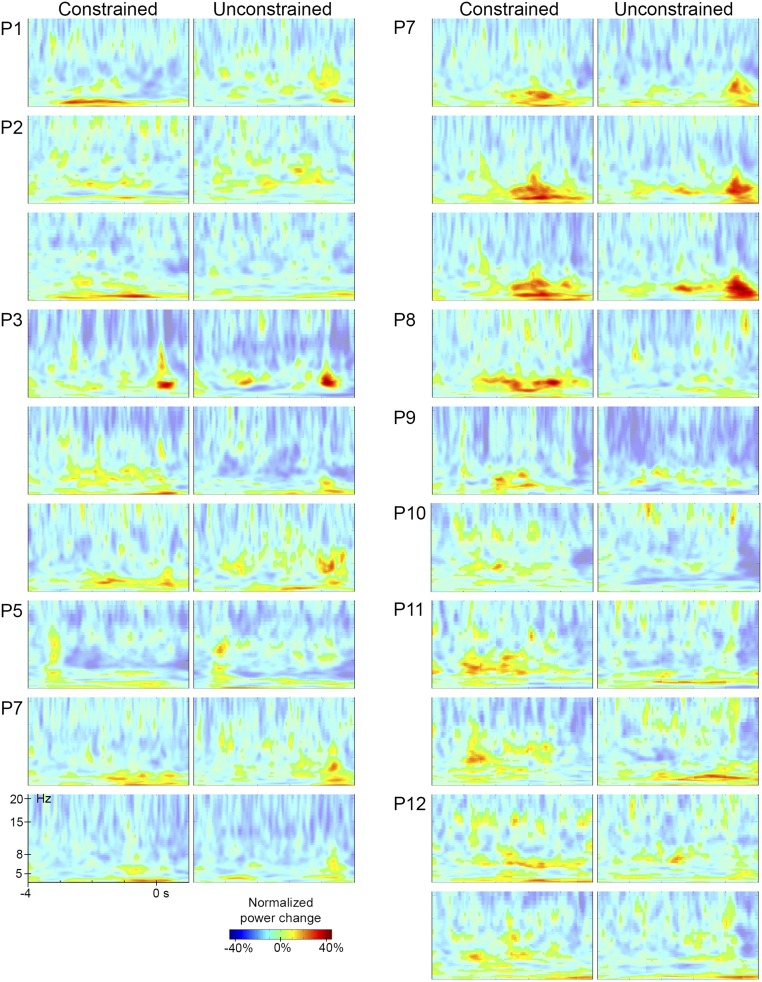
Fig. S2.
Time-frequency representations. Time-resolved spectra for each condition time-locked to picture onset for bipolar contacts of all patients with significant theta effects. Each row of a patient presents the data of one bipolar contact. The color scale represents normalized power changes. Power changes below 0.8% increases are shown with less-strong colors.
Fig. 3.
Context effect. Time-resolved spectra of the context effect (constrained vs. unconstrained) time locked to picture presentation in 10 patients, with significant effects in stronger colors (multiple comparisons corrected). The location of these contacts is given in Table 1. Trial events are shown at the bottom (from left to right: the words of the sentence, picture presentation, and response). The timing of each word position is indicated by the continuous lines. The left end of each line indicates the earliest possible word onset. The right end indicates the latest possible word offset (and next word onset). Median word onset (and previous word offset) is indicated by the orange vertical bars.
Fig. 3 shows that the contextual theta-power increases were sustained consistently across patients and occurred before picture onset (starting on average around 2.5 s before picture presentation). No hippocampal-complex contact showed theta-power increases exclusively after picture onset, indicating that the theta effect is related to semantic associations present in the sentence context.
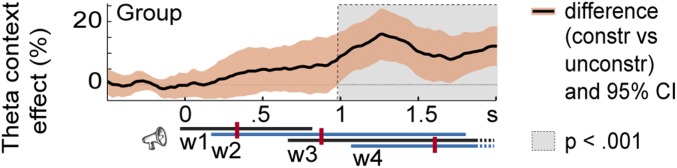
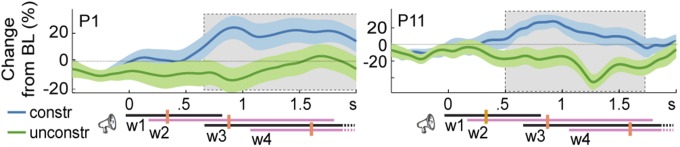
In the bipolar contacts examined for patients P1 and P11, the theta-context effect was evident relatively early. However, given that the words across the sentences differed in their timing (onset, offset, and duration), picture-locked analyses do not provide information about the theta effect relative to sentence onset. Moreover, time-resolved power estimations are inherently temporally smoothed, especially for low frequencies (in the order of hundreds of milliseconds), which could contribute to smearing effect onsets earlier in time. Fig. 4 shows the theta time-series for the constrained and unconstrained contexts and corresponding 95% confidence intervals (CI) for all 19 contacts showing a significant theta effect in the picture-locked analyses. On the group level, sustained power increases in hippocampal-complex contacts started around 1 s after sentence onset (cluster-based permutation P < 0.001), which is slightly later than the median onset of the third word of the sentence. For the two patients with apparent early effects (P1 and P11), sustained power increases in hippocampal-complex contacts started slightly earlier than the median onset of the third word of the sentence (Fig. S3) (cluster-based permutation tests Ps < 0.05, Bonferroni-corrected for number of contacts tested per patient). Thus, the theta-context effect does not start too early in the sentence, especially when the inherent temporal smoothing of spectral estimations is taken into account.
Fig. 4.
Context effect from sentence onset. The theta-context effect and 95% CI for the group level (19 contacts with significant effects in the picture-locked analyses). Gray area indicates the significant interval (from cluster-based permutation tests on the group-level, P < 0.001 at an α-level of 0.05). Trial events are shown at the bottom right. The timing of each word (w) position is indicated by the continuous lines. The left end of each line indicates the earliest possible word onset. The right end indicates the latest possible word offset (and next word onset). Median word onset (and previous word offset) is indicated by the dark red vertical bars. Dashed lines for word3 and word4 indicate that the words lasted beyond the time point shown in the figure. Constr, constrained; unconstr, unconstrained.
Fig. S3.
Context effect from sentence onset. Sentence-locked normalized theta-power changes from baseline (BL) in constrained and unconstrained contexts for one bipolar contact of patient P1 and P11. Shaded areas indicate the SEM. Gray areas indicate the significant intervals (from cluster-based permutation tests Bonferroni-corrected for the number of contacts tested). Trial events are shown at the bottom right. The timing of each word (w) position is indicated by the continuous lines. The left end of each line indicates the earliest possible word onset. The right end indicates the latest possible word offset (and next word onset). Median word onset (and previous word offset) is indicated by the orange vertical bars. Dashed lines for word3 and word4 indicate that the words lasted beyond the time point shown in the figure.
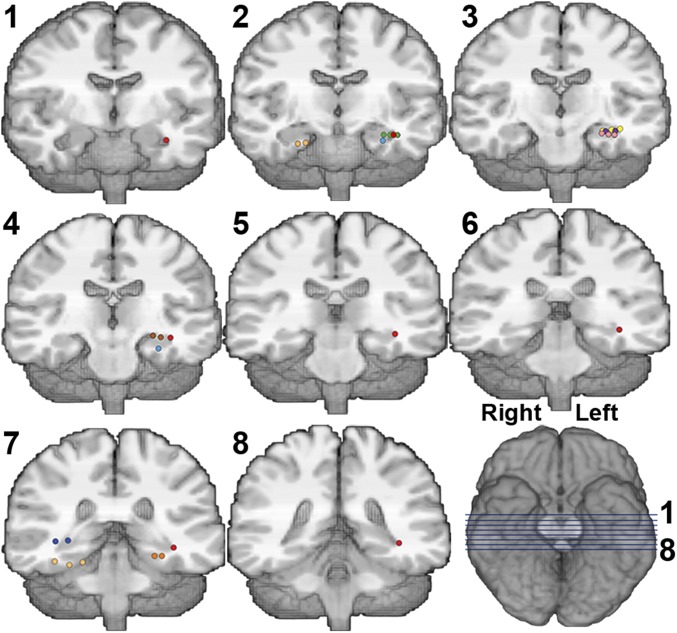
Fig. 5 indicates the bipolar contacts showing significant theta-power increases (in left and right hippocampal complexes). We found the context theta effect not only in the hippocampus proper (74% of the significant contacts), but also in the hippocampal complex, including the parahippocampal gyrus (seven available, three with a theta effect), and entorhinal cortex (two available, two with an effect) (Table 1), with each region showing a similar spectro-temporal profile (Fig. S1).
Fig. 5.
Anatomical locations of bipolar contacts with significant contextual theta-power increases. Each color represents one participant, after normalization to the Montreal Neurological Institute template (anatomical localization was performed on the patients’ original scans). Each bipolar contact consists of two immediately adjacent monopolar contacts, indicated as colored dots in this figure. Note that contacts can be adjacent in any spatial orientation. Location of each coronal slice (numbered 1–8) is indicated by blue lines Right Bottom.
theta-Power Increases with Strong Semantic Associations.
When processing sentences, listeners use syntactic (i.e., grammatical; for example, a noun follows a determiner) and semantic information. To elucidate the functional role of the theta oscillations in our study, we examined a syntactic measure (i.e., syntactic probability) (18) and a semantic measure (i.e., latent semantic analysis, LSA) (19) of our context manipulation. Syntactic probability of the words did not differ between the two conditions (P = 0.343). Thus, syntactic processing cannot explain the theta-context effect.
For the semantic-processing measure, we calculated the strength between each word in the sentence and the picture name using LSA. LSA provides a statistical measure of the semantic relationship between words. Associative strength was stronger for constrained than unconstrained contexts from the second word onward (Ps < 0.034) (see Analysis of Semantic Associations, below). The word with the largest semantic contribution to defining the sentence context was determined as the word that had the strongest LSA association with the picture name.
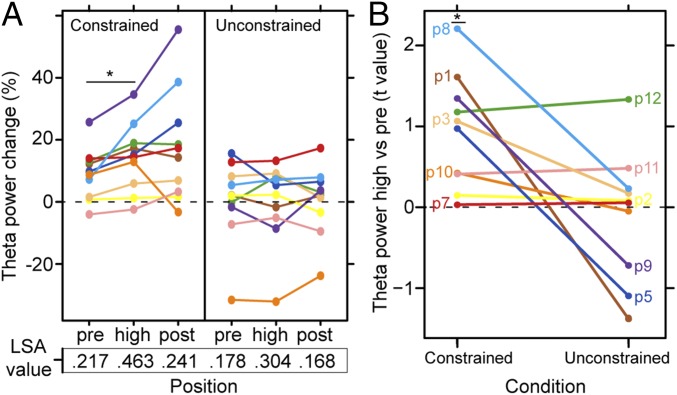
The theta power for the context-defining words are shown in Fig. 6A, as well as theta power to the preceding and following word. The average LSA association values are shown in the bottom part of Fig. 6A. The defining context theta-power increases were more robust in the constrained than in the unconstrained condition (group-level Wilcoxon signed-rank z = 1.99, P = 0.047). For the constrained condition, all patients showed increased theta power at the context-defining words relative to the preceding word, shown in Fig. 6B (group-level Wilcoxon signed-rank test z = 2.803, P = 0.005). Note that for all but one patient, theta power was already increased relative to baseline so the theta-power increases at the context-defining word consist of a further increase in the ongoing theta oscillations. For the unconstrained condition, this pattern was not observed (group-level Wilcoxon signed-rank z = 0.153, P = 0.879). There was no evidence for theta-power differences between the context-defining word and the following word (group-level Wilcoxon signed-rank z = −1.27, P = 0.203).
Fig. 6.
Semantic-association theta power. (A) theta-Power change from baseline for one bipolar contact per patient at the context-defining word (i.e., highest semantic association, high), the preceding word (pre), and the following word (post). The LSA strength for each word with the target picture name is shown below. (B) Participants’ t-values for the comparison between theta power at the context-defining word (“high” in A) and the previous word (“pre” in A) for one bipolar contact per patient. Positive values indicate that theta power increases at the context-defining word. An asterisk indicates that the power changes were significant at P = 0.005.
Discussion
Our study demonstrates that the same neuronal computations used by the hippocampus for memory function, as measured through theta-oscillatory activity, also subserve online language use. Specifically, theta power in the human hippocampal complex increased with the establishment of a meaningful context in a sentence. These theta effects were observed well before picture presentation, providing evidence that the hippocampal complex is actively involved in language processing.
We tested the relationship between theta power and semantic, association-governed processing. Specifically, association strength of the context-defining words differed between the two conditions and theta power increased in all patients at the context-defining words relative to the preceding word in the constrained condition. Regarding rule-governed processing, syntactic probabilities of the words were matched between the two conditions. Thus, rule-governed processing cannot explain the theta-context effect.
Our results are in line with the hippocampal literature related to retrieval of associative memories. When correctly recollecting the context in which an item has been learned (i.e., a specific association), hippocampal theta-power increases are observed (20). Recent human lesion evidence also supports a role for the hippocampal-complex structures in retrieving associations in the domain of semantic memory. Patients with bilateral hippocampal-complex damage showed impairment in semantic-association tasks testing words learned before the brain damage (21). These lesion findings implicate the hippocampal structures in maintaining and strengthening existing associations between words learned through life. Together with this neuropsychological evidence, our physiological findings of increased theta power for sentences with stronger semantic associations indicate that the hippocampal complex contributes to language in an active fashion, relating incoming words to stored semantic knowledge.
Hippocampal theta power also increases during episodic memory retrieval (22). For our sentences, the constrained contexts allow the picture name to be retrieved in an anticipatory fashion, which could support the interpretation that the theta-power increases reflect retrieval from memory (12). Under this retrieval account, the theta-power increases would reflect a lexical retrieval instance (i.e., of the picture name) and be briefly present. Contrary to this retrieval account, however, we found that the theta-power increases were sustained before picture presentation. More importantly, it usually takes speakers about 0.4 s to articulate a word after retrieving it (1). If the theta-power increases during sentence processing reflect the retrieval of the picture name per se, picture-naming response latencies should be relatively short (see ref. 1). Patients’ response times were generally longer than 0.7 s and the theta effect was observed in all 10 patients, regardless of their naming speed. Finally, neither theta power at the context-defining word nor at the following word predicted picture-naming times on the group level (z = −0.87, Ps = 0.386). Thus, the theta-power increases are best explained by the active, ongoing relational processing of the incoming words to stored semantic knowledge.
Hippocampal theta power also increases with working-memory demands (23). Working-memory demands are lower when items maintained in working memory have a stronger associative relation (24, 25). Following this reasoning, working-memory demands were likely lower in the constrained contexts because of the strong associations between the words in the sentence. This fact would predict theta-power decreases for constrained relative to unconstrained contexts, contrary to what we observed. Thus, it is unlikely that the theta-power increases reflect increased working-memory load.
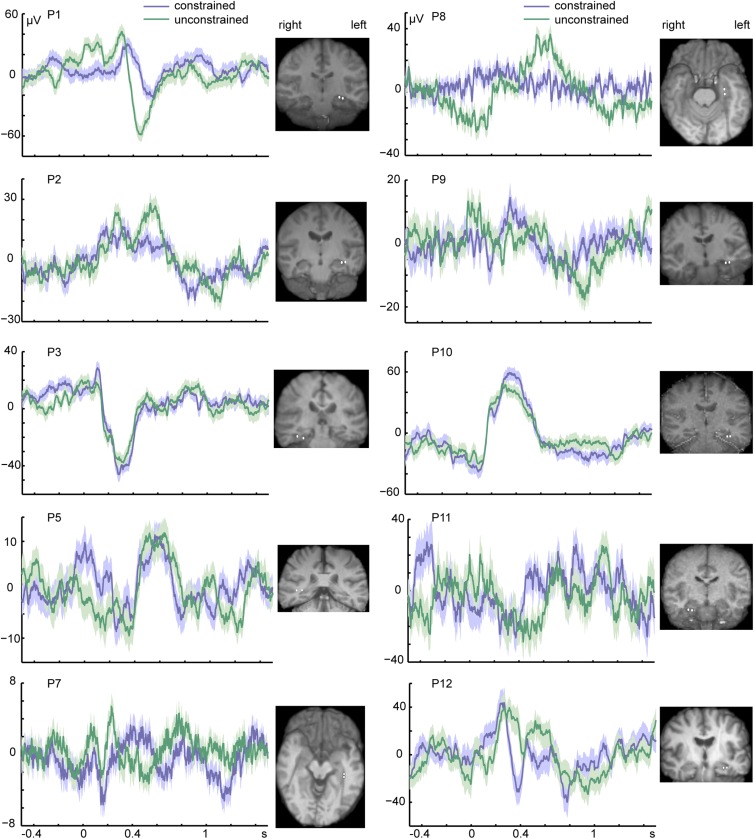
To date, no study has related hippocampal theta oscillations to language function. Previous studies have found a negative event-related potential (ERP), the N400 (26), in the medial temporal lobe for semantically and syntactically anomalous sentences (27, 28). However, these ERP findings came from an aggregate signal of low- and high-frequency bands. Furthermore, these studies only reported N400 modulations to the last word of the sentence, which could be a correct ending for the sentence or a word that rendered the sentence syntactically or semantically incorrect. In contrast, in our study, theta modulations occurred through the course of the sentence, before picture presentation. Fig. S4 shows the ERPs of each patient and the corresponding bipolar contacts. We did not find a significant difference in the N400 amplitude as a function of context [mean constrained = 4.59 µV, mean unconstrained = 1.64 µV, t(9) = 0.445, P = 0.669, 95% CI = (−12.059, 17.967)].
Fig. S4.
Event-related potentials. Event-related potentials time-locked to the picture (the 0-s time point) from the bipolar contact showing the strongest theta effect (i.e., largest t-values and widest frequency band) of each participant. Shaded areas around the waveforms indicate the SEM. The location of the bipolar contacts is shown for each patient as two white circles.
Previous studies have demonstrated hippocampal theta involvement in the predictive processing of sequences using mismatch paradigms (29, 30). Importantly, the contextual information in our study was not experimentally induced, but rather derived from knowledge acquired through life. Moreover, in contrast to previous studies of hippocampal function using violation paradigms (29, 31), our hippocampal theta was modulated by the processing of naturalistic sentences. The theta effect was observed during active processing, rather than reactive to an unexpected stimulus.
Previous theta-oscillatory effects related to language have been found over cortical areas (32) and are argued to support lexical-semantic retrieval processes (33). We cannot directly relate our hippocampal findings to the cortical theta oscillations with the present study. In the episodic memory literature, theta oscillations are thought to dynamically connect the hippocampus with neocortical regions, binding memory representations that are distributed over the cortex. For language, we can speculate that the hippocampus provides the associative links between different lexical-semantic representations in cortical areas, such as the left lateral temporal lobe (1, 2).
In support of accumulating lesion evidence on the role of the hippocampus in language (3–5, 21), our findings point to a neuronal mechanism that is used by the brain in the service of memory and language. This finding constitutes an important step in bridging the gap between the two fields. On the one hand, language researchers could use memory models to constrain theorizing and guide experimentation in language research. On the other hand, memory researchers could benefit from the experimental materials that language has to offer. As an example, in the present study the associative information was not created within an experimental setting, but rather existed already in the participants’ memory system.
Conclusions
This study reports neurophysiological evidence for the theory that the hippocampus plays an important role in language (3). We demonstrate that the same neuronal computations used by the hippocampus for memory function, as measured through theta oscillations, also subserve online language use. Our findings specify that the hippocampal complex contributes to language in an active, ongoing fashion, relating incoming words to stored semantic knowledge, a necessary process in the generation of sentence meaning.
Methods
Approval.
The study protocol was approved by the Office for the Protection of Human Subjects of the University of California, Berkeley. All patients gave written informed consent before participating.
Participants.
Twelve participants (six female, mean age = 39 y, SD = 12, range = 25–69 y) were implanted stereotactically with depth electrodes to localize the seizure onset zone for subsequent surgical resection. The electrodes were placed at the University of California, Irvine Medical Center (11 patients) or at Stanford University, Medical Center (1 patient) with 5-mm interelectrode spacing. All participants had normal hearing and normal vision. Only two participants had an IQ lower than 80 (P5 and P6) but they performed the task without any difficulty and within the range of the other participants. No seizures occurred during task administration. Only contacts in the hippocampal complex of the nonepileptic hemisphere were included for analysis. Furthermore, these contacts were inspected by two independent neurologists to confirm that none presented with interictal epileptiform activity.
Materials.
Fifty-one words were chosen along with color images depicting each word. Each word was paired with two sentences for which the target word was the last word of the sentence, presented as a picture. Following the sentence, either no specific word was expected as the final word of the sentence (unconstrained condition) or the target word was highly expected (constrained condition). All 102 sentences had six syllables and were between four and six words. For each target picture, the associated sentences had the same two last words (e.g., “She locked the door with the,” “She came in here with the”). More information on the materials is reported in SI Methods. A pretest confirmed that the sentences in the two conditions differed in the degree of expectancy for the final word, P < 0.001 (reported in SI Methods).
Procedure.
Stimulus presentation was controlled by Presentation (Neurobehavioral Systems).
Participants were instructed to listen to the sentences attentively and name the pictures as soon as they appeared on the screen. The sentences were presented via stereo loudspeakers, after participants confirmed that the volume was optimal for comprehension. A trial began with a white fixation cross on a black background, displayed continuously during sentence presentation. After 1 s, the sentence was presented auditorily. After sentence offset, the fixation cross remained on the screen for another 0.5 s before the picture was displayed for 1.5 s. A black screen was then presented for an interval varying between 0.8 and 1.5 s. One unique randomized list of materials was used for each participant.
Behavioral Analysis.
The experimenter monitored naming responses online. Trials with disfluent responses, omissions, or unrelated responses were excluded from analysis. A microphone connected to the computer recorded the vocal responses, which were manually analyzed using Praat (34) for the detection of speech onset before trials were split into two conditions. Thus, when determining speech onset, the experimenter was blind to the condition label of each trial. Response times were mean-averaged for each participant and condition, and tested on the group-level for the effect of context using a paired-sample t test at an α level of 0.05.
EEG Data Collection, Preprocessing, and Statistical Testing.
Intracranial EEG data were acquired using the Nihon Khoden recording system, analog-filtered above 0.01 Hz, and digitally sampled at 5 or 10 KHz, depending on the patient.
The loudspeakers and a photodiode were recorded as analog channels to mark the beginning of the sentences and the presentation of the picture, respectively. A neurologist selected the contacts that were in the hippocampal complex for analysis: that is, hippocampus proper, parahippocampal gyrus, subiculum, entorhinal and perirhinal cortex.
All EEG analyses were run in Matlab 2014a using in-house scripts, EEGLAB (35), and Fieldtrip (36). Offline, the EEG of these contacts was detrended, high-pass–filtered at 0.5 Hz using a zero-phase delay finite impulse response filter of order 10,000 with Hamming window (fir1 in Matlab), and then down-sampled to 1 Khz using Matlab’s resample() function. All contacts were then rereferenced to their adjacent contact, yielding bipolar contacts of the hippocampal complex. A multitaper regression method (37) was used to filter 60-Hz line power noise and harmonics.
Artifact rejection was performed over raw data segments in the following way. A baseline segment was created from the 500-ms preceding sentence onset. Picture-locked segments of 500 ms each were created between 4 s preceding picture presentation to 1 s following picture onset. Artifact rejection was then performed for each of the 12 segments of each trial. First, using the raw signal, any trial segment with a data point exceeding 5.8 SDs from the mean of all other trial segments at the same time point was excluded. Next, to look for fast changes in the signal, adjacent time points were subtracted in a sequential fashion, and trial segments with any points exceeding 8 SD were excluded. These thresholds were chosen to remove segments with outlier data points while keeping as many trials as possible (38). Trials with incorrect responses were also removed. Artifact- and error-free picture-locked segments summed to an average of 49 trials per condition per participant. Spectral decomposition was performed over the entire recording between 2 and 20 Hz with center frequencies logarithmically spaced and a fractional bandwidth of 25% of the center frequency. The band-passed signal was then transformed using the Hilbert transform to obtain the power envelope. The artifact-free picture-locked time-frequency segments were normalized against their baseline (−0.5 s presentence onset to sentence onset) by subtracting the baseline from the data and then dividing it by the sum of the data and baseline at each time point. This way of normalizing the data is less sensitive to differences in noise between baseline and the other segments.
Differences in power for the picture-locked segments as a function of sentence context were evaluated using a cluster-based permutation approach (39) for each bipolar contact and each patient separately. The largest cluster of adjacent time points and frequencies exhibiting a similar difference between the two experimental conditions was identified by means of dependent-samples t tests thresholded at an α level of 0.025 (two-tailed) for each patient and contact separately. A null distribution was estimated by randomly shuffling trials 1,000 times between conditions before averaging, followed by the same clustering procedure. Using a Monte Carlo method with 1,000 random permutations, P values of the observed clusters were calculated as the proportion of random partitions that yielded a larger effect than the observed experimental effect. Those P values were then Bonferroni-corrected for the number of hippocampal-complex contacts in each patient (see Table 1 for the number of contacts). We note that no reliable effects were found in higher frequencies and we did not observe any clear cross-frequency coupling effects between the theta- and high-γ bands.
Sentence-Locked Analyses.
From the significant theta range identified in the picture-locked analysis for each patient, a band-pass filter was created to filter the data, followed by the Hilbert transform, yielding single-trial theta-power time series. Segments were created between −0.5 and 2 s relative to sentence onset. The temporal smearing around the center time point was on average around 255 ms. Baseline correction, artifact rejection, and statistical analyses were performed as reported for the picture-locked segments.
Analysis of Semantic Associations.
We investigated how the context manipulation related to a syntactic and a semantic measure. For the syntactic measure, we calculated the syntactic probability of each word in the sentence (18). There was no difference in syntactic probability of the words in the two conditions [t(542) = −0.959, P = 0.343], thus the context effect is not related to syntactic probability of the words. For the semantic measure, we calculated the strength between each word in the sentence (first to fourth word because all sentences had at least four words and some sentences had only four words) and the picture name using latent semantic analysis (19) (lsa.colorado.edu/). The first word of the sentences did not differ in strength with the picture name as a function of context [mean constrained = 0.236, mean unconstrained = 0.227, t(50) = 0.421, P = 0.675]. In contrast, the second, third, and fourth words of the sentences were more strongly related to the picture name in the constrained than in the unconstrained condition [second: mean constrained = 0.317, mean unconstrained = 0.178, t(50) = 4.768, P < 0.001; third: mean constrained = 0.288, mean unconstrained = 0.193, t(50) = 3.041, P = 0.004; fourth: mean constrained = 0.298, mean unconstrained = 0.237, t(50) = 2.178, P = 0.034]. For each sentence, the context-defining word was determined as the word that had the strongest LSA association with the picture name. Then, for each patient, one bipolar contact was chosen as the contact with the strongest theta effect (i.e., largest t-values and widest frequency band) in the picture-locked analysis. From the significant theta range identified in the picture-locked analysis for each patient, a band-pass filter was created to filter the data of the bipolar contact time-locked to sentence onset, followed by the Hilbert transform, yielding single-trial theta-power time series. Baseline correction, artifact rejection, and statistical analyses were performed as reported above for the picture-locked segments. We then averaged the theta-power time series for each word of the sentences for the bipolar contact of each patient. On the participant level, theta power for the context-defining word was compared with theta power for the preceding word by means of a t test over trials. The resulting t value of each participant was taken as a measure of the direction and robustness of the theta-power changes. This is a more robust measure than using average theta power. To test whether theta power remained sustained after increasing, we compared theta power for the context-defining word to the following word in the same fashion. All patterns were considered reliable on the group level if all participants showed the same relationship between the two variables (i.e., all positive or negative t-values). This was tested with group-level Wilcoxon signed rank tests on the participants’ values against zero.
ERP Analysis.
For the ERP analysis, the raw, artifact-free signal was baseline-corrected by subtracting a 0.5-s interval preceding sentence onset from the picture-locked signal. For each patient, we then averaged the signal between 0.3- and 0.5-s postpicture onset from the bipolar contact showing the strongest theta effect (i.e., largest t-values and widest frequency band) for each condition separately. We tested the N400 amplitude as a function of context across patients with a paired-samples t test (two-tailed).
SI Methods
Materials.
The sentences were spoken by a female native speaker of American English and recorded with a sampling frequency of 44.1 KHz. The sentences were spoken at a regular pace, guided by a metronome set at 127 beats per minute (i.e., 2.11 syllables per second). The mean duration of the sentences was equal across conditions (constrained: mean = 2.8 s, SD = 0.10, range = 2.6–3.1; unconstrained: mean = 2.8 s, SD = 0.13, range = 2.6–3.1), paired-samples t(50) < 1. The sound files were normalized for volume using the speech waveform editor Praat (33). Prosody was similar across sentences and did not differ between conditions, all Ps > 0.05.
Degree of Expectancy for the Final Word (i.e., Cloze Probability).
Fourteen participants were presented with the sentences up to, but excluding, the last word of the sentence. Cloze probability was calculated as the proportion of participants who used the target picture-name as their completion. Our target words had a mean cloze probability of 0.83 for the constrained sentences and of 0.04 for the unconstrained sentences. The sentences in the unconstrained condition did not have a high cloze probability for any word. The two conditions differed in the degree of expectancy for the final word, t(50) = 45.928, P < 0.001.
Semantic Associations from Behavioral Data.
We collected predictability measures for each word in the sentence given the ongoing context in the following way. Participants (n = 7) performed a sentence-completion task. For each sentence, participants were given the first word of the sentence and asked to complete the sentence as they felt appropriate. Then, for a same sentence, the first and second words were presented for completion. Next, the first, second, and third words were presented for completion, and so on. From these results, we computed the probability of the last word being completed as well as the probability of the next content word (i.e., a noun, adjective, or verb) being completed at all points in the sentence for the constrained condition.
We found that for 14% of our sentences, half of the participants completed the sentence with the same words as in our materials upon seeing the second word of the sentence (starting on average at 0.37 s from sentence onset and at −3.2 s from picture onset). Upon seeing the third word (starting on average at 0.94 s from sentence onset and −2.8 s from picture onset), half of the participants completed 35% of the remaining sentences with the same words as in our materials. Upon seeing the fourth word (starting on average at 1.6 s from sentence onset and at −2.2 s from picture onset), 67% of the remaining sentences were completed with the same words as in our materials. The remaining sentences were completed at the fifth word (starting on average at 2.2 s from sentence onset and at −1.4 s from picture onset).
Hemispheric Lateralization.
We examined whether the probability of having a theta-context effect in the left hippocampal-complex was higher than in the right by using a mixed-effect logistic regression with hemisphere as a fixed effect and random intercept for participants. There was a descriptive difference between hemispheres in that 56% of the left contacts were significant versus only 21% of the right contacts. However, this difference was not significant on the group level, b = 1.996, SE = 1.211, z = 1.649, P = 0.0992.
Acknowledgments
We thank the patients and their families for their participation; Kristoffer Dahlslätt and Anna Jafarpour for invaluable discussions; Selvi Paulraj and Amber Moncrief for help with the materials; Laura Agee and Paige Mumford for help with audio recordings; Victor Kuperman for assistance with the syntactic probability measures; the members of the R.T.K. laboratory for help with data collection; and Juliana Baldo, Krista Schendel Parker, and Janet Patterson for feedback on the text. This work is supported by grants from the Netherlands Organization for Scientific Research (446-13-009) and the National Institutes of Health [National Institute of Neurological Disorders and Stroke (NINDS) R37 NS21135, K23 NS060993, F32 NS082065, R01 NS078396]; a US Department of Veterans Affairs Clinical Sciences Research and Development Program Research Scientist award; the Stanford NeuroVentures Program; US National Science Foundation (BCS1358907); and the Nielsen Corporation. This article was also prepared within the framework of the Basic Research Program at the National Research University Higher School of Economics (HSE) and supported within the framework of a subsidy by the Russian Academic Excellence Project “5-100.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.C. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603312113/-/DCSupplemental.
References
- 1.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front Hum Neurosci. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKay DG, Burke DM, Stewart R. H.M.’s language production deficits: Implications for relations between memory semantic binding and the hippocampal system. J Mem Lang. 1998;38:28–69. [Google Scholar]
- 5.MacKay DG, Stewart R, Burke DM. H.M. revisited: Relations between language comprehension, memory, and the hippocampal system. J Cogn Neurosci. 1998;10(3):377–394. doi: 10.1162/089892998562807. [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 7.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 8.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs J. Hippocampal theta oscillations are slower in humans than in rodents: Implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2013;369(1635):20130304. doi: 10.1098/rstb.2013.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watrous AJ, Fell J, Ekstrom AD, Axmacher N. More than spikes: Common oscillatory mechanisms for content specific neural representations during perception and memory. Curr Opin Neurobiol. 2015;31:33–39. doi: 10.1016/j.conb.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin ZM, Bock KJ. Constraint word frequency and the relationship between lexical processing levels in spoken word production. J Mem Lang. 1998;38:313–338. [Google Scholar]
- 12.Piai V, Roelofs A, Maris E. Oscillatory brain responses in spoken word production reflect lexical frequency and sentential constraint. Neuropsychologia. 2014;53:146–156. doi: 10.1016/j.neuropsychologia.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Smith NJ, Levy R. The effect of word predictability on reading time is logarithmic. Cognition. 2013;128(3):302–319. doi: 10.1016/j.cognition.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins A, Loftus E. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82(6):407–428. [Google Scholar]
- 15.McRae K, de Sa VR, Seidenberg MS. On the nature and scope of featural representations of word meaning. J Exp Psychol Gen. 1997;126(2):99–130. doi: 10.1037//0096-3445.126.2.99. [DOI] [PubMed] [Google Scholar]
- 16.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Yushkevich PA, et al. Hippocampal Subfields Group (HSG) Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. Neuroimage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boston MF. 2012. A computational model of cognitive constraints in syntactic locality. PhD dissertation (Cornell University, Ithaca, NY)
- 19.Landauer TK, Foltz PW, Laham D. Introduction to latent semantic analysis. Discourse Process. 1998;25:259–284. [Google Scholar]
- 20.Guderian S, Düzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15(7):901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- 21.Klooster NB, Duff MC. Remote semantic memory is impoverished in hippocampal amnesia. Neuropsychologia. 2015;79(Pt A):42–52. doi: 10.1016/j.neuropsychologia.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22(4):748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 23.Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA. 2000;97(2):919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deese J. Influence of inter-item associative strength upon immediate free recall. Psychol Rep. 1959;5:305–312. doi: 10.2466/pr0.1965.16.2.451. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins JJ, Mink WD, Russell WA. Associative clustering as a function of verbal association strength. Psychol Rep. 1958;4:127–136. [Google Scholar]
- 26.Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. J Neurosci. 1995;15(2):1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer P, et al. Language processing within the human medial temporal lobe. Hippocampus. 2005;15(4):451–459. doi: 10.1002/hipo.20070. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, et al. Human hippocampal increases in low-frequency power during associative prediction violations. Neuropsychologia. 2013;51(12):2344–2351. doi: 10.1016/j.neuropsychologia.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrido MI, Barnes GR, Kumaran D, Maguire EA, Dolan RJ. Ventromedial prefrontal cortex drives hippocampal theta oscillations induced by mismatch computations. Neuroimage. 2015;120:362–370. doi: 10.1016/j.neuroimage.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383(6597):256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 32.Dikker S, Pylkkänen L. Predicting language: MEG evidence for lexical preactivation. Brain Lang. 2012;127(1):55–64. doi: 10.1016/j.bandl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Bastiaansen MCM, Oostenveld R, Jensen O, Hagoort P. I see what you mean: Theta power increases are involved in the retrieval of lexical semantic information. Brain Lang. 2008;106(1):15–28. doi: 10.1016/j.bandl.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Boersma P, Weenink D. 2013 Praat: Doing phonetics by computer (version 5.4) Available at www.fon.um.uva.nl/praat. Accessed October 12, 2014.
- 35.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen T. 2012 Cleanline. Available at: www.nitrc.org/projects/cleanline/. Accessed November 1, 2014.
- 38.Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20(7):1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- 39.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]