Significance
Cellulose is an abundant natural polymer synthesized primarily by vascular plants in which it forms the load-bearing component of the cell wall. It is a linear polymer of glucose molecules synthesized by membrane-embedded cellulose synthases that couple polymer synthesis with its secretion across the plasma membrane. Plants express multiple cellulose synthase isoforms that are organized into large macromolecular assemblies of varying composition that are likely responsible for aligning the cellulose strands into microfibrils. Here we show that recombinantly expressed and purified Populus tremula x tremuloides (hybrid aspen) cellulose synthase-8 is sufficient for cellulose biosynthesis and produces cellulose microfibrils in vitro. Our results demonstrate that no other plant-derived factors are required for cellulose microfibril biosynthesis.
Keywords: biopolymer, cellulose, glycosyltransferase, plant cell wall, membrane transport
Abstract
Plant cell walls are a composite material of polysaccharides, proteins, and other noncarbohydrate polymers. In the majority of plant tissues, the most abundant polysaccharide is cellulose, a linear polymer of glucose molecules. As the load-bearing component of the cell wall, individual cellulose chains are frequently bundled into micro and macrofibrils and are wrapped around the cell. Cellulose is synthesized by membrane-integrated and processive glycosyltransferases that polymerize UDP-activated glucose and secrete the nascent polymer through a channel formed by their own transmembrane regions. Plants express several different cellulose synthase isoforms during primary and secondary cell wall formation; however, so far, none has been functionally reconstituted in vitro for detailed biochemical analyses. Here we report the heterologous expression, purification, and functional reconstitution of Populus tremula x tremuloides CesA8 (PttCesA8), implicated in secondary cell wall formation. The recombinant enzyme polymerizes UDP-activated glucose to cellulose, as determined by enzyme degradation, permethylation glycosyl linkage analysis, electron microscopy, and mutagenesis studies. Catalytic activity is dependent on the presence of a lipid bilayer environment and divalent manganese cations. Further, electron microscopy analyses reveal that PttCesA8 produces cellulose fibers several micrometers long that occasionally are capped by globular particles, likely representing PttCesA8 complexes. Deletion of the enzyme’s N-terminal RING-finger domain almost completely abolishes fiber formation but not cellulose biosynthetic activity. Our results demonstrate that reconstituted PttCesA8 is not only sufficient for cellulose biosynthesis in vitro but also suffices to bundle individual glucan chains into cellulose microfibrils.
Cellulose is an abundant biopolymer primarily produced by vascular plants and also by some bacteria, algae, and tunicates (1). It is a polymer of glucose molecules connected between their C1 and C4 carbons via β-glycosidic linkages. The unbranched polymer is further stabilized by intramolecular hydrogen bonds between the ring oxygen and C2 hydroxyl of one sugar and the C3 and C6 hydroxyl groups, respectively, of a neighboring sugar unit.
Cellulose is synthesized by cellulose synthase, CesA (1), a membrane-integrated processive family-2 glycosyltransferase that shares significant similarity with chitin, hyaluronan, and alginate synthases (2). CesA combines two functions: the enzyme polymerizes UDP-activated glucose (UDP-Glc) in an SN2-like nucleophilic displacement reaction (3) and translocates the growing cellulose polymer across the plasma membrane through a pore formed by its own transmembrane (TM) region. Depending on the tissue, the synthesized polymers can be of astonishing lengths, some containing tens of thousands of sugar units (1).
In most plant tissues, cellulose polymers are bundled into cable-like structures that are wrapped around the cell to form the load-bearing component of the cell wall (1). The smallest repeating unit, the cellulose microfibril, consists of a currently unknown [but most likely 18–24 (4, 5)] number of cellulose polymers. The microfibrils are stabilized by van der Waals interactions between the polymer’s glucopyranose rings and by hydrophilic intermolecular contacts mediated by the sugars’ equatorial hydroxyl groups.
The mechanism by which CesA synthesizes and secretes individual glucan chains has been delineated recently for bacterial cellulose synthase (6–8); however, the process by which plant CesAs bundle these glucan chains into cellulose microfibrils is currently unknown. An intriguing hypothesis is that the oligomeric state of CesAs in the plasma membrane dictates the spontaneous association of the extruded glucan chains into microfibrils (9, 10). Indeed, plant cellulose synthases have been shown to form large membrane-embedded complexes displaying pseudo-sixfold symmetry referred to as “CesA rosettes” (11, 12). In some species these rosettes are organized further into 2D arrays from which cellulose fibrils originate (13). Thus it seems likely that close proximity of CesAs during cellulose biosynthesis is sufficient for microfibril formation. This notion is supported by the observation that cellulose produced by monomeric bacterial cellulose synthase is amorphous, with no detectable higher-order structure (14).
Despite many years of study, cellulose biosynthesis has been reconstituted in vitro only recently from purified components from Rhodobacter sphaeroides (14). In these studies, the cellulose synthase was BcsA, which requires a membrane-anchored, periplasmic subunit (BcsB) for catalytic activity. Plant cellulose biosynthetic activity has been demonstrated in membrane extracts from several plant tissues (15–17), but none of the catalytically active synthases has been purified to date. Because of these difficulties, it was speculated that the plant enzymes also might require an additional, weakly associated factor for catalytic activity, similar to the BcsA–B complex in bacteria.
Plants produce several CesA isoforms; some are required for primary and others for secondary cell wall formation (1). To determine whether a single CesA isoform is catalytically active, we heterologously expressed and purified Populus tremula × tremuloides (hybrid aspen) CesA isoform 8 (PttCesA8), which is implicated in secondary cell wall formation, in Pichia pastoris. The enzyme was reconstituted into proteoliposomes where it synthesizes cellulose microfibrils in an UDP-Glc– and manganese-dependent manner, thereby demonstrating that a single CesA isoform suffices for cellulose biosynthesis and microfibril formation.
Results
Heterologous Expression and Purification of PttCesA8.
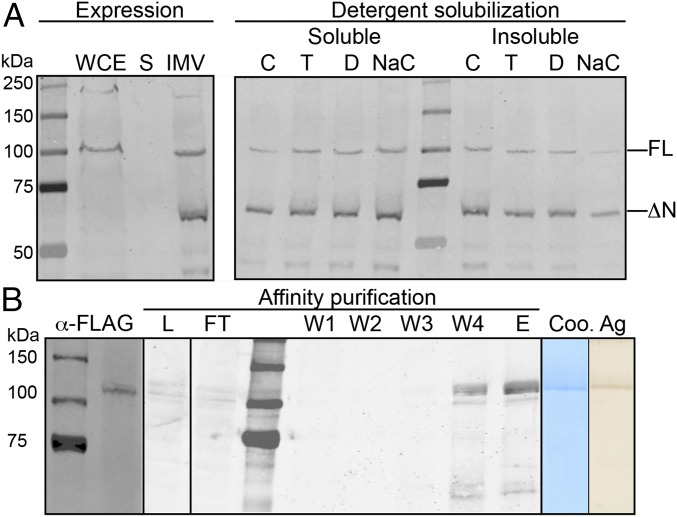
CesA is an integral membrane protein predicted to contain an N-terminal cytosolic domain preceding two TM helices, an intracellular glycosyltransferase domain, and five C-terminal TM helices (6). If this prediction is correct, the odd number of TM helices would place the enzyme’s N and C termini on opposing sides of the plasma membrane. To facilitate detection and purification of the recombinant enzyme, we engineered an N-terminal FLAG and C-terminal His-tag on PttCesA8 (Fig. S1). The construct was expressed in P. pastoris upon induction with methanol, leading to the formation of an immunoreactive protein species migrating near the 100-kDa marker on SDS/PAGE, consistent with the calculated PttCesA8 molecular mass of 110.5 kDa (Fig. 1A). Fractionation of the cell lysate into soluble and membrane fractions revealed that the recombinant protein is membrane-integrated, a prerequisite for proper PttCesA8 folding and function (Fig. 1A). Approximately 50% of the enzyme can be solubilized from the membrane in several nondenaturing detergents, including Triton X-100, dodecyl-maltoside (DDM), sodium cholate, and polyoxyethylene(8)dodecyl ether (C12E8). For all subsequent PttCesA8 purifications, DDM was used for solubilization and LysoFoscholine-Ether14 for purification (see Methods). However, membrane isolation and solubilization generated a smaller PttCesA8 species migrating at ∼60 kDa. This species was detectable only with an antibody directed against the C-terminal His-tag and not against the N-terminal FLAG-tag, suggesting that the N-terminal region of PttCesA8 is prone to degradation, likely because of conformational flexibility or loose association of the N-terminal RING-finger (Fig. 1B). Interestingly, cobalt-affinity chromatography primarily enriched the full-length PttCesA8 protein, perhaps because of weaker interactions with the affinity matrix or aggregation of the truncated species (Fig. 1B). Although SDS/PAGE of the affinity-purified fraction followed by Coomassie and silver staining revealed the presence of only one major protein species (Fig. 1B), we determined its total protein composition (arising from contaminating Pichia proteins) by tandem mass spectrometry fingerprinting (Table S1). Apart from a small number of peptides matching a Pichia β-1,3 glucan synthase, none of the contaminating proteins has any documented or predicted function in polysaccharide biosynthesis. PttCesA8 was represented by 61 unique peptides covering most of the enzyme’s hydrophilic regions.
Fig. S1.
Primary sequence of recombinantly expressed Populus tremula x tremuloides CesA8. The protein contains an N-terminal FLAG and HA tag, shown in blue and red, respectively, and a C-terminal dodeca-His tag (green).
Fig. 1.
Expression and purification of recombinant PttCesA8. (A, Left) Western blot analysis of C-terminally His-tagged PttCesA8 expressed in P. pastoris. IMV, inverted membrane vesicles; S, soluble fraction; WCE, whole-cell extract. (Right) Solubilization of IMVs with different nondenaturing detergents. C, C12E8; D, DDM; NaC, sodium cholate; T, Triton X-100. FL and ΔN: Full-length and truncated CesA8, respectively. (B) Cobalt-affinity purification of PttCesA8. (Left) Western blot against the N-terminal FLAG-tag of PttCesA8. (Right) Coomassie (Coo)- and silver (Ag)-stained SDS/PAGE of the eluted fraction. E, eluted fraction; FT, flow-through; L, load; W1–4, wash steps 1–4 with imidazole concentrations increasing from 20–60 mM.
Table S1.
P. pastoris proteins identified in the purified PttCesA8 preparation by MS-MS fingerprinting
| Unique | Total | Gene symbol | kDa | Function |
| 103 | 124 | FAS1 | 229.6 | Fatty acid synthase subunit beta, fungi type |
| 85 | 779 | PP7435_Chr3-0372 | 94.55 | Protein of unknown function involved in vacuolar protein sorting |
| 84 | 99 | PP7435_Chr1-1078 | 206.02 | Alpha subunit of fatty acid synthetase |
| 67 | 70 | PP7435_Chr1-1029 | 250.09 | Biotin carboxylase |
| 50 | 60 | AMD1 | 92.88 | AMP deaminase |
| 47 | 65 | PP7435_Chr1-0897 | 80.85 | Heat shock cognate protein HSP 90-beta |
| 45 | 77 | PP7435_Chr1-1209 | 76.64 | Phosphatidylinositol 4,5-bisphosphate effector protein SLM2 |
| 44 | 54 | CEF3 | 116.55 | Elongation factor EF-3 |
| 43 | 85 | PMA1 | 97.81 | Plasma membrane ATPase |
| 43 | 53 | PP7435_Chr4-0120 | 170.52 | ATP-dependent permease PDR15 |
| 43 | 47 | PP7435_Chr2-0436 | 133.47 | DIS3-like exonuclease 2 |
| 42 | 59 | EEF2 | 212.47 | Classical protein kinase C |
| 42 | 48 | GSL2 | 215.08 | 1,3-β-glucan synthase |
| 42 | 45 | Chc | 187.14 | Clathrin heavy chain |
| 41 | 59 | AOX1 | 73.85 | Alcohol oxidase 1 |
| 40 | 47 | PP7435_Chr1-0901 | 65.26 | Signal recognition particle subunit SRP68 |
| 38 | 110 | atpD | 53.99 | ATP synthase subunit beta |
| 35 | 92 | atpA | 58.73 | ATP synthase subunit alpha |
| 35 | 63 | PP7435_Chr2-0840 | 79.5 | NADH dehydrogenase (Ubiquinone) Fe-S protein 1 |
| 35 | 49 | PP7435_Chr3-0996 | 70.72 | Heat shock 70 kDa protein |
| 33 | 45 | CPNA | 73.9 | Heat shock protein 60 |
| 32 | 41 | NUC1 | 54.83 | NADH dehydrogenase (Ubiquinone) Fe-S protein 2 |
| 32 | 41 | SNF1 | 62.69 | Carbon catabolite-derepressing protein kinase |
| 32 | 41 | PP7435_Chr1-0413 | 79.17 | X-Pro aminopeptidase |
| 31 | 122 | RGI1 | 21.16 | Respiratory growth induced protein 1 |
| 30 | 79 | RPL3 | 43.64 | 60S ribosomal protein L3 |
| 30 | 53 | NUE1 | 85.35 | NADH dehydrogenase (Ubiquinone) 1 alpha subcomplex 9 |
| 30 | 30 | ARO1 | 169.95 | Penta functional AROM polypeptide |
“Unique” and “Total” refer to the number of peptides identified for each listed protein. Gene symbols refer to the identified Pichia genes in the MEROPS database (https://merops.sanger.ac.uk).
Recombinant PttCesA8 Synthesizes Cellulose.
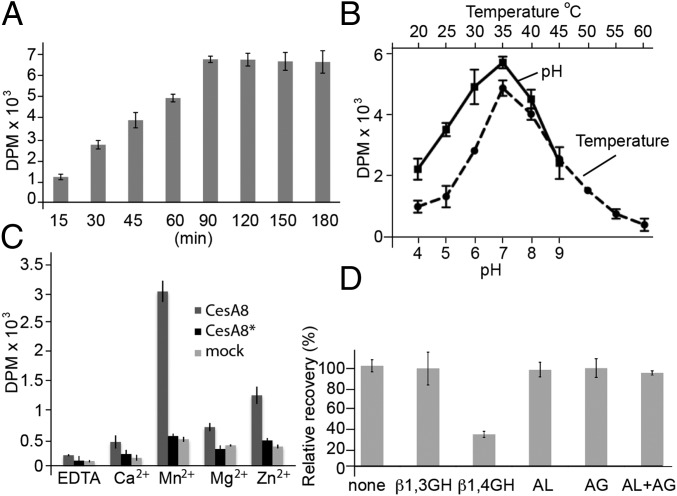
Following previous successes with bacterial cellulose synthase (14), we reconstituted PttCesA8 into Saccharomyces cerevisiae total lipid extract proteoliposomes and analyzed the enzyme’s catalytic activity in the presence of UDP-Glc, Mn2+, and UDP-[3H]-Glc as tracer. Because cellulose is water insoluble past a degree of polymerization of about 8 (18), insoluble reaction products were sedimented and further purified by descending paper chromatography as described for bacterial cellulose synthase (14). As shown in Fig. 2 A and B, PttCesA8 continued to form a water-insoluble glucan over 90 min at an optimal pH and temperature of 7.0 and 35 °C, respectively. The catalytic activity stalls after 90 min of incubation, likely because of product inhibition or cosedimentation of the synthase and polymeric product.
Fig. 2.
In vitro cellulose biosynthesis by reconstituted PttCesA8. (A) Time course of cellulose biosynthesis using UDP-[3H]-glucose as tracer. DPM, disintegrations per minute. (B and C) pH and temperature (B) and cation dependence (C) of PttCesA8’s catalytic activity. Synthesis reactions were performed in the presence of 20 mM of the indicated divalent cations. A catalytically inactive PttCesA8 mutant (CesA8*) and material obtained from a mock purification do not produce detectable glucan amounts. (D) Enzymatic degradation of the PttCesA8-synthesized glucan. All experiments were performed at least in triplicate; error bars represent the SDs from the means. AG, amyloglucosidase; AL, amylase; GH, glycoside hydrolase.
Processive and nonprocessive inverting glycosyltransferases require a divalent cation, often a magnesium or manganese ion, for catalytic activity (3). Accordingly, PttCesA8’s catalytic activity depends on the presence of Mn2+; no significant enzymatic activity was observed in the presence of Ca2+ or Mg2+, and only modest activity was seen in the presence of Zn2+ (Fig. 2C).
To confirm that the observed in vitro catalytic activity is indeed caused by recombinant PttCesA8 and not by a minor contamination with a Pichia protein, we generated a catalytically inactive PttCesA8 mutant. Cellulose biosynthesis requires a general base to deprotonate the acceptor’s hydroxyl group during glycosyl transfer (3, 7, 19). This base is formed by the Asp residue of an invariant TED motif, which we replaced in PttCesA8 with an Asn (D676N) to render the enzyme catalytically inactive. The PttCesA8 mutant (PttCesA8*) showed no significant cellulose biosynthetic activity, comparable to material obtained from a mock purification in which Pichia had been transformed with an empty pPICZA vector (Fig. 2C), confirming that the observed biosynthetic activity results from PttCesA8. Further, to establish that the in vitro-synthesized material indeed represents a β-1,4 glucan, the reaction product was hydrolyzed with glucanases specifically degrading β-1,3–, β-1,4–, or α-1,4–linked glucans. Consistent with the formation of authentic cellulose, the PttCesA8-produced material is degraded only by a β-1,4–specific glucanase, a cellulase (Fig. 2D).
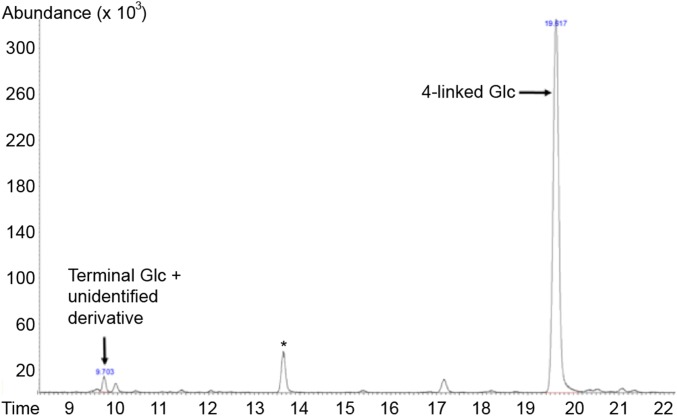
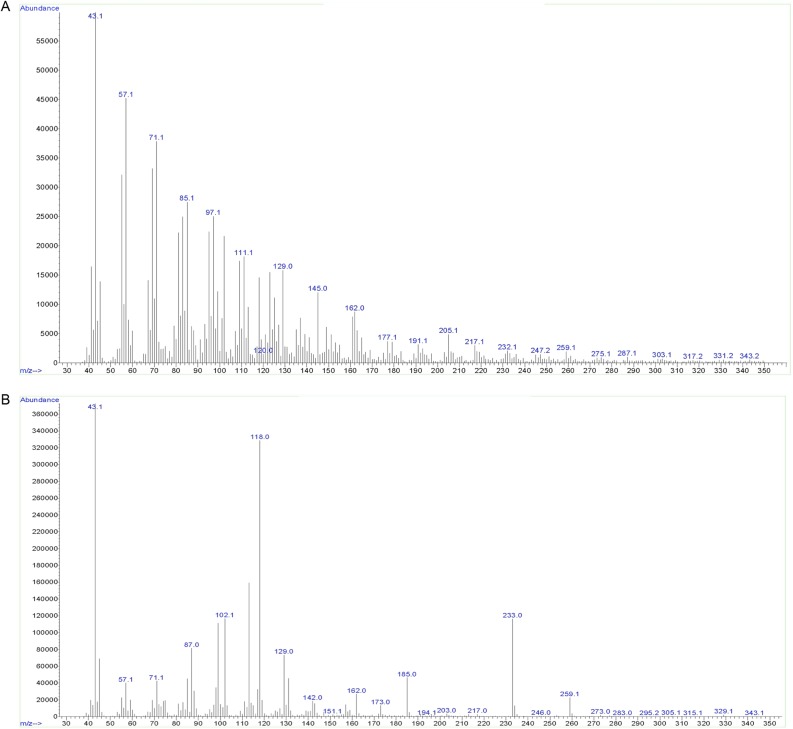
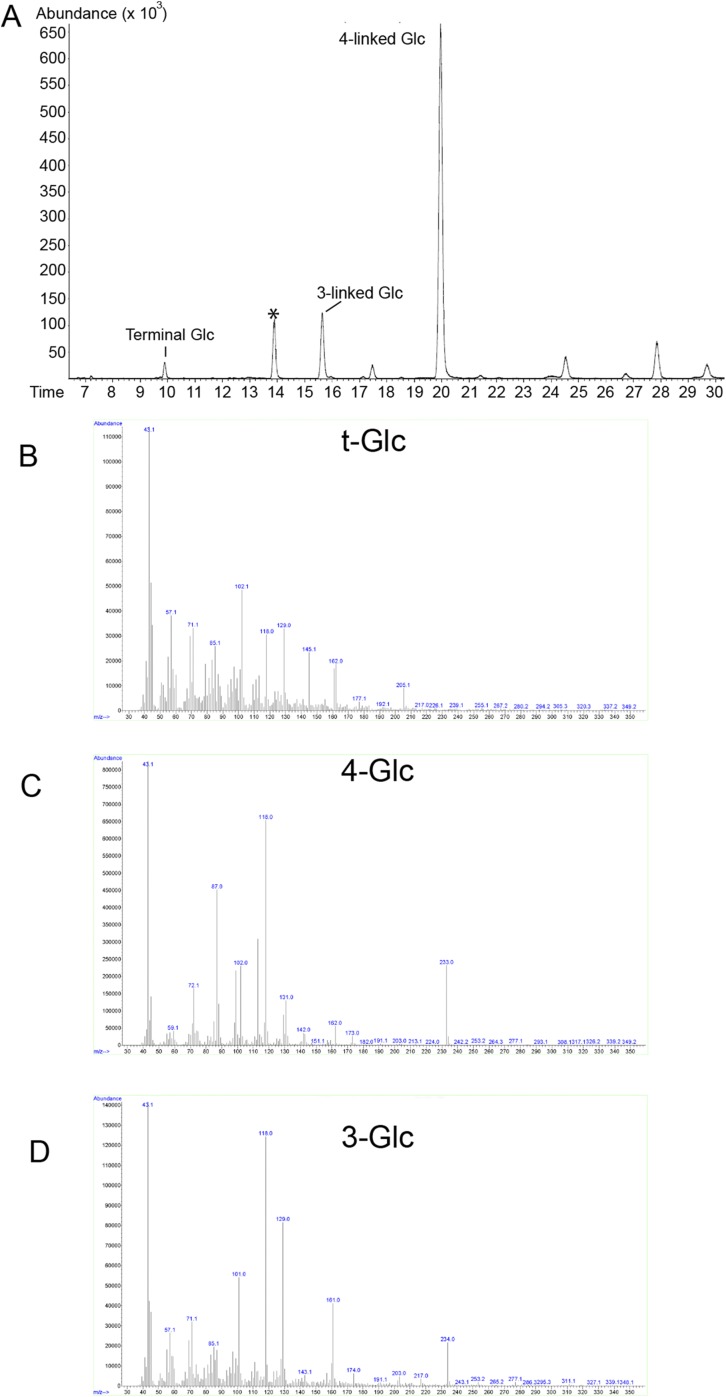
In addition to testing the polymer’s sensitivity to enzymatic degradation, we also characterized it by permethylation glycosyl linkage analysis. The product obtained from a 60-min synthesis reaction contained primarily 1,4-linked glucose together with a small amount of terminal glucose (Fig. 3 and Fig. S2). The identity of the derivatives was confirmed by electron-impact mass spectrometry (Fig. S2). However, the peak corresponding to terminal glucose also contained another unidentified compound that was not a sugar derivative. This compound prevents the calculation of an accurate degree of polymerization, because the fraction of the peak arising from terminal glucose cannot be determined accurately.
Fig. 3.
Glycosyl linkage analysis of PttCesA8-synthesized cellulose. Gas chromatogram corresponding to the separated permethylated alditol acetates. The identity of the derivatives corresponding to 4-linked and terminal glucose was confirmed by electron-impact mass spectrometry (Fig. S2). All other peaks of the chromatogram, except for the peak marked with an asterisk, correspond to unidentified derivatives that are not alditol acetates, as evidenced by the corresponding fragmentation data. The peak marked with an asterisk corresponds to 4-linked xylose, a known instrument contamination at the time of analysis.
Fig. S2.
Electron-impact MS fragmentation spectra of the permethylated alditol acetates corresponding to terminal glucose (A) and 4-linked glucose (B) arising from PttCesA8-synthesized cellulose. The fragmentation spectrum shown in A (GC retention time of 9.7 min) contains all the signals indicative of 1,5-di-O-acetyl, 2,3,4,6-tetra-O-methyl-d-glucitol, i.e., terminal Glc residues, but it also is contaminated by a rather high number of additional signals arising from an unidentified derivative. The fragmentation spectrum shown in B (GC retention time of 19.6 min) is characteristic of 1,4,5-tri-O-acetyl, 2,3,6-tri-O-methyl-d-glucitol, corresponding to 1,4-Glc.
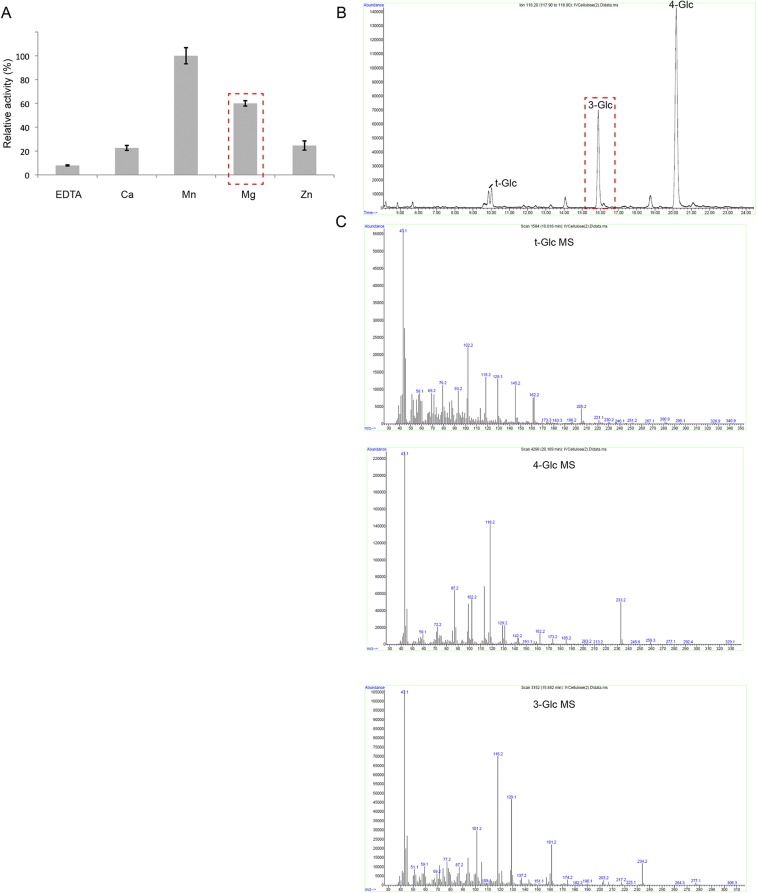
Some PttCesA8 preparations also showed minor product accumulation in the presence of Mg2+ in addition to the Mn2+-stimulated PttCesA8 activity (Fig. S3). In this case, glycosyl linkage analysis of the product also revealed the presence of a 1,3-linked glucan, most likely arising from a contaminating Mg2+-dependent β-1,3-glucan synthase and not from PttCesA8. As expected for a contamination, the presence of the β-1,3-glucan synthase activity varied among purifications, and we used only enzyme preparations exhibiting negligible catalytic activity in the presence of magnesium (Fig. 2C) for further analyses.
Fig. S3.
Glycosyl linkage analysis of glucans obtained from a PttCesA8 preparation contaminated with a putative P. pastoris 1,3-glucan synthase. (A) Cation dependence of glucan formation. The contaminated PttCesA8 preparation exhibits usually high activity in the presence of Mg2+, correlating with the formation of a 1,3–linked glucan detected in B. (B) Gas chromatogram corresponding to the separated permethylated alditols acetates. (C) Electron-impact mass spectrometry of the permethylated alditol acetates.
Kinetic Characterization, Product Inhibition, and Lipid Dependence.
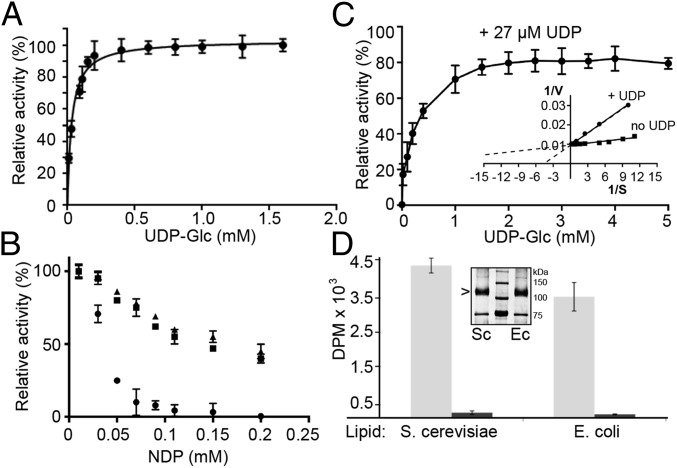
Cellulose synthase binds the substrate UDP-Glc via its cytosolic glycosyltransferase domain and transfers the activated glucose to the C4 hydroxyl of the nascent cellulose chain, thereby extending the cellulose polymer and forming UDP as a second reaction product (8, 14, 20). Monitoring PttCesA8’s catalytic activity under increasing UDP-Glc concentrations and at a constant Mn2+ concentration suggests monophasic Michaelis–Menten kinetics with an apparent Km of 30 µM (Fig. 4A). If we assume that k−1 is negligible compared with k2, Km can be assimilated to an affinity constant. This affinity compares to an apparent Km for UDP-Glc of about 500 μM for R. sphaeroides BcsA (14), perhaps reflecting different cytosolic UDP-Glc levels in plant and bacteria. Performing cellulose synthesis reactions at a constant UDP-Glc but increasing UDP concentrations reveals a profound inhibitory effect of UDP, with an apparent inhibitory constant (Ki) of 27 µM, matching the enzyme’s affinity (Km) for the substrate UDP-Glc (Fig. 4B). Other nucleoside diphosphates, such as ADP and GDP, do not inhibit PttCesA8, suggesting that UDP, a reaction product of PttCesA8, rebinds to the active site and inhibits the enzyme competitively. Indeed, UDP inhibition is overcome at a UDP-Glc concentration about 30 times above the Km, as expected for competitive inhibition (Fig. 4C). A similar product inhibition has been observed for bacterial cellulose and hyaluronan synthases and is in accordance with a conserved reaction mechanism of these processive family-2 glycosyltransferases (14, 21).
Fig. 4.
Kinetic analyses of PttCesA8. (A) Titration of UDP-Glc and quantification of the synthesized cellulose. The data were fit to monophasic Michaelis–Menten kinetics, yielding a Km of 30 µM. (B) Cellulose biosynthesis in the presence of 30 µM UDP-Glc and increasing concentrations of UDP (circles), ADP (triangles), and GDP (squares). UDP inhibits cellulose synthesis with a Ki of about 27 µM. (C) UDP competitively inhibits PttCesA8. The catalytic activity of PttCesA8 in the presence of 27 µM UDP and increasing UDP-Glc concentrations is shown relative to its activity in the absence of UDP. (Inset) Lineweaver Burk plot of data shown in A and C. (D) Detergent inhibition of PttCesA8’s catalytic activity. Comparison of PttCesA8’s catalytic activity in intact (light gray bars) and solubilized (dark gray bars) proteoliposomes formed from S. cerevisiae or E. coli total lipid extracts. (Inset) Western blot of S. cerevisiae (Sc) and E. coli (Ec) proteoliposomes. All experiments were performed at least in triplicate; error bars represent SDs from the means.
The heterologously expressed PttCesA8 was solubilized from Pichia membranes and purified in the detergent DDM. Several attempts failed to enrich the enzyme in a detergent-solubilized state by the product entrapment method used previously to isolate for example chitin synthases (22). The method relies on the catalytic activity of the synthase in a detergent-solubilized state, so that it cosediments with the synthesized water-insoluble polysaccharide. To test whether PttCesA8 is catalytically active in a detergent micelle, we solubilized PttCesA8-containing proteoliposomes with DDM and analyzed the enzyme’s catalytic activity according to our established protocol. As shown in Fig. 4D, PttCesA8 almost completely loses its catalytic activity upon detergent solubilization of the vesicles, suggesting that enzymatic activity requires an intact lipid bilayer. This loss of catalytic activity is in contrast to bacterial BcsA, which is catalytically active in several nondenaturing detergents (14). Lack of catalytic activity in detergents could be caused either by a particular sensitivity of the enzyme toward solubilizing detergents and/or by the disruption of quaternary structures required for proper function. Despite this dependence, the enzyme exhibits comparable activity in proteoliposomes formed from either S. cerevisiae or E. coli total lipid extracts (Fig. 4D), indicating that no eukaryote-specific lipid, unless copurifying with the enzyme, is required for catalytic activity.
PttCesA8’s N-Terminal Cytosolic Domain Is Dispensable for Catalytic Activity.
Primarily plant CesAs contain extended cytosolic N termini of about 170 residues that precede the first TM helix (Fig. 5A). The first ∼65 residues share significant homology with RING-finger domains, which are specialized Zn-binding domains coordinating two Zn2+ ions via a Cys-rich motif. The following ∼100 residues are less well conserved and have no known function (Fig. S4). Because RING-fingers are often implicated in mediating protein–protein interactions, in particular dimerization (23), and isolated CesA RING-fingers have been shown to form homo- and heterodimers in vitro (24), it is likely that these domains stabilize membrane-embedded CesA oligomers.
Fig. 5.
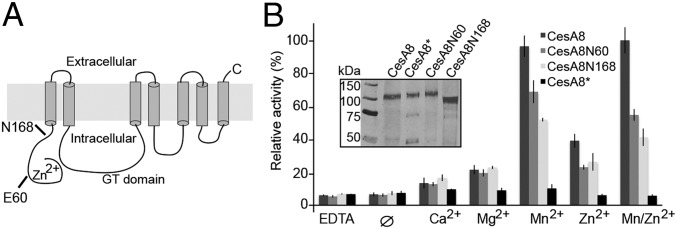
PttCesA8’s N terminus is dispensable for catalytic activity. (A) TM topology diagram of PttCesA8 as predicted by TOPCONS (41). The N termini of the generated PttCesA8 truncations are indicated. GT, glycosyltransferase. (B) Comparison of the catalytic activities of all PttCesA8 variants in the presence of 20 mM of the indicated divalent cations or EDTA. ∅ denotes reactions in the absence of additional cations or EDTA. (Inset) Western blot analysis of proteoliposomes containing the indicated PttCesA8 variants.
Fig. S4.
Sequence alignment of CesA N-terminal cytosolic regions. Sequences were aligned in CLUSTALW and colored according to sequence identity. The black dashed line and green cylinder indicate the predicted N-terminal RING-finger and first TM helix, respectively. The first residues of the N-terminally truncated PttCesA8 constructs used in this study are indicated by red arrows. At, Arabidopsis thaliana; Bd, Brachypodium distachyon; Gh, Gossypium hirsutum; Md, Micrasterias denticulata; Pp, Physcomitrella patens; Pt, Populus tremula x tremuloides.
To test whether PttCesA8’s N terminus is required for catalytic activity, we generated two N-terminally truncated mutants, one devoid of only the RING-finger (CesA8N60, starting with Glu60) and the other missing the entire N-terminal cytosolic domain (CesA8N168, starting with Asn168) (Fig. 5A and Fig. S4). Both mutants exhibit significant catalytic activity relative to the wild-type enzyme, albeit reduced by about 25 and 50% for CesA8N60 and CesA8N168, respectively (Fig. 5B). The lower activity of CesA8N168 likely results from improper folding and/or membrane integration, because this mutant expresses at a reduced level and is prone to proteolytic degradation. No significant change in catalytic activity was observed for any of the enzymes in the presence of both Mn2+ and Zn2+, as compared with Mn2+ only, suggesting that an intact and Zn2+-coordinating RING-finger does not directly affect CesAs catalytic activity. Further, permethylation linkage analysis of cellulose produced by PttCesA8N60 shows a ratio of terminal to internal glucose similar to that observed for the wild-type product (Fig. 3), indicating that the two enzymes produce cellulose polymers of similar lengths (Fig. S5). Although in both analyses the exact amount of terminal glucose is obscured by the presence of either unidentified or 1,3-glucan contaminations, the results suggest a degree of polymerization of at least 25–35 and most likely significantly higher (Fig. 3 and Fig. S5).
Fig. S5.
Glycosyl linkage analysis of glucans synthesized by PttCesA8N60. (A) A gas chromatogram corresponding to the separated permethylated alditol acetates. The asterisk denotes a peak corresponding to 4-linked xylose, a known instrument contamination at the time of analysis. (B–D) Electron-impact mass spectrometry of the permethylated alditol acetates corresponding to terminal (B), 4-linked (C), and 3-linked (D) Glc.
PttCesA8-Synthesized Cellulose Is Partially Resistant to Acid Hydrolysis.
Because of its organization into cellulose micro- and macrofibrils, native plant cellulose exhibits a remarkable resistance to acid hydrolysis. In particular, treatment of cellulosic tissues with Updegraff reagent [80% acetic acid and concentrated nitric acid at a 10:1 (vol:vol) ratio] (25) has been shown to hydrolyze noncellulosic cell wall materials efficiently as well as isolated glucan chains not organized into fibrils (16, 26). Hence, resistance to acid hydrolysis correlates with a higher-order organization of cellulose.
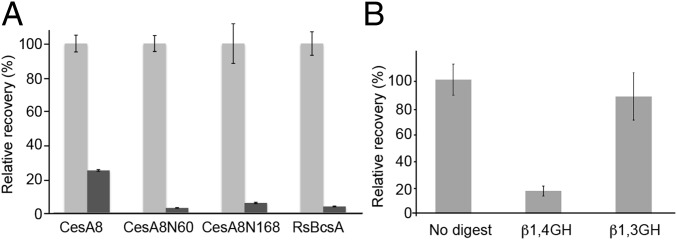
We compared the resistance to acid hydrolysis of PttCesA8 and bacterial BcsA-synthesized cellulose, the latter forming noncrystalline cellulose expected to be susceptible to acid hydrolysis. To this end, cellulose synthesized in vitro by PttCesA8 and BcsA was resuspended in Updegraff reagent and incubated for 20 min at 40 °C before quantification. Although the reagent completely hydrolyzed bacterial cellulose, ∼25% of the PttCesA8-synthesized cellulose was resistant to hydrolysis, suggesting a different organization, at least in part, for BcsA- and PttCesA8-produced cellulose (Fig. 6A). To confirm that the PttCesA8-produced material resistant to acid hydrolysis indeed represents cellulose, the remaining material was washed several times in deionized water and subsequently incubated with β-1,3– and β-1,4–specific glucanases of which only the cellulase was able to degrade the product (Fig. 6B).
Fig. 6.
PttCesA8-synthesized cellulose is partially acid resistant. (A) Quantification of cellulose resistant to acid hydrolysis. The amount of cellulose resistant to hydrolysis by 80% acetic acid and concentrated nitric acid [10:1 (vol:vol)] is shown relative to the initial cellulosic material produced by each cellulose synthase variant (dark and light gray bars, respectively). (B) Enzymatic degradation of the acid-resistant cellulose produced by PttCesA8. GH, glycoside hydrolase.
Interestingly, cellulose produced by PttCesA8 lacking either the N-terminal RING-finger (CesA8N60) or the entire N-terminal cytosolic domain (CesA8N168) was completely hydrolyzed by the Updegraff reagent, perhaps because of the formation of primarily noncrystalline cellulose (Fig. 6A).
PttCesA8 Forms Fiber-Like Cellulosic Structures.
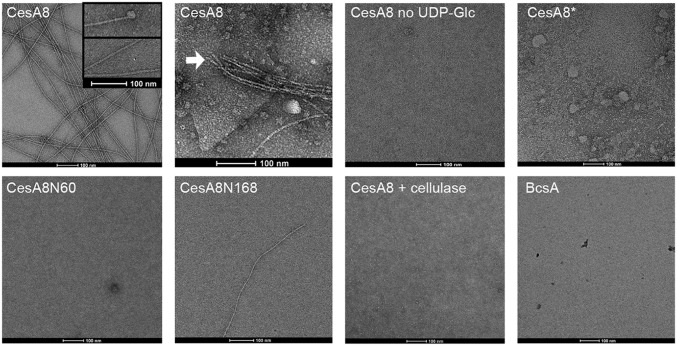
The partial resistance of PttCesA8-synthesized cellulose to acid hydrolysis (Fig. 6A), not observed for noncrystalline bacterial cellulose, is consistent with a higher-order organization of the glucan chains. To analyze the organization of the PttCesA8 cellulose product, we visualized the polymers by transmission electron microscopy (TEM) after negative staining. Cellulose was synthesized from proteoliposome-reconstituted PttCesA8, and the vesicles were solubilized in the detergent Triton X-100, after which the sample was diluted at least 100-fold before preparing EM grids. Proteoliposomes containing wild-type PttCesA8 produced fiber-like polymers several micrometers long only when incubated in the presence of substrate. Control reactions in the absence of UDP-Glc did not contain any polymeric material (Fig. 7). Some samples contained apparently incompletely solubilized membrane fragments, clearly visible by negative-stain EM, from which cellulose fibrils originated, suggesting that microfibril formation occurs directly after the release of the nascent glucans from their site of synthesis.
Fig. 7.
PttCesA8 produces cellulose microfibrils in vitro. Cellulose synthesized by reconstituted PttCesA8 was visualized by negative-stain EM. Shown are representative images of cellulosic material formed by the indicated PttCesA8 constructs. Cellulose microfibrils are seen and in some cases are associated with globular particles that may be cellulose synthase complexes. Cellulose microfibrils originating from a membrane sheet are indicated by a white arrow. Negative controls in the absence of UDP-Glc or with the inactive PttCesA8* mutant result in no fiber formation. Infrequent fiber formation is seen with the N-terminal truncation mutants. Treatment of the sample with cellulase for 10 min before grid preparation results in the loss of cellulose fibers. BcsA-produced cellulose was not detectable.
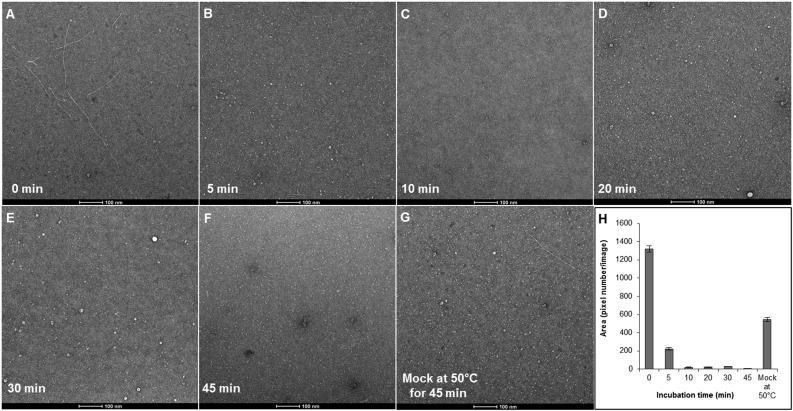
The apparent cellulose microfibrils were produced only by wild-type PttCesA8 and not by the catalytically inactive PttCesA8* mutant, confirming that fiber formation indeed is a result of PttCesA8’s catalytic activity (Fig. 7). Additionally, the synthesized fibers could be degraded with cellulase, resulting in almost complete loss of visible fibers within 10 min of incubation (Fig. 7 and Fig. S6).
Fig. S6.
Time-course of cellulose fiber degradation by cellulase. (A–F) In vitro-synthesized cellulose fibers were subjected to cellulase treatment using a mix of highly purified cellulases including Cel6A, Cel7A, and Cel7B in a time series of 0 (A), 5 (B), 10 (C), 20 (D), 30 (E), and 45 (F) min at 50 °C. (G) As a mock control, fibers without cellulase were incubated at 50 °C for 45 min. (H) Average areas of fibers in 36 images at each time point were plotted. Error bars indicate SEs.
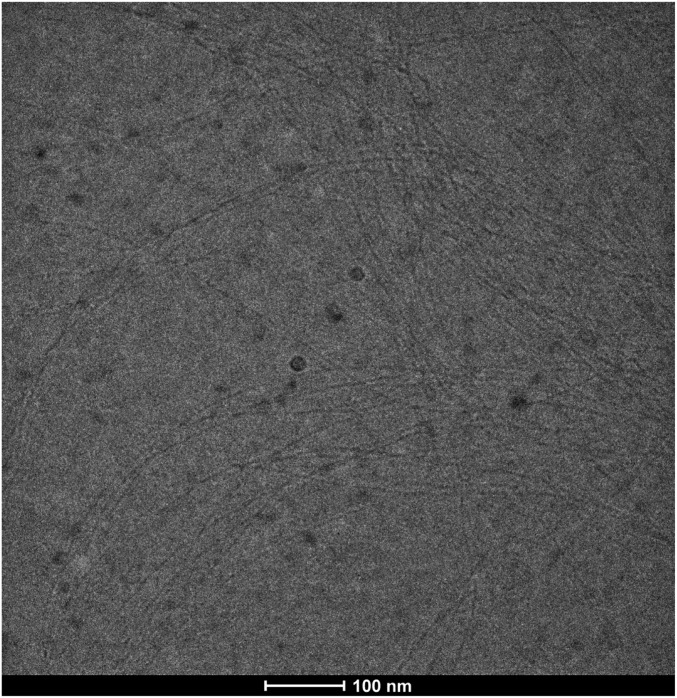
Analysis of frozen hydrated fibers by cryo-electron microscopy (cryoEM) suggests a diameter of 48 ± 10Å (Fig. S7), which is in agreement with diameter estimates for cellulose microfibrils obtained from spruce wood and celery collenchyma (27, 28). Assuming a stacking distance of about 5 Å between glucan chains within a cellulose microfibril, the estimated width of the PttCesA8-produced fiber is sufficient to accommodate at least 18–24 glucan chains. Indeed, comparable cellulose fibers have been synthesized from solubilized Physcomitrella patens CesA5 (17), with a similar estimated diameter of 20–30 Å, as well as from blackberry membrane extracts (16).
Fig. S7.
CryoEM of cellulose fibers. In vitro-synthesized cellulose fibers were treated as described, applied to a Quantifoil grid, and flash cooled in liquid ethane. Fiber dimensions were estimated from ∼400 fibers using ImageJ.
Some fibers are capped at one end by globular structures ∼250 Å in diameter (Fig. 7). Similar structures have been observed on cellulose fibers produced by CesAs solubilized from blackberry and P. patens membranes and most likely represent polymer-attached CesAs (16, 17). The strong interaction of cellulose synthase with the nascent cellulose polymer is exemplified by Rhodobacter BcsA, which, upon heterologous expression in E. coli and detergent solubilization, copurifies with a cellulose chain (7). The width of the fibril-attached particle is consistent with size estimates for plant CesA oligomers, referred to as “rosettes,” observed by freeze-fracture EM (11, 29, 30). CesA rosettes have recently been interpreted as hexamers of CesA trimers, yielding a total of 18 CesA molecules per complex and, accordingly, a maximum of 18 glucan chains per cellulose microfibril (31, 32).
Interestingly, cellulose produced by the N-terminally truncated PttCesA8s contained very little to no fibrous material (Fig. 7), despite significant in vitro catalytic activity (Fig. 5B) and an apparent polymer length similar to that obtained from wild-type PttCesA8 (Fig. 3 and Fig. S5). Given also that the mutant’s cellulosic product is particularly sensitive to acid hydrolysis (Fig. 6A), our data suggest that N-terminally truncated PttCesA8 cannot form cellulose microfibrils. Of note, our cellulose biosynthesis assay reports only on the formation of water-insoluble glucans, irrespective of their supramolecular organization. Negative-stain EM, as used in this work, is able to visualize only fibrous material and not noncrystalline cellulose, as confirmed by the observation that BcsA-produced noncrystalline cellulose is not detected (Fig. 7). Taken together, our data suggest that cellulose microfibril formation is not an intrinsic property of CesA and more likely is a secondary effect perhaps arising from a particular CesA quaternary structure supported by the enzyme’s N-terminal cytosolic domain.
Discussion
CesA functions as polymer synthase and translocase because the enzyme synthesizes glucan chains several hundred to thousand sugar units long and translocates the polymer across the plasma membrane during its synthesis. In addition, cellulose polymers synthesized by vascular plants and many other eukaryotic species are assembled into fibrillar structures, thereby establishing their physical properties as the load-bearing component of the cell wall.
Plant CesAs have been shown to interact with several components, including cytosolic microtubule-binding proteins, and also with membrane-integrated or -attached factors, such as the β-1,4 glucanase KORRIGAN (33). However, the cellulose biosynthetic activity of recombinantly expressed and reconstituted CesA8 from hybrid aspen demonstrates that no other plant-specific proteinaceous or lipidic factors are required for its catalytic activity.
Recent advances in bacterial cellulose biosynthesis provide detailed insights into how the synthase elongates the nascent cellulose polymer one glucose unit at a time with translocation steps between each round of polymer extension (8). However, it is currently unknown how cellulose biosynthesis is initiated and in particular whether a specific primer is required to form the first acceptor of glycosyl transfer. Several proposed models for bacterial and plant cellulose biosynthesis include the involvement of lipid-linked oligosaccharides or glycolipids, such as sitosterol-glucoside, which has been found only in plants so far (34–36). However, the catalytic activity of Pichia-expressed PttCesA8 suggests that the enzyme either initiates cellulose biosynthesis in vivo using an expression host-provided factor [as observed for Rhodobacter BcsA upon expression in E. coli (14)] or that cellulose biosynthesis initiates in vitro upon incubation with UDP-Glc and Mn2+. Based on the Rhodobacter BcsA structure, priming of cellulose biosynthesis with monomeric glucose (derived from UDP-Glc hydrolysis) seems likely, as recently discussed (6, 37).
In contrast to BcsA, PttCesA8-synthesized cellulose appears as long fibers that are partially resistant to acid hydrolysis. It is tempting to speculate that in vivo these microfibrils could coalesce to form macrofibrils, similar to those isolated from native tissues and giving rise to X-ray powder diffraction (28). The limited amount and random orientation of the PttCesA8-produced microfibrils currently preclude a similar analysis of our product. However, the observations that recombinant PttCesA8 forms cellulose microfibrils and BcsA does not suggest that microfibril formation is caused by CesA-specific features. An attractive model is that CesA oligomerization suffices to drive glucan chain alignment, consistent with the large globular densities capping some of the cellulose microfibrils (Fig. 7). The observations that N-terminally truncated PttCesA8s are catalytically active but fail to form cellulose microfibrils further supports this hypothesis. CesA’s N-terminal Zn2+-binding region has strong similarity to RING-fingers and likely mediates CesA–CesA interactions. Further, plant CesAs contain two prominent insertions within the evolutionarily conserved glycosyltransferase domain, referred to as “plant-conserved” and “class-specific” regions (38), which have been proposed to be involved in CesA oligomerization as well (9).
CesA oligomers (rosettes) contain different isoforms; for example, in Arabidopsis and Populus CesA1, 3, and 6 are involved in primary and CesA4, 7, and 8 are involved in secondary cell wall biosynthesis (1). Stoichiometry estimates of CesA rosettes formed during primary or secondary cell wall formation suggest an equal number of isoforms per complex (39, 40). Although the arrangements and functions of the individual CesAs in a rosette are currently unknown, our data demonstrate that a single isoform suffices to form a cellulose microfibril. This finding raises the possibility that CesA rosettes represent higher-order complexes of catalytically active CesA homooligomers, perhaps preformed dimers or trimers, as recently observed for an isolated glycosyltransferase domain from Arabidopsis CesA1 (31). Rosette formation then could be driven by interactions between preformed CesA homooligomers of different isoforms.
The reconstitution of cellulose biosynthesis from heterologously expressed and purified PttCesA8 provides a powerful tool to delineate many crucial steps during cellulose microfibril formation. These steps include interaction studies of PttCesA8 and its cellulosic product with other proteins and complex carbohydrates, thereby providing an in vitro platform for investigating the many steps implicated in plant cell wall formation.
Methods
Populus tremula × tremuloides CesA8 was expressed as a C-terminally His-tagged species in P. pastoris and was purified in the detergent LysoFoscholine-Ether14 by metal-affinity chromatography. The purified protein was reconstituted into proteoliposomes, in which it synthesized cellulose upon incubation with the substrate UDP-glucose and Mn2+ cations. The synthesized polymer was quantified by scintillation counting upon incorporation of trace amounts of 3H-labeled glucose as described (14). The synthesis of authentic cellulose was confirmed by enzymatic degradation and permethylation glycosyl linkage analysis as described for bacterial cellulose synthase (14). Uranyl formate-stained electron micrographs were obtained from in vitro-synthesized cellulose after the proteoliposomes were solubilized in the detergent Triton X-100. Detailed methods are provided in SI Methods.
SI Methods
Cloning of Populus tremula × tremuloides CesA8.
The Populus tremula × Populus tremuloides cesA8 gene was cloned as described (42). Briefly, the full-length cDNA was obtained by the rapid amplification of cDNA ends (FirstChoice RLM-RACE; Thermo Fisher Scientific) using total RNA from hybrid aspen cambial tissue. The full-length cDNA then was cloned into the pGEM-T Easy vector (Promega) and sequenced. PttcesA8 was amplified by PCR from the pGEM-T Easy-PttcesA8 construct using the following primers: forward: ATCTGCTCGAGATGATGGAATCTGGGGCTC and reverse: ACAATAGCGGCCGCTCAGTGATGGTGATGGTGATGGTGATGGTGATGGTGATGGCAATCTATAGAAATGCAGGTTTC. N-terminal FLAG and HA tags were sequentially introduced into the obtained construct using the following forward primers: HA-tag: TACCCTTATGATGTTCCAGATTACGCTATGATGGAATCTGGGGCTCCTATATG and FLAG-HA-tag: GTCATCTG CTCGAGATGGATTACAAGGACGACGACGATAAATACCCTTATGATGTTCCAGATTACGCT. The gene was cloned into the XhoI and NotI sites (underlined) of the P. pastoris expression vector pPICZA (Invitrogen) with a C-terminal dodeca-His tag. The construct was sequenced to confirm the correct gene sequence.
Electrocompetent P. pastoris strain SMD1168H was transformed with PmeI linearized plasmid using the Easyselect Pichia Expression kit (Invitrogen) according to the manufacturer’s specifications. The cells were plated at low densities on YPDS plates [1% yeast extract, 2% peptone, 2% dextrose, 1 M sorbitol, 2% agar (wt/wt)] containing 100 µg/mL zeocin and were incubated for 3 d at 30 °C.
PttCesA8N60 and PttCesA8N168 were generated using the forward primers GTCATCTGCTCGAGATG GAGAACTTGCTGGACGATGTAG and GTCATCTGCTCGAGATGCGCAACAAACTCACACCATAC, respectively. The catalytically inactive PttCesA8* construct was generated using QuikChange mutagenesis with the forward and reverse primers GGGTCGGTCACTGAGAATATCTTAAGT and ACTTAAGATATTCTCAGTGACCGACCC. The correct gene sequence was confirmed by DNA sequencing.
Expression and Purification of PttCesA8.
Transformants were cultured overnight at 30 °C in 300 mL BMGY medium (1% yeast extract, 2% peptone, 1.34% yeast nitrogen base, 1% glycerol) to an OD600 of 10–15. Cells were collected and diluted to an OD600 of 0.4 in buffered methanol-complex (BMMY) medium containing 0.5% methanol and were cultured. Expression was carried out in 1-L volumes using 2.5-L baffled flasks at 28 °C for 24–32 h with vigorous shaking. The cells were harvested and stored at −80 °C. Cells were resuspended in 180 mL of lysis buffer [20 mM Tris (pH 7.5), 0.6 M sorbitol] and were lysed by two passes through a microfluidizer at ∼30,000 psi in the presence of one EDTA-free complete protease inhibitor tablet (Roche) per 50-mL sample volume. The lysate was centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatant was centrifuged for 2 h at 110,000 × g and 4 °C to pellet the membrane fraction. The membrane pellet was solubilized in 120 mL membrane resuspension buffer (MRB) [20 mM Tris (pH 7.5), 100 mM NaCl, 40 mM dodecyl-maltoside, 10% (vol/vol) glycerol, and one EDTA-free complete protease inhibitor tablet per 50-mL volume] and incubated at 4 °C for 60 min with agitation. Insoluble material was removed by centrifugation at 110,000 × g for 30 min at 4 °C. The supernatant was incubated with 5 mL preequilibrated Talon metal-affinity resin for 1 h at 4 °C. The resin was packed into a gravity flow column and sequentially washed with MRB containing 1 mM LysoFoscholineEther-14 instead of DDM and 20, 40, or 60 mM imidazole. PttCesA8 was eluted in the above buffer containing 300 mM imidazole. All fractions were analyzed by 10% SDS/PAGE and Western blotting.
Western Blot Analysis.
After SDS/PAGE the protein was transferred to nitrocellulose membranes at 100 V with constant current (350 mA) for 60 min at 4 °C in a Bio-Rad Mini-Transfer Cell according to the manufacturer’s specifications. The nitrocellulose membrane was blocked with 5% (wt/vol) milk/TBS-Tween solution for 1 h and was incubated for 1 h with anti–penta-His or FLAG primary mouse antibodies at room temperature (25 °C). The membranes were washed once in TBS-Tween before incubation with an IRDye800-conjugated anti-mouse secondary antibody (Rockland) for 1 h at room temperature. After washing, the membrane was scanned on an Odyssey Infrared Imager (LI-COR).
Detergent Screening for Solubilization of PttCesA8.
Inverted-membrane vesicles (IMVs) were prepared from PttCesA8-expressing Pichia cells as described (14). IMVs were resuspended in MRB containing 2% C12E8, 2% (vol/vol) Triton X-100, 40 mM DDM, or 80 mM sodium cholate and were incubated for 1 h at 4 °C with agitation. The insoluble material was separated by centrifugation at 228,000 × g for 15 min at 4 °C. The soluble and insoluble fractions were analyzed by Western blotting.
PttCesA8 Reconstitution into Proteoliposomes.
Chloroform-solubilized S. cerevisiae and E. coli total lipid extracts were dried in glass vials under an N2 stream and were incubated overnight under vacuum to remove residual solvent. The lipids were solubilized at a concentration of 10 mg/mL in 120 mM N,N-dimethyldodecylamine N-oxide (LDAO) or 40 mM DDM for S. cerevisiae and E. coli lipids, respectively.
The affinity-purified PttCesA8 was concentrated to 1 mL using a 100-kDa molecular weight cutoff concentrator (Amicon) and was mixed with 4 mg/mL of lipids. The detergent was removed by the addition of SM-2 Bio-Beads (Bio-Rad) until turbidity indicated the formation of vesicles. Proteoliposomes were stored in aliquots at −80 °C until use.
PttCesA8 Activity Assay.
Standard cellulose synthase activity assays were performed as described (14) with modifications. Twenty microliters of PttCesA8-containing proteoliposomes were incubated in the presence of 20 mM MnCl2, 3 mM UDP-Glc, and 0.25 μCi UDP-[3H]-Glc in a buffer containing 20 mM Tris (pH 7.5), 0.1 M NaCl, and 10% (vol/vol) glycerol. After incubation at 37 °C for 3 h, the synthesis reaction was terminated by the addition of 0.1% Triton X-100, and the water-insoluble polymer was pelleted by centrifugation at 16,000 × g at room temperature for 20 min. The obtained pellet was resuspended in 20 μL of 20 mM Tris (pH 7.5), 0.1 M NaCl, and 10% glycerol and was spotted at the origin of a descending Whatman-2MM chromatography paper, which was developed in an aqueous solution of 60% (vol/vol) ethanol. For enzymatic degradation, the pellet was resuspended in 20 μL of 20 mM sodium phosphate (pH 6.0) and was digested with 10 U of endo-β-1,4 or endo-β-1,3 glucanase, amylase, or amyloglucosidase (Megazyme) for 1 h at 37 °C.
Kinetic Analysis of PttCesA8.
Kinetic analyses were performed in the presence of 20 mM MnCl2 and 0–5 mM UDP-Glc. A fourfold concentrated stock solution of 20 mM UDP-Glc and 1.0 μCi UDP-[3H]-Glc was diluted to the final substrate concentration required for the individual experiments. After synthesis for 30 min, the reactions were treated as described above.
Acid Hydrolysis of in Vitro-Synthesized Cellulose.
In vitro-generated cellulose was incubated at 40 °C for 20 min in the presence of premixed 80% (vol/vol) acetic acid and concentrated nitric acid at a volume ratio of 10:1. Then the sample was pelleted at room temperature for 20 min at 16,000 × g. The pellet was washed two times with deionized water before spotting onto chromatography paper or resuspension in 20 mM sodium phosphate (pH 6.0) for enzymatic degradation.
Optimal Temperature and pH of Cellulose Biosynthesis.
Standard PttCesA8 cellulose biosynthesis reactions were incubated in the presence of 0.03 mM UDP-Glc, 0.25 μCi UDP-[3H]-Glc, and 20 mM MnCl2 at different temperatures in a thermocycler for 30 min. The synthesized cellulose was quantified as described above. A continuous 4–9 pH range was achieved by dissolving 2.68 g DL-malic acid, 8.53 g MES, and 4.85 g Tris base in 100 mL. The pH of the 10-times concentrated buffer stock solution was adjusted with NaOH or HCl. pH screens were performed in the presence of 0.03 mM UDP-Glc, 0.25 μCi UDP-[3H]-Glc, and 20 mM MnCl2 for 30 min at 37 °C as described above.
Product Inhibition of PttCesA8.
Cellulose biosynthesis assays were performed in the presence of increasing concentrations of UDP, ADP, or GDP and 0.03 mM UDP-Glc, 0.25 μCi UDP-[3H]-Glc, and 20 mM MnCl2 for 30 min at 37 °C. The synthesized product was quantified relative to the polymer formed in the absence of any nucleoside diphosphate.
The catalytic activity of PttCesA8 in the presence of UDP and increasing UDP-Glc concentrations was analyzed in the presence of 0.027 mM UDP and 20 mM MnCl2 and incubation at 37 °C for 30 min. The obtained products were quantified relative to the amount formed in the absence of UDP and in the presence of 5 mM UDP-Glc. A fourfold concentrated stock solution of 20 mM UDP-Glc and 1.0 μCi UDP-[3H]-Glc was diluted to the final substrate concentrations required for the individual experiments.
Error Estimates.
All experiments were performed at least in triplicate; error bars represent the SDs from the means.
Linkage Analysis.
Linkage analysis of in vitro-synthesized cellulose was performed by permethylation and GC/MS as described (14). Before derivatization for linkage analysis, the cellulose samples were treated with 2% SDS and 1 mg/mL proteinase K; the samples were washed with water twice after each treatment, followed by two washes with hexane. The samples then were freeze-dried and used for linkage analysis.
Negative Staining and TEM Analysis.
Reconstituted proteoliposomes (5 μL) were incubated with 1 μL of 10 mM UDP-Glc and 4 μL reaction buffer containing 20 mM Tris (pH 7.5), 100 mM NaCl, 20 mM MnCl2, and 10% (vol/vol) glycerol at 37 °C for 3 h, as described for standard activity assays. Following cellulose synthesis, the sample was mixed with Triton-X 100 to a final concentration of 0.1% (vol/vol) and then was diluted 100-fold into buffer containing sodium taurocholate [50 mM 3-(N-morpholino)propanesulfonic acid (pH 6.8), 8 mM MgCl2, 0.625% sodium taurocholate, 20 mM cellobiose]. After incubation overnight at 4 °C, 3.5 µL was applied for 1 min to a glow-discharged carbon-coated copper grid (Ted Pella) that then was negatively stained with 10 serial drops of 0.7% uranyl formate on parafilm, followed by air drying. Electron micrographs (3.55 Å per pixel) were taken using a Tecnai 12 Spirit BioTWIN electron microscope (FEI) [120 kV; 6.3 mm spherical aberration (Cs); 56 K magnification; 4 × 4K Eagle CCD camera] at the Huck Institutes of the Life Sciences, Pennsylvania State University.
For fiber analysis after cellulase treatment, in vitro-synthesized fibers were incubated with 30 ng of a highly purified cellulase mix including Cel6A, Cel7A, and Cel7B (17) at 50 °C in a time series (0, 5, 10, 20, 30, and 45 min). As a mock control, fiber without cellulase was incubated at 50 °C for 45 min. A 3.5-µL sample was collected at each time point, followed by negative staining and TEM analysis. Thirty-six images were randomly taken from each sample. Areas occupied by fibers were measured using ImageJ (NIH).
cryoEM of Cellulose Fibers.
Fiber samples were produced by the same method used for negative staining, and cryoEM was performed following the previously described method (17). The thickness of 400 individual fibers was measured using ImageJ with the scale bars in the micrographs used as reference. Widths of individual fibers were measured using the measurement function provided in the program.
Acknowledgments
We thank Chao Fang and Caitlin Hubbard for assistance with negative-stain EM and total internal reflection fluorescence microscopy, respectively. Protein MS-MS fingerprinting was performed by the Taplin Mass Spectrometry Facility, Harvard Medical School. P.P., S.H.C., M.K., B.T.N., and J.Z. are supported by the Center for Lignocellulose Structure and Formation, Energy Frontier Research Center, US Department of Energy, Office of Science Award DE-SC0001090. S.M.D.-M. and V.B. are supported by the Australian Research Council Centre of Excellence in Plant Cell Walls and matching funding from the Royal Institute of Technology, Stockholm.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606210113/-/DCSupplemental.
References
- 1.McFarlane HE, Döring A, Persson S. The cell biology of cellulose synthesis. Annu Rev Plant Biol. 2014;65:69–94. doi: 10.1146/annurev-arplant-050213-040240. [DOI] [PubMed] [Google Scholar]
- 2.Bi Y, Hubbard C, Purushotham P, Zimmer J. Insights into the structure and function of membrane-integrated processive glycosyltransferases. Curr Opin Struct Biol. 2015;34:78–86. doi: 10.1016/j.sbi.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 4.Newman RH, Hill SJ, Harris PJ. Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol. 2013;163(4):1558–1567. doi: 10.1104/pp.113.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis MC. Cellulose biosynthesis: Counting the chains. Plant Physiol. 2013;163(4):1485–1486. doi: 10.1104/pp.113.231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara J, Morgan JLW, Zimmer J. A molecular description of cellulose biosynthesis. Annu Rev Biochem. 2015;84:17.11–17.27. doi: 10.1146/annurev-biochem-060614-033930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan JL, Strumillo J, Zimmer J. Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature. 2013;493(7431):181–186. doi: 10.1038/nature11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan JL, et al. Observing cellulose biosynthesis and membrane translocation in crystallo. Nature. 2016;531(7594):329–334. doi: 10.1038/nature16966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethaphong L, et al. Tertiary model of a plant cellulose synthase. Proc Natl Acad Sci USA. 2013;110(18):7512–7517. doi: 10.1073/pnas.1301027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slabaugh E, Davis JK, Haigler CH, Yingling YG, Zimmer J. Cellulose synthases: New insights from crystallography and modeling. Trends Plant Sci. 2014;19(2):99–106. doi: 10.1016/j.tplants.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kimura S, et al. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell. 1999;11(11):2075–2086. doi: 10.1105/tpc.11.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RM. Cellulose structure and biosynthesis: What is in store for the 21st century? J Polym Sci A Polym Chem. 2003;42(3):487–495. [Google Scholar]
- 13.Giddings TH, Jr, Brower DL, Staehelin LA. Visualization of particle complexes in the plasma membrane of Micrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary cell walls. J Cell Biol. 1980;84(2):327–339. doi: 10.1083/jcb.84.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omadjela O, et al. BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc Natl Acad Sci USA. 2013;110(44):17856–17861. doi: 10.1073/pnas.1314063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cifuentes C, Bulone V, Emons AMC. Biosynthesis of callose and cellulose by detergent extracts of tobacco cell membranes and quantification of the polymers synthesized in vitro. J Integr Plant Biol. 2010;52(2):221–233. doi: 10.1111/j.1744-7909.2010.00919.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai-Kee-Him J, et al. In vitro versus in vivo cellulose microfibrils from plant primary wall synthases: Structural differences. J Biol Chem. 2002;277(40):36931–36939. doi: 10.1074/jbc.M203530200. [DOI] [PubMed] [Google Scholar]
- 17.Cho SH, et al. In vitro synthesis of cellulose microfibrils by a membrane protein from protoplasts of the non-vascular plant Physcomitrella patens. Biochem J. 2015;470(2):195–205. doi: 10.1042/BJ20141391. [DOI] [PubMed] [Google Scholar]
- 18.Gray MC, Converse AO, Wyman CE. 2003. Sugar monomer and oligomer solubility: Data and predictions for application to biomass hydrolysis. Appl Biochem Biotechnol 105 -108:179–193.
- 19.Yang H, Zimmer J, Yingling YG, Kubicki JD. How cellulose elongates--a QM/MM study of the molecular mechanism of cellulose polymerization in bacterial CESA. J Phys Chem B. 2015;119(22):6525–6535. doi: 10.1021/acs.jpcb.5b01433. [DOI] [PubMed] [Google Scholar]
- 20.Brown C, Leijon F, Bulone V. Radiometric and spectrophotometric in vitro assays of glycosyltransferases involved in plant cell wall carbohydrate biosynthesis. Nat Protoc. 2012;7(9):1634–1650. doi: 10.1038/nprot.2012.089. [DOI] [PubMed] [Google Scholar]
- 21.Tlapak-Simmons VL, Baron CA, Weigel PH. Characterization of the purified hyaluronan synthase from Streptococcus equisimilis. Biochemistry. 2004;43(28):9234–9242. doi: 10.1021/bi049468v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MS, et al. Isolation of chitin synthetase from Saccharomyces cerevisiae. Purification of an enzyme by entrapment in the reaction product. J Biol Chem. 1984;259(23):14966–14972. [PubMed] [Google Scholar]
- 23.Liew CW, Sun H, Hunter T, Day CL. RING domain dimerization is essential for RNF4 function. Biochem J. 2010;431(1):23–29. doi: 10.1042/BJ20100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurek I, Kawagoe Y, Jacob-Wilk D, Doblin M, Delmer D. Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc Natl Acad Sci USA. 2002;99(17):11109–11114. doi: 10.1073/pnas.162077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maji S, Mehrotra R, Mehrotra S. Extraction of high quality cellulose from the stem of Calotropis procera. South Asian J Exp Biol. 2013;3(3):113–118. [Google Scholar]
- 26.Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- 27.Thomas LH, et al. Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol. 2013;161(1):465–476. doi: 10.1104/pp.112.206359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes AN, et al. Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA. 2011;108(47):E1195–E1203. doi: 10.1073/pnas.1108942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herth W. Arrays of plasma-membrane “rosettes” involved in cellulose microfibril formation of Spirogyra. Planta. 1983;159(4):347–356. doi: 10.1007/BF00393174. [DOI] [PubMed] [Google Scholar]
- 30.Roberts AW, Roberts EM, Haigler CH. Moss cell walls: Structure and biosynthesis. Front Plant Sci. 2012;3:166. doi: 10.3389/fpls.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandavasi VG, et al. A structural study of CESA1 catalytic domain of Arabidopsis cellulose synthesis complex: Evidence for CESA trimers. Plant Physiol. 2016;170(1):123–135. doi: 10.1104/pp.15.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon BT, et al. Comparative structural and computational analysis supports eighteen cellulose synthases in the plant cellulose synthesis complex. Sci Rep. 2016;6:28696. doi: 10.1038/srep28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vain T, et al. The cellulase KORRIGAN is part of the cellulose synthase complex. Plant Physiol. 2014;165(4):1521–1532. doi: 10.1104/pp.114.241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthysse AG, Thomas DL, White AR. Mechanism of cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1995;177(4):1076–1081. doi: 10.1128/jb.177.4.1076-1081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng L, Kawagoe Y, Hogan P, Delmer D. Sitosterol-beta-glucoside as primer for cellulose synthesis in plants. Science. 2002;295(5552):147–150. doi: 10.1126/science.1064281. [DOI] [PubMed] [Google Scholar]
- 36.Grillitsch K, et al. Isolation and characterization of the plasma membrane from the yeast Pichia pastoris. Biochim Biophys Acta. 2014;1838(7):1889–1897. doi: 10.1016/j.bbamem.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Morgan JLW, McNamara JT, Zimmer J. Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat Struct Mol Biol. 2014;21(5):489–496. doi: 10.1038/nsmb.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergara CE, Carpita NC. Beta-d-glycan synthases and the CesA gene family: Lessons to be learned from the mixed-linkage (1-->3),(1-->4)beta-D-glucan synthase. Plant Mol Biol. 2001;47(1-2):145–160. [PubMed] [Google Scholar]
- 39.Hill JL, Jr, Hammudi MB, Tien M. The Arabidopsis cellulose synthase complex: A proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell. 2014;26(12):4834–4842. doi: 10.1105/tpc.114.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonneau M, Desprez T, Guillot A, Vernhettes S, Höfte H. Catalytic subunit stoichiometry within the cellulose synthase complex. Plant Physiol. 2014;166(4):1709–1712. doi: 10.1104/pp.114.250159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernsel A, Viklund H, Hennerdal A, Elofsson A. TOPCONS: Consensus prediction of membrane protein topology. Nucleic Acids Res. 2009;37(Web Server issue):W465–468. doi: 10.1093/nar/gkp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djerbi SAH, et al. Identification and expression analysis of genes encoding putative cellulose synthases (CesA) in the hybrid aspen, Populus tremula (L.) x P-tremuloides (Michx.) Cellulose. 2004;11:301–312. [Google Scholar]