Significance
Many cell types have been observed to migrate toward stiffer regions of mechanical gradients in a process termed durotaxis. Tissue stiffness gradients are being discovered in an increasing number of diseases, including cancer and fibrotic diseases. However, the role of ECM composition, which often changes in diseased tissues with stiffness, in directing this behavior has not previously been investigated. To understand how stiffness gradients and changing matrix composition may affect cell migration in disease, we have designed a mechanical gradient platform that allows for independent control of absolute stiffness, gradient steepness, and ECM coating. We demonstrate that smooth muscle cells will undergo durotaxis on mechanical gradients coated with fibronectin but not laminin.
Keywords: durotaxis, cell migration, extracellular matrix, polyacrylamide
Abstract
Mechanical compliance has been demonstrated to be a key determinant of cell behavior, directing processes such as spreading, migration, and differentiation. Durotaxis, directional migration from softer to more stiff regions of a substrate, has been observed for a variety of cell types. Recent stiffness mapping experiments have shown that local changes in tissue stiffness in disease are often accompanied by an altered ECM composition in vivo. However, the importance of ECM composition in durotaxis has not yet been explored. To address this question, we have developed and characterized a polyacrylamide hydrogel culture platform featuring highly tunable gradients in mechanical stiffness. This feature, together with the ability to control ECM composition, allows us to isolate the effects of mechanical and biological signals on cell migratory behavior. Using this system, we have tracked vascular smooth muscle cell migration in vitro and quantitatively analyzed differences in cell migration as a function of ECM composition. Our results show that vascular smooth muscle cells undergo durotaxis on mechanical gradients coated with fibronectin but not on those coated with laminin. These findings indicate that the composition of the adhesion ligand is a critical determinant of a cell’s migratory response to mechanical gradients.
Cell migration is essential to numerous biological processes, including development, angiogenesis, wound healing, and cancer metastasis (1–4). The movement of cells in these processes is determined by a complex assessment of environmental cues that include soluble factors, ECM composition, orientation, and stiffness. Numerous experiments have demonstrated that directional cell migration can result from gradients in these environmental cues: for example, chemotaxis (cell migration in response to gradients of soluble signals) and haptotaxis (cell migration in response to gradients of bound ligands) have been established in both in vitro and in vivo experimental systems (5–7). More recently, it has been demonstrated that cells are also capable of directed migration in response to gradients in substrate stiffness, a process termed durotaxis (8). Though there have been limited reports of specifically measured in vivo gradients (9), a number of recent stiffness mapping measurements imply the presence of stiffness gradients in both healthy and diseased tissues spanning a wide range of stiffnesses (10–13). In vitro experiments have demonstrated that directed migration in response to stiffness gradients can be observed in numerous cell types, using various materials as substrates, and across various stiffness levels (8, 14–19). However, the role of ECM composition in mediating this behavior has not been thoroughly investigated.
The interplay between mechanical stiffness and matrix composition in normal and pathological physiology is only now becoming appreciated. Recent studies in which tissue stiffness was mapped by atomic force microscopy (AFM) indentation have identified heterogeneities that indicate the presence of mechanical stiffness gradients in both healthy and diseased tissues. These measurements indicate the presence of a wide range of absolute stiffnesses and gradient strengths in vivo (9–13, 20). Importantly, such stiffness gradients have been demonstrated to accompany changes in ECM composition in a number of diseases. For instance, in lung fibrosis, local increases in lung parenchymal tissue stiffness are accompanied by an increase in collagen I concentration (21), and in breast cancer an increase in stiffness from the tumor core to the periphery is associated with increased levels of collagen I and laminin (12). In atherosclerosis, a disease characterized by the thickening of the intimal region of the arterial wall, changes in the mechanics and composition of the intimal matrix occur in conjunction with the accumulation of smooth muscle and inflammatory cells (22–24). Stiffness mapping experiments have shown that plaque stiffness is spatially heterogeneous, and that these changes can be histologically related to the ECM composition of the plaque (20, 25). Given the increasing number of examples for which changes in ECM composition in disease are coupled to changes in mechanical properties of the diseased tissue, there is a need for in vitro experimental systems that allow for systematic exploration of how the cellular response to stiffness gradients is modulated by ECM composition.
Recent experimental work has demonstrated that the effect of matrix stiffness on cell behaviors such as differentiation, spreading, and motility is modulated by the composition of the ECM on which the cells are grown (26–30). Though the role of matrix composition has been investigated on uniformly stiff substrates, previous work investigating the cellular response to mechanical gradients has been limited to substrates coated with a single type of matrix protein, typically collagen or fibronectin (31), and the behavioral response of a given cell type to different matrix compositions has yet to be explored in the same study. Previous work in our laboratory has demonstrated that vascular smooth muscle cell (VSMC) adhesion rate, spread area, cytoskeletal assembly, and focal adhesion signaling undergo opposing responses to substrate stiffness depending on whether they are seeded on fibronectin- or laminin- coated substrates (27), and furthermore that VSMCs will preferentially migrate toward stiffer regions (durotaxis) when exposed to mechanical gradients on fibronectin substrates (14, 16). Additionally, there is evidence that both stiffness and ECM composition can modulate VSMCs between a quiescent, contractile phenotype typical of healthy tissue and a synthetic, proliferative, and migratory phenotype observed in vascular disease (32–34). Fibronectin and laminin have opposing effects on VSMC phenotype, with fibronectin driving cells away from the contractile phenotype in vitro, whereas laminin has been shown to conserve it (35–38). A loss of laminin and an increase in fibronectin surrounding smooth muscle cells has been observed during the progression of neointima formation in vascular disease, suggesting these proteins may play an important role in regulation of cell phenotype in disease (39). Additionally, increasing stiffness has been shown to drive smooth muscle cells toward the synthetic, migratory phenotype observed in atherosclerosis (40, 41), This evidence that VSMC phenotype is modulated by both matrix stiffness and composition, coupled with our previous observations of matrix type-dependent responses to stiffness by VSMCs, led us to hypothesize that the durotactic migratory behavior of VSMC s on substrates with mechanical gradients may also be differentially modulated by fibronectin and laminin.
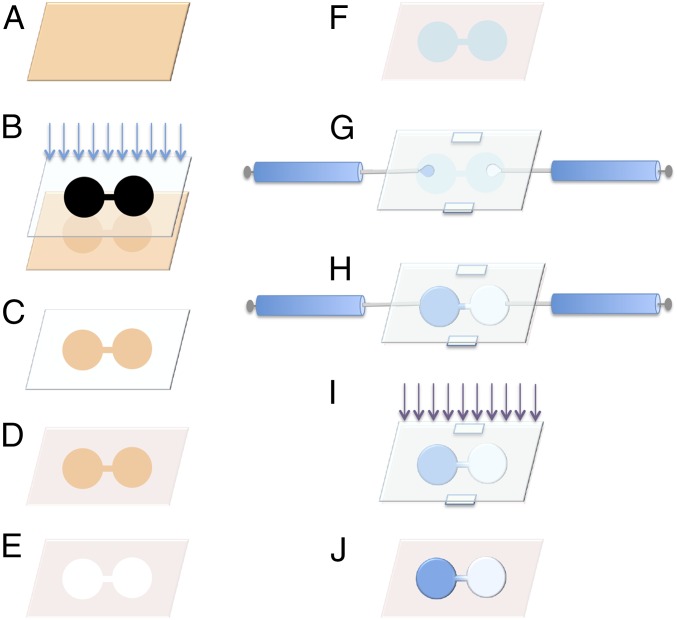
To test this hypothesis, we have developed a method of generating polyacrylamide hydrogels with tunable gradients in substrate stiffness and independent control of matrix composition. Previously described methods to fabricate polyacrylamide mechanical gradient gels have been limited due to the difficulty of independently controlling the absolute stiffness range and gradient steepness (8, 14–16, 19, 42–44). However, by carefully controlling hydrogel geometry, cross-linker diffusion, and UV photopolymerization (Fig. 1), we are able to easily and independently adjust both absolute stiffness and gradient steepness in our system. Maskless lithography, which unlike traditional lithographic techniques does not require a custom-made, physical photomask and thus allows one to rapidly modify pattern dimensions with ease, is used to micropattern glass slides with hydrophobic and hydrophilic silanes (45); this difference in hydrophobicity gives the geometric constraint necessary for fine control over the location and degree of pregel solution mixing before polymerization. Accordingly, a dumbbell- shape pattern was developed in which the “weights” of the dumbbell consisted of large reservoir regions filled with a high-concentration cross-linker solution on one side and low-concentration cross-linker solutions on the other (Fig. 1), and the “bar” of the dumbbell acted as a mixing region for cross-linker diffusing between the two weights. After polymerization, the resultant polyacrylamide gels were then functionalized with either fibronectin or laminin through an NHS–ester linker. The advantage of this design is that the cell movements observed in the constant stiffness regions at the ends can serve as controls for cell migration observed in the stiffness gradient region between them. Atomic force microscope nanoindentation of the end-product cell substrates produced in this manner demonstrated the presence of linear and highly reproducible mechanical gradients, and fluorescence imaging demonstrated that homogeneous surface coatings of Alexa Fluor 488-labeled fibronectin or laminin could be achieved.
Fig. 1.
Schematic of gradient generator device preparation and use. (A) Glass slide coated in S1818 photoresist (orange). (B) Maskless lithography with visible light exposes device pattern onto photoresist-coated slide. (C) Photoresist removed by development in TMAOH. (D) Exposed glass is coated with OTS to form a hydrophobic barrier. (E) Remaining photoresist is removed with acetone. (F) Exposed glass is backfilled with hydrophilic and adhesive silane mixture (teal). (G) The gradient generator device is assembled with 250-µm Teflon spacers between the silane-coated generator slide and a top sacrificial slide coated in DCDM. Solutions of acrylamide solutions with high (blue) and low (white) concentrations of bis-acrylamide cross-linker are injected into the reservoir regions through 32-gauge needles. (H) The two acrylamide solutions meet in the middle of the device with their boundary constrained by silane patterning. The needles are removed and diffusion of cross-linker proceeds until (I) the acrylamide solutions are polymerized by exposure to UV light. (J) The sacrificial slide and spacers are removed leaving a gradient hydrogel with a well-defined linear gradient in elasticity.
We then used this system to directly compare the durotactic migration of VSMCs on mechanical gradients coated with either fibronectin or laminin. The extent to which cells exhibited biased migration in the direction of increasing substrate stiffness was compared across all conditions by calculating a durotactic index, defined as the length of migration in the direction of increasing substrate stiffness divided by the total migration path distance, for each cell track. We found that cells seeded on fibronectin-coated gradient substrates exhibited durotactic behavior, whereas cells seeded on laminin-coated gradient substrates were capable of random movement but did not exhibit durotaxis. This finding indicates that ECM composition is capable of modulating a cell’s motile response to mechanical gradients.
Results
Generation and Characterization of Hydrogels with Defined Stiffness Gradients.
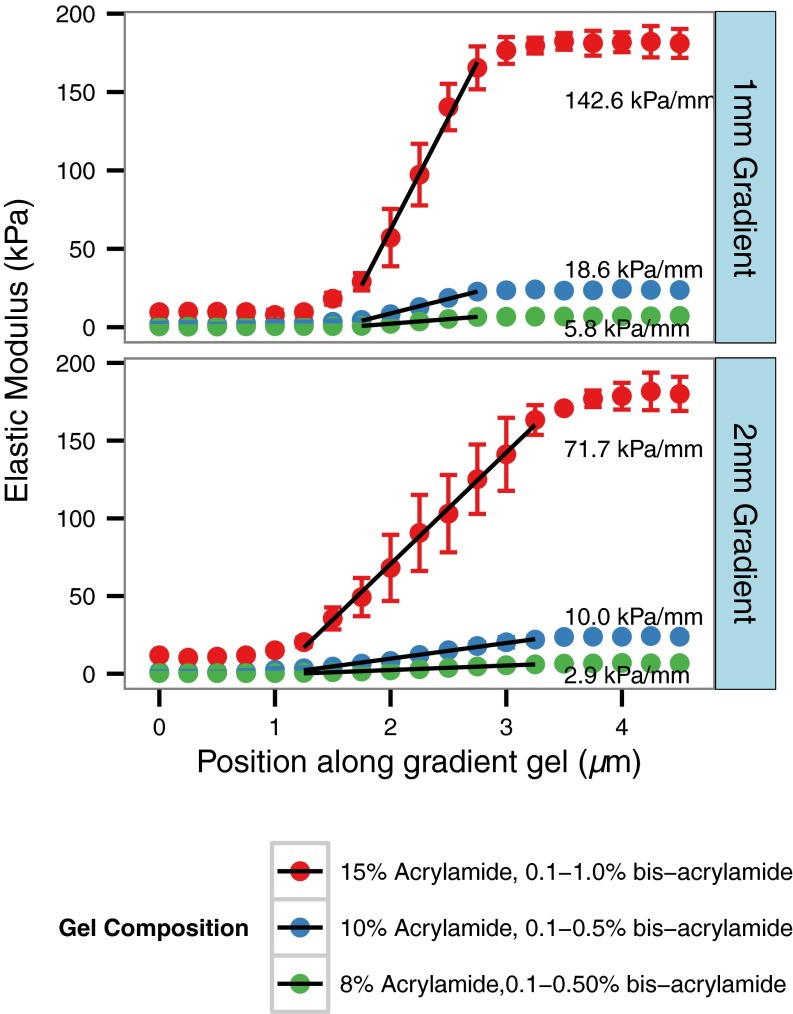
Polyacrylamide hydrogel stiffness is determined by the concentration of acrylamide monomer in solution and the extent of cross-linking, which can be controlled by the concentration of bis-acrylamide cross-linker. In preliminary studies, we produced uniform stiffness gels with 8–15% (wt/vol) acrylamide and bis-acrylamide cross-linker concentrations ranging from 0.1–1%. We found that after polymerization by photoinitiation, the elastic modulus of the resulting gels was linearly proportional to the bis-acrylamide concentration over these ranges. We took advantage of this linear proportionality to generate hydrogels with linear gradients in elastic modulus (Fig. 2). Briefly, the time allowed for diffusion of cross-linker between reservoirs of high and low bis-acrylamide concentration solutions was adjusted to yield linear gradients in bis-acrylamide across the region connecting the two reservoirs. Nanoindentation was performed using an AFM in force contact mode to measure the stiffness of the gels as a function of position. It was determined that for gradient gels with 2- and 1-mm-long gradient regions, 40 and 10 min of diffusion time, respectively, was sufficient to generate a linear gradient in cross-linker concentration between the two reservoir regions. Results from the gels tested demonstrate that the gradient steepness can be changed both by altering the composition of the pregel solutions while maintaining the same gradient geometry and adjusting the gradient length. The stiffness gradients between the high- and low-stiffness regions (0.5–10 and 7–170 kPa, respectively, for the range of gel solutions tested) ranged from 2.9 to 142.6 kPa/mm (Fig. 2). Notably, an effective doubling of the gradient steepness was achieved by halving the distance between the reservoir regions, demonstrating that the stiffness gradient of the gels can be tuned by adjusting the gel dimensions.
Fig. 2.
Mechanical characterization of polyacrylamide gradient gels with differing gel compositions and dimensions by AFM nanoindentation as a function of position along the gradient. Gel compositions tested were as follows: 15% (wt/vol) acrylamide:0.1–0.1% bis-acrylamide, 10% (wt/vol) acrylamide:0.1–0.5% bis-acrylamide, 8% (wt/vol) acrylamide:0.1–0.5% bis-acrylamide with gradient lengths of 1 and 2 mm for each gel composition. Gel elastic modulus was calculated as a function of position from a linearized Hertzian contact model, and the elastic modulus profile was fitted to a linear model in across the gradient region of the gel to determine the steepness of the gradient.
Functionalization of Gradient Hydrogels with ECM Substrates.
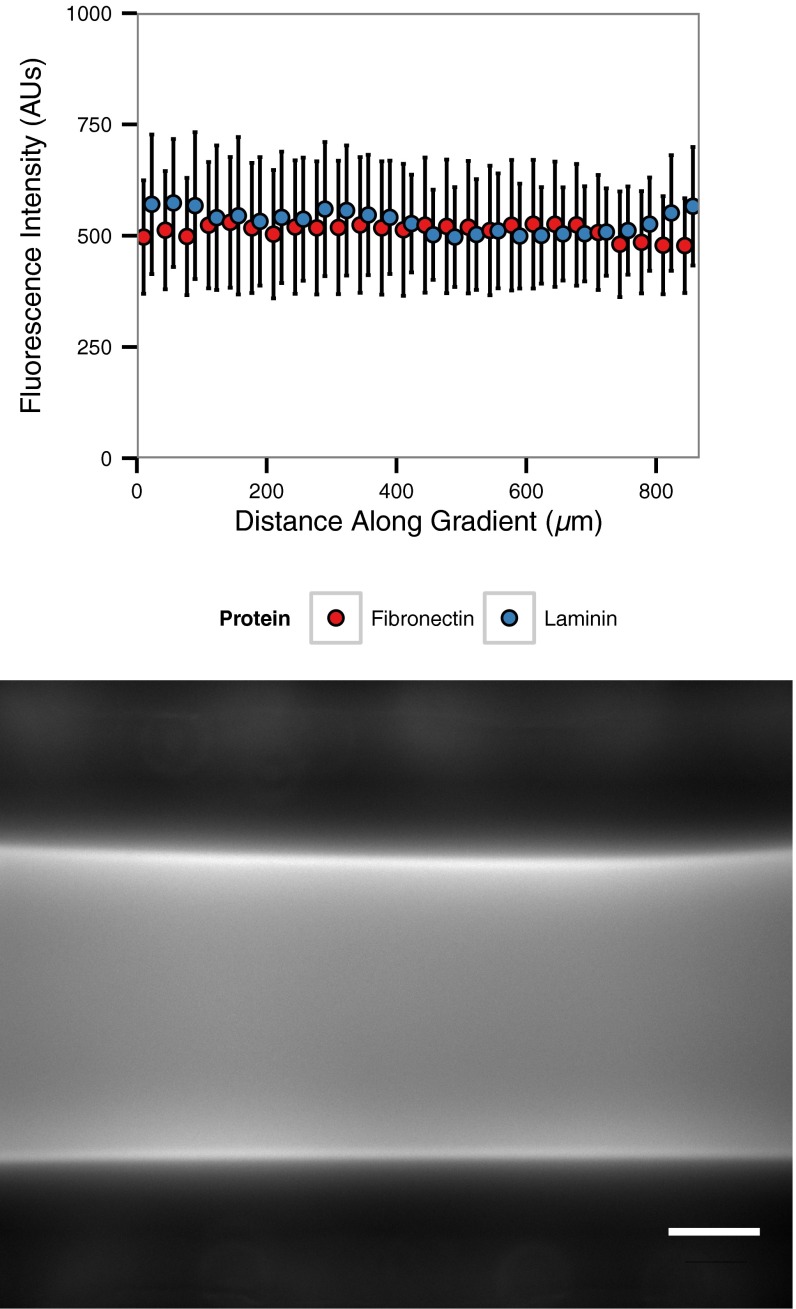
To ensure cell migration on gradient gel substrates was not biased by stiffness-induced changes in coating density, substrates with stiffness gradients between 10- and 170-kPa reservoir regions were functionalized with Alexa Fluor 488-labeled fibronectin or laminin-1 and were imaged under epifluorescence for quantification. The results indicate the average fluorescence intensity of attached fibronectin did not change either as a function of position along gradient gels or as a function of stiffness (Fig. S1).
Fig. S1.
Characterization of ECM attachment to gradient hydrogels. (Upper) Fluorescence intensity of Alexa Fluor 488-labeled fibronectin (red) and laminin (blue) is uniform across polyacrylamide gradient gels. Values are presented as mean ± SD, n = 3 gels per condition. (Lower) Representative image of gradient gel functionalized with Alexa 488-labeled fibronectin. (Scale bar: 100 µm.)
Effect of Substrate Stiffness on Cell Migration.
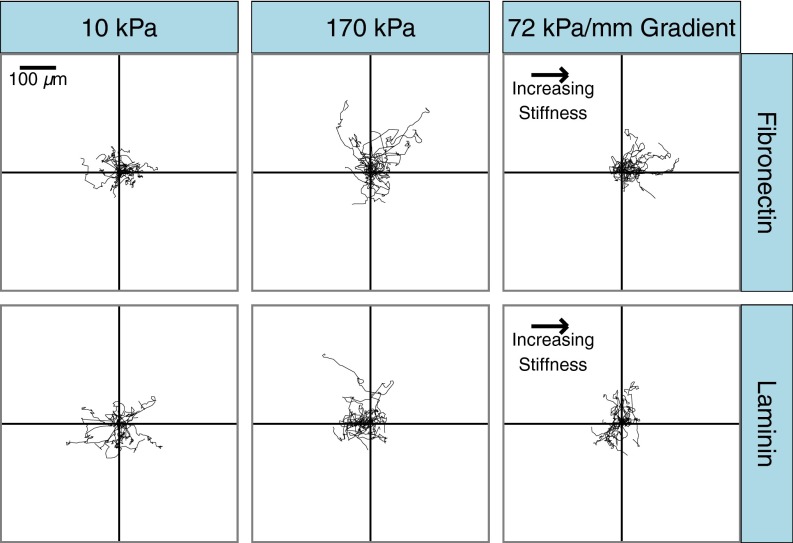
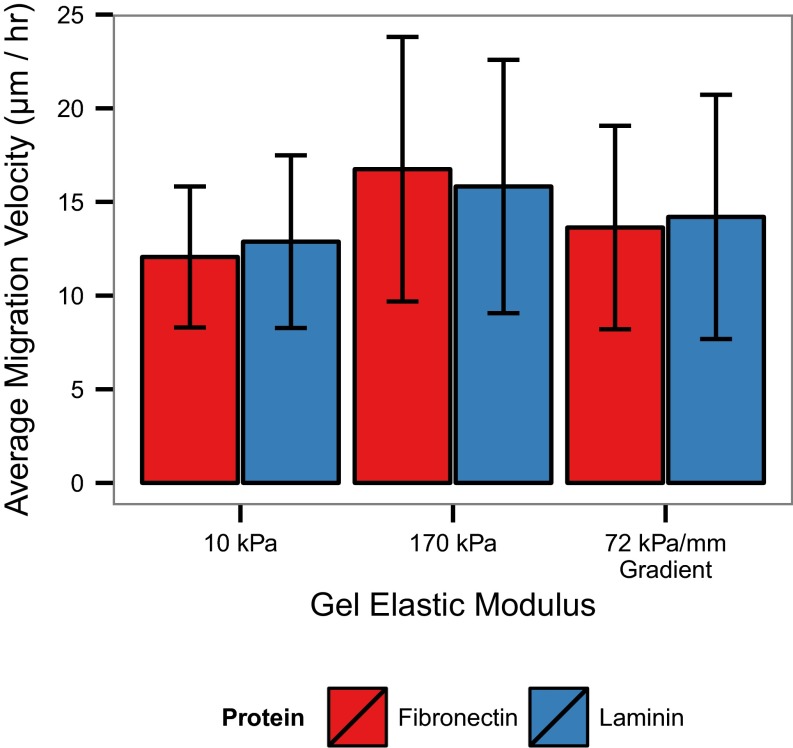
Cell migration on gradient gels and uniform control gels coated with either fibronectin or laminin was qualitatively assessed by plotting cell trajectories relative to a common origin (Fig. 3). On gels of uniform stiffness, there is a stiffness-dependent increase in the average migration velocity on both fibronectin- and laminin-coated gels, but there was no apparent directionality to the movement. Additionally, there were no significant differences in the migration velocities of cells on fibronectin compared with on laminin for both the control and gradient conditions (Fig. S2). In contrast to the uniform stiffness controls, cells migrating on fibronectin-coated gradient gels appear to migrate preferentially toward the stiffer end of the gradient, whereas cells on laminin-coated gradients do not display a bias in migration to any direction.
Fig. 3.
Representative migration tracks for VSMCs migrating on mechanical gradient and uniform stiffness gels coated with fibronectin or laminin. Cell centroid position was tracked at 20-min intervals for 18 h. n = 15 randomly selected cells per condition.
Fig. S2.
Average VSMC migration velocity on uniform control and gradient hydrogels. Values are presented as mean ± SD, n = 120 cells from n > 5 gels for each condition.
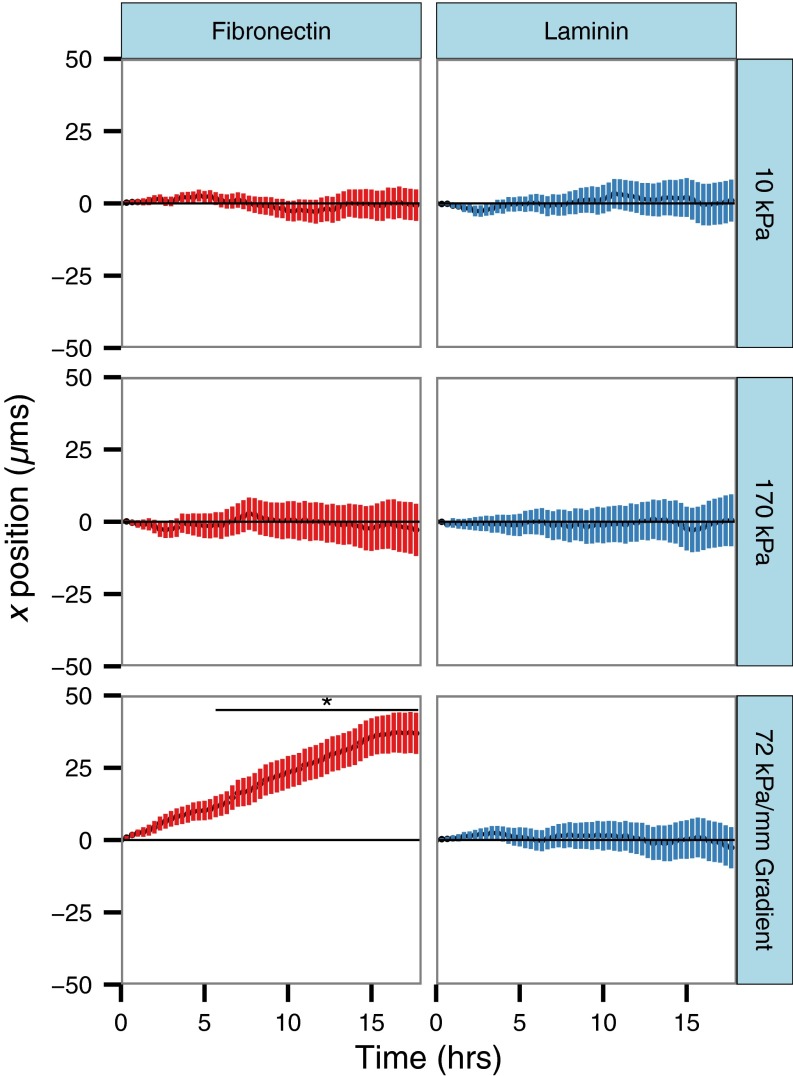
To more quantitatively assess whether there is biased migration of cells in the direction of the gradient, the average x-direction trajectory, where x is defined as parallel to the direction of increasing gradient stiffness (or a random direction in the case of uniform controls), was calculated from migration tracks as a function of time (Fig. 4). Cells on uniform stiffness control gels experienced an average x-directional displacement of zero as time increased, with a wider range of displacements reflected in an increase in the SD of displacement as stiffness is increased. Cells on gradient gels coated with fibronectin displayed a steady increase in x-directional displacement as a function of time, reaching an average value of 37 µm after 18-h migration, whereas cells on laminin-coated gradients migrated to an average displacement of −2.63 µm after 18 h. Statistical testing of the displacements of cells at all time points by ANOVA and Tukey’s honest significant difference test showed that the mean displacement of cells on fibronectin-coated gradient gels was significantly different from the mean displacement of cells in all other experimental conditions after 6 h (P < 0.05) of migration.
Fig. 4.
Average x displacement of cells migrating on polyacrylamide gels coated with fibronectin (red) or laminin (blue) as a function of time. Values presented as mean ± 95% confidence interval.
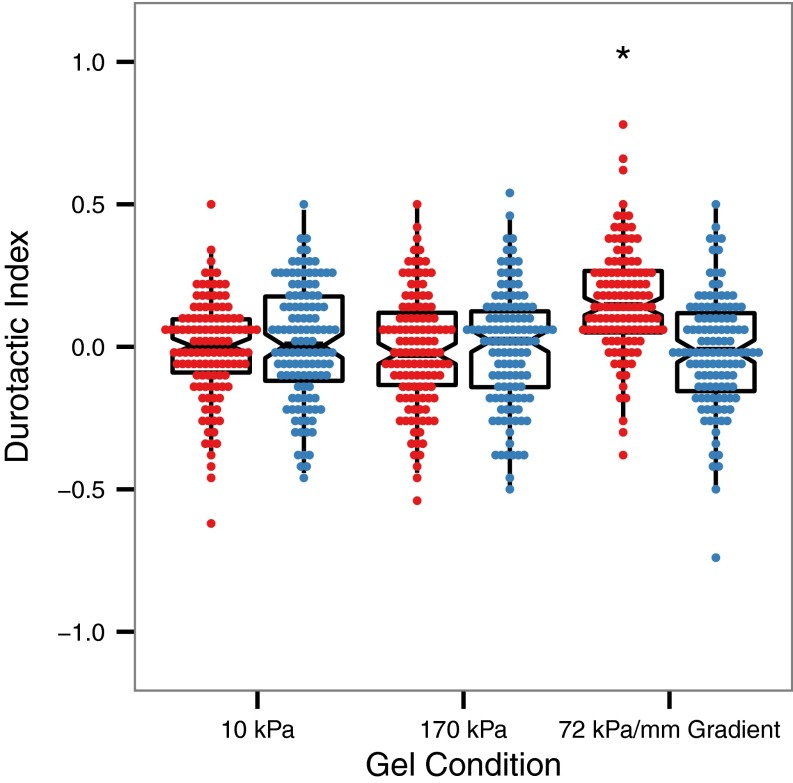
The extent to which each cell exhibited biased migration in the direction of the gradient can be further quantified using a durotactic index (16), defined as the displacement of a cell in the gradient direction divided by its cumulative displacement (i.e., total path length). The distribution of durotactic indices for each population of cells observed is plotted in Fig. 5. For both cells on uniform stiffness control gels and laminin-coated gradients, the mean durotactic index is zero, whereas for the population of cells migrating on fibronectin-coated gradients, the distribution of durotactic indices is significantly shifted to a mean of 0.17 (P < 0.005). There is thus a clear shift toward biased migration in the cell population when these cells are seeded on fibronectin-coated substrates having a stiffness gradient.
Fig. 5.
Distribution of durotactic index values for VSMCs migrating on substrates coated with fibronectin (red) or laminin (blue) with overlaid boxplots representing median and quartile values for each distribution. n = 120 cells from n > 5 gels for each condition. *P < 0.05 relative to all other conditions.
Discussion
Generation of Hydrogels with Controlled Gradients in Substrate Rigidity.
Here we present a method to generate gradients in mechanical compliance in hydrogels based on rapid prototyping and UV-initiated photopolymerization. An important feature of this method is that it allows for independent selection of absolute high and low stiffness values and gradient steepness, accomplished by varying gel solution composition and gel dimensions, respectively. In previous studies where hydrogels with gradients in mechanical compliance were used to investigate durotaxis (31), the methods of generating mechanical gradients relied on juxtaposition of pregel solutions with varying concentrations of cross-linker (8, 15, 16), varying UV exposure in photopolymerization with a photomask (14, 19, 43), or overlaying a uniform gel solution on a rigid material with varying height to achieve a gradient in apparent rigidity at the surface (42, 44). Though these methods effectively generate stiffness gradients, they are limited because they do not easily allow independent control of both the absolute stiffness range of the gels and the steepness of the generated gradient. We overcame this limitation by using an approach to gradient generation based on carefully controlled hydrogel geometry, cross-linker diffusion time, and UV photopolymerization time. As demonstrated in Fig. 2, the absolute stiffness range of the gradient gels produced using the method presented here can be tuned by changing the composition of the acrylamide gel, and the steepness of the mechanical gradients produced using this method can be predictably controlled by adjusting the length of the gradient region and cross-linker diffusion time. This flexibility will allow gradient profiles and material elasticity to be tailored to mimic stiffness gradients between tissues or cellular layers in vivo and also allows for simple modulation of gradient properties to further study the roles of absolute stiffness and gradient steepness in the cellular response to mechanical gradients (16, 46). In addition to the ease of gradient customization, gels produced using this method contain built-in uniform stiffness regions on the same sample to act as controls, allowing for easier experimental setup and greater throughput from a smaller number of independent samples. For this set of experiments we have optimized the gradient profile generated to be linear between two reservoir solutions, but it is also possible to produce gels with step-like gradients or sigmoid shaped gradients by reducing the time allowed for cross-linker diffusion. Additionally, this method is compatible with photomask-based techniques of stiffness modulation, theoretically allowing for any desired gradient profile to be produced in easily customized geometries (43).
Though this method of gradient gel production is highly tunable, there are practical limits on the mechanical properties and dimensions of gels that may be produced. Polyacrylamide gels have been produced with stiffnesses ranging from ∼200 Pa to 350 kPa, consistent with the mechanical data presented in this study (43, 47). The length of the gradient region can be made longer or shorter to alter the gradient steepness, but for extremely low gradient rates, limited directional migration is expected (16, 46), whereas at extremely high gradient steepness the system mimics step gradient methods (8, 15) and the observation region of interest becomes extremely small. The absolute stiffness range and gradient rate range is sufficient to model the stiffnesses of a wide variety of soft tissues that are likely to contain mechanical gradients (9–13). Gels can be made thinner or thicker as desired by changing the size of the spacers used, but there are practical limits on the achievable upper and lower thicknesses. A gel that is too thin will begin to appear stiffer to a cell due to mechanical coupling to the underlying glass substrate (42). Additionally, because the gradient generator system relies on surface tension between two slides to control the gel geometry, using spacers that are too thick can result in surface tension failing to maintain the pregel solution between both slides, and thus the gel dimensions and contact point between the two solutions cannot be controlled. The limits of the width of the gradient region are related to the size of the reservoir region and imaging area. As the gradient region is made thinner, the available area for cells to migrate on the gradient region is reduced, and it becomes less likely that cells will migrate without interacting with the sides of the gel, which greatly reduces the areas in the gel from which data can be reliably acquired. The width of the gradient area can be increased provided there is also an increase in the size of the reservoir regions to compensate and prevent depletion of cross-linker from the reservoir during diffusion. The volume of a reservoir region needs to be sufficiently large such that it is largely unaffected by diffusion from the much smaller gradient region and thus maintains a nearly constant cross-linker concentration; this ensures that a pseudosteady gradient profile can form in the gradient region.
VSMC Durotaxis Is ECM-Type Dependent.
Though durotaxis behavior has been observed in numerous experimental systems, to our knowledge, the role of matrix composition in durotactic behavior has not yet been investigated. To that end, we evaluated the migration of VSMCs on gradient substrates coated with either fibronectin or laminin, matrix proteins that have previously been demonstrated to evoke opposing responses to stiffness changes in smooth muscle cells and drive smooth muscle cells toward different phenotypes (27, 35–38). This model system was chosen both because of its potential relevance to atherosclerosis and its use in previous studies in our laboratory. Interestingly, directed migration up mechanical gradients was observed when cells were seeded on fibronectin, but no bias in migration was observed on gradient substrates coated with laminin (Figs. 3–5). To verify that the lack of observable biased migration on gradient substrates coated with laminin was not due to a decrease in the overall migration rates of VSMCs on laminin relative to those observed on fibronectin, the velocities of cells migrating on gradients, as well as uniform high- and low-stiffness control gels, were measured. The matrix selection was not found to have a significant effect on the cell migration velocities (Fig. S2) and, as expected, randomly directed migration was observed on all uniform stiffness control regions (Fig. 4). Collectively, these results indicate that VSMCs were capable of adhering on substrates coated with either fibronectin or laminin and that the ability of cells to move on these substrates in regions of uniform stiffness did not vary significantly with matrix choice. However, though we observed biased migration toward the stiffer region of gradient gels coated with fibronectin, migration on laminin-coated gradients appeared to be random (Fig. 4), with cells exhibiting a distribution of tactic indices similar to that observed for migration on the uniform stiffness control gels (Fig. 5).
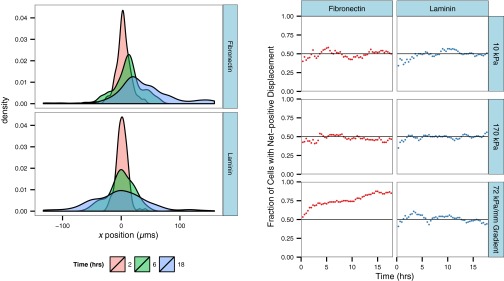
The distribution of tactic indices for cells on fibronectin-coated gels was found to shift to a mean of 0.17, but it is interesting to note that after 18 h of migration, ∼20% of cells still had a negative tactic index. To assess whether this might indicate a subpopulation of cells was not able to sense or respond to the mechanical gradient by exhibiting directed migration, we looked at the change in the distribution of x displacements over time (Fig. S3). For cells migrating randomly, we expect the distribution to widen over time but remain centered about x = 0, as observed for cells on laminin-coated gradients. If a subpopulation of cells on fibronectin exhibited this random migration behavior, a peak in the distribution centered at x = 0 should emerge at later time points. Rather, as time increases, there is a shift in the distribution toward positive x displacements corresponding to biased migration. Additionally, the left tail of the distribution does not continue to spread to more negative values, but rather moves toward more positive values than observed at earlier time points. A similar trend is observed for the fraction of cells with positive net displacements as a function of time. For randomly migrating cells, the fraction of cells with positive x displacements fluctuates around 0.5, whereas for cells exhibiting directed migration, this value increases from over time. The rate of this increase slows at later time points, but has not leveled off to an asymptote at a fraction <1. Taken together, these observations suggest that the negative tactic indices calculated for some cells are not necessarily indicative of a subpopulation of cells that does not respond to the gradient, but rather that migration would need to be observed for a longer duration to see the remainder of the population approach a positive x displacement.
Fig. S3.
The distribution of x displacements and fraction of cells exhibiting positive x displacements vary as a function of time. (Left) Kernel density estimation of x displacement of cells migrating on gradient substrates coated with fibronectin or laminin at t = 2, 6, and 18 h. (Right) Fraction of cells exhibiting net positive x displacement as a function of time on control and gradient substrates. n = 120 cells from n > 5 gels for each condition.
Collectively, the data presented here indicate that the ability of VSMCs to detect and respond to a gradient in substrate stiffness may be subject to the type of matrix they are seeded on, and that matrix composition could therefore be an important determinant of how cells respond to variations in stiffness in their local environment in vivo. Assessing the manner in which cells respond to these variations in stiffness when presented with different ECM molecules will be critical in understanding diseases in which the presence of a gradient in local tissue mechanical compliance is accompanied by alterations in the ECM composition, such as breast cancer, lung fibrosis, and atherosclerosis (12, 20, 21).
Future Directions.
The observation that VSMCs are capable of undergoing durotaxis on mechanical gradients coated with fibronectin but do not exhibit this behavior on laminin indicates that the ability of cells to sense and respond to variations in substrate stiffness may be ECM-type dependent, but the mechanisms underlying this matrix-dependent response are currently unknown. It is well documented that different integrin subtypes engage different matrix proteins, and thus the mechanical interaction between cells and the underlying substrates are mediated by different integrin–ECM pairings, which can vary in their bond strengths and dynamics of adhesion formation and turnover (48–52). Though beyond the scope of this present study, it is possible that such differences in the dynamics of integrin–ECM bond pairs could lead to variations in the response of VSMCs to stiffness gradients coated with fibronectin or laminin. Recent work by Elosegui-Artola et al. (53) has shown that, even for a single type of matrix, cell attachment via different integrins can result in changes in the traction forces exerted by cells as a function of stiffness, leading to the hypothesis that different integrins may be “tuned” to different environmental stiffnesses. Future experiments to evaluate whether VSMCs exhibit this integrin-type–dependent behavior and whether differences in a cell’s ability to generate tension on different types of matrix could lead to matrix-dependent changes in a cell’s sensitivity to stiffness gradients would help clarify the mechanisms underlying the results reported here.
Additionally, the experimental platform detailed here can be used to determine whether this phenomenon can be observed in other cell types and what the effect of additional matrix proteins beyond fibronectin and laminin are on directed migration; it could also potentially play a significant role in generating a library of cell responses to aid in the design of complex engineered tissues, for which control over the positioning and migration of one or more cell types would be desirable. Moreover, one can use this system to better understand complex dynamic environments in vivo. For example, the changing matrix environment in diseased states in terms of both composition and mechanics could work in concert to modulate cell migratory behavior. This experimental platform is ideally suited to assess whether such changes induce a migratory response and allows for the testing of therapeutics targeted to prevent or diminish such behavior.
Materials and Methods
Substrate Preparation.
Polyacrylamide gels featuring gradients in mechanical compliance between uniform stiffness control regions were prepared using surface tension-based glass microfluidic device (Fig. 1). Briefly, clean glass slides were coated with Microposit S1818 photoresist (Shipley) and were micropatterned to feature a dumbbell shape (Fig. 1) using maskless lithography (45). The pattern was developed in 2.5% (wt/wt) tetramethylammonium hydroxide in water (TMAOH; Sigma), and the exposed region was then treated with octadecyltrichlorosilane (OTS; Sigma) to form a hydrophobic region. The remaining photoresist was removed using acetone, and newly exposed portion of the surface was backfilled with a 2:1 ratio of 2[methoxy(poly(ethyleneoxy)n = 6–9)propyl]trimethoxysilane (Gelest) and 3-(trimethoxysilyl)propyl methacrylate (Sigma) to provide a hydrophilic, gel-adhesive surface. A micropatterned slide and a sacrificial glass slide coated with dichlorodimethylsilane (DCDM; Sigma) were stacked and separated by 250-µm spacers. Acrylamide solutions with varying monomer and cross-linker concentrations, adjusted to pH 6.0, consisting of acrylamide (Bio-Rad), bisacrylamide (Bio-Rad), amine-reactive cross-linker NHS–ester acrylic acid (Sigma), I2959 photoinitiator (Irgacure), and hydrochloric acid were injected into each end of the gradient device until the solutions contacted in the center of the device. The solutions were allowed to diffuse for 40 min for 2-mm gradient gels and 10 min for 1-mm gradient gels to establish a linear gradient in cross-linker concentration before polymerization under UV light for 240 s. Hydrogels were stored PBS adjusted to pH 6.0 until protein functionalization was performed.
Mechanical Characterization.
Characterization of mechanical gradient gels was performed as previously described (16); complete details of the method are given in SI Materials and Methods.
Substrate Functionalization.
Polyacrylamide gradient gels were functionalized with ECM proteins via NHS–ester acrylic acid, which incorporates into the hydrogel backbone during polymerization and can subsequently be reacted with primary amines to covalently attach proteins to the gel surface. Polyacrylamide gels were incubated in a solution of fibronectin (Millipore) or laminin-1 (Sigma) at 5 µg/cm2 in PBS adjusted to pH 8.0 for 2 h at room temperature. The uniformity of ECM attachment across gradient gels was assessed from fluorescent micrographs of gradient gels functionalized with Alexa Fluor 488 NHS ester (Life Technologies)-labeled fibronectin or laminin. The average fluorescence intensity was measured in 33-µm-wide bins across the gel oriented parallel to the direction of the gradient.
Cell Culture and Motility.
Cell culture and tracking was performed as described in SI Materials and Methods.
SI Materials and Methods
Mechanical Characterization.
Polyacrylamide gels with gradients in stiffness were characterized by atomic force microscopy. Nanoindentation measurements were performed using an AFM (MFP-3D; Asylum Research) with a micromanipulator-controlled stage and a silicon nitride cantilever (0.06 N/m) functionalized with a 10-μm borosilicate bead (Novascan). Gels were indented in PBS at room temperature in 250-µm steps parallel to the direction of the gradient at an indentation rate of 500 nm/s. The elastic modulus was calculated by fitting force vs. indentation depth data to a linearized Hertz model (54, 55).
Cell Culture and Motility.
Bovine aortic VSMCs were cultured in DMEM (Gibco) supplemented with l-glutamine (Gibco) and 10% (vol/vol) bovine calf serum (HyClone) at 37 °C and 5% CO2. Cells were seeded at a density of 1,500/cm2 on polyacrylamide gels coated with either fibronectin or laminin and were allowed to attach for 6 h before imaging. A minimum of five gels per experimental condition were examined in independent experiments. For migration experiments, we chose to use hydrogels with a relatively steep gradient stiffness profile of 72 kPa/mm, which was found to be sufficient to induce durotaxis in VSMCs between regions of uniform stiffness comparable to those found in vascular tissue in vivo (56). A gradient steepness of 72 kPa/mm allowed for directional migration to be induced on fibronectin-coated gels while still allowing an area of observation sufficient for 5–10 noncontacting cells per gel gradient region. Cells adhered to the low stiffness, high stiffness, and gradient stiffness regions of each gel were imaged in 20-min intervals over a period of 18 h using an inverted optical microscope (Zeiss) equipped with a motorized stage (Ludl Electronic Products) and a custom-built incubator to control temperature, CO2, and humidity. Cell centroid positions were tracked in ImageJ, and tracks were assembled and analyzed using a custom R script. The durotactic index was calculated as the migration distance in the direction of the gradient divided by the cumulative migration distance. Statistical analysis of mean cell displacement and durotactic index was performed using two-factor ANOVA and Tukey’s honest significant difference test for post hoc analysis where appropriate.
Acknowledgments
This work was supported by NIH Grants R01 HL072900 and R01 HL124280 (to J.Y.W.); NIH Predoctoral Training Grant NIGMS 5T32 GM008764 (to C.D.H.); and a Lutchen fellowship (to S.G.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611324113/-/DCSupplemental.
References
- 1.Trinkaus JP. Further thoughts on directional cell movement during morphogenesis. J Neurosci Res. 1985;13(1-2):1–19. doi: 10.1002/jnr.490130102. [DOI] [PubMed] [Google Scholar]
- 2.Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol. 2010;341(1):20–33. doi: 10.1016/j.ydbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11(8):573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber M, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339(6117):328–32. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 7.Carter SB. Principles of cell motility: The direction of cell movement and cancer invasion. Nature. 1965;208(5016):1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- 8.Lo CM, Wang HB, Dembo M, Wang Y-L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry MF, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp. 2011;(54):2911. doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez JI, Kang I, You W-K, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol (Camb) 2011;3(9):910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plodinec M, et al. The nanomechanical signature of breast cancer. Nat Nanotechnol. 2012;7(11):757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- 13.Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98(12):2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19(5):1908–1913. [Google Scholar]
- 15.Gray DS, Tien J, Chen CS. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J Biomed Mater Res A. 2003;66(3):605–614. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg BC, Dimilla PA, Walker M, Kim S, Wong JY. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J. 2009;97(5):1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6(1):e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung YK, et al. Microscale control of stiffness in a cell-adhesive substrate using microfluidics-based lithography. Angew Chem Int Ed Engl. 2009;48(39):7188–7192. doi: 10.1002/anie.200900807. [DOI] [PubMed] [Google Scholar]
- 19.Kidoaki S, Matsuda T. Microelastic gradient gelatinous gels to induce cellular mechanotaxis. J Biotechnol. 2008;133(2):225–230. doi: 10.1016/j.jbiotec.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Tracqui P, et al. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J Struct Biol. 2011;174(1):115–123. doi: 10.1016/j.jsb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akyildiz AC, Speelman L, Gijsen FJH. Mechanical properties of human atherosclerotic intima tissue. J Biomech. 2014;47(4):773–783. doi: 10.1016/j.jbiomech.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81(3):173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebenstein DM, Coughlin D, Chapman J, Li C, Pruitt LA. Nanomechanical properties of calcification, fibrous tissue, and hematoma from atherosclerotic plaques. J Biomed Mater Res A. 2009;91(4):1028–1037. doi: 10.1002/jbm.a.32321. [DOI] [PubMed] [Google Scholar]
- 26.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295(4):C1037–C1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 27.Sazonova OV, et al. Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol. 2015;41:36–43. doi: 10.1016/j.matbio.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Gilchrist CL, Darling EM, Chen J, Setton LA. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell-cell interactions. PLoS One. 2011;6(11):e27170. doi: 10.1371/journal.pone.0027170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calve S, Simon H-G. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 2012;26(6):2538–2545. doi: 10.1096/fj.11-200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shebanova O, Hammer DA. Biochemical and mechanical extracellular matrix properties dictate mammary epithelial cell motility and assembly. Biotechnol J. 2012;7(3):397–408. doi: 10.1002/biot.201100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roca-Cusachs P, Sunyer R, Trepat X. Mechanical guidance of cell migration: Lessons from chemotaxis. Curr Opin Cell Biol. 2013;25(5):543–549. doi: 10.1016/j.ceb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 33.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol (1985) 2005;98(6):2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 34.Louis SF, Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. Exp Clin Cardiol. 2010;15(4):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 35.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Yamamoto K, Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res. 1993;204(1):121–129. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 37.Thyberg J, Hultgårdh-Nilsson A. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 1994;276(2):263–271. doi: 10.1007/BF00306112. [DOI] [PubMed] [Google Scholar]
- 38.Qin H, et al. Effects of extracellular matrix on phenotype modulation and MAPK transduction of rat aortic smooth muscle cells in vitro. Exp Mol Pathol. 2000;69(2):79–90. doi: 10.1006/exmp.2000.2321. [DOI] [PubMed] [Google Scholar]
- 39.Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45(6):837–846. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- 40.Timraz SBH, Rezgui R, Boularaoui SM, Teo JCM. Stiffness of extracellular matrix components modulates the phenotype of human smooth muscle cells in vitro and allows for the control of properties of engineered tissues. Procedia Eng. 2015;110:29–36. [Google Scholar]
- 41.Newby AC, Zaltsman AB. Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41(2):345–360. [PubMed] [Google Scholar]
- 42.Kuo C-HR, Xian J, Brenton JD, Franze K, Sivaniah E. Complex stiffness gradient substrates for studying mechanotactic cell migration. Adv Mater. 2012;24(45):6059–6064. doi: 10.1002/adma.201202520. [DOI] [PubMed] [Google Scholar]
- 43.Sunyer R, Jin AJ, Nossal R, Sackett DL. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS One. 2012;7(10):e46107. doi: 10.1371/journal.pone.0046107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng P, Di Carlo D. Substrates with patterned extracellular matrix and subcellular stiffness gradients reveal local biomechanical responses. Adv Mater. 2014;26(8):1242–1247. doi: 10.1002/adma.201304607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoga K, Kobayashi J, Tsuda Y, Yamato M, Okano T. Second-generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal Chem. 2008;80(4):1323–1327. doi: 10.1021/ac702208d. [DOI] [PubMed] [Google Scholar]
- 46.Kawano T, Kidoaki S. Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels. Biomaterials. 2011;32(11):2725–2733. doi: 10.1016/j.biomaterials.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Tse JR, Engler AJ. 2010. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol, Chapter 10:Unit 10.6,
- 48.Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: Regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8(2):51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 49.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385(6616):537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 50.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3(9):a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88(1):39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 52.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66(2):247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 53.Elosegui-Artola A, et al. Rigidity sensing and adaptation through regulation of integrin types. Nat Mater. 2014;13(6):631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domke J, Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14(12):3320–3325. [Google Scholar]
- 55.Guo S, Akhremitchev BB. Packing density and structural heterogeneity of insulin amyloid fibrils measured by AFM nanoindentation. Biomacromolecules. 2006;7(5):1630–1636. doi: 10.1021/bm0600724. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto T, Abe H, Ohashi T, Kato Y, Sato M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas. 2002;23(4):635–648. doi: 10.1088/0967-3334/23/4/304. [DOI] [PubMed] [Google Scholar]