Significance
β-Tubulin is a validated target for Taxol. There are seven β-tubulin isotypes present in distinct quantities in mammalian cells of different origin. Altered expression of βIII-tubulin is found in cancer cell lines and in human tumors resistant to Taxol. A tritium-labeled Taxol analog was used to determine that βIII-tubulin binds the least amount of drug, compared with other isotypes. Sequence analysis near the Taxol binding site revealed the presence of a unique Ala218 in βIII-tubulin. Molecular dynamics simulations indicated that this residue could influence the ability of Taxol to interact with its binding site. These results link drug response with the β-tubulin isotypes present in a tumor and emphasize the importance of designing Taxol analogs that specifically interact with βIII-tubulin.
Keywords: Taxol, microtubule stabilizing agents, tubulin isotypes, drug binding, photolabeling
Abstract
There are seven β-tubulin isotypes present in distinct quantities in mammalian cells of different origin. Altered expression of β-tubulin isotypes has been reported in cancer cell lines resistant to microtubule stabilizing agents (MSAs) and in human tumors resistant to Taxol. To study the relative binding affinities of MSAs, tubulin from different sources, with distinct β-tubulin isotype content, were specifically photolabeled with a tritium-labeled Taxol analog, 2-(m-azidobenzoyl)taxol, alone or in the presence of MSAs. The inhibitory effects elicited by these MSAs on photolabeling were distinct for β-tubulin from different sources. To determine the exact amount of drug that binds to different β-tubulin isotypes, bovine brain tubulin was photolabeled and the isotypes resolved by high-resolution isoelectrofocusing. All bands were analyzed by mass spectrometry following cyanogen bromide digestion, and the identity and relative quantity of each β-tubulin isotype determined. It was found that compared with other β-tubulin isotypes, βIII-tubulin bound the least amount of 2-(m-azidobenzoyl)taxol. Analysis of the sequences of β-tubulin near the Taxol binding site indicated that, in addition to the M-loop that is known to be involved in drug binding, the leucine cluster region of βIII-tubulin contains a unique residue, alanine, at 218, compared with other isotypes that contain threonine. Molecular dynamic simulations indicated that the frequency of Taxol-accommodating conformations decreased dramatically in the T218A variant, compared with other β-tubulins. Our results indicate that the difference in residue 218 in βIII-tubulin may be responsible for inhibition of drug binding to this isotype, which could influence downstream cellular events.
The tubulin/microtubule system is a validated target for a number of important antitumor drugs; examples are the vinca alkaloids, the taxanes, and the epothilones, all of natural product origin. Our laboratory has studied extensively the microtubule stabilizing agent (MSA) Taxol (paclitaxel), which has been approved for treatment of a variety of malignancies, including ovarian, breast, and lung carcinomas, and Ixempra (ixabepilone), approved for metastatic breast cancer. MSAs abrogate normal microtubule function by stabilizing the polymer, leading to inhibition of cell division and cell death. By altering the dynamicity of microtubules, Taxol also exerts a variety of effects in interphase cells, such as interfering with microtubule trafficking (1, 2).
Microtubules are made up of α- and β-tubulin, the latter containing the binding site for Taxol (3, 4). There are seven β- and eight α-tubulin isotypes present in distinct quantities in mammalian cells of different origin (5). Each isotype is the product of an individual gene. Expression of different tubulin isotypes, as well as altered posttranslational modifications (PTMs) of tubulin, have been found in cancers (6, 7). It has been reported that in several cancers, overexpression of βIII-tubulin is associated with resistance to Taxol and other classes of antitumor drugs (8, 9). βIII-Tubulin–induced Taxol resistance has been associated with reduced effects on microtubule dynamic instability (10).
We have demonstrated that the expression level of β-tubulin isotypes is altered in cancer cell lines resistant to MSAs, such as Taxol, epothilone B (EpoB), and ixabepilone (Ixab), all of which bind in the taxane binding site in β-tubulin (11, 12). For example, the level of βIII-tubulin is very low in the human ovarian cancer cell line, Hey, but increased 8- to 15-fold in drug-resistant daughter cell lines (12). It has been reported that βIII-tubulin present in the cytoskeleton is phosphorylated and glycosylated, and associated with the resistant phenotype in ovarian cancer cells (13). Although many factors play a role in drug resistance, binding of drugs to their primary cellular target is of fundamental importance. Therefore, it is worthwhile to study the binding of MSAs to different β-tubulin isotypes, particularly to βIII-tubulin, and determine if there is a correlation between isotype expression, drug binding, and drug sensitivity. To study binding of MSAs to human tubulin, in particular to different human tubulin isotypes, sufficient quantities of soluble tubulin is necessary. However, the expression of significant levels of human tubulin in a cell-free system has been difficult to accomplish, although recently recombinant αIβIII-tubulin has been expressed in a baculovirus system (14). As a first step, we have performed binding studies using tubulin from three eukaryotic sources, bovine brain (BBT), porcine brain (PBT), and chicken erythrocytes (CET). BBT and PBT contain mainly βI-, βII-, βIII-, and βIV isotypes, whereas CET contains only βVI-tubulin.
We previously demonstrated that [3H]Taxol binding to BBT was almost completely inhibited by equal molar discodermolide, another MSA, but its binding to CET was not inhibited (15). These results suggested that the binding affinity of discodermolide (Disco) was different for tubulins with distinct β-tubulin isotype content. In this report, we investigated the relative binding affinities of MSAs to β-tubulin isolated from different eukaryotic sources, using a radiolabeled photoaffinity Taxol analog, [3H]2-(m-azidobenzoyl)taxol (2-m-AzTax). We also determined the amount of [3H]2-m-AzTax binding to different β-tubulin isotypes resolved by high-resolution isoelectrofocusing (IEF), followed by identification with mass spectrometry (16).
Our studies demonstrated that, compared with other β-tubulin isotypes, βIII-tubulin binds the least amount of [3H]2-m-AzTax. Analysis of sequences of β-tubulin near the Taxol binding site indicated that there is a unique alanine residue at 218 in the leucine cluster region (17) of βIII-tubulin, whereas all other isotypes have a threonine at this position. Subsequent computational molecular simulation studies indicated that the frequency of Taxol-accommodating conformations decreased dramatically in the T218A variant compared with the other isotypes.
Results
MSAs Have Distinct Effects on Photolabeling of β-Tubulin from Different Eukaryotic Sources.
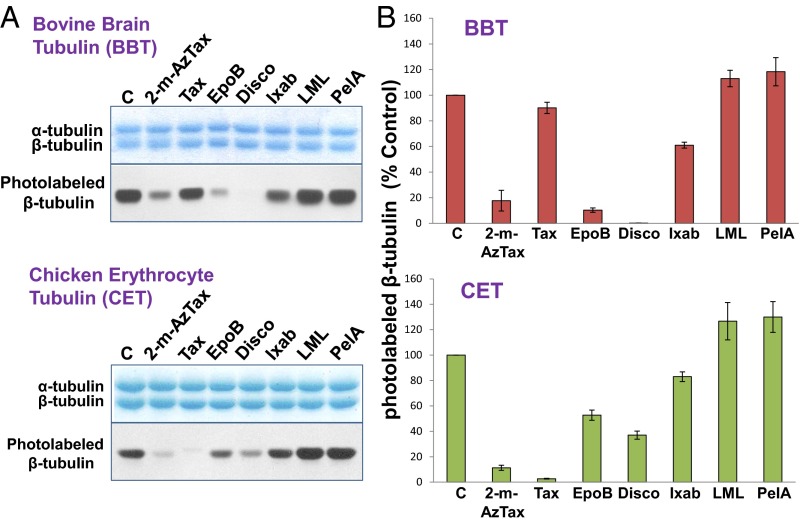
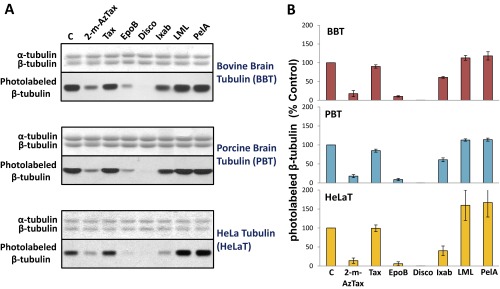
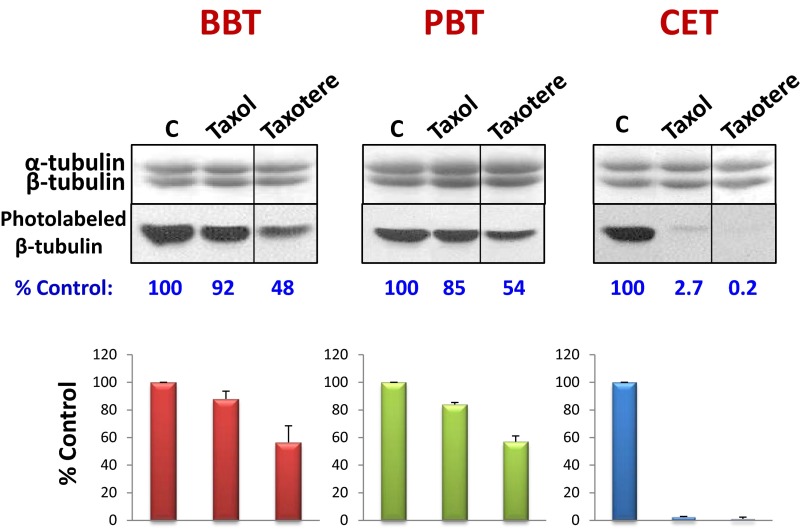
To study the relative binding affinities of MSAs, tubulin from bovine brain, porcine brain, HeLa cells, and chicken erythrocytes was specifically photolabeled with [3H]2-m-AzTax, in the presence and absence of a fourfold molar excess of either Taxol, EpoB, Disco, Ixab, laulimalide (LML), or peloruside A (PelA). GTP (1 mM) was included in all experiments. β-Tubulin isotype content from these sources are different, particularly for chicken erythrocytes that contain only one β-tubulin isotype, βVI. The inhibitory effects elicited by Taxol, EpoB, Disco, and Ixab on photolabeling were distinct for β-tubulin from BBT and CET (Fig. 1A). Image quantification of each band indicated that a fourfold molar excess of unlabeled 2-m-AzTax inhibited the photolabeling of β-tubulin by 80–90%, demonstrating the specificity of this photolabeling. Taxol had a minimal inhibitory effect on BBT, but a strong inhibitory effect on CET. In contrast, EpoB and Disco exhibited strong inhibitory effects on BBT but moderate effects on CET (Fig. 1). This finding is most likely a result of the presence of distinct tubulin isotypes in BBT and CET (Table S1) (18–21). Ixab had moderate effects on both systems. It is to be noted that fourfold molar excess of LML or PelA had a stimulatory effect (∼15–30% increase) on photolabeling of tubulin from these two sources (Fig. 1). When BBT, PBT, and HeLa cell tubulin (HeLaT) were compared, all MSAs exhibited similar effects on photolabeling, except that Ixab had a stronger inhibitory effect on HeLaT, compared with BBT and PBT (Fig. S1). Although Taxol’s effects on BBT and PBT are minimal, Taxotere (docetaxel) appeared to have a stronger inhibitory effect than Taxol on photolabeling of β-tubulin, indicating that structural modifications of Taxol can alter its interaction with β-tubulin (Fig. S2).
Fig. 1.
MSAs exhibit distinct effects on 2-m-AzTax photoaffinity labeling of β-tubulin from bovine brain and chicken erythrocytes. Four micromolars soluble BBT or CET were incubated with 5 µM [3H]2-m-AzTax and 1 mM GTP, in the absence or presence of a fourfold molar excess of MSAs, followed by UV irradiation as described in Materials and Methods. (A) Analysis by 9% SDS/PAGE and fluorography. Tax: Taxol. (B) Each band in A was image quantified by ImageJ. Results from three to four experiments are presented as mean ± SE. C, control.
Table S1.
Isotype composition of isolated tubulins
| Tubulin source | Tubulin Isotype (%) | Source | |||||
| βI | βII | βIII | βIV | βV | βVI | ||
| Bovine brain | 3 | 58 | 25 | 13 | ND | 0 | (18) |
| HeLa cells* | 90 | 0 | 0 | 10 | ND | 0 | (19) |
| Chicken erythrocytes | 0 | 0 | 0 | 0 | 0 | 100 | (20, 21) |
Isotype composition of porcine brain tubulin is very similar to bovine brain tubulin (see Fig. S7). It should be noted that βI- and βII-tubulins have a very similar sequence, and it was found that the available βII-tubulin antibody cross-reacts with βI-tubulin (16). They are often grouped together (βI/βII). ND, not determined.
We have estimated that βIII-tubulin and βV-tubulin content in HeLa cells is ≤1% and ≤5%, respectively.
Fig. S1.
MSAs exhibit similar effects on 2-m-AzTax photoaffinity labeling of β-tubulins from BBT, PBT, and HeLaT. (A) Four micromolars tubulin from different eukaryotic sources was incubated with 5 µM [3H]2-m-AzTax in the absence or presence of fourfold molar excess of different MSAs, followed by UV irradiation, SDS/PAGE, and fluorography as described in Fig. 1. The bands in the upper panels represent α- and β-tubulin stained with Coomassie Blue. (B) Each band in the lower panels of A was image quantified by ImageJ and plotted. Results were analyzed from three to four experiments and data were presented as mean ± SE.
Fig. S2.
Inhibition of photolabeling by Taxotere. Four micromolar BBT, PBT, and CET were incubated with 5 µM [3H]2-m-AzTax in the absence or presence of fourfold molar excess (20 µM) Taxol or Taxotere. Samples were UV-irradiated and analyzed by SDS/PAGE as described in Materials and Methods. The bands in the upper panels represent α- and β-tubulin stained with Coomassie Blue. Photolabeling data were plotted as %Control ± SE, from 2 independent experiments.
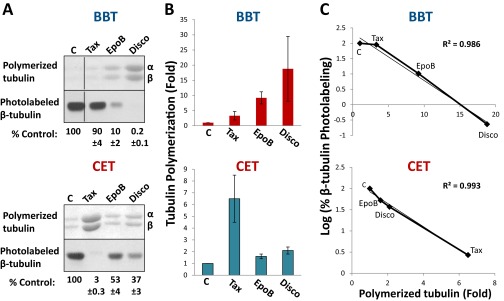
The Stimulatory Effect of Taxol, EpoB, and Disco on Tubulin Polymerization Correlates with Their Inhibitory Effect on Photolabeling of β-Tubulin.
Tubulin from BBT and CET was treated with Taxol, EpoB, or Disco and the polymerized microtubules isolated. The protein levels of microtubules after each treatment were compared with the photolabeling data, and it was found that there was an excellent correlation between the stimulatory effect of Taxol, EpoB, and Disco on tubulin polymerization and their inhibitory effect on photolabeling of β-tubulin (r = 0.99) (Fig. S3). These results suggest that binding affinities of MSAs correlate with their effects on tubulin polymerization.
Fig. S3.
The stimulatory effect of Taxol, epoB, and Disco on tubulin polymerization correlates well with their inhibitory effect on photolabeling of β-tubulin. (A) Tubulin polymerization assays and photolabeling of tubulin with 2-m-AzTax in the absence or presence of MSAs were carried out as described in Materials and Methods. Tax-, EpoB- or Disco-induced polymerized tubulins were stained with Coomassie Blue (upper panels). Photolabeled β-tubulin (lower panels) was presented as %Control (mean ± SE, n=3-4). (B) Tax-, EpoB- or Disco-induced tubulin polymerization indicated in the upper panels of A was image quantified and plotted. Results were analyzed from three to four experiments and data are presented as mean ± SE. (C) The stimulatory effect of the drugs on tubulin polymerization and their inhibitory effect on photolabeling of β-tubulin were compared and the correlation coefficients determined.
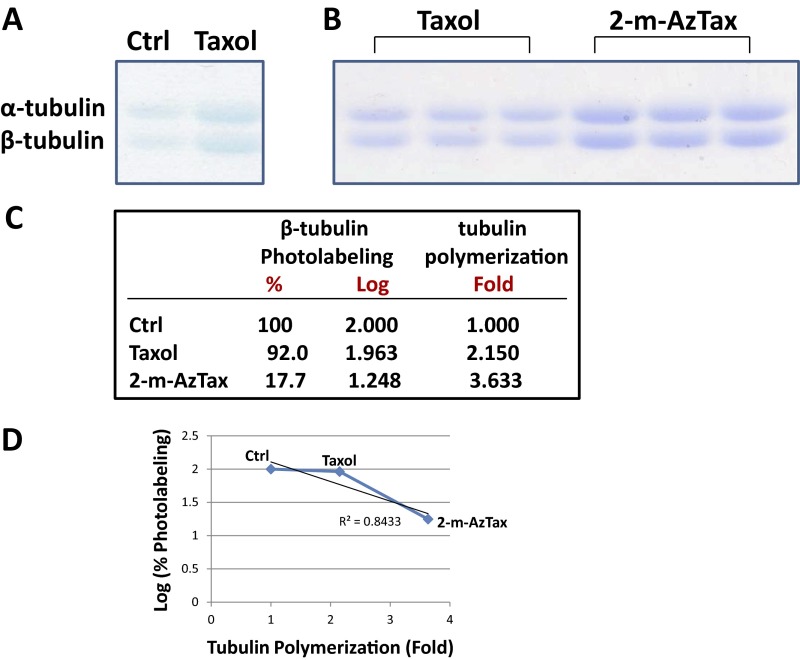
It is noted that Taxol inhibits photolabeling of BBT only ∼8 ± 5%, whereas 2-m-AzTax inhibits 80–90%. To determine if this difference is a result of different quantities of polymer formed with the two compounds, a polymerization experiment was performed using 4 µM BBT and 5 µM Taxol or 5 µM 2-m-AzTax (stoichiometry: ∼1:1). It was found that 2-m-AzTax induced approximately twice as much polymer compared with Taxol (Fig. S4). The correlation between the effect of these two drugs on tubulin polymerization and on the logarithm of β-tubulin photolabeling was plotted, and a correlation coefficient of 0.843 was obtained.
Fig. S4.
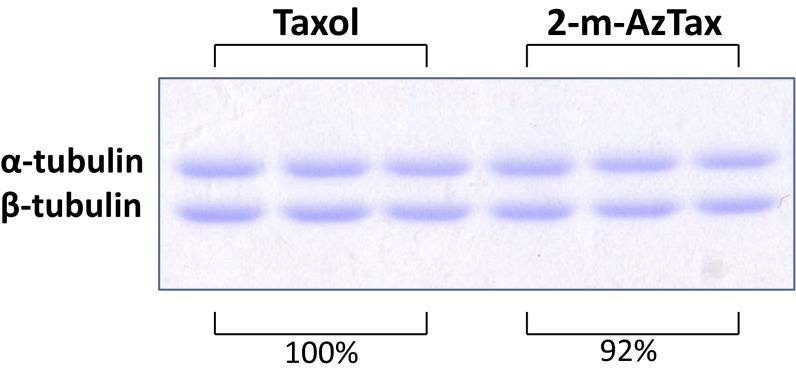
Effect of Taxol and 2-m-AzTax on BBT polymerization and photolabeling. (A) Four micromolars BBT was incubated with 5 µM Taxol for 30 min at 37 °C, in the presence of 1 mM GTP. Samples were centrifuged at 100,000 × g and the pellets were solubilized in sample buffer and resolved by SDS/PAGE. (B) Polymerization of tubulin was done using 5 µM Taxol or 5 µM 2-m-AzTax. (C) Level of polymerized tubulin in A and B was quantified by ImageJ and fold-increase in tubulin polymerization was calculated and normalized. Data for the effect of Taxol and 2-m-AzTax on β-tubulin photolabeling was obtained from Fig. 1B. (D) Tubulin polymerization (fold) was plotted against the logarithm values of percent β-tubulin photolabeling, and correlation coefficient determined.
Unlike the minimal effect on 2-m-AzTax photolabeling elicited by Taxol in BBT, both Taxol and 2-m-AzTax significantly inhibited photolabeling in CET. These two compounds do not induce a major difference in polymerization of CET (Fig. S5). Taxol actually causes a slightly higher level of polymerization, compared with 2-m-AzTax. These polymerization results correlated well with the CET photolabeling pattern (Fig. 1).
Fig. S5.
Effect of Taxol and 2-m-AzTax on CET polymerization. Four micromolars chicken erythrocyte tubulin was incubated with 5 µM Taxol or 5 µM 2-m-AzTax for 30 min at 37 °C, in the presence of 1 mM GTP. Samples were centrifuged at 100,000 × g and the pellets were solubilized in sample buffer and resolved by SDS/PAGE.
Taxol Analog 2-m-AzTax Binds Differentially to Distinct Tubulin Isotypes.
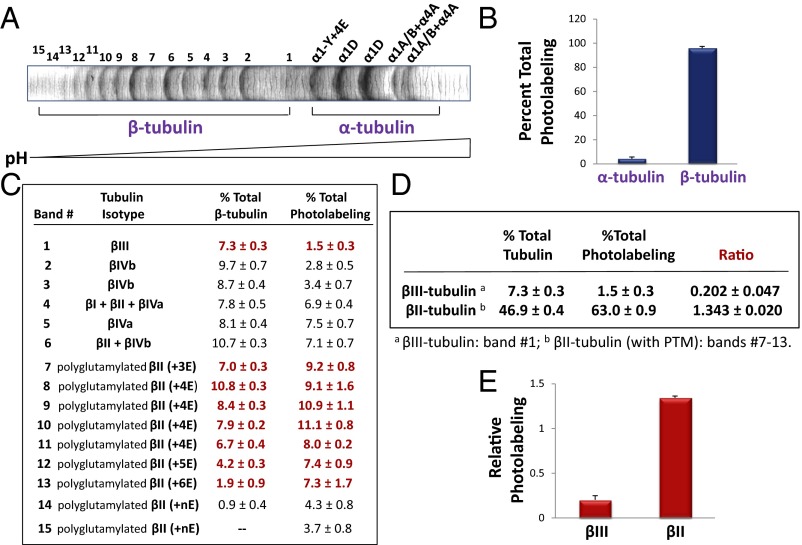
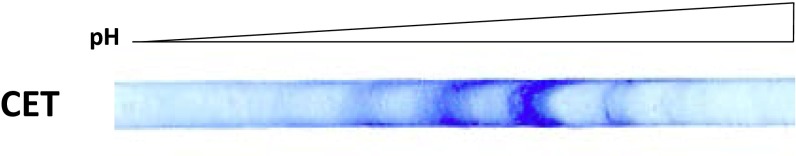
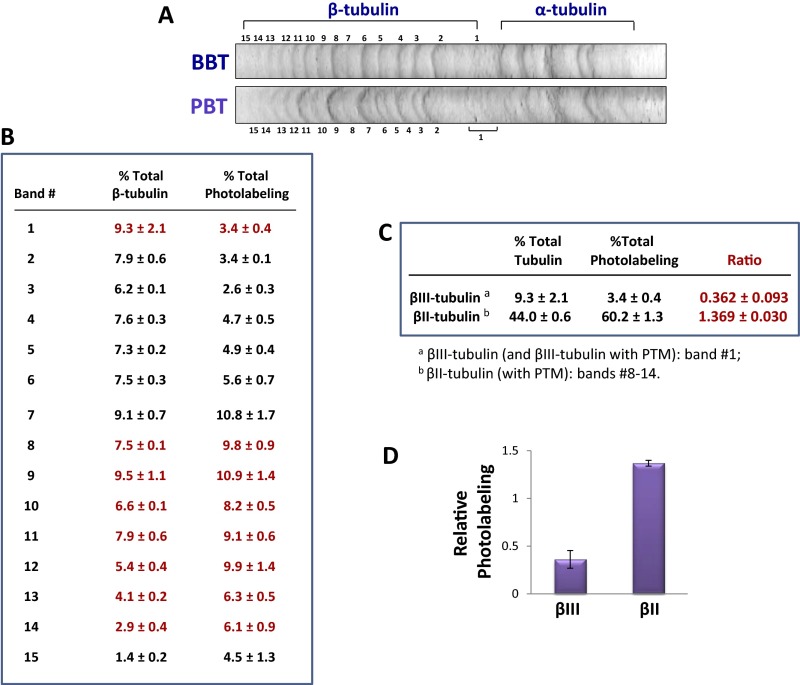
To determine the amount of drug that binds to different β-tubulin isotypes, tubulin from different sources was photolabeled and the isotypes resolved by high-resolution IEF. Approximately 20 bands were seen with BBT (Fig. 2A), but only 3–5 bands were resolved with CET (Fig. S6). Tubulin is known to be extensively PTM, so many of the bands represent PTM tubulin (22). [3H]2-m-AzTax bound essentially only to β-tubulin and not α-tubulin (Fig. 2B). Each band was isolated and analyzed by mass spectrometry following cyanogen bromide (CNBr) digestion, and the identity and relative quantity of each β-tubulin isotype determined (Fig. 2C). After examining the identity of each band, it was found that some bands contained more than one isotype. Therefore, the exact amount of drug that binds to each individual tubulin isotype, except for βIII- and PTM βII-tubulin, could not be determined. After measuring the radioactivity associated with each band and normalizing the values to the relative protein level of the corresponding band, it was found that all βII-tubulin containing different PTMs bind approximately sixfold more drug than βIII-tubulin (Fig. 2 C–E). βIII-Tubulin bound the lowest amount of 2-m-AzTax, compared with other β-tubulin isotypes. The data also indicate that βIVb-tubulin binds a relatively low level of the drug. IEF patterns for BBT and PBT were very similar (Fig. S7A) and similar results were obtained when photolabeling and IEF experiments were performed with PBT (Fig. S7 B–D).
Fig. 2.
Taxol analog 2-m-AzTax differentially photoaffinity labels distinct tubulin isotypes. Five micromolar BBT was incubated with 6 µM [3H]2-m-AzTax, in the presence of 1 mM GTP, at 37 °C for 30 min, followed by UV irradiation and centrifugation as described in Materials and Methods. (A) Separation of BBT by high-resolution IEF. Tubulin isotypes were identified by mass spectrometry. (B) Photolabeling of total α-tubulin and β-tubulin. (C) β-Tubulin isotypes were identified by CNBr digestion and mass spectrometry. Level of each isotype was determined by ImageJ and the amount of radioactivity associated with each band was determined by liquid scintillation counting as described in Materials and Methods. (D and E) Amount of radioactivity associated with βII- and βIII-tubulin (red bolded values in C) was normalized to their protein levels, and relative photolabeling of βII- and βIII-tubulin was plotted.
Fig. S6.
Separation of CET by high-resolution IEF. Forty micrograms CET was resolved by IEF using an IEF strip with pH range of 3–5.6.
Fig. S7.
Binding of [3H]2-m-AzTax to PBT isotypes. (See Fig. 2 for BBT results). (A) Resolution of PBT by IEF using pH 3–5.6 IEF strips. (B) Protein level of each band was determined by ImageJ and the amount of radioactivity associated with each band was determined by liquid scintillation counting. (C and D) Amount of radioactivity associated with βII- and βIII-tubulin was normalized to their protein levels (red values in B) and plotted.
Unique Alanines at Residues 218 and 275 Are Present in the Leucine Cluster and the M-Loop Domains of βIII-Tubulin.
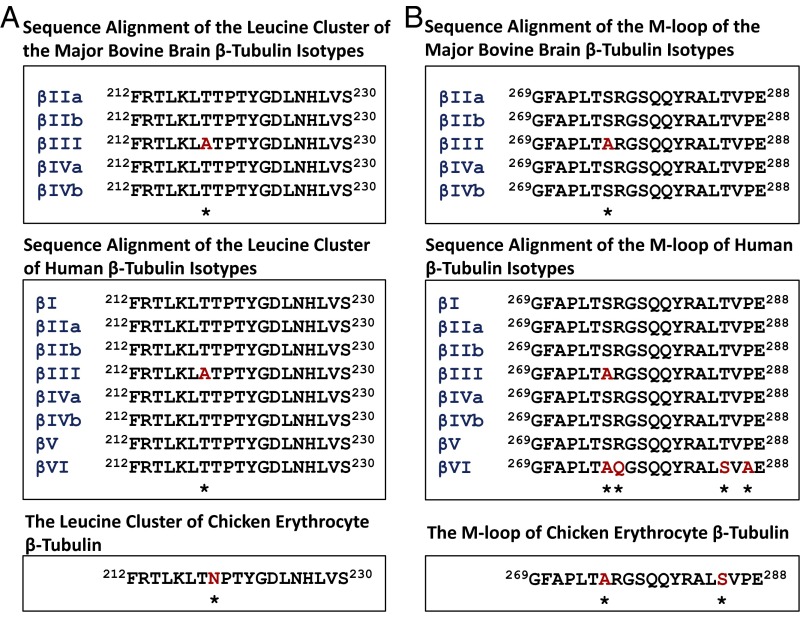
Sequence alignment of the leucine cluster region of bovine brain and human β-tubulin indicated that βIII-tubulin harbors a unique alanine at residue 218 that is different from all other β-tubulin isotypes that contain a threonine at this residue (Fig. 3A). Murine βIII-tubulin also has this unique Ala218. In the M-loop of human β-tubulin, βIII- as well as βVI-tubulin each contains an alanine at residue 275, whereas other isotypes harbor serine at this position (Fig. 3B). Both of these domains have been shown to be important for the binding of taxanes to β-tubulin (4, 17, 23) in microtubules.
Fig. 3.
Sequence alignment of the leucine cluster (A) and the M-loop (B) of β-tubulin isotypes from bovine brain, human, and chicken erythrocytes. The asterisks denote altered residues (in red).
The Frequency of Taxol-Accommodating Conformations Is Reduced Dramatically in the T218A Variant.
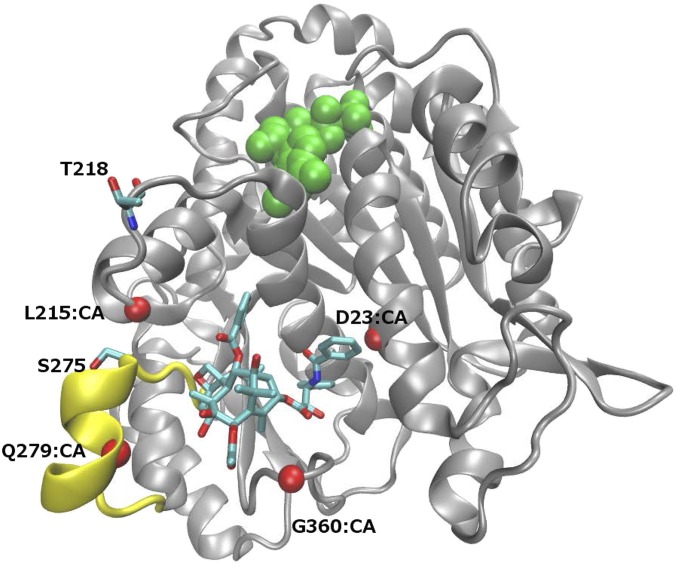
To gain insight into possible structural and functional differences between human β-tubulin isotypes I and III and their binding to Taxol, we built molecular models for these proteins in complex with Taxol and ran molecular dynamics simulations. To establish whether residues 218 and 275 are involved in drug binding and have contacts that could explain the differences observed in binding, comparative protein structure models were built for the βI-tubulin monomer with Taxol in the binding pocket and analyzed (Fig. 4). After running 20 independent 30-ps-long molecular dynamic simulations and analyzing 600 snapshots, no contacts between T218 and Taxol were found. S275 makes contacts with Taxol, but none are energetically favorable (hydrogen bond, hydrophobic, or aromatic). Hence, the lower binding affinity of βIII-tubulin cannot be attributed to loss of direct interaction between Taxol and residues 218 and 275.
Fig. 4.
Class III β-tubulin subunit contains unique residue 218 (T218A) in the leucine cluster and residue 275 (S275A) in the M-loop. β-Tubulin subunit (gray) in complex with Taxol (stick representation) and GDP (green). M-loop (yellow) is involved in both Taxol binding and interaction with lateral β-tubulin in the microtubule. Four Cα atoms (CA, red spheres) are used to define the binding pocket dimensions. T218 and S275, which are alanines in β-tubulin isotype III, have negligible interactions with Taxol.
To establish if differences in binding pocket accessibility, if any, could explain the difference in binding, molecular dynamic simulations were performed on the βI-tubulin monomer with GDP (Fig. 4). For each 1-ps snapshot, a representative set of six distances were monitored across the binding pocket (Fig. 4 and Table 1). Both the average distance across all snapshots and the percentage of snapshots that have a distance equal or greater than that in the corresponding βI-tubulin complex with Taxol inside the binding pocket are shown (Fig. 4).
Table 1.
Distances across binding pocket in monomers of βI-tubulin and variants
| Distance definition | Distance in complex | Average distance [Å] (% of snapshots with distance ≥ distance in complex) | ||
| βI-Tubulin | T218A | T218A.S275A | ||
| 23–215 | 18.39 | 20.1 ± 0.9 (97.3) | 18.9 ± 1.4 (63.5) | 19.3 ± 1.4 (76.0) |
| 23–279 | 23.34 | 26.5 ± 1.3 (99.2) | 23.1 ± 2.6 (51.5) | 23.8 ± 3.4 (73.3) |
| 23–360 | 11.34 | 12.4 ± 1.6 (73.3) | 12.0 ± 1.4 (67.2) | 13.2 ± 1.4 (91.2) |
| 215–279 | 9.8 | 11.3 ± 1.0 (95.2) | 12.8 ± 2.5 (85.3) | 12.6 ± 1.5 (97.6) |
| 215–360 | 16.43 | 17.6 ± 1.4 (80.3) | 17.5 ± 1.3 (78.7) | 17.8 ± 1.8 (79.5) |
| 279–360 | 16.06 | 18.5 ± 1.7 (90.7) | 14.7 ± 2.7 (36.3) | 14.9 ± 3.4 (38.9) |
Distance is measured between the Cα atoms of the two residues in the definition.
Three sets of distances were significantly decreased in the variants (βI-tubulin containing T218A): the distances between residues 23 and 215, 23 and 279, and that between 279 and 360. Although the βI-tubulin maintains these pocket distances in greater than 90% of the time, the variants have a smaller fraction of time in which these pocket distances are large enough to accommodate Taxol. In particular, in the case of residues 279 and 360, the frequency of Taxol-accommodating conformations dropped from 90.7% in βI-tubulin to 36.3% and 38.9% in the variants T218A and T218A.S275A, respectively.
The same analysis was extended to a lateral dimer of β-tubulin with two molecules of GDP. The lateral interaction between the two β-tubulin subunits caused compaction around the M-loop. Indeed, a slight deformation in the binding pocket in our complex of the lateral dimer with Taxol bound was observed, resulting in different values of the six distances. The compaction also resulted in reduced flexibility around the binding pocket, leading to smaller differences in the distances between βI-tubulin and the variant. Nonetheless, the difference in distance between residues 279 and 360 persisted: the βI-tubulin maintained a distance equal or greater than the complex distance (13.05 Å) in 23% of the time, whereas the variants rarely had this portion of the pocket opened (0.8% for T218A; 7.2% for T218A.S275A).
Discussion
Development of resistance to antitumor drugs is a complex process and it is for this reason that it has proven to be so difficult to overcome in patients. An increase in βIII-tubulin has been associated with a reduced sensitivity to Taxol and other drugs, and there are a variety of interpretations for this in the literature (8, 9). In our study, we demonstrated that MSAs, such as Taxol, EpoB, Disco, and Ixab exhibited differential inhibitory effects on binding of a Taxol analog to tubulin from different eukaryotic sources that have different β-tubulin isotype content. It is noted that Taxol inhibits 2-m-AzTax photolabeling of β-tubulin in BBT minimally (Fig. 1 and Fig. S1). Similar results have been reported previously (23). In addition, it has been demonstrated that, whereas baccatin is inactive, 2-m-azido-baccatin has some Taxol-like activity (24), thereby suggesting that the 2-m-azido substituent in baccatin or Taxol is capable of enhancing the interaction between the drug and tubulin. Overall, it is thought that the differences in photolabeling results are due to: (i) the structure of the drug, because Taxotere has a stronger inhibitory effect than Taxol; and (ii) the content of tubulin isotypes, as it has been shown that Taxol has a strong effect on CET that contains only βVI-tubulin.
Interestingly, two MSAs of marine origin, LML and PelA, showed a stimulatory effect on photolabeling of tubulin from all sources tested (Fig. 1 and Fig. S1). These two MSAs have been reported to bind to a different site than Taxol in β-tubulin (25–27) and exhibit synergism with the taxane site binding drugs (28). Based on X-ray crystallography, LML and PelA have been shown to bind to a unique nontaxane site on β-tubulin. These drugs use their macrolide core structures to interact with a second tubulin dimer across protofilaments (29). Our results are consistent with the finding that these two drugs allosterically stabilize the taxane binding site in the M-loop that establishes lateral tubulin contacts in microtubules (29).
To answer a fundamental question related to differential drug binding to tubulin isotypes, we used [3H]2-m-AzTax (23) to photolabel tubulin, followed by separation of β-tubulin isotypes by high-resolution IEF with identification by mass spectrometry. Although good resolution of tubulin isotypes was obtained, some bands still represent more than one isotype. Therefore, a complete analysis of the exact amount of Taxol analog binding to each β-tubulin isotype is not feasible. However, compared with other β-tubulin isotypes, we were able to determine that βIII-tubulin binds the least amount of 2-m-AzTax. This result correlates with the finding that MSAs inhibit dynamic instability more effectively with microtubules that lack βIII-tubulin (30). Recently, it has been shown that another microtubule-interacting drug, eribulin, is able to strengthen microtubule binding and dynamic instability in the absence of βIII-tubulin (31).
All β-tubulin isotypes share high sequence identity with the majority of differences residing in the C terminus. Two domains in β-tubulin are known to have a role in drug binding, microtubule assembly, and drug resistance (4, 17, 23, 32–35): the M-loop and the leucine cluster (Fig. 3). The leucine cluster has received less attention as part of an important domain in β-tubulin influencing Taxol binding. There is evidence that this cluster is involved in Taxol binding and resistance: (i) several Taxol-resistant Chinese hamster ovary cell lines exhibit a cluster of mutations, in the H6–H7 loop region, affecting Leu-215, Leu-217, and Leu-228 (17); (ii) expression of the transfected mutant genes (L215H, L217R, or L228F) conferred Taxol resistance (17, 35); (iii) the Taxol analog 2-m-AzTax photolabels a peptide, amino acids 217–231 of β-tubulin (23); and (iv) hydrogen/deuterium exchange mass spectrometry (HDX-MS) studies have demonstrated that peptide 212–230 of β-tubulin is very strongly protected by Taxol (15), in fact, more so than the M-loop. βIII-Tubulin has a residue, Ala218, in this region that is unique to this isotype and appears to influence its interaction with Taxol.
It is also known that the M-loop of tubulin has a substantial role in interacting with the H1–S2 loop of an adjacent β-tubulin molecule. In addition, it has been reported that the M-loop of β-tubulin has a role in drug resistance (33, 34). βIII-Tubulin of BBT differs from other major isotypes (βII- and βIV-tubulin) in the M-loop, with a difference at residue 275 (S275A). βI-Tubulin of BBT also harbors S275A, R276Q, as well as P287A alterations in the M-loop, but because βI-tubulin accounts for only 3% of total BBT (18), this isotype is not included in our comparison. The M-loop sequences of human β-tubulins are identical to those of BBT (Fig. 3). It was proposed that, because of the presence of S275A, the M-loop of βIII-tubulin becomes much more flexible than other isotypes, leading to a weaker interaction with Taxol (36). However, our molecular dynamics results indicate that S275A appears not to have a significant influence on drug entrance into the binding pocket (Table 1). Unique alterations are found in the M-loop of human βVI-tubulin: R276Q, T285S, and P287A. These alterations may explain why the photolabeling competition results were so different between BBT/PBT and CET, the latter containing only one β-tubulin isotype, βVI. It is noted that neither T218A nor S275A make direct contact with the bound drug; however, our computational simulation studies suggest that the presence of T218A has a strong influence on the shape of the binding pocket, down-modulating drug accessibility to the binding pocket.
We have demonstrated previously that some MSA-resistant cell lines express increased levels of βIII-tubulin (12). In intact cells, binding of microtubule interacting agents to the polymer is the primary event occurring when cells are challenged with such drugs, resulting in microtubule disarray that in turn influences downstream signaling cascades. Therefore, a difference in the extent of drug binding could easily lead to alterations in cellular events. It may also alter the expression of distinct tubulin isotypes and influence drug sensitivity in resistant cells. In addition, our laboratory has demonstrated, using HDX analysis, that Taxol binding to microtubules can influence the interaction of the endogenous dynamics regulator MAP4 with microtubules (37), suggesting that the presence of less Taxol in βIII-tubulin could alter a variety of signaling processes.
Several investigators have considered βIII-tubulin as a drug target for overcoming drug resistance and have designed drugs based on computational modeling of the Taxol binding site of βIII-tubulin, with the focus on the M-loop. Many secondary taxanes were synthesized and have demonstrated cytotoxicity in βIII-tubulin–overexpressing ovarian cell lines (36, 38, 39). In the future, the T218A variant should be considered when designing drugs for the binding site in βIII-tubulin.
In addition to the finding that suppression of βIII-tubulin increased drug sensitivity to tubulin binding agents in nonsmall cell lung cancer (NSCLC) cell lines (40), many observations have been reported concerning the effects of βIII-tubulin in cells. For example, maspin, an adhesion-associated tumor suppressor protein, is directly regulated by βIII-tubulin in NSCLC (41, 42). Suppression of βIII-tubulin caused a reduction of tumor spheroid outgrowth and an increase in sensitivity to anoikis. Phosphatase and tensin homolog (PTEN)/AKT signaling has been demonstrated to be regulated by βIII-tubulin in NSCLC (42). In addition, a high percentage of breast cancer patients develop brain metastasis and βIII-tubulin overexpression is associated with brain metastasis (43). Recently, it was demonstrated that βIII-tubulin is involved in a complex, prosurvival molecular pathway induced by hypoxia (44). Roles for βIII-tubulin in stress-response signaling and glucose metabolism have been proposed (45). Therefore, βIII-tubulin is not only involved in drug sensitivity, but also in a variety of other cellular events. Increased expression of βIII-tubulin in drug-resistant ovarian cancer cells is likely to be the consequence of a combination of drug binding to tubulin and subsequent cellular processes.
In summary, this study demonstrates that less 2-m-AzTax, a Taxol analog, binds to βIII-tubulin than to other β-tubulin isotypes. We noted the presence of a unique alanine at residue 218 in the leucine cluster of βIII-tubulin. To extend our understanding of this observation, we used molecular dynamic simulation that strongly suggested that the alanine residue 218 is likely responsible for the reduced binding of the drug to βIII-tubulin. The low drug-binding characteristic of βIII-tubulin may be compensated with an increased expression of this isotype that leads to reduced drug sensitivity.
Materials and Methods
Drugs and Tubulin.
Taxol, EpoB, Disco, Ixab, LML, and PelA were obtained as described previously (46). [3H]2-m-AzTax was provided by GlaxoSmithKline. BBT, PBT, and HeLaT were purchased from Cytoskeleton. CET was isolated from the marginal bands of chicken erythrocytes as previously described (20). Reagents for IEF and IEF strips were from GE Healthcare.
Photoaffinity Labeling of Tubulin and Drug Competition Assays.
Four micromolars of soluble tubulin in MEM buffer (0.1 M Mes, 1 mM EGTA, 1 mM MgCl2, pH 6.7) containing 3 M glycerol and 1 mM GTP was incubated with 5 µM [3H]2-m-AzTax (specific activity: 5.5 Ci/mmol) in the absence or presence of a fourfold molar excess of competing MSAs (20 µM) at 37 °C for 30 min (23). The samples were irradiated for 10 min at 4 °C with a UV lamp (254 nm) at a distance of 6 cm. The entire sample containing photolabed microtubules was analyzed by SDS/PAGE on 9% gels, using a cathode buffer that was titrated to pH 9, and detected by fluorography.
Photolabeling of Tubulin and Resolution of Tubulin Isotypes.
Five micromolars of BBT or PBT (200 µg in 400 µL MEM-glycerol buffer containing 1 mM GTP) was incubated with 6 µM [3H]2-m-AzTax at 37 °C for 30 min, followed by 10 min UV irradiation at 4 °C. The irradiated samples were layered over a 5% (wt/vol) sucrose pad in MEM-glycerol buffer and centrifuged at 120,000 × g for 30 min at 37 °C. Pellets were resuspended in 450 µL solubilization buffer [7 M urea, 2 M thiourea, 4% (wt/vol) CHAPS, 0.5% Triton X-100, 0.5% Pharmalyte pH 4.5–5.4 (GE), 20 mM DTT and Bromophenol blue], loaded onto 24-cm IPG strips [pH 3–5.6 NL (nonlinear)], and run on an IPGphor IEF system for a total of 75,000 V/h.
Identification of Tubulin Isotypes and Determination of Radioactivity Associated with Each Isotype.
IPG strips were fixed in 20% (wt/vol) trichloroacetic acid, stained with GelCode Blue Stain Reagent (Thermo Scientific), and destained with water. The stained bands were excised, destained with 50% (vol/vol) acetonitrile and 200 mM ammonium bicarbonate, pH 8.9, treated with 100 mg/mL CNBr and 70% formic acid for 16 h, followed by mass spectrometry (MALDI-TOF). Parallel IPG strips were destained, scanned, and bands quantified by ImageJ. The stained bands were excised, treated with 90% hydroxide of Hyamine 10-X (PerkinElmer) and radioactivity associated with each band determined by liquid scintillation counting.
Tubulin Polymerization Assays.
BBT or CET (2 µM) in MEM-glycerol buffer containing 2 mM GTP was incubated with 10 µM of the indicated MSAs at 37 °C for 30 min. Samples were centrifuged at 120,000 × g at 37 °C for 1 h, and the pellet containing the polymerized tubulin was dissolved in SDS sample buffer and analyzed by SDS/PAGE.
Molecular Dynamics Studies.
Structure of human β-tubulin (isotype I) in apo and Taxol-complexed states were modeled with MODELER (47, 48) using the structure of bovine tubulin (1TUB and 1JFF) as templates. Molecular dynamics simulations were performed using the AMBER Molecular Dynamics Packages (49), at 300 K with implicit solvent, and snapshots collected at 1-ps intervals. To determine the contacts between Taxol and βI-tubulin, we collected 600 snapshots (30 snapshots from 20 trajectories) and extracted contacts between β-tubulin and Taxol using the software LPC (50). For the analysis of pocket opening, molecular dynamics simulations were performed on βI-tubulin monomer with GDP, and 375 snapshots (25 snapshots from 15 trajectories) were collected. Interresidue distances were computed between Cα atoms using the program ptraj from the AMBER package.
Acknowledgments
We thank Profs. D. G. I. Kingston [unlabeled 2-(m-azidobenzoyl)taxol], A. Smith III (discodermolide), and P.T. Northcote and J.H. Miller (laulimalide and peloruside A) for providing drugs. This work was supported by the Breast Cancer Research Foundation, the National Foundation for Cancer Research, National Cancer Institute Grant CA077263, and the Albert Einstein Cancer Center Support Grant of the NIH Award P30CA013330.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613286113/-/DCSupplemental.
References
- 1.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8(4):244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 2.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: Boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72(18):4611–4615. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 4.Rao S, et al. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in beta-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274(53):37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 5.Verdier-Pinard P, et al. Detection of human betaV-tubulin expression in epithelial cancer cell lines by tubulin proteomics. Biochemistry. 2005;44(48):15858–15870. doi: 10.1021/bi051004p. [DOI] [PubMed] [Google Scholar]
- 6.Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. 2014;4:153. doi: 10.3389/fonc.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao SK, et al. Characterization of a human βV-tubulin antibody and expression of this isotype in normal and malignant human tissue. Cytoskeleton (Hoboken) 2012;69(8):566–576. doi: 10.1002/cm.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10(3):194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 9.Mariani M, et al. Class III β-tubulin in normal and cancer tissues. Gene. 2015;563(2):109–114. doi: 10.1016/j.gene.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath K, Wilson L, Cabral F, Jordan MA. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280(13):12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 11.Kavallaris M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100(5):1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrethsen J, Angeletti RH, Horwitz SB, Yang CP. Proteomics of cancer cell lines resistant to microtubule-stabilizing agents. Mol Cancer Ther. 2014;13(1):260–269. doi: 10.1158/1535-7163.MCT-13-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicchillitti L, et al. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol Cancer Ther. 2008;7(7):2070–2079. doi: 10.1158/1535-7163.MCT-07-2370. [DOI] [PubMed] [Google Scholar]
- 14.Ti SC, et al. Mutations in human tubulin proximal to the kinesin-binding site alter dynamic instability at microtubule plus- and minus-ends. Dev Cell. 2016;37(1):72–84. doi: 10.1016/j.devcel.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khrapunovich-Baine M, et al. Distinct pose of discodermolide in Taxol binding pocket drives a complementary mode of microtubule stabilization. Biochemistry. 2009;48(49):11664–11677. doi: 10.1021/bi901351q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdier-Pinard P, et al. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry. Biochemistry. 2003;42(18):5349–5357. doi: 10.1021/bi027293o. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A beta-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274(34):23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee A, Roach MC, Trcka P, Luduena RF. Preparation of a monoclonal antibody specific for the class IV isotype of β-tubulin. Purification and assembly of α β II, α β III, and α β IV tubulin dimers from bovine brain. J Biol Chem. 1992;267(8):5625–5630. [PubMed] [Google Scholar]
- 19.Davis A, Martinez S, Nelson D, Middleton K. A tubulin polymerization microassay used to compare ligand efficacy. Meth Cell Biol. 2009;95(Ch 18):327–347. doi: 10.1016/S0091-679X(10)95018-8. [DOI] [PubMed] [Google Scholar]
- 20.Murphy DB, Wallis KT. Isolation of microtubule protein from chicken erythrocytes and determination of the critical concentration for tubulin polymerization in vitro and in vivo. J Biol Chem. 1983;258(13):8357–8364. [PubMed] [Google Scholar]
- 21.Rüdiger M, Weber K. Characterization of the post-translational modifications in tubulin from the marginal band of avian erythrocytes. Eur J Biochem. 1993;218(1):107–116. doi: 10.1111/j.1432-1033.1993.tb18357.x. [DOI] [PubMed] [Google Scholar]
- 22.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 23.Rao S, Orr GA, Chaudhary AG, Kingston DG, Horwitz SB. Characterization of the Taxol binding site on the microtubule. 2-(m-Azidobenzoyl)Taxol photolabels a peptide (amino acids 217-231) of beta-tubulin. J Biol Chem. 1995;270(35):20235–20238. doi: 10.1074/jbc.270.35.20235. [DOI] [PubMed] [Google Scholar]
- 24.He L, et al. A common pharmacophore for Taxol and the epothilones based on the biological activity of a taxane molecule lacking a C-13 side chain. Biochemistry. 2000;39(14):3972–3978. doi: 10.1021/bi992518p. [DOI] [PubMed] [Google Scholar]
- 25.Pryor DE, et al. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41(29):9109–9115. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 26.Gaitanos TN, et al. Peloruside A does not bind to the taxoid site on beta-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res. 2004;64(15):5063–5067. doi: 10.1158/0008-5472.CAN-04-0771. [DOI] [PubMed] [Google Scholar]
- 27.Huzil JT, et al. A unique mode of microtubule stabilization induced by peloruside A. J Mol Biol. 2008;378(5):1016–1030. doi: 10.1016/j.jmb.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamel E, et al. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol Pharmacol. 2006;70(5):1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 29.Prota AE, et al. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew Chem Int Ed Engl. 2014;53(6):1621–1625. doi: 10.1002/anie.201307749. [DOI] [PubMed] [Google Scholar]
- 30.Lopus M, et al. Mechanism of action of ixabepilone and its interactions with the βIII-tubulin isotype. Cancer Chemother Pharmacol. 2015;76(5):1013–1024. doi: 10.1007/s00280-015-2863-z. [DOI] [PubMed] [Google Scholar]
- 31.Wilson L, et al. Effects of eribulin on microtubule binding and dynamic instability are strengthened in the absence of the βIII tubulin isotype. Biochemistry. 2015;54(42):6482–6489. doi: 10.1021/acs.biochem.5b00745. [DOI] [PubMed] [Google Scholar]
- 32.Löwe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313(5):1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 33.He L, Yang CP, Horwitz SB. Mutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol Cancer Ther. 2001;1(1):3–10. [PubMed] [Google Scholar]
- 34.Verrills NM, et al. Microtubule alterations and mutations induced by desoxyepothilone B: Implications for drug-target interactions. Chem Biol. 2003;10(7):597–607. doi: 10.1016/s1074-5521(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, et al. Mutations at leucine 215 of beta-tubulin affect paclitaxel sensitivity by two distinct mechanisms. Biochemistry. 2006;45(1):185–194. doi: 10.1021/bi051207d. [DOI] [PubMed] [Google Scholar]
- 36.Pepe A, et al. Novel C-seco-taxoids possessing high potency against paclitaxel-resistant cancer cell lines overexpressing class III beta-tubulin. Bioorg Med Chem Lett. 2009;19(12):3300–3304. doi: 10.1016/j.bmcl.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, et al. Structural evidence for cooperative microtubule stabilization by Taxol and the endogenous dynamics regulator MAP4. ACS Chem Biol. 2012;7(4):744–752. doi: 10.1021/cb200403x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferlini C, et al. The seco-taxane IDN5390 is able to target class III beta-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65(6):2397–2405. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 39.Matesanz R, et al. Taxanes with high potency inducing tubulin assembly overcome tumoural cell resistances. Bioorg Med Chem. 2014;22(18):5078–5090. doi: 10.1016/j.bmc.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 40.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67(19):9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 41.McCarroll JA, Gan PP, Liu M, Kavallaris M. BetaIII-tubulin is a multifunctional protein involved in drug sensitivity and tumorigenesis in non-small cell lung cancer. Cancer Res. 2010;70(12):4995–5003. doi: 10.1158/0008-5472.CAN-09-4487. [DOI] [PubMed] [Google Scholar]
- 42.McCarroll JA, et al. TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2015;75(2):415–425. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- 43.Kanojia D, et al. βIII-Tubulin regulates breast cancer metastases to the brain. Mol Cancer Ther. 2015;14(5):1152–1161. doi: 10.1158/1535-7163.MCT-14-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raspaglio G, et al. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3′ flanking region. Gene. 2008;409(1-2):100–108. doi: 10.1016/j.gene.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Parker AL, Turner N, McCarroll JA, Kavallaris M. βIII-Tubulin alters glucose metabolism and stress response signaling to promote cell survival and proliferation in glucose-starved non-small cell lung cancer cells. Carcinogenesis. 2016;37(8):787–798. doi: 10.1093/carcin/bgw058. [DOI] [PubMed] [Google Scholar]
- 46.Khrapunovich-Baine M, et al. Hallmarks of molecular action of microtubule stabilizing agents: Effects of epothilone B, ixabepilone, peloruside A, and laulimalide on microtubule conformation. J Biol Chem. 2011;286(13):11765–11778. doi: 10.1074/jbc.M110.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9(9):1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 49.Case DA, et al. The Amber biomolecular simulation programs. J Comput Chem. 2005;26(16):1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobolev V, Sorokine A, Prilusky J, Abola EE, Edelman M. Automated analysis of interatomic contacts in proteins. Bioinformatics. 1999;15(4):327–332. doi: 10.1093/bioinformatics/15.4.327. [DOI] [PubMed] [Google Scholar]