Significance
During pregnancy, nutrients are required for fetal growth and for the mother to maintain the pregnancy. The placenta is central to this tug-of-war over nutrients, as it is responsible for materno-fetal resource allocation. Failure to allocate resources appropriately can lead to pregnancy complications and abnormal fetal development, with long-term health consequences for both mother and baby. Here we use manipulation of a growth-regulatory protein, p110α, in mice to compare the importance of the maternal environment and genotype with fetal genotype in determining placental resource allocation. This study shows that the placenta fine-tunes the supply of maternal resources to the fetus via p110α in accordance with both the fetal drive for growth and the maternal ability to supply the required nutrients.
Keywords: resource allocation, fetus, placenta, PI3K, nutrient transport
Abstract
Pregnancy success and life-long health depend on a cooperative interaction between the mother and the fetus in the allocation of resources. As the site of materno-fetal nutrient transfer, the placenta is central to this interplay; however, the relative importance of the maternal versus fetal genotypes in modifying the allocation of resources to the fetus is unknown. Using genetic inactivation of the growth and metabolism regulator, Pik3ca (encoding PIK3CA also known as p110α, α/+), we examined the interplay between the maternal genome and the fetal genome on placental phenotype in litters of mixed genotype generated through reciprocal crosses of WT and α/+ mice. We demonstrate that placental growth and structure were impaired and associated with reduced growth of α/+ fetuses. Despite its defective development, the α/+ placenta adapted functionally to increase the supply of maternal glucose and amino acid to the fetus. The specific nature of these changes, however, depended on whether the mother was α/+ or WT and related to alterations in endocrine and metabolic profile induced by maternal p110α deficiency. Our findings thus show that the maternal genotype and environment programs placental growth and function and identify the placenta as critical in integrating both intrinsic and extrinsic signals governing materno-fetal resource allocation.
The successful outcome of mammalian pregnancy depends on balancing resource allocation between the genetically determined fetal drive for growth and the maternal nutrient requirements to support pregnancy and lactation. Failure to achieve the right balance can lead to pregnancy complications and abnormal fetal development with long-term consequences for maternal and offspring health (1, 2). As the interface between the mother and fetus, the placenta is central to this tug-of-war over nutrient allocation. Genetic and dietary manipulations during pregnancy as well as reciprocal crosses between breeds of different sizes indicate that the placenta can adapt dynamically to both fetal signals of nutrient demand and maternal signals of nutrient availability to ensure appropriate allocation of available resources (3–9). The fetus and mother, therefore, have to cooperate to optimize both offspring and maternal fitness, but to date, little is known about the relative importance of fetal versus maternal genomes in balancing resource allocation at the placental level.
The phosphoinositol 3-kinase (PI3K) pathway is highly conserved and evolved to regulate growth in relation to nutrient supply (10). In adult tissues, the PI3K p110α isoform is primarily responsible for mediating the metabolic effects of insulin and insulin-like growth factors (IGFs), which are major feto-placental growth factors (11–14). Altered placental PI3K signaling, particularly downstream of p110α, accompanies adaptations in placental phenotype when mismatches occur between maternal resource availability and the fetal genetic drive for growth during mouse development (15–18). These findings suggest that p110α may be a critical sensor integrating the various signals involved in placental adaptation. In mice, inactivating p110α kinase genetically causes embryonic lethality of p110αD933A/D933A (α/α) homozygotes (13). In contrast, heterozygotes (p110αD933A/+, α/+) are viable although growth-restricted near term (13, 19). However, nothing is known about the placenta in these α/+ mutants. Postnatally, α/+ mice remain lighter and are insulin-resistant, glucose-intolerant, and obese, but not overtly diabetic, as adults (13). During pregnancy the consequences of these metabolic and endocrine alterations for placental phenotype and materno-fetal resource allocation remain unknown. Therefore, this study sought to answer the following three questions: (i) What role does feto-placental p110α inactivation have in determining placental phenotype and resource allocation to fetal growth? (ii) What effect does maternal α/+ phenotype have on feto-placental development? (iii) Do fetal and maternal genomes interact at the level of the placenta to modify resource allocation during late mouse pregnancy? To achieve this, we established reciprocal crosses of WT and α/+ mice bearing litters of mixed genotype.
Results
p110α Deficiency Affects Feto-Placental Growth.
To unravel the role of p110α at the feto-maternal interface, conceptus growth and placental phenotype were assessed on day (D) 16 and D19 of pregnancy in WT and α/+ dams that had been mated with α/+ and WT males, respectively. Both crosses, therefore, generated two fetal genotypes within a litter (i.e., WTs and heterozygous p110α mutants, α/+) (Fig. S1). This breeding strategy allows assessment of the effects of the p110α mutation in the feto-placental unit (13) compared with WT littermates (referred to as fetal genotype and statistically as PFet) as well effects of the p110α mutation in the mother compared with WT dams (PMat). Effects of both maternal and fetal genotypes are referred throughout as fetal and maternal interaction (PFet⋅Mat).
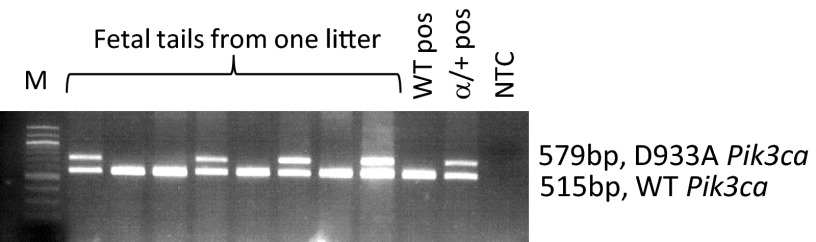
Fig. S1.
Film of agarose gel showing WT and α/+ fetal DNA PCR amplified with primers against WT and p110α mutant allele (Pik3ca-D933A). WT Pik3ca allele is 515 bp, and knock-in Pik3ca mutant is 579 bp. The targeting approach to create the α/+ mice was devised and described previously (13). M, marker; NTC, no DNA template control; pos, positive.
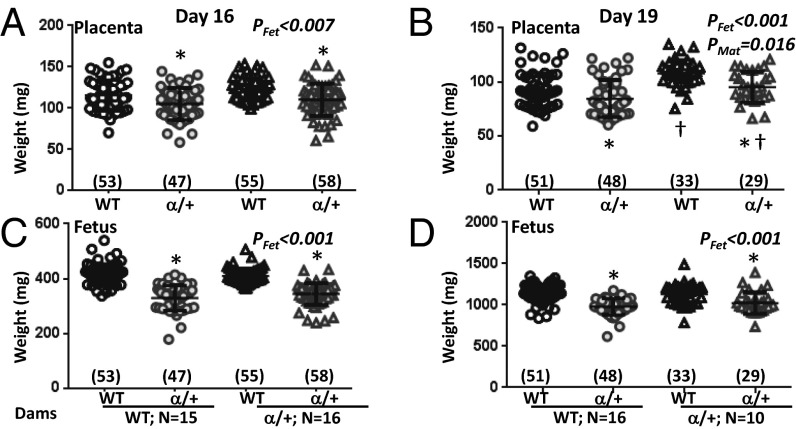
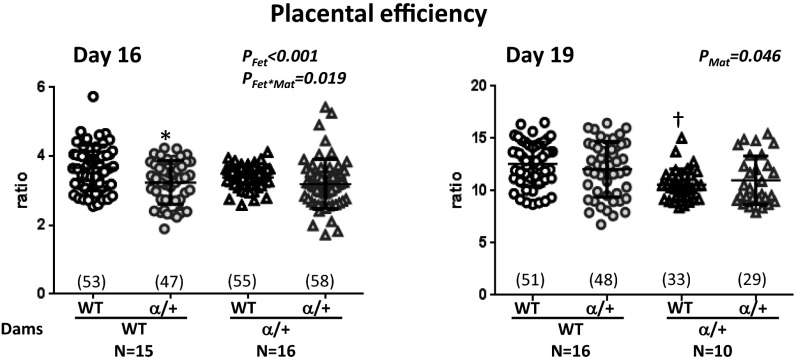
There was no significant difference in the litter size of WT and α/+ dams at either D16 or D19 (mean ± SEM: D16, 6.5 ± 0.3 and 6.7 ± 0.4; D19, 6.3 ± 0.4 vs. 6.2 ± 0.3, respectively) or in the ratio of WT to α/+ fetuses per litter (mean ± SEM: 0.49 ± 0.03 vs. 0.51 ± 0.03 for litters in WT and α/+ dams at both days of pregnancy). Fetuses and α/+ placentas were significantly lighter than WT at both ages (Fig. 1) (PFet < 0.01). The average growth restriction of α/+ placentas across maternal genotypes was 9% and 12% of WT, at D16 and D19, respectively, with corresponding fetal growth restriction of 19% and 11% of WT. Consequently, placental efficiency (measured as weight ratio of fetus to placenta) in α/+ fetuses was reduced at D16 but not D19 (Fig. S2) (PFet < 0.001). Both placental weight and efficiency, but not fetal weight, were significantly influenced by maternal genotype at D19 (Fig. 1B and Fig. S2) (both PMat < 0.05). Accordingly, placentas of α/+ dams were heavier (∼+15%; PMat < 0.05) (Fig. 1B) and less efficient relative to WT dams (Fig. S2) (PMat < 0.05), irrespective of fetal genotype. These findings reveal that fetal p110α deficiency causes placental growth restriction and that maternal p110α genotype affects placental weight and efficiency but not fetal weight, particularly at D19. The latter findings suggest a role for maternal genotype in programming placental phenotype.
Fig. 1.
Feto-placental growth in response to p110α deficiency. Placental (A and B) and fetal weights (C and D) on D16 (A and C) and D19 (B and D) of pregnancy. Shown are data from crosses where the mother is WT (circle symbols) and α/+ (triangle symbols) with mean denoted as a horizontal line with SEM. Number of fetuses is in parentheses. *Significantly different to WT littermates (P < 0.05, pairwise comparison). †Significantly different to WT dams (P < 0.05, pairwise comparison).
Fig. S2.
Placental efficiency in response to p110α deficiency, calculated by the ratio of fetal weight to placental weight. Shown are data from crosses where the mother is WT (circle symbols) and α/+ (triangle symbols) with mean denoted as a horizontal line with SEM. Number of fetuses shown in parentheses. *Significantly different to WT littermates (P < 0.05, pairwise comparisons). †Significantly different to WT dams (P < 0.05, pairwise comparisons).
p110α Deficiency Alters Female Endocrine and Metabolic Profile.
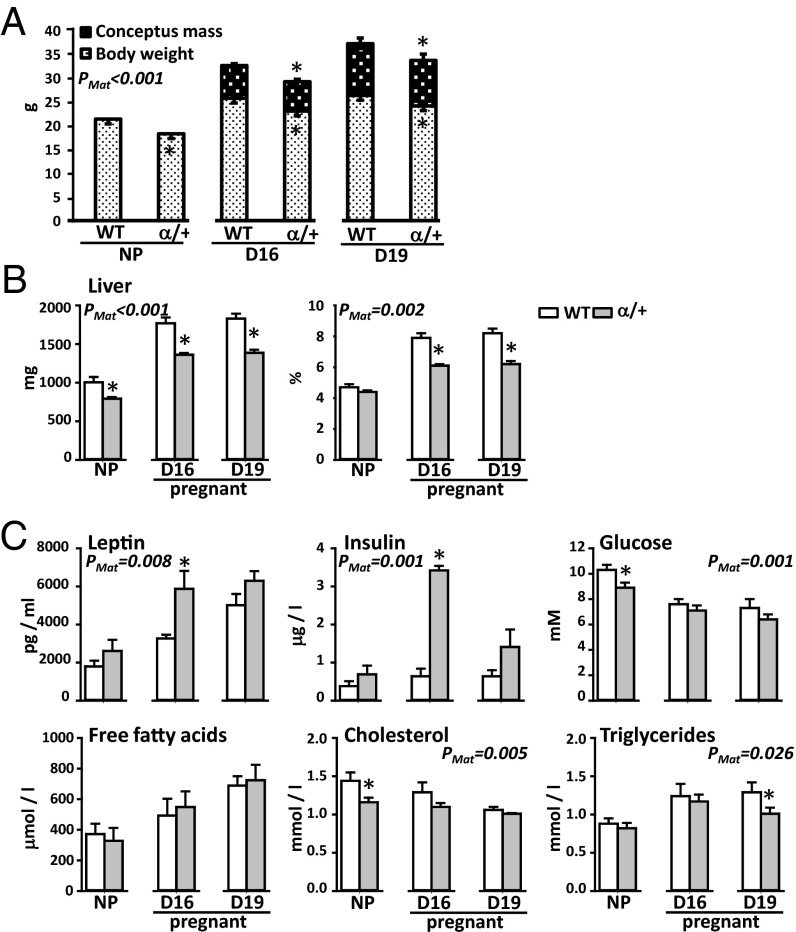
We therefore determined whether p110α genotype affected the metabolic and endocrine profile of nonpregnant (NP) and pregnant female mice. Age-matched α/+ NP females were 14% lighter than WT (Fig. 2A), in line with previous observations of postnatal growth for these mice (13). Consistent with lower starting D1 weights (mean ± SEM: 21.8 ± 0.3 g and 19.4 ± 0.3 g for WT and α/+ dams, P < 0.05, respectively), age-matched, time-mated α/+ females were ∼10% lighter than their WT counterparts at D16 and D19 in whole body and hysterectomised weight (Fig. 2A). Gravid uterus mass, however, was similar in the two maternal genotypes (Fig. 2A). Absolute liver weight was 20–25% less in α/+ than WT females. In NP females, this reduction was in line with the reduced body weight, whereas during pregnancy, the liver accounted for a smaller proportion of hysterectomised weight in α/+ than WT dams (Fig. 2B). At D16, α/+ females were hyperinsulinemic, with concentrations 5.3-fold higher than WT values (Fig. 2C, trends only observed for NP and D19 pregnant dams). Compared with WTs, NP α/+ females were hypoglycemic (glucose concentration, –14%); however, during pregnancy, they were normoglycemic, suggesting insulin resistance of the α/+ dam particularly at D16 (Fig. 2C). Although there was no difference in plasma leptin with genotype before pregnancy, D16 α/+ pregnant females were hyperleptinemic (+80%), with concentrations returning to WT values by D19 (Fig. 2C). Plasma concentrations of cholesterol in NP α/+ females were 19% lower than WT but did not differ between genotypes during pregnancy (Fig. 2C). α/+ females had lower plasma triglycerides specifically on D19 (–22%), although free fatty acid concentrations were unaffected by genotype either before or during pregnancy (Fig. 2C). Thus, p110α deficiency altered body composition and the endocrine and metabolic state in both NP and pregnant states but with more pronounced effects during pregnancy. These metabolic changes are likely to impact on placental phenotype, which we examined next.
Fig. 2.
The effect of p110α deficiency on maternal body weight (A), hepatic weight (B), and circulating metabolic indicators (C). Mean ± SEM values from age-matched nonfasted WT and α/+ littermates. *Significantly different to WT dams (P < 0.05, t test comparison). Numbers of mice used for body composition and blood glucose were 7–11 and for plasma metabolites and hormones were 5–8 samples per genotype, per age.
p110α Deficiency Impairs the Formation of the Placenta.
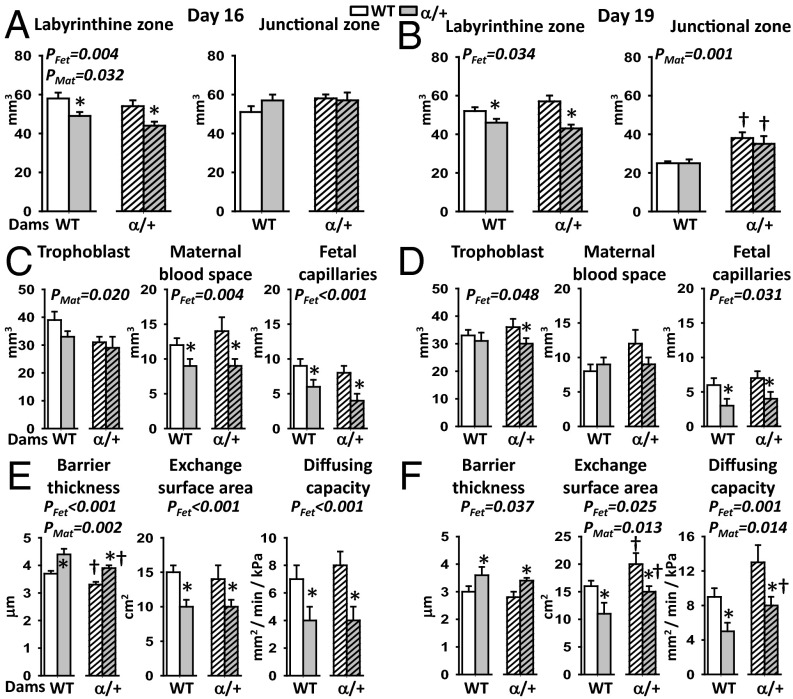
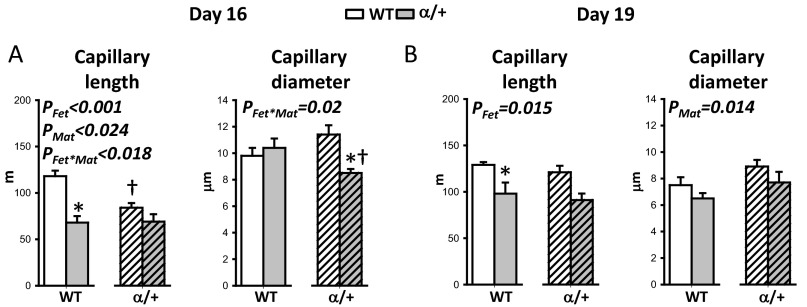
We assessed whether changes in placental growth with fetal and maternal p110α genotype were accompanied by morphological alterations using stereology. With respect to fetal genotype, the placental exchange labyrinthine zone (Lz) was 18% smaller in α/+ than WT littermates (Fig. 3 A and B) (PFet < 0.05) and related to reductions in the fetal capillary volume (–41% and –47%), exchange surface area (–22% and –27%), and diffusion capacity (–47% and –36%), at D16 and D19, respectively (Fig. 3 C–F) (PFet < 0.01). These changes were accompanied by a ∼20% thicker barrier between the fetal and maternal blood spaces and a ∼25% reduction in fetal capillary length at both ages (Fig. 3 E and F and Fig. S3). α/+ placentas had 30% less maternal blood space volume at D16 and 12% less trophoblast at D19 compared with WT littermates (Fig. 3 C and D) (PFet < 0.05).
Fig. 3.
Placental morphology in response to p110α deficiency. Placental structure (A and B), labyrinthine composition (C and D), and substrate exchange characteristics (E and F) on D16 (A, C, and E) and 19 (B, D, and F) of pregnancy. Mean ± SEM values for >5 litters per maternal genotype per gestational age. From each litter, 1–2 placentas per genotype were analyzed for gross structure. Across 4–5 litters, a representative placenta per genotype, per cross, was taken for labyrinthine zone analysis. *Significantly different to WT littermates (P < 0.05, pairwise comparisons). †Significantly different to WT dams (P < 0.05, pairwise comparisons).
Fig. S3.
The effect of p110α deficiency on placental fetal capillary length and diameter on D16 (A) and D19 (B). Shown are mean ± SEM values for more than five litters per maternal genotype at D16 and D19 of pregnancy. Across four to five litters, a representative placenta per genotype, per cross was taken for analysis. *Significantly different to WT littermates (P < 0.05, pairwise comparisons). †Significantly different to WT dams (P < 0.05, pairwise comparisons).
Maternal genotype affected placental Lz morphology at both ages (PMat < 0.04) but with opposite effects at the two ages. At D16 in α/+ dams, the volumes of the placental Lz and trophoblast were reduced (–9% and –17%, respectively), fetal capillaries were 14% shorter, and the exchange barrier was 11% thinner compared with WT dams (Fig. 3 A and E and Fig. S3A). At D19 in α/+ dams, the volume of the placental endocrine junctional zone (Jz) was 46% greater, the fetal capillaries were 18% larger in diameter, the diffusing capacity was 52% greater, and the exchange surface was 34% greater (Fig. 3 B and F and Fig. S3B).
There were significant interactions between maternal and fetal genomes in determining fetal capillary length and diameter on D16 (PFet⋅Mat < 0.03). Placental capillaries were significantly shorter (–29%) in WT fetuses of α/+ dams specifically, whereas the diameter was reduced (–18%) in α/+ fetuses of α/+ dams only (Fig. S3A). There were also interactions between fetal and maternal genotypes at the level of placental nutrient storage at D16. There was no difference in glycogen content between WT and α/+ placentas in WT dams (mean ± SEM: 9.7 ± 0.4 mg/g vs. 9.9 ± 0.7 mg/g) whereas in α/+ dams glycogen content was reduced by 38% in α/+ placentas compared with their WT littermates (mean ± SEM: 8.7 ± 0.7 mg/g vs. 5.7 ± 0.4 mg/g for WT and α/+, respectively, P < 0.001). Collectively, these data indicate that fetal and maternal genotypes interact to modify the morphology and composition of the placenta.
p110α Deficiency Affects Placental Nutrient Transport.
To assess whether placental transport function was altered by lack of p110α in either the fetus and/or mother, we determined the in vivo uni-directional, materno-fetal transfer of the nonmetabolisable analogs of glucose and a neutral amino acid using 3H-methyl d-glucose (MeG) and14C-methyl amino-isobutyric acid (MeAIB), respectively, at D16 and at D19. Because the mutant placentas were smaller relative to their WT littermates at these ages, we measured absolute accumulation of radioisotopes by the fetus and also expressed fetal counts relative to estimated surface area for transport and/or fetal weight, which provide indices of the placental capacity for nutrient transfer and fetal growth relative to supply.
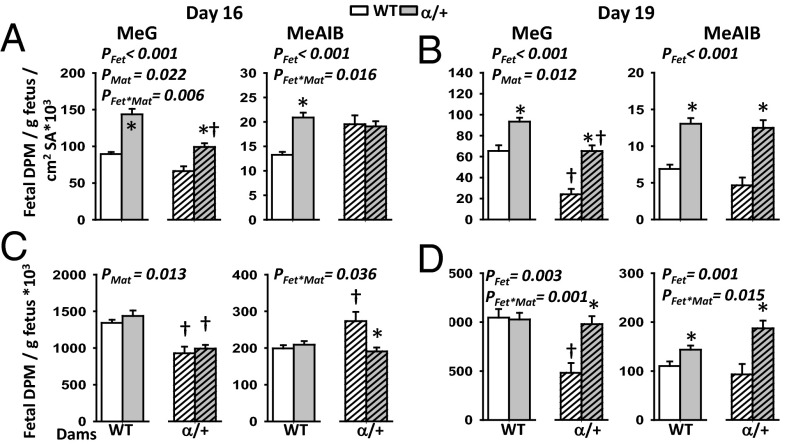
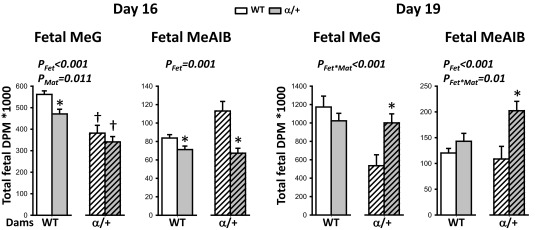
Placental transfer of both MeG and MeAIB relative to estimated surface area shows that α/+ mutant placentas transferred more of both solutes compared with WT at both gestational ages (+56% and +107% for MeG and +30 and +122% for MeAIB at D16 and D19, respectively; PFet < 0.001) (Fig. 4 A and B). This suggests the α/+ placenta transfers the solutes with increased efficiency in compensation for the reduced surface area. In agreement with these findings, mutant fetuses received either normal or increased amount of solutes for their size (Fig. 4 C and D) (PFet < 0.004), even though the absolute amount of MeG and MeAIB solute received by the α/+ fetus is reduced at D16 (Fig. S4). The increase in solute transfer per surface area is most evident at D19 (Fig. 4B) and is related to less severe growth restriction of α/+ fetuses than at D16 (–19% D16 vs. –11% D19).
Fig. 4.
Placental nutrient transfer in response to p110α deficiency. Materno-fetal transfer of MeG and MeAIB on D16 (A and C) and D19 (B and D) of pregnancy expressed relative to the exchange surface area (A and B) and/or per gram of fetus (C and D). Mean ± SEM for 8 and 10 litters on D16 (n = 23–36 per fetal genotype, per cross) and 5 and 5 litters on D19 (n = 15–19) for WT and α/+ dams, respectively. *Significantly different to WT littermates (P < 0.05, pairwise comparisons). †Significantly different to WT dams (P < 0.05, pairwise comparisons). SA, exchange surface area.
Fig. S4.
The effect of p110α deficiency on the absolute MeG and MeAIB received by the fetus via the placenta. Shown are the means ± SEM for 8 and 10 litters on D16 (n = 23–36 per fetal genotype, per cross) and 5 and 5 litters on D19 (n = 15–19 per fetal genotype, per cross) for WT and α/+ dams, respectively. *Significantly different to WT littermates (P < 0.05, pairwise comparisons). †Significantly different to WT dams (P < 0.05, pairwise comparisons). DPM, disintegrations per minute.
Maternal genotype also exerted a significant effect on placental MeG transfer (Fig. 4; PMat < 0.05). Overall placental transfer of this solute estimated per unit surface area was reduced in α/+ compared with WT dams at both ages (63% average of WT) (Fig. 4 A and B), with ∼30% less fetal accumulation of MeG irrespective of fetal genotype at D16 (PMat < 0.05) (Fig. 4C and Fig. S4). At D19, reduced fetal accumulation of MeG was only seen in WT fetuses of α/+ dams (–55%; PFet⋅Mat < 0.01) (Fig. 4D). There were also fetal–maternal genotype interactions modulating fetal MeAIB accumulation at both ages (PFet⋅Mat < 0.05) (Fig. 4 C and D). In particular, the WT fetus accumulated more MeAIB in α/+ dams on D16 compared with WT (Fig. 4C). Furthermore, in α/+ but not WT dams, α/+ fetuses accumulated less MeAIB than their WT littermate on D16 (Fig. 4C). Thus, placental transfer capacity is modified by both fetal and maternal genotypes, however the specific nature of the changes depends on gestational age and the nutrient.
p110α Deficiency Alters Placental Expression of Nutrient Transporters and Lineage Genes.
Finally, we sought to identify the molecular mechanisms altering placental MeG and MeAIB transport in α/+ fetuses in WT and α/+ dams. We assessed placental expression of genes encoding the glucose (SLC2A; Slc2a1 and Slc2a3) and system A amino acid (SLC38A; Slc38a1, Slc38a2, and Slc38a4) transporter proteins. We also measured expression of placental cell lineage genes, which in some cases have been implicated in regulating materno-fetal nutrient allocation (20–22).
Maternal genotype had the strongest effect on placental expression of the transporter and cell lineage genes, although fetal genotype determined the specific nature of the effect (Table S1). In particular, at D16, the placental expression of Slc38a1 and the giant cell gene Hand1 was reduced in α/+ dams by ∼40% and ∼35%, respectively, compared with WT dams (Table S1) (PMat < 0.01). In α/+ dams, Slc38a1 continued to be expressed at lower levels at D19, with expression of Slc2a1 and Slc38a2 transporter genes also now reduced by ∼18% (PMat < 0.001). There was no effect of fetal genotype on placental expression of any of the nutrient transporters at either age studied (Table S1).
Table S1.
The effect of p110α inactivation on placental expression of nutrient transporter and cell lineage genes
| Day 16 | Day 19 | |||||||||||||
| WT | α/+ | WT | α/+ | |||||||||||
| Genes | WT | α/+ | WT | α/+ | Fet genotype | Mat genotype | Con×Mat | WT | α/+ | WT | α/+ | Fet genotype | Mat genotype | Fet⋅Mat |
| Lz trophoblast | ||||||||||||||
| Ctsq | 1.00 ± 0.09 | 0.89 ± 0.09 | 1.01 ± 0.14 | 0.87 ± 0.12 | 1.00 ± 0.07 | 0.88 ± 0.03 | 0.77 ± 0.05† | 0.81 ± 0.04 | 0.020 | |||||
| Syna | 1.00 ± 0.08 | 0.95 ± 0.08 | 0.75 ± 0.07 | 1.03 ± 0.08* | 0.037 | 1.00 ± 0.07 | 1.12 ± 0.06 | 1.08 ± 0.08 | 0.78 ± 0.07*† | 0.016 | 0.019 | |||
| Gcm1 | 1.00 ± 0.06 | 0.79 ± 0.11 | 0.86 ± 0.10 | 0.88 ± 0.08 | 1.00 ± 0.16 | 1.15 ± 0.19 | 0.49 ± 0.07† | 0.33 ± 0.05† | <0.001 | |||||
| Jz trophoblast | ||||||||||||||
| Prl3d1 | 1.00 ± 0.11 | 0.84 ± 0.11 | 0.77 ± 0.06 | 0.73 ± 0.05 | 1.00 ± 0.12 | 0.95 ± 0.08 | 0.91 ± 0.24 | 1.43 ± 0.26* | ||||||
| Prl8a8 | 1.00 ± 0.16 | 0.79 ± 0.06 | 0.67 ± 0.08 | 0.84 ± 0.07 | 1.00 ± 0.11 | 1.31 ± 0.14 | 0.78 ± 0.09 | 0.78 ± 0.07† | 0.001 | |||||
| Prl3b1 | 1.00 ± 0.08 | 0.99 ± 0.09 | 0.77 ± 0.09 | 1.07 ± 0.07 | 1.00 ± 0.05 | 1.17 ± 0.09 | 0.66 ± 0.06† | 0.87 ± 0.06*† | 0.009 | <0.001 | ||||
| Hand1 | 1.00 ± 0.15 | 0.87 ± 0.09 | 0.60 ± 0.04† | 0.64 ± 0.04 | 0.005 | 1.00 ± 0.05 | 1.18 ± 0.05* | 1.08 ± 0.07 | 1.11 ± 0.05 | |||||
| Endothelial cells | ||||||||||||||
| Mest | 1.00 ± 0.12 | 0.99 ± 0.14 | 0.88 ± 0.12 | 1.04 ± 0.08 | 1.00 ± 0.10 | 0.95 ± 0.07 | 0.55 ± 0.06† | 0.41 ± 0.03*† | 0.046 | <0.001 | ||||
| Nutrient transporters | ||||||||||||||
| Slc2a1 | 1.00 ± 0.14 | 0.77 ± 0.07 | 1.04 ± 0.13 | 1.01 ± 0.17 | 1.00 ± 0.04 | 0.98 ± 0.05 | 0.83 ± 0.05† | 0.81 ± 0.05† | <0.001 | |||||
| Slc2a3 | 1.00 ± 0.13 | 0.80 ± 0.10 | 1.06 ± 0.17 | 0.83 ± 0.14 | 1.00 ± 0.09 | 0.96 ± 0.11 | 1.01 ± 0.07 | 0.94 ± 0.03 | ||||||
| Slc38a1 | 1.00 ± 0.21 | 0.69 ± 0.10 | 0.49 ± 0.07 | 0.50 ± 0.06 | 0.008 | 1.00 ± 0.05 | 1.06 ± 0.05 | 0.55 ± 0.02† | 0.53 ± 0.03† | <0.001 | ||||
| Slc38a2 | 1.00 ± 0.21 | 0.74 ± 0.12 | 0.77 ± 0.08 | 0.74 ± 0.08 | 1.00 ± 0.05 | 0.97 ± 0.04 | 0.85 ± 0.04† | 0.75 ± 0.04† | <0.001 | |||||
| Slc38a4 | 1.00 ± 0.16 | 1.05 ± 0.13 | 0.99 ± 0.04 | 1.15 ± 0.07 | 1.00 ± 0.06 | 1.05 ± 0.05 | 1.02 ± 0.05 | 1.02 ± 0.05 | ||||||
Data were normalized to the expression of Gapdh, are expressed relative to values for WT placentas in WT dams, and are presented as mean ± SEM. Data from 8 to 13 placentas per genotype per cross. Gapdh expression remained constant between groups.
Significantly different to WT littermates (P < 0.05, pairwise comparison).
Significantly different to values in WT dams (P < 0.05, pairwise comparison). Fet, effect of fetal genotype; Fet×Mat, interaction of fetal and maternal genotypes; Mat, effect of maternal genotype.
At D19, the fetal endothelium Mest gene, Jz spongiotrophoblast genes Prl3b1 and Prl8a8, Lz sinusoidal giant cell gene Ctsq, and syncytial trophoblast genes Gcm1 and Syna were also lower in expression (ranging from –15% to –60%) in litters carried by α/+ compared with WT dams (Table S1). Mest and Prl3b1 expression was also affected by fetal genotype at D19, with lower and higher expression, respectively, in α/+ than WT placentas (–12% and +24%, respectively; PFet < 0.05) (Table S1). There was also fetal–maternal genotype interactions in regulating Syna gene expression at both ages (PFet⋅Mat < 0.05) (Table S1). Thus, fetal and maternal genomes interact to modulate placental expression of several cell lineage genes, particularly at D19 of pregnancy.
Discussion
This study shows that: (i) signaling via PI3K-p110α regulates the formation and function of the mouse placenta, (ii) placental phenotype is influenced by maternal p110α genotype, and (iii) there is an interplay between fetal and maternal genotypes in determining specific placental phenotypes. Using genetic manipulation of p110α (13), this study demonstrates that development of the placental exchange region was impaired by D16 of pregnancy in association with reduced growth of α/+ fetuses. However, despite its compromised morphology, the α/+ placenta adapted functionally to increase the supply of maternal nutrients to the fetus, an effect that was more pronounced near term. The specific nature of these changes, however, depended on whether the mother was WT or mutant, which probably relates, in part, to the altered endocrine and metabolic profile of α/+ dams. Because of these maternal genotype-driven adaptations in placental phenotype, fetal growth was unaffected by maternal genotype. Thus, by acting on placental phenotype, signaling via p110α kinase is critical in integrating intrinsic and extrinsic signals governing the balance of materno-fetal resource allocation to fetal growth.
Signaling via PI3K-p110α Regulates Placental Formation.
We found that the α/+ placenta was lighter at both D16 and D19 of pregnancy and there were defects in the formation of the Lz, irrespective of maternal genotype. At both ages, Lz vascularization was impaired; the fetal capillaries were reduced in volume and shorter in length in the α/+ placentas. Reduced vascularisation of the α/+ placenta is consistent with previous work showing impaired developmental angiogenesis and vascular patterning in these mutant fetuses (19).
At D16, but not D19, the volume of Lz maternal blood spaces was also decreased in α/+ placenta. This may reflect delayed trophoblast-induced dilation of the uterine spiral arteries, which normally occurs by D14.5 of pregnancy (23) as PI3K signaling also regulates the differentiation and invasive properties of trophoblast (24). At both ages, the trophoblast interhemal membrane was thicker and the surface area for exchange was reduced in the α/+ placenta, which resulted in a decreased theoretical diffusing capacity. Changes in Lz morphology of the α/+ mutants were similar to those reported previously for mice deficient in Igf2, as well as the PI3K effector protein, protein kinase b (Akt1) (25, 26). Together, these data suggest that p110α is a main mediator of IGF2 actions, operating primarily via AKT1 to attain normal placental weight and Lz structure in mice.
As expected, the changes in Lz morphology of the α/+ placentas impaired the normal capacity to deliver nutrients from mother to fetus; the absolute amount of MeG (significant in WT dams) and MeAIB solute received by the α/+ fetus was reduced at D16, compared with WT littermates. However, estimating the placental transfer of these solutes per unit exchange surface area showed greater transport for α/+ fetuses at both ages, suggesting compensatory adaptive mechanisms of both facilitated and active transport in the α/+ placenta. This up-regulation of placental transport achieved greater amounts of solute per gram of fetal mass in α/+ mutants compared with their WT littermates at D19 and was associated with an improved fetal growth trajectory of α/+ compared with WT. Alterations in nutrient transport capacity were, however, unrelated to expression of Slc2a and Slc38a glucose and amino acid transporter genes in the α/+ placenta at either age. Adaptive up-regulation of placental transport function has been seen when the fetal demand for maternal resources exceeds the supply capacity of the placenta following deletion of the Slc2a3 glucose transporter gene, in naturally small placentas within litters and when placental growth is impaired by a deficiency in the Lz-specific Igf2 transcript or the 11-β hydroxysteroid dehydrogenase gene (27–31). Moreover, system A amino acid transporter activity is adaptively up-regulated in the small human placenta of infants at the lower end of the normal birthweight range (32) but is diminished in pathological pregnancies with severe fetal growth restriction (33, 34). Further work is required to determine the mechanism by which the α/+ placenta adapts, largely independent of maternal genotype. Further work should also identify whether the fetal sex influences the observed alterations in placental phenotype with fetal genotype and their possible interaction with the maternal environment (35).
Placental Phenotype Is Modified by Maternal p110α Genotype.
Placental transport for both WT and α/+ fetuses was additionally influenced by the phenotype of the α/+ dam. α/+ female mice were lighter and exhibited an altered endocrine and metabolic profile before pregnancy. However, some of these changes were exacerbated (fractional liver weight, hyperinsulinemia, hyperleptinemia, and hypotriglyceridemia) and others mitigated (hypocholesterolemia) by pregnancy in these mutants.
At D16 of pregnancy, Lz volume and its volume of trophoblast were less and fetal capillaries shorter particularly for WT placentas, in α/+ dams, even though absolute placental weight was not altered by maternal genotype at this age. Reduced placental vascularization has been observed in embryos transferred to recipients of a smaller breed (8, 36), and thus, the current findings suggest that the smaller sized α/+ dam may be attempting to constrain fetal growth via actions on the placental supply capacity. Indeed, the placental ability to transport MeG per gram of fetus was less for both WT and α/+ fetuses in α/+ dams at D16 compared with WT. These observations are also consistent with reduced placental glucose transport capacity in cows of small maternal size (9), sheep of young maternal age (37) as well as mouse pregnancies where fetal demand for growth exceeds the maternal ability to supply nutrients via the placenta (38). Reduced MeG transfer may also be related to elevated insulin concentrations in α/+ dams, as chronic exposure to maternal hyperinsulinemia reduces placental uptake and fetal supply of glucose (39) and diminished placental glucose transfer occurs in late gestation in association with hyperinsulinemia and other changes in maternal-nutrient partitioning in pregnant mice treated with glucocorticoids (17). In contrast to MeG transport, there was no independent effect of maternal genotype on MeAIB transfer. This occurred despite reduced placental expression of the amino acid transporter gene Slc38a1 in litters of α/+ dams (also at D19 along with decreased Slc38a2). Similar disparity between Slc38a gene expression and transport of MeAIB in vivo has been observed in placentas in which growth regulatory genes (15) or the maternal environment have been affected in rodents (3–7). These findings suggest that there are temporal differences between the expression, translation, and membrane trafficking of amino acid transporters in the placenta and that measuring placental expression of Slc38a genes provides little information with respect to actual transport function in vivo. Further work should investigate the contribution of other transporters that are responsible for facilitating amino acid accumulation, exchange, and efflux in the placenta (40). In α/+ dams, the placental barrier to exchange was, however, thinner at D16 irrespective of fetal genotype. This structural alteration would have optimized passive diffusion of molecules, such as oxygen, which could be beneficial for fetal growth, particularly given the compromised glucose transfer and maternal environment in α/+ dams.
There were also changes in placental morphology specific to α/+ dams on D19. Placentas were heavier and the exchange surface area larger for both fetal genotypes in α/+, compared with WT dams. Expression of the Gcm1 gene, which is involved in early patterning of the Lz vasculature, was reduced and fetal capillaries were larger in caliber and showed reduced Mest expression, irrespective of fetal genotype, in placentas of α/+ dams, which taken together may indicate a more advanced Lz vascular phenotype (22, 23). Increased placental growth and maturation of the exchange area would have enhanced the placental capacity to supply nutrients and oxygen to the fetus and suggest that the placenta adapts morphologically in response to the altered maternal environment induced by maternal p110α deficiency. Beneficial changes in placental morphology have also been observed in response to suboptimal maternal environments, including poor nutrition, hypoxia, and glucocorticoid overexposure in many species (3–7).
Interplay Between Fetal and Maternal Genotypes in Determining Specific Placental Phenotypes.
Fetal and maternal genomes also interacted at the level of placental nutrient storage and endocrine capacity. In α/+ but not WT dams, glycogen storage in the α/+ placenta was diminished on D16. Placental glycogen content is reduced in dams fed diets high in sugar and fat and in dams carrying Igf2P0 null litters, both conditions in which there are signs of maternal insulin resistance and altered placental PI3K signaling (15, 16). α/+ dams were normoglycemic despite their hyperinsulinemia at D16, indicative of maternal insulin resistance in this mutant too. However, in contrast to the α/+ placenta, glycogen content of the WT placenta was not affected by maternal genotype, which suggests that signaling via p110α in the mouse placenta is required to maintain placental glycogen under conditions of maternal insulin resistance. Future investigations should determine whether the differentiation and/or lysis of glycogen cells in the Jz (41) is altered in response to fetal and maternal p110α deficiency, especially as Jz volume was greater for both fetal genotypes in mutant dams at D19. Maternal genotype also affected the expression of different cell lineage genes in the placental Lz (Syna, Mest, Gcm1, and Ctsq) and the Jz (Prl8a8 and Prl3b1). In some instances, fetal genotype determined the extent of the effect. For instance, spongiotrophophoblast Prl8a8 expression was significantly decreased in α/+ but not WT fetuses in mutant dams at D19. Moreover, depending on maternal genotype, the α/+ placenta exhibited increased expression of the Prl3b1/chorionic somatomammotropin-2 gene compared with WT littermates at D19. Insulin secretion and β-cell proliferation are increased by placental production of the prolactin/chorionic somatomammotropin family of hormones (PRL/CSH) (42). Future work should determine the interrelationship between changes in the endocrine Jz and the metabolic state of the α/+ dams.
Due to overall differences in placental weight, structure, and functional capacity, the outcome for fetal growth was the same in α/+ and WT dams (i.e., there was no interaction of fetal and maternal genomes on fetal weight). These findings suggest that the placenta acts as a “buffer” to fine-tune the supply of maternal resources to the fetus in accordance with both the genetically determined fetal drive for growth and the maternal ability to supply the nutrients required for fetal growth. However, the adaptive signals modifying placental transport capacity in α/+ mutants in late pregnancy remain to be identified, although they are likely to originate from both the mother and the growth-restricted conceptus (Fig. 5). The relative contribution of the fetus and placenta to placental phenotype and adaptive responses could not be determined as both the fetus and placenta carry the mutation in α/+ conceptuses. Future work could use cell-selective manipulation of the p110α gene to examine the specific contribution of these tissues in the regulation of placental phenotype.
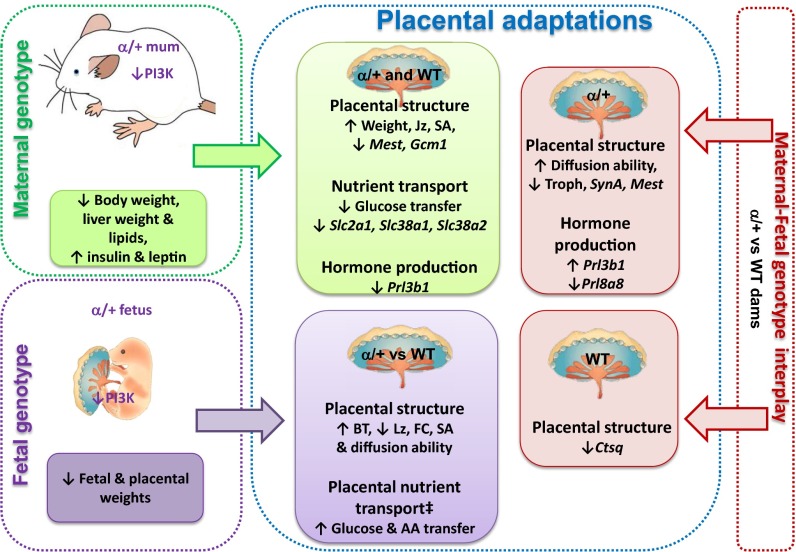
Fig. 5.
Summary illustration showing the effects of maternal genotype (green text boxes), fetal genotype (purple text boxes), and their interplay (pink text boxes) in determining placental phenotype at D19 of pregnancy. ‡Transfer per unit SA. AA, amino acid; BT, barrier thickness; FC, fetal capillaries; Jz, junctional zone; Lz, labyrinthine zone; SA, surface area for exchange; Troph, trophoblast.
In the present study, the fetal weight to placental weight ratio, which is often used as a proxy measure of placental efficiency (3), did not always track with nutrient transfer estimated per unit of placental surface area. Numerous factors determine the ability of the placenta to support fetal nutrient provision and growth beyond simply its wet weight, including surface area, vascularity, and activity of nutrient transporters (43). This work, therefore, highlights that caution is warranted when interpreting changes in the fetal weight to placental weight ratio, particularly if there is no information on the morphological or functional characteristics of the placenta.
In summary, our data show that the WT and α/+ placenta adopt different strategies at the level of nutrient supply capacity to cope with the altered metabolic state of the α/+ dam and, in a broader sense, emphasize the interplay between maternal and fetal genomes in determining the amount of materno-fetal resource allocation (Fig. 5). These findings are important in the context of human pregnancy as dysregulated expression of both up- and down-stream components of the PI3K pathway has been reported in human placenta associated with abnormal fetal growth (44–50). Furthermore, our study highlights the usefulness of the PI3K α/+ mouse as a tool for investigating the role and mechanisms of maternal–fetal genomic interactions in determining feto-placental phenotype and the programming of subsequent offspring health.
Materials and Methods
Mice were housed in the University of Cambridge Animal Facility in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and the University of Cambridge ethics committee. Virgin 10-wk-old C57BL/6 WT and α/+ female mice were mated to α/+ and WT males, respectively. α/+ mice were backcrossed to C57BL/6 for >8 generations. On D16 or D19 of pregnancy, dams were anesthetized (fentanyl-fluanisone:midazolam in sterile water at 1:1:2; Jansen Animal Health) and underwent a placental nutrient transfer assay or were used for maternal blood collection by cardiac puncture and liver retrieved following cervical dislocation. Virgin 8–10-wk-old WT and α/+ female mice (littermates) were anesthetized, blood collected, and liver weighed as for pregnant mice. The α/+ mice were previously generated as described in ref. 13 and genotyped using primers 5′-TTCAAGCACTGTTTCAGCT-3′ and 5′-TTATGTTCTTGCTCAAGTCCTA-3′. Blood glucose and plasma concentrations of leptin, insulin, triglycerides, cholesterol, and free fatty acids were determined as reported (51). Materno-fetal clearance of MeAIB (NEN NEC-671; specific activity 1.86 GBq/mmol; Perkin-Elmer) and MeG (NEN NEC-377; specific activity 2.1G Bq/mmol) was measured 2 min after maternal tracer injection as described (52). Entire litters of placentas were collected for morphological analyses using the Computer Assisted Stereological Toolbox program as previously described (16) or snap-frozen for quantification of gene expression by qPCR (Table S2) or tissue glycogen content using amyloglucosidase as reported (51). Data were analyzed using SPSS 21 using t test (effect of genotype on maternal body composition) or two-way ANOVA (effect of maternal and fetus genotype) with pairwise comparisons. Data from individual litters (fetal and placental weights and transport) were analyzed using a two-factor generalized linear mixed model (maternal and fetus genotypes as factors), with litter identified as a random effect, followed by sequential pairwise comparisons to assess the effect of maternal and/or fetal genotype. Data were significant when P < 0.05.
Table S2.
List of the Taqman probes used for quantifying the expression of placental cell lineage and transporter genes
| Gene | Taqman probe |
| Ctsq | Mm01349948_m1 |
| Syna | Mm02744887_s1 |
| Gcm1 | Mm00492310_m1 |
| Prl3d1 | Mm04213281_g1 |
| Prl8a8 | Mm00452401_m1 |
| Prl3b1 | Mm00435852_m1 |
| Hand1 | Mm00433931_m1 |
| Mest | Mm00485003_m1 |
| Slc2a1 | Mm00441473 m1 |
| Slc2a3 | Mm00441483 m1 |
| Slc38a1 | Mm00506391 m1 |
| Slc38a2 | Mm00628416 m1 |
| Slc38a4 | Mm00459056 m1 |
| Gapdh | Mm99999915_g1 |
Taqman probes were purchased from Life Technologies.
SI Materials and Methods
Circulating Metabolites and Hormones.
Blood glucose concentrations in pregnant and NP mice were measured on a hand-held glucometer (One Touch). Whole blood was collected into EDTA-coated tubes and centrifuged at 3,000 rpm for 10 min (MSE Mistral 3000i centrifuge, DJB labcare, Newport Pagnell, UK) and plasma recovered. Plasma leptin and insulin were measured simultaneously by a two-plex electrochemical luminescence immunoassay (MesoScale Discovery) with intra-assay coefficients of variation of 10.8% and 12.4%, respectively. Plasma triglycerides and total cholesterol concentrations were determined using enzymatic assays (Siemens Healthcare) with intra-assay coefficients of variation of 4.0% and 3.5%, respectively. Plasma free fatty acids were determined using an enzymatic assay (Roche) with an intra-assay coefficient of variation of 7.3%.
Placental Nutrient Transport Assays.
Materno-fetal clearance of MeAIB (NEN NEC-671; specific activity 1.86 GBq/mmol; Perkin-Elmer) and MeG (NEN NEC-377; specific activity 2.1 GBq/mmol) was measured as described previously (52). In particular, a 200 μL bolus containing 3.5 µCi of MeAIB and 3.5 µCi of MeG in physiological saline [0.9% (wt/vol)] was injected into the maternal jugular vein. Two minutes after tracer injection, the dam was killed, uteri were collected, and fetal and placental weights recorded. Entire litters of placentas were collected for morphological analyses or snap-frozen in liquid nitrogen for quantification of gene expression or glycogen content. Fetuses were decapitated, fetal tails taken for DNA genotyping, and then fetuses minced and lysed at 55 °C in Biosol (National Diagnostics). Fetal lysates were measured for beta emissions by liquid scintillation counting (Optiphase Hisafe II and Packard Tri-Carb, 1900; Perkin-Elmer USA), and radioactivity (DPM) in the fetuses was used to calculate placental transfer of MeAIB or MeG per unit of placental surface area and/or per milligram of fetus.
Placental Morphology.
Placentas were bisected. One placental half was fixed in 4% (wt/vol) paraformaldehyde, paraffin-embedded, exhaustively sectioned at 7 µm, and stained with hematoxylin and eosin to determine gross placental structure. The absolute and percentage volumes of the placental Lz and Jz were determined by point counting using the Computer Assisted Stereological Toolbox (CAST v2.0; Olympus), as described previously (16). The other placental half was fixed in 4% (wt/vol) glutaraldehyde, embedded in Spurr’s epoxy resin and a single, and 1-µm midline section was cut and then stained with toluidine blue for detailed analysis of Lz structure using the CAST system as described (16). Briefly, to determine the volume densities of each Lz component (FC, MBS, trophoblast), point counting was used and their densities were multiplied by the estimated volume of the Lz to obtain estimated component volumes. Surface densities of maternal-facing and fetal-facing interhaemal membrane surfaces were then determined by recording the number of intersection points along cycloid arcs in random fields of view. These were converted to absolute surface areas and the total surface area for exchange calculated (averaged surface area of MBS and FC). Labyrinthine fetal capillary length density, total capillary length, and diameter were obtained using counting frames, and the mean orthogonal interhaemal membrane thickness was determined by measuring the shortest distance between FC and the closest MBS at random starting locations within the Lz. Per Lz, 200 measurements were made. The harmonic mean thickness (barrier thickness) was calculated from the reciprocal of the mean of the reciprocal distances. The theoretical diffusion capacity (diffusing capacity) was calculated using the total surface area for exchange divided by the mean barrier thickness and multiplied by Krogh’s constant for oxygen diffusion.
Placental Gene Expression.
RNA was extracted from placentas using the RNeasy Plus Mini Kit (Qiagen) and the quantity of RNA determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Inc.). From each sample, 2.5 µg of total RNA was reverse-transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit with random primers (Applied Biosystems). Gene expression was assessed by quantitative real-time PCR (7500 Fast Real-Time PCR System, Applied Biosystems) in duplicate using Taqman assays (Table S2) and the 2–ΔΔCT method for quantification.
Placental Glycogen Content.
Placental glycogen content was measured indirectly using amyloglucosidase as previously described (51). Briefly, placentas were homogenized in cold, deionized water and assayed in duplicate by adding acetate buffer (0.05 M, pH 4.5) with amyloglucosidase (70U; Sigma Aldrich) or an equivalent volume of water. Samples were incubated at 55 °C for 10 min before deproteinization with 0.3 M zinc sulfate and 0.3 M barium hydroxide. Samples were then centrifuged at 3,000 rpm for 10 min (MSE Mistral 3000i centrifuge, DJB labcare, Newport Pagnell, UK) and supernatant glucose concentrations determined using a Yellow Springs glucose analyzer. Glycogen concentration was determined by subtracting the glucose concentration of the sample with enzyme from the sample without enzyme (endogenous glucose only).
Acknowledgments
We thank Melanie Monk, Nuala Daw, Emma Eastwell, and staff at the Combined Animal Facility for their technical help; Dr. Gavin Jarvis for statistical advice; and Dr. Klaus Okkenhaug for providing the α/+ mice. This study was supported in part by a Centre for Trophoblast Research award of a Next Generation Fellowship (to A.N.S.-P.) and the Erasmus Exchange scheme for a scholarship (to J.L.-T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11066.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602012113/-/DCSupplemental.
References
- 1.Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58(2):114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petry CJ, Ong KK, Dunger DB. Does the fetal genotype affect maternal physiology during pregnancy? Trends Mol Med. 2007;13(10):414–421. doi: 10.1016/j.molmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: Endocrine regulation. J Physiol. 2009;587(Pt 14):3459–3472. doi: 10.1113/jphysiol.2009.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimasuay KG, Boeuf P, Powell TL, Jansson T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol. 2016;7:12. doi: 10.3389/fphys.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. doi: 10.3389/fphys.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(Pt 1):25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, et al. Placental adaptations in growth restriction. Nutrients. 2015;7(1):360–389. doi: 10.3390/nu7010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson ME, Biensen NJ, Youngs CR, Ford SP. Development of Meishan and Yorkshire littermate conceptuses in either a Meishan or Yorkshire uterine environment to day 90 of gestation and to term. Biol Reprod. 1998;58(4):905–910. doi: 10.1095/biolreprod58.4.905. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell CL. Maternal and fetal influences on uterine and conceptus development in the cow: II. Blood flow and nutrient flux. J Anim Sci. 1991;69(5):1954–1965. doi: 10.2527/1991.6951954x. [DOI] [PubMed] [Google Scholar]
- 10.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 11.Knight ZA, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125(4):733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grey A, et al. Evidence for a role for the p110-alpha isoform of PI3K in skeletal function. Biochem Biophys Res Commun. 2010;391(1):564–569. doi: 10.1016/j.bbrc.2009.11.099. [DOI] [PubMed] [Google Scholar]
- 13.Foukas LC, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441(7091):366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 14.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2011;589(Pt 1):7–20. doi: 10.1113/jphysiol.2010.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sferruzzi-Perri AN, et al. Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology. 2011;152(8):3202–3212. doi: 10.1210/en.2011-0240. [DOI] [PubMed] [Google Scholar]
- 16.Sferruzzi-Perri AN, et al. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J. 2013;27(10):3928–3937. doi: 10.1096/fj.13-234823. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan OR, et al. Corticosterone alters materno-fetal glucose partitioning and insulin signalling in pregnant mice. J Physiol. 2015;593(5):1307–1321. doi: 10.1113/jphysiol.2014.287177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JS, Vaughan OR, Fernandez de Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594(5):1341–1356. doi: 10.1113/JP271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graupera M, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 20.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284(1):12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304(2):567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Mayer W, et al. Expression of the imprinted genes MEST/Mest in human and murine placenta suggests a role in angiogenesis. Dev Dyn. 2000;217(1):1–10. doi: 10.1002/(SICI)1097-0177(200001)217:1<1::AID-DVDY1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Adamson SL, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250(2):358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 24.Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10(1):97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coan PM, et al. Disproportional effects of Igf2 knockout on placental morphology and diffusional exchange characteristics in the mouse. J Physiol. 2008;586(20):5023–5032. doi: 10.1113/jphysiol.2008.157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z-Z, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 27.Coan PM, et al. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008;586(18):4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Constância M, et al. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci USA. 2005;102(52):19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dilworth MR, et al. Placental-specific Igf2 knockout mice exhibit hypocalcemia and adaptive changes in placental calcium transport. Proc Natl Acad Sci USA. 2010;107(8):3894–3899. doi: 10.1073/pnas.0911710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganguly A, et al. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007;292(5):E1241–E1255. doi: 10.1152/ajpendo.00344.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150(3):1287–1293. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey KM, et al. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83(9):3320–3326. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- 33.Glazier JD, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42(4):514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: Does the placenta function as a nutrient sensor? A review. Placenta. 2006;27(Suppl A):S91–S97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Clifton VL. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Biensen NJ, Wilson ME, Ford SP. The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J Anim Sci. 1998;76(8):2169–2176. doi: 10.2527/1998.7682169x. [DOI] [PubMed] [Google Scholar]
- 37.Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW., Jr Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003;547(Pt 1):85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angiolini E, et al. Developmental adaptations to increased fetal nutrient demand in mouse genetic models of Igf2-mediated overgrowth. FASEB J. 2011;25(5):1737–1745. doi: 10.1096/fj.10-175273. [DOI] [PubMed] [Google Scholar]
- 39.Aldoretta PW, Carver TD, Hay WW., Jr Ovine uteroplacental glucose and oxygen metabolism in relation to chronic changes in maternal and fetal glucose concentrations. Placenta. 1994;15(7):753–764. doi: 10.1016/0143-4004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 40.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20(4):419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 41.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235(12):3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- 42.Brelje TC, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: Implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132(2):879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 43.Hayward CE, et al. Placental adaptation: What can we learn from birthweight:placental weight ratio? Front Physiol. 2016;7:28. doi: 10.3389/fphys.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laviola L, et al. Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling. Endocrinology. 2005;146(3):1498–1505. doi: 10.1210/en.2004-1332. [DOI] [PubMed] [Google Scholar]
- 45.Yung HW, et al. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173(2):451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Street ME, et al. Impairment of insulin receptor signal transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal growth. Eur J Endocrinol. 2011;164(1):45–52. doi: 10.1530/EJE-10-0752. [DOI] [PubMed] [Google Scholar]
- 47.Abu-Amero SN, Ali Z, Bennett P, Vaughan JI, Moore GE. Expression of the insulin-like growth factors and their receptors in term placentas: A comparison between normal and IUGR births. Mol Reprod Dev. 1998;49(3):229–235. doi: 10.1002/(SICI)1098-2795(199803)49:3<229::AID-MRD2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 48.Jansson N, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scioscia M, et al. Insulin resistance in human preeclamptic placenta is mediated by serine phosphorylation of insulin receptor substrate-1 and -2. J Clin Endocrinol Metab. 2006;91(2):709–717. doi: 10.1210/jc.2005-1965. [DOI] [PubMed] [Google Scholar]
- 50.Iñiguez G, et al. IGF-IR signal transduction protein content and its activation by IGF-I in human placentas: Relationship with gestational age and birth weight. PLoS One. 2014;9(7):e102252. doi: 10.1371/journal.pone.0102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musial B, et al. Proximity to delivery alters insulin sensitivity and glucose metabolism in pregnant mice. Diabetes. 2016;65(4):851–860. doi: 10.2337/db15-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Constância M, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417(6892):945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]