Significance
Invasive mammalian predators are arguably the most damaging group of alien animal species for global biodiversity. Thirty species of invasive predator are implicated in the extinction or endangerment of 738 vertebrate species—collectively contributing to 58% of all bird, mammal, and reptile extinctions. Cats, rodents, dogs, and pigs have the most pervasive impacts, and endemic island faunas are most vulnerable to invasive predators. That most impacted species are insular indicates that management of invasive predators on islands should be a global conservation priority. Understanding and mitigating the impact of invasive mammalian predators is essential for reducing the rate of global biodiversity loss.
Keywords: extinction, feral cat, island, invasive mammal, trophic cascade
Abstract
Invasive species threaten biodiversity globally, and invasive mammalian predators are particularly damaging, having contributed to considerable species decline and extinction. We provide a global metaanalysis of these impacts and reveal their full extent. Invasive predators are implicated in 87 bird, 45 mammal, and 10 reptile species extinctions—58% of these groups’ contemporary extinctions worldwide. These figures are likely underestimated because 23 critically endangered species that we assessed are classed as “possibly extinct.” Invasive mammalian predators endanger a further 596 species at risk of extinction, with cats, rodents, dogs, and pigs threatening the most species overall. Species most at risk from predators have high evolutionary distinctiveness and inhabit insular environments. Invasive mammalian predators are therefore important drivers of irreversible loss of phylogenetic diversity worldwide. That most impacted species are insular indicates that management of invasive predators on islands should be a global conservation priority. Understanding and mitigating the impact of invasive mammalian predators is essential for reducing the rate of global biodiversity loss.
Invasive mammalian predators (“invasive predators” hereafter) are arguably the most damaging group of alien animal species for global biodiversity (1–3). Species such as cats (Felis catus), rats (Rattus rattus), mongoose (Herpestes auropunctatus), and stoats (Mustela erminea) threaten biodiversity through predation (4, 5), competition (6), disease transmission (7), and facilitation with other invasive species (8). The decline and extinction of native species due to invasive predators can have impacts that cascade throughout entire ecosystems (9). For example, predation by feral cats and red foxes (Vulpes vulpes) has led to the decline or extinction of two thirds of Australia’s digging mammal species over the past 200 y (10, 11). Reduced disturbance to topsoil in the absence of digging mammals has led to impoverished landscapes where little organic matter accumulates and rates of seed germination are low (10). In the Aleutian archipelago, predation of seabirds by introduced Arctic foxes (Alopex lagopus) has lowered nutrient input and soil fertility, ultimately causing vegetation to transform from grasslands to dwarf shrub/forb-dominated systems (12).
Mitigating the negative impacts of invasive mammalian predators is a primary goal of conservation agencies worldwide (1, 13, 14). Regardless, there remains no global synthesis of the role of invasive predators in species declines and extinctions (but see refs. 3 and 15). Here, we quantify the number of bird, mammal, and reptile species threatened by, or thought to have become extinct (since AD 1500) due to, invasive mammalian predators. We use metaanalysis to examine taxonomic and geographic trends in these impacts and show how the severity of predator impacts varies according to species endemicity and evolutionary distinctiveness.
Results and Discussion
In total, 596 threatened and 142 extinct species (total 738) have suffered negative impacts from 30 species of invasive mammalian predators from 13 families and eight orders. These species include three canids, seven mustelids, five rodents, two procyonids, three viverrids, two primates, two marsupials, two mongooses, and single representatives from four other families, with 60% from the order Carnivora (Table S1). The 738 impacted species consist of 400 bird species from 78 families, 189 mammal species from 45 families, and 149 reptile species from 26 families (Dataset S1). Invasive mammalian predators emerge as causal factors in the extinction of 87 bird, 45 mammal, and 10 reptile species, which equates to 58% of modern bird, mammal, and reptile species extinctions globally (including those species classed as “extinct in the wild”). Invasive predators also threaten 596 species classed as “vulnerable” (217 species), “endangered” (223), or “critically endangered” (156), of which 23 are classed as “possibly extinct.”
Table S1.
List of invasive mammalian predators identified as negatively affecting threatened or extinct bird, mammal, and reptile species
| Order | Family | Species | Name in Dataset S1 “Predator” column |
| Afrosoricida | Tenrecidae | Tenrec ecaudatus | Tenrec |
| Artiodactyla | Suidae | Sus scrofa | Pig* |
| Carnivora | Canidae | Canis familiaris | Dog* |
| Lycalopex culpaeus/griseus | Chile fox | ||
| Vulpes vulpes | Red fox* | ||
| Felidae | Felis catus | Cat* | |
| Herpestidae | Herpestes fuscus | Brown mongoose | |
| Herpestes auropunctatus | Indian mongoose* | ||
| Mustelidae | Martes martes | Pine marten | |
| Mustela erminea | Stoat* | ||
| Mustela itatsi | Japan weasel | ||
| Mustela nivalis | Least weasel | ||
| Mustela putorius furo | Ferret | ||
| Mustela sibirica | Siberian weasel | ||
| Neovison vison | Mink | ||
| Procyonidae | Nasua nasua | Coati | |
| Procyon lotor | Raccoon | ||
| Viverridae | Civettictis civetta | African civet | |
| Genetta genetta | Genet | ||
| Viverricula indica | Indian civet | ||
| Didelphimorphia | Didelphidae | Didelphis marsupialis | Opossum |
| Diprotodontia | Phalangeridae | Trichosurus vulpecula | Possum |
| Erinaceomorpha | Erinaceidae | Erinaceus europaeus | Hedgehog |
| Primates | Cercopithecidae | Cercopithecus mona | Monkey |
| Macaca fascicularis | Macaque | ||
| Rodentia | Muridae | Mus musculus | Rodent* |
| Rattus argentiventer | Rodent* | ||
| Rattus exulans | Rodent* | ||
| Rattus norvegicus | Rodent* | ||
| Rattus rattus | Rodent* |
Predators that were included in the predator model (Statistical Analyses) are indicated with an asterisk (*).
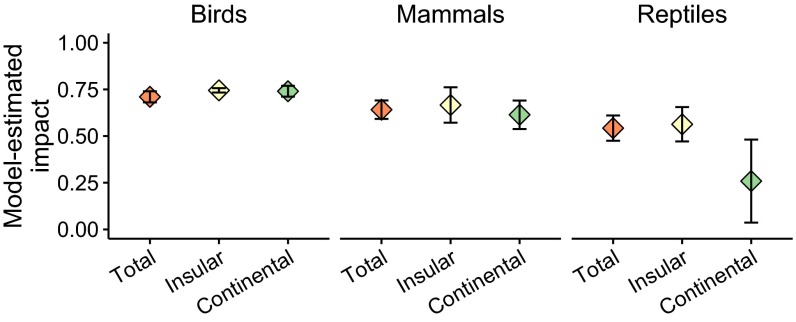
To assess the comparative severity of predator impacts, we assigned each of 1,439 predator-threatened species cases a value of either 0.25 (secondary cause of species decline), 0.75 (primary cause of species decline), or 1.0 (species extinction attributed to the predator), and we weighted these values by the strength of evidence available, drawing on a total of 996 supporting references (Methods). The severity of predator impacts and the strength of evidence supporting them [the inverse of the width of confidence intervals (CIs)] was higher for bird and mammal species compared with reptile species (Fig. 1).
Fig. 1.
Model-estimated severity of impact of invasive predators on birds, mammals, and reptiles for all species combined (Total), insular endemics (Insular), and species found on continents (Continental). Error bars are 90% confidence intervals. Model estimates and confidence intervals are weighted by the strength of evidence available. See Table S5 for model estimates.
Table S5.
Metaanalysis model estimates for the effect of taxonomic class, insularity, and predator species on the severity of predator impacts on threatened species (Figs. 1 and 3)
| Moderator variables | Birds | Mammals | Reptiles | |||
| Estimate | CI | Estimate | CI | Estimate | CI | |
| Taxonomic class | 0.71 | 0.68, 0.74 | 0.64 | 0.59, 0.69 | 0.54 | 0.47, 0.61 |
| Insularity | ||||||
| Insular endemic | 0.75 | 0.73, 0.76 | 0.67 | 0.57, 0.76 | 0.56 | 0.47, 0.66 |
| Continental | 0.74 | 0.71, 0.77 | 0.61 | 0.54, 0.69 | 0.26 | 0.04, 0.48 |
| Predators | ||||||
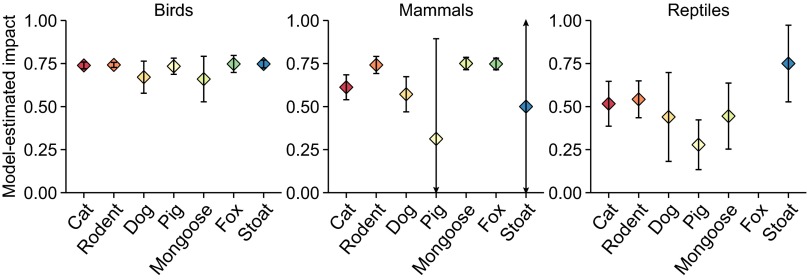
| Cat | 0.74 | 0.72, 0.76 | 0.61 | 0.54, 0.68 | 0.52 | 0.39, 0.65 |
| Rodent | 0.74 | 0.73, 0.76 | 0.74 | 0.69, 0.79 | 0.54 | 0.44, 0.65 |
| Dog | 0.67 | 0.58, 0.76 | 0.57 | 0.47, 0.67 | 0.44 | 0.18, 0.70 |
| Pig | 0.73 | 0.69, 0.78 | 0.31 | −0.27, 0.89 | 0.28 | 0.13, 0.42 |
| Mongoose | 0.66 | 0.53, 0.79 | 0.75 | 0.71, 0.79 | 0.44 | 0.25, 0.64 |
| Fox | 0.75 | 0.70, 0.80 | 0.75 | 0.71, 0.78 | — | — |
| Stoat | 0.75 | 0.72, 0.77 | 0.5 | −0.66, 1.66 | 0.75 | 0.53, 0.97 |
Em dashes (—) indicate that there were no cases where foxes impacted reptiles.
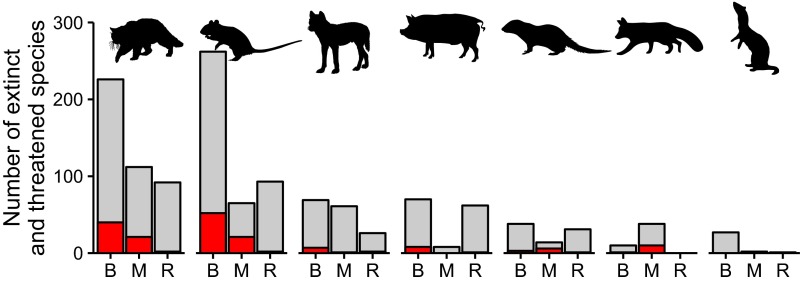
Rodents are linked to the extinction of 75 species (52 bird, 21 mammal, and 2 reptile species; 30% of all extinctions) and cats to 63 extinctions (40, 21, and 2 species, respectively; 26%) whereas red foxes, dogs (Canis familiaris), pigs (Sus scrofa), and small Indian mongoose (H. auropunctatus) are implicated in 9–11 extinctions each (Fig. 2). For all threatened and extinct species combined, cats and rodents threaten similar numbers of species (430 and 420 species, respectively), followed by dogs (156 species), pigs (140 species), mongoose (83 species), red foxes (48 species), stoats (30 species) (Fig. 2), and the remaining predators (range 1–14 species). The lower number of species impacted by some predators, such as red foxes and stoats, reflects the limited number of locations in which these predators have established alien populations (16). The frequency of impacted species in each taxonomic class differed among predators (χ2 = 112.27, P < 0.001). Cats, rodents, and stoats threaten more bird than mammal or reptile species whereas red foxes threaten more mammal species (Fig. 2). Dogs threaten fewer reptile species, and pigs and mongoose threaten fewer mammal species, compared with other taxonomic classes (Fig. 2). Although cats and rodents negatively affect the most bird species, birds experience similar impact across predator species (Fig. 3). Mammals experience lower, but more variable, impacts from pigs and stoats compared with the other predators (Fig. 3). The greatest impact on reptile species is from stoats, and the lowest from foxes (no impact) and pigs (Fig. 3). The “significance” of differing relationships between invasive predators and impacted species classes is uncertain, however, because confidence intervals overlapped in most cases.
Fig. 2.
Numbers of threatened and extinct bird (B), mammal (M), and reptile (R) species negatively affected by invasive mammalian predators. Gray bars are the total number of extinct and threatened species, and red bars are extinct species (including those classed as extinct in the wild). Predators affecting <15 species are not shown here. Predators (L to R) are the cat, rodents, dog, pig, small Indian mongoose, red fox, and stoat.
Fig. 3.
Severity of model-estimated impacts of invasive predator species on birds, mammals, and reptiles. Error bars are 90% confidence intervals. Model estimates and confidence intervals are weighted by the strength of evidence available. See Table S5 for model estimates. To aid visual interpretation across all estimates, the error bars for the effects of pigs and stoats on mammals are truncated at the limits of the y axis, but the values can be found in Table S5.
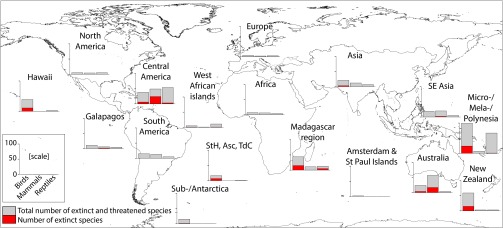
Central America (including the Caribbean) has experienced the most extinctions (33 species), followed by Micro-/Mela-/Polynesia (25), Australia (21), the Madagascar region (20), New Zealand (15), and Hawaii (11), with the remaining regions having 0–7 species extinctions each (Fig. 4). The taxonomy of impacted species varied among regions, with the highest numbers of impacted mammal species occurring in Australia and Central America, and most of the impacted reptile species occurring in Micro-/Mela-/Polynesia and Central America (Fig. 4). Most impacted bird species are in Micro-/Mela-/Polynesia, New Zealand, the Madagascar region, Central America, and Hawaii (Fig. 4).
Fig. 4.
Numbers of threatened and extinct bird, mammal, and reptile species impacted by invasive predators in 17 regions (Fig. S3 and Table S2). Gray bars represent the total number of extinct and threatened species, and red bars represent the number of extinct species (including those classed as extinct in the wild). StH, Asc, and TdC indicate the islands of St. Helena, Ascension, and Tristan da Cunha, respectively.
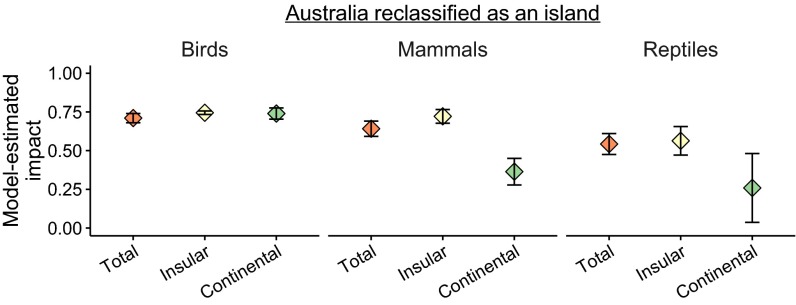
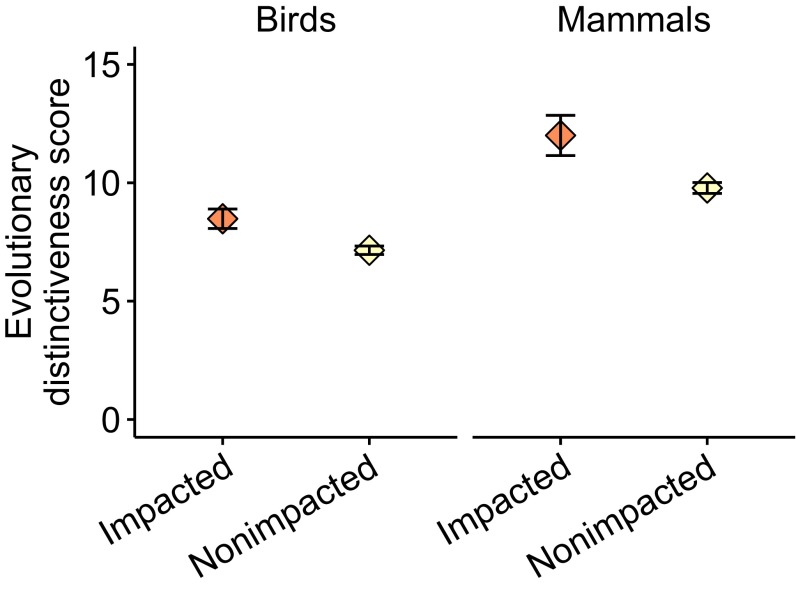
Insular endemics accounted for 87% of extinct species (124 species) and 81% of the sum of all threatened/extinct species (601 species). The proportions of total threatened/extinct species that were insular endemics varied between taxonomic classes (χ2 = 117.29, P < 0.001; birds 90%, mammals 55%, reptiles 91%). Insular endemic reptile species were more negatively affected by invasive mammalian predators than continental species, whereas mammal and bird species experienced similar impacts between the two groups (Fig. 1). If Australia is reclassified as an island, insular endemic mammals experience more severe predator impacts than continental species (Fig. S1). We sourced evolutionary distinctiveness scores from published databases (Methods) to show that species negatively affected by invasive predators were more evolutionarily distinct than “nonimpacted” species for both bird (t = 3.32, P = 0.001) and mammal species (t = 3.31, P = 0.001) (Fig. S2).
Fig. S1.
Model-estimated severity of impact of invasive predators on birds, mammals, and reptiles for all species combined (Total), insular endemics (Insular), and species found on continents (Continental) with Australia reclassified as insular instead of continental. Error bars are 90% confidence intervals. Model estimates and confidence intervals are weighted by the strength of evidence available.
Fig. S2.
Mean evolutionary distinctiveness scores for extant threatened birds and mammals. Impacted, identified as being negatively affected by invasive predators (Dataset S1); Nonimpacted, not identified as being negatively affected by invasive predators. Error bars are SEs.
Although it is often stated that invasive predators have contributed to many modern extinctions (1, 2, 11, 17), our findings reveal the magnitude and pervasiveness of their impacts and link them to the majority (58%) of modern bird, mammal, and reptile species extinctions. This figure is likely an underestimate because 23 critically endangered species negatively affected by invasive predators are currently classed as possibly extinct. Evolutionarily distinct species are most affected, meaning that invasive predators are drivers of irreversible loss of global phylogenetic diversity, affecting both mainland and island-endemic species.
Introduced rodents and cats are major agents of extinction, collectively being listed as causal factors in 44% of modern bird, mammal, and reptile species extinctions. We pooled the impacts of rodents across five species, but previous studies indicate that R. rattus has negatively affected the most species, followed by Rattus norvegicus and Rattus exulans (18–20). The role of the house mouse (Mus musculus) is less well understood, but there is emerging evidence of severe predatory impacts on insular seabird (21) and lizard species (22). We found that cats, rodents, dogs, and pigs have had the most pervasive effects across regions and taxonomic classes, supporting recent work by Bellard et al. (3), who identified these four taxa as the invasive species affecting the greatest number of threatened vertebrates globally, after chytrid fungus (Batrachochytrium dendrobatidis). However, other predators have had large impacts in particular regions; stoats remain a major threat to New Zealand bird and reptile species (23), and the red fox, along with the feral cat, is an important driver of Australian mammal species extinctions (11).
Fewer reptile species were negatively affected by invasive mammalian predators, compared with bird and mammal species. Reptiles also had a lower average impact score, which may be because reptiles are less studied than birds and mammals (9), with only 40% of the world’s reptiles having been assessed for the Red List thus far (compared with ∼99% for birds and mammals) (24). Further insights will likely emerge once the conservation status of most reptiles has been determined. Detailed studies from individual regions nonetheless demonstrate that invasive predators can have severe impacts on local reptile assemblages (e.g., ref. 25). Evolutionary exposure to native mammalian predators might moderate such effects; few Australian reptiles are threatened by cat and fox predation whereas more than 100 reptile species in the Caribbean/Central America and Micro-/Mela-/Polynesia are threatened with extinction by rodents, cats, pigs, dogs, and mongoose (25, 26).
Insular regions are most affected by invasive predators, and insular endemic reptile species, but not bird and mammal species, are more heavily affected than continental species. This last finding contrasts with Blackburn et al. (13), who reported such an effect for birds, as did Medina et al. (1) for all three taxonomic classes. The difference in our results could arise because both previous studies assessed insular species only and used individual populations (species × island) as the experimental unit whereas we assessed all species across their entire geographic ranges. The isolation of many islands and a lack of natural predators mean that insular species often lack appropriate defensive traits, thus making them naive to the threat of invasive predators (9, 27). The high extinction rates of ground-dwelling birds in Hawaii (28) and New Zealand (29)—both of which lack native mammalian predators—are cases in point.
That most impacted species are insular indicates that management of invasive predators on islands should be a global conservation priority. Given the many islands on which invasive predators occur and the high costs involved in controlling or eradicating them, prioritization of islands for eradications is an important exercise (30–33). Facilitation between multiple invasive species (e.g., rodents providing abundant food for cats, thus maintaining high densities of the latter) can exacerbate their respective impacts on native species (1, 9). Thus, it is essential that eradications adopt a whole-ecosystem approach to avoid the ecological release of undesirable species (5, 34). Modeling can help determine the order in which multiple species should be eradicated (35) and how best to allocate resources (36). On continents or large islands where eradications are difficult, alternative approaches are needed, such as predator-proof fencing (37), improved land management (38, 39), restoration of top predators (40, 41), and lethal control (42).
Although we have documented the comparative severity of impacts of invasive mammalian predators, we note that the strength of evidence available to quantify predator impacts was often low (Dataset S1), particularly for reptile species. While invasive predators are named as causal factors in large numbers of extinctions and as key threats to many threatened species, the lack of strong evidence suggests that there remains an urgent need for research on the impacts of invasive predators relative to other threats (e.g., habitat loss). Teasing apart the impacts of different threatening processes is challenging for extinct species and for those that have suffered historical declines, have small populations, and/or inhabit remote islands but should be more feasible for many other threatened species. Understanding and mitigating the impact of invasive mammalian predators is essential for reducing the rate of global biodiversity loss.
Methods
Data Collation.
For all threatened species in the taxonomic classes Aves, Mammalia, and Reptilia, we downloaded data on taxonomy and conservation status from the International Union for Conservation of Nature and Natural Resources (IUCN) Red List in December 2014 (version 2014.3) using the inbuilt search and export functions (n = 3,745 species) (Dataset S2). We did not assess amphibians here because our preliminary research indicated that the invasive predators impacting them are mostly nonmammalian (e.g., snakes, fish, crayfish, and other amphibians). Threatened species were those listed as vulnerable, endangered, critically endangered, extinct, or extinct in the wild. We then used a custom R script (Dataset S3) to download additional Red List information on each species’ range and major threats.
We filtered this database (n = 3,745 species) in Microsoft Access by searching the “major threats” section for any of the following keywords: predator*, predation, cat, cats, fox*, dog, dogs, rat, rats, rodent*, Rattus, mouse, mice, stoat*, mongoose*, pig, pigs, mink, ferret*, weasel*, mustelid*, possum*, macaque*, coati*, and civet*. These predators were chosen based on consultation of the Global Invasive Species Database (43) and Long (16). This search returned 771 records, which we inspected to determine whether invasive alien predators were identified as a known or likely threat to each species (n = 703 species identified as negatively impacted by invasive predators). We cross-checked this list against previous reviews (1, 18, 20, 44–48) and added 35 additional threatened species recorded as being negatively affected by invasive predators, but not revealed in our Red List search. Given the small number of additional species identified and the broad geographic coverage of the previous studies used for cross-checking, we do not consider that this exercise brings any systematic bias to our analyses.
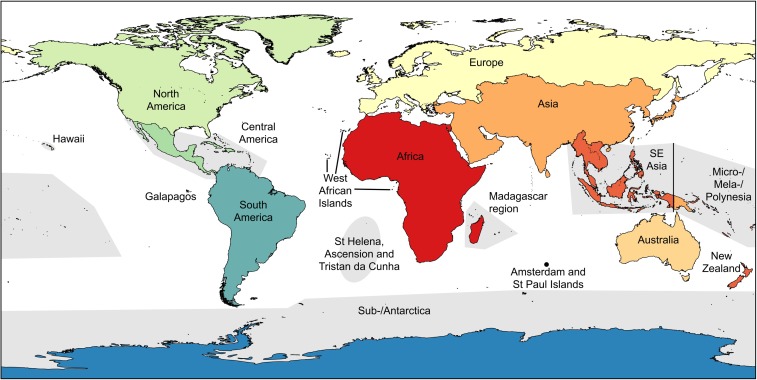
For each of the 738 study species, we recorded information on taxonomic classification (class, order, family), Red List status, insularity (insular endemic or found on continents also), and region (Fig. S3 and Table S2). Information on species distributions was sourced primarily from the Red List although other sources were consulted in a small number of cases. For the analyses, we included in the extinct category four species classed as extinct in the wild.
Fig. S3.
Classification of regions (Table S2).
Table S2.
Classification of regions (see Fig. S1)
| Region ID | Region |
| N_AMER | North America (excluding Hawaii) |
| HAWAII | Hawaii |
| CEN_AMER | Central America (including the Caribbean) |
| GALAP | Galapagos |
| S_AMER | South America (excluding Galapagos) |
| AFRI | Africa (excluding islands listed below) |
| W_AFRI_IS | West African islands (Canary Islands, Cape Verde, Sao Tome, Equatorial Guinea) |
| MADA | Madagascar region (Madagascar, Comoros, Mayotte, Seychelles, Mauritius, Reunion Islands) |
| EUR | Europe |
| ASIA_EW | Asia (excluding Southeast Asia) |
| SE_ASIA | Southeast Asia (including Christmas Island) |
| AUS | Australia (Australia, excluding Christmas Island, Macquarie Island, and Heard and McDonald Islands) |
| MICRO | Micro-/Mela-/Polynesia |
| NZ | New Zealand (excluding sub-Antarctic islands) |
| AMSTER | Amsterdam and St. Paul Islands |
| SUB_ANT | Antarctica and sub-Antarctic islands (Antipodes, Auckland, Bounty, Bouvet, Campbell, Crozet, Heard and McDonald, Kerguelen, Macquarie, Prince Edward, South Georgia, South Sandwich, Snares) |
| StHEL | St. Helena, Ascension, and Tristan da Cunha |
| MULTI | Species occurs within multiple regions. |
Items in column 1 refer to the region IDs used in Dataset S1. We did not include the nonbreeding ranges of pelagic seabirds and turtles in regional classifications.
To find information on the impact of invasive predators on each of the study species, we initially searched the Red List and Scopus database for relevant material using species names and synonyms, followed by consultation of primary and gray literature cited therein. We defined impact as any inference that an invasive predator had caused a decline in the abundance or distribution of a species. In most cases, predation was inferred as the primary mechanism of predator impacts although competition, disease transmission, and habitat disturbance were also cited in some cases. For accounts that referred only to “introduced/invasive predators” and not a specific species, we assigned the impact to a generic predator group. We took any reference to “domestic predators/carnivores/pets” to mean cats (F. catus) and dogs (C. familiaris). We did not distinguish the impacts of individual rodent species because many accounts did not provide sufficient information to allow discrimination of individual species effects and because the relative impacts of the different rodent species have been reviewed elsewhere (18–20, 49, 50).
Given the difficulties in attributing causation in species declines and extinctions, most inferences regarding the impact of invasive predators were based on observational evidence, rather than experimental data. For this reason, we used a similar approach to that of previous studies (1, 19) and coded the degree of predator impacts as follows: mixed (0.25, when the predator was a secondary cause of species decline); high (0.75, when the predator was a primary cause of species decline); and strong (1.0, when the extinction of the species was attributed to the predator). Unlike previous studies (1, 19), however, we did not include a “nil impact” level (e.g., 0.01) because such information is not systematically reported in the literature. Other threats may have contributed to the species’ declines/extinctions although assessing their relative importance was beyond the scope of this study. We assessed species across their entire geographic ranges and thus did not code predator impacts for individual populations (e.g., multiple islands). This exercise was conducted between March and September 2015, and it revealed 1,381 individual predator-threatened species cases, plus an additional 58 cases where the predator species were not named. The 996 references supporting the rankings are listed in Dataset S4.
Statistical Analyses.
We first summarized numbers of extinct and threatened species impacted by invasive predators, based on taxonomic classes and geographic regions where they occur, or occurred. We then used metaanalysis in the metafor package version 1.9-6 in R version 3.1.2 (51, 52) to analyze these trends based on three categorical variables: (i) taxonomic class model (levels: Aves, Mammalia, Reptilia); (ii) insularity model [levels: insular endemic, or continental (either wholly or partially)]; and (iii) predator model [levels: rodent (Rodentia), cat, dog, red fox (V. vulpes), stoat (M. erminea), small Indian mongoose (H. auropunctatus), and pig (S. scrofa)].
For the predator model, we excluded 19 predator species that impacted fewer than 15 threatened species each (range 30–430 threatened species impacted by each of the seven remaining predators). We conducted separate tests for each of these variables using the restricted maximum-likelihood estimator. We pooled impacts across all predators for the taxonomic class and insularity models; if a threatened species was impacted by multiple predators, we used the highest impact and its associated weight. For example, if a bird species was impacted by both cats (impact = 0.75, weight = 10) and rodents (impact = 0.25, weight = 100), we used the former pair of values for the pooled category, which means that the models estimate the strongest predator impacts across taxonomic classes and insularity. To examine individual responses of the three taxonomic classes, we conducted separate analyses for birds, mammals, and reptiles across insular endemism and predators. The response variable was the impact rankings described above, such that higher effect sizes represented greater predator impacts. We inferred “significant” effects where the 90% confidence intervals of the different predictor variable levels did not overlap. Data used in the analyses are available as Dataset S1 (see also Table S3).
Table S3.
Structure of Dataset S1
| Column name | Explanation |
| Species.ID.number | Unique species ID number in the IUCN Red List |
| Class | Taxonomic class |
| Order | Taxonomic order |
| Family | Taxonomic family |
| Genus.species | Species name |
| Red.List.status | Status on the Red List v2014.3 (VU, vulnerable; EN, endangered; CR, critically endangered; EX, extinct; EW, extinct in the wild) |
| Island.endemic | 1, species is an island endemic; 0, species is found on continents, either wholly or partially. |
| Region | See Table S2 |
| Predator | See Table S1 for species codes. “Generic” refers to an unidentified species, and “Pooled” is the strongest impact ranking and its associated weight for each threatened species. |
| Impact | Predator impact rankings taking values of 0.25, 0.75, 1.0 (Data Collation). |
| Weight | Weighting of predator impacts taking values of 1, 10, 100, 1000 (Statistical Analyses). |
| Inverse.Weight | The inverse of weight values. |
| References | Reference numbers corresponding to the reference list in Dataset S4. |
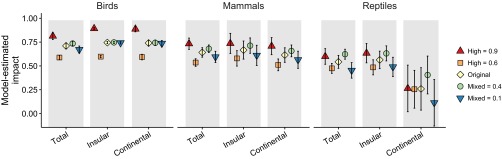
Metaanalysis traditionally weights effect sizes based on each study’s sample variance and/or size. However, these data do not exist for our database because each case consists of a predator × threatened species combination that is assigned a categorical level of impact. Instead, we used a weighting system similar to that of Jones et al. (19) and Medina et al. (1) that weights individual cases based on the type and strength of evidence provided in each case. Assigned weights were as follows: 1 (lowest: no evidence provided apart from stating that the predator is thought to be a cause of species decline or extinction), 10 (single line of correlative evidence), 100 (multiple lines of correlative evidence), or 1,000 (highest: experimental evidence in a before–after and/or control–impact design). We used the inverse of the weights as the variance component in the metaanalysis. Examples of correlative evidence included artificial nest experiments, correlation between species decline and predator introduction, absence of a species from parts of its historical range now inhabited by predators, monitoring of predation events, and analysis of predator diet. Examples of experimental evidence included monitoring of population parameters in response to predator removal, and comparison of islands with and without predators. The weights were assigned during the impact ranking exercise described above. We conducted a fail-safe analysis to determine the number of cases showing no effect that would be needed to eliminate a significant overall effect size (SI Text). We also conducted a sensitivity analysis to determine how the selection of impact values and the use of weights influenced the results (SI Text and Figs. S4 and S5).
Fig. S4.
Comparison of weighted and unweighted model-estimated severity of impact of invasive predators on birds, mammals, and reptiles for all species combined (Total), insular endemics (Insular), and species found on continents (Continental). Error bars are 90% confidence intervals.
Fig. S5.
Influence of different impact-ranking values on the model-estimated severity of impact of invasive predators on birds, mammals, and reptiles for all species combined (Total), insular endemics (Insular), and species found on continents (Continental). Error bars are 90% confidence intervals.
We used χ2 analyses to determine (i) whether the proportion of impacted species that were insular endemics varied among taxonomic classes and (ii) whether the proportion of impacted species in each taxonomic class differed among predators. We restricted the second analysis to those seven predators included in the predator model described above. Significant effects were inferred at the 0.05 level.
Evolutionary Distinctiveness.
We used evolutionary distinctiveness (ED) scores to examine whether invasive predators have had a disproportionate impact on evolutionarily distinct species. ED scores were calculated based on the “fair proportion” metric: i.e., the weighted sum of branch lengths along phylogenetic tree roots to tips, with weights based on the number of tips sharing that branch (see refs. 53–55 for detailed descriptions). This analysis was restricted to extant birds (53) and mammals (54, 55) because data limitations currently prevent ED scores being calculated for reptiles and extinct taxa from all classes. We used general linear models to compare the ED scores of the impacted species against threatened species for which invasive predators were not identified as a threat (“nonimpacted” species hereafter). We used a gamma error distribution because the data were positive, continuous, and skewed. Significant effects were inferred at the 0.05 level. Taxonomic differences between the Red List version 2014.3 and the source databases (53, 55) are detailed in Table S4. Because ED scores were not available for extinct species, the values presented here are likely to be an underestimate of the true effect sizes.
Table S4.
Taxonomic differences between Red List version 2014.3 and source databases (53, 55) for analysis of evolutionary distinctiveness in extant birds and mammals
| Species name in Red List v2014.3 | Comment |
| Birds | |
| Laterallus spilonota | Name changed from L. spinolotus to L. spinulota |
| Siphonorhis americana | Excluded, classed as extinct by Jetz et al. (53) |
| Hydrobates macrodactylus | Excluded, classed as extinct by Jetz et al. (53) |
| Gallirallus lafresnayanus | Excluded, classed as extinct by Jetz et al. (53) |
| Pareudiastes pacificusw | Excluded, classed as extinct by Jetz et al. (53) |
| Alopecoenas erythropterus | Used value for Gallicolumba erythroptera (former genus) |
| Alopecoenas kubaryi | Used value for Gallicolumba kubaryi (former genus) |
| Alopecoenas rubescens | Used value for Gallicolumba rubescens (former genus) |
| Alopecoenas sanctaecrucis | Used value for Gallicolumba sanctaecrucis (former genus) |
| Alopecoenas stairi | Used value for Gallicolumba stairi (former genus) |
| Pareudiastes silvestris | Used value for Gallinula silvestris (former genus) |
| Hypotaenidia okinawae | Used value for Gallirallus okinawae (former genus) |
| Hypotaenidia owstoni | Used value for Gallirallus owstoni (former genus) |
| Hypotaenidia sylvestris | Used value for Gallirallus sylvestris (former genus) |
| Anthropoides paradiseus | Used value for Grus paradisea (former genus) |
| Antigone antigone | Used value for Grus antigone (former genus) |
| Fregetta maoriana | Used value for Oceanites maorianus (former genus) |
| Hydrobates homochroa | Used value for Oceanodroma homochroa (former genus) |
| Hydrobates matsudairae | Used value for Oceanodroma matsudairae (former genus) |
| Ardenna creatopus | Used value for Puffinus creatopus (former genus) |
| Chlidonias albostriatus | Used value for Sterna albostriata (former genus) |
| Sternula nereis | Used value for Sterna nereis (former genus) |
| Thalasseus bernsteini | Used value for Sterna bernsteini (former genus) |
| Aerodramus elaphrus | Used value for Collocalia elaphra (former genus) |
| Aerodramus sawtelli | Used value for Collocalia sawtelli (former genus) |
| Antrostomus noctitherus | Used value for Caprimulgus noctitherus (former genus) |
| Geobiastes squamiger | Used value for Brachypteracias squamiger (former genus) |
| Rhyticeros narcondami | Used value for Aceros narcondami (former genus) |
| Puffinus bryani | Excluded, new species |
| Puffinus bannermani | Used value for nonthreatened Puffinus lherminieri (recent split) |
| Coracopsis barklyi | Used value for nonthreatened Coracopsis nigra (recent split) |
| Hemiphaga chathamensis | Used value for nonthreatened Hemiphaga novaeseelandiae (recent split) |
| Gallinula comeri | Used value for Gallinula nesiotis (recent split) |
| Actenoides excelsus | Excluded, recent split from threatened Actenoides bougainvillei |
| Eurostopodus exul | Used value for nonthreatened Eurostopodus mystacalis (recent split) |
| Treron griveaudi | Used value for nonthreatened Treron australis (recent split) |
| Chasiempis ibidis | Excluded, recent split from threatened Chasiempis sandwichensis |
| Chasiempis sclateri | Excluded, recent split from threatened Chasiempis sandwichensis |
| Pomarea mira | Excluded, recent split from threatened Pomarea mendozae |
| Icterus northropi | Used value for nonthreatened Icterus dominicensis (recent split) |
| Turnix novaecaledoniae | Used value for nonthreatened Turnix varius (recent split) |
| Prosobonia parvirostris | Used value for Prosobonia cancellata (recent split) |
| Apteryx rowi | Excluded, recent split from threatened Apteryx mantelli |
| Synthliboramphus scrippsi | Excluded, recent split from threatened Synthliboramphus hypoleucus |
| Thinornis cucullatus | Name changed from Thinornis rubricollis to T. cucullatus |
| Geotrygon leucometopia | Excluded, recent split from threatened Geotrygon caniceps |
| Mammals | |
| Phascogale pirata | Used value for nonthreatened Phascogale tapoatafa (recent split) |
| Dipodomys insularis, Dipodomys margaritae | Used value for nonthreatened Dipodomys merriami (recent split) |
| Tokudaia tokunoshimensis | Excluded, not listed by Collen et al. (55) |
| Pseudocheirus occidentalis | Used value for nonthreatened Pseudocheirus peregrinus (formerly considered conspecific) |
| Pipistrellus murrayi | Used value for nonthreatened Pipistrellus tenuis (recent split) |
| Neomonachus schauinslandi | Used value for Monachus schauinslandi (former genus) |
| Eubalaena japonica | Excluded, recent split from threatened Eubalaena glacialis |
| Neophocaena asiaeorientalis | Excluded, recent split from threatened Neophocaena phocaenoides |
SI Text
Explanatory Text for Dataset S1.
Dataset S1 contains a CSV file with information on species taxonomy, endemicity, regional occurrence, and the predator impact rankings that were used in the metaanalysis (Table S3); there is also a PDF file (Dataset S4) listing the 996 references that support the impact rankings.
Explanatory Text for Dataset S2.
Dataset S2 is a CSV file containing information on the taxonomy and conservation status of 3,745 birds, mammals, and reptiles classified as threatened in the IUCN Red List version 2014.3 (Data Collation).
Fail-Safe and Sensitivity Analyses.
We conducted a fail-safe analysis (56) to show that 3,203,182 cases showing an effect size of zero would be needed to eliminate a significant overall effect size. We also conducted a sensitivity analysis to determine how the selection of impact values (0.25, 0.75, 1) and the use of weights (1, 10, 100, 1,000) (Methods) influenced the results. We reran the taxonomic class and insularity models five times and changed a different variable each time, as follows: (i) an unweighted model; (ii) 0.25 decreased to 0.1; (iii) 0.25 increased to 0.4; (iv) 0.75 decreased to 0.6; and (v) 0.75 increased to 0.9. We plotted the effect sizes and confidence intervals (Figs. S4 and S5) to identify any qualitative changes in the results relative to the original results (i.e., the pattern changed across taxonomic classes or insular endemicity). We did not rerun the predator model because the results are unlikely to be informative, given the large number of models involved (5 models × 7 predators × 3 taxonomic classes = 105 sets of parameter estimates). The unweighted analysis revealed no systematic qualitative changes, except that the effect size decreased for continental birds, although the confidence intervals were wide and overlapped with those for insular species (Fig. S4). Varying the impact rankings likewise revealed no systematic qualitative differences relative to the original results, except that effect sizes for birds and mammals were more greatly influenced by changes in the “high” than the “mixed” level, whereas the opposite was true for reptiles (Fig. S5). This result is to be expected, given that reptiles on average had lower impact scores, but it does not change the interpretation of the results.
Supplementary Material
Acknowledgments
The IUCN and its many contributors are acknowledged for maintaining the Red List, which provided information on species taxonomy, status, threats, and range. Grant Williamson is thanked for writing and testing the custom R script. Comments from Corey Bradshaw, Chris Johnson, and three anonymous reviewers greatly improved earlier versions of this manuscript. T.S.D. was supported by scholarships from Edith Cowan University and Earthwatch Institute Australia during the initial stages of this study, and C.R.D. by a fellowship from the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602480113/-/DCSupplemental.
References
- 1.Medina FM, et al. A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol. 2011;17(11):3503–3510. [Google Scholar]
- 2.Szabo JK, Khwaja N, Garnett ST, Butchart SHM. Global patterns and drivers of avian extinctions at the species and subspecies level. PLoS One. 2012;7(10):e47080. doi: 10.1371/journal.pone.0047080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellard C, Genovesi P, Jeschke JM. Global patterns in threats to vertebrates by biological invasions. Proc Biol Sci. 2016;283(1823):20152454. doi: 10.1098/rspb.2015.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty TS, et al. A continental-scale analysis of feral cat diet in Australia. J Biogeogr. 2015;42(5):964–975. [Google Scholar]
- 5.Rayner MJ, Hauber ME, Imber MJ, Stamp RK, Clout MN. Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci USA. 2007;104(52):20862–20865. doi: 10.1073/pnas.0707414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris DB, Macdonald DW. Interference competition between introduced black rats and endemic Galápagos rice rats. Ecology. 2007;88(9):2330–2344. doi: 10.1890/06-1701.1. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt KB, et al. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One. 2008;3(11):e3602–e3609. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simberloff D. How common are invasion-induced ecosystem impacts? Biol Invasions. 2011;13(5):1255–1268. [Google Scholar]
- 9.Courchamp F, Chapuis J-L, Pascal M. Mammal invaders on islands: Impact, control and control impact. Biol Rev Camb Philos Soc. 2003;78(3):347–383. doi: 10.1017/s1464793102006061. [DOI] [PubMed] [Google Scholar]
- 10.Fleming PA, et al. Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function? Mammal Rev. 2014;44(2):94–108. [Google Scholar]
- 11.Woinarski JCZ, Burbidge AA, Harrison PL. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci USA. 2015;112(15):4531–4540. doi: 10.1073/pnas.1417301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307(5717):1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305(5692):1955–1958. doi: 10.1126/science.1101617. [DOI] [PubMed] [Google Scholar]
- 14.Doherty TS, Ritchie EG. Stop jumping the gun: A call for evidence-based invasive predator management. Conserv Lett. May 16, 2016 doi: 10.1111/conl.12251. [DOI] [Google Scholar]
- 15.Bellard C, Cassey P, Blackburn TM. Alien species as a driver of recent extinctions. Biol Lett. 2016;12(2):20150623–20150624. doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long J. Introduced Mammals of the World. CSIRO Publishing; Melbourne: 2003. [Google Scholar]
- 17.Tershy BR, Shen KW, Newton KM, Holmes ND, Croll DA. The importance of islands for the protection of biological and linguistic diversity. Bioscience. 2015;65(6):592–597. [Google Scholar]
- 18.Towns DR, Atkinson IAE, Daugherty CH. Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions. 2006;8(4):863–891. [Google Scholar]
- 19.Jones HP, et al. Severity of the effects of invasive rats on seabirds: A global review. Conserv Biol. 2008;22(1):16–26. doi: 10.1111/j.1523-1739.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 20.Harris DB. Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions. 2009;11(7):1611–1630. [Google Scholar]
- 21.Cuthbert RJ, Louw H, Parker G, Rexer-Huber K, Visser P. Observations of mice predation on dark-mantled sooty albatross and Atlantic yellow-nosed albatross chicks at Gough Island. Antarct Sci. 2013;25(06):763–766. [Google Scholar]
- 22.Norbury G, et al. Impacts of invasive house mice on post-release survival of translocated lizards. N Z J Ecol. 2014;38(2):322–327. [Google Scholar]
- 23.O’Donnell C, Clapperton BK, Monks JM. Impacts of introduced mammalian predators on indigenous birds of freshwater wetlands in New Zealand. N Z J Ecol. 2015;39(1):19–33. [Google Scholar]
- 24.Meiri S, Chapple DG. Biases in the current knowledge of threat status in lizards, and bridging the “assessment gap.”. Biol Conserv. March 17, 2016 doi: 10.1016/j.biocon.2016.03.009. [DOI] [Google Scholar]
- 25.Hedges SB, Conn CE. A new skink fauna from Caribbean islands (Squamata, Mabuyidae, Mabuyinae) Zootaxa. 2012;3288:1–244. [Google Scholar]
- 26.Hunt GR, Hay R, Veltman CJ. Multiple kagu Rhynochetos jubatus deaths caused by dog attacks at a high-altitude study site on Pic Ningua, New Caledonia. Bird Conserv Int. 2010;6(04):295–306. [Google Scholar]
- 27.Banks PB, Dickman CR. Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol Evol. 2007;22(5):229–230, author reply 230–231. doi: 10.1016/j.tree.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Boyer AG. Extinction patterns in the avifauna of the Hawaiian islands. Divers Distrib. 2008;14(3):509–517. [Google Scholar]
- 29.Duncan RP, Blackburn TM. Extinction and endemism in the New Zealand avifauna. Glob Ecol Biogeogr. 2004;13(6):509–517. [Google Scholar]
- 30.Dawson J, et al. Prioritizing islands for the eradication of invasive vertebrates in the United Kingdom overseas territories. Conserv Biol. 2015;29(1):143–153. doi: 10.1111/cobi.12347. [DOI] [PubMed] [Google Scholar]
- 31.Jones HP, et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc Natl Acad Sci USA. 2016;113(15):4033–4038. doi: 10.1073/pnas.1521179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell JC, et al. Importance of lethal control of invasive predators for island conservation. Conserv Biol. 2016;30(3):670–672. doi: 10.1111/cobi.12666. [DOI] [PubMed] [Google Scholar]
- 33.McGeoch MA, et al. Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion. Biol Invasions. 2015;18(2):299–314. [Google Scholar]
- 34.Bergstrom DM, et al. Indirect effects of invasive species removal devastate World Heritage Island. J Appl Ecol. 2009;46(1):73–81. [Google Scholar]
- 35.Bode M, Baker CM, Plein M. Eradicating down the food chain: Optimal multispecies eradication schedules for a commonly encountered invaded island ecosystem. J Appl Ecol. 2015;52(3):571–579. [Google Scholar]
- 36.Helmstedt KJ, et al. Prioritizing eradication actions on islands: It’s not all or nothing. J Appl Ecol. 2016;53(3):733–741. [Google Scholar]
- 37.Moseby KE, Hill BM, Read JL. Arid recovery: A comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Austral Ecol. 2009;34(2):156–169. [Google Scholar]
- 38.McGregor HW, Legge S, Jones ME, Johnson CN. Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS One. 2014;9(10):e109097. doi: 10.1371/journal.pone.0109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty TS, Dickman CR, Nimmo DG, Ritchie EG. Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biol Conserv. 2015;190:60–68. [Google Scholar]
- 40.Hunter DO, Britz T, Jones M, Letnic M. Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated. Biol Conserv. 2015;191:428–435. [Google Scholar]
- 41.Ritchie EG, et al. Ecosystem restoration with teeth: What role for predators? Trends Ecol Evol. 2012;27(5):265–271. doi: 10.1016/j.tree.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Reardon JT, et al. Predator control allows critically endangered lizards to recover on mainland New Zealand. N Z J Ecol. 2012;36(2):141–150. [Google Scholar]
- 43.IUCN 2014 Global Invasive Species Database. Available at www.iucngisd.org/gisd/. Accessed February 5, 2015.
- 44.Woinarski JCZ, Burbidge AA, Harrison P. The Action Plan for Australian Mammals 2012. CSIRO Publishing; Melbourne: 2014. [Google Scholar]
- 45.Hilton GM, Cuthbert RJ. The catastrophic impact of invasive mammalian predators on birds of the UK Overseas Territories: A review and synthesis. Ibis. 2010;152(3):443–458. [Google Scholar]
- 46.Duffy DC, Capece P. Biology and impacts of Pacific Island invasive species. 7. The domestic cat (Felis catus) Pac Sci. 2012;66(2):173–212. [Google Scholar]
- 47.Hays WS, Conant S. Biology and impacts of Pacific Island invasive species. 1. A worldwide review of effects of the small Indian mongoose, Herpestes javanicus (Carnivora: Herpestidae) Pac Sci. 2007;61(1):3–16. [Google Scholar]
- 48.Shiels AB, Pitt WC, Sugihara RT, Witmer GW. Biology and impacts of Pacific island invasive species. 11. Rattus rattus, the black rat (Rodentia: Muridae) Pac Sci. 2014;68(2):145–184. [Google Scholar]
- 49.Capizzi D, Bertolino S, Mortelliti A. Rating the rat: Global patterns and research priorities in impacts and management of rodent pests. Mammal Rev. 2014;44(2):148–162. [Google Scholar]
- 50.Banks PB, Hughes NK. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildl Res. 2012;39(1):78–88. [Google Scholar]
- 51.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 52.R Core Team 2014 R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna), Version 3.1.2. Available at www.R-project.org/
- 53.Jetz W, et al. Global distribution and conservation of evolutionary distinctness in birds. Curr Biol. 2014;24(9):919–930. doi: 10.1016/j.cub.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Isaac NJB, Turvey ST, Collen B, Waterman C, Baillie JEM. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS One. 2007;2(3):e296–e297. doi: 10.1371/journal.pone.0000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collen B, et al. Investing in evolutionary history: Implementing a phylogenetic approach for mammal conservation. Philos Trans R Soc Lond B Biol Sci. 2011;366(1578):2611–2622. doi: 10.1098/rstb.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg MS. The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59(2):464–468. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.