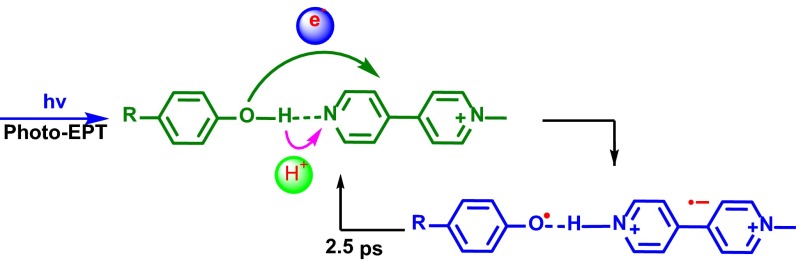
Significance
Concerted proton-coupled electron transfer (EPT) reactions in which both electrons and protons transfer in tandem are at the heart of many chemical and biological conversions including photosystem II. We report here the direct observation of absorption bands arising from photoEPT transitions, in this case, in H-bonded complexes between N-methyl-4,4′-bipyridinium cation and biologically relevant donors including tyrosine. The importance of these observations follows from the earlier experimental observations by Taube and coworkers on intervalence transfer in mixed-valence complexes. The observation of these photoEPT transitions and the appearance of reactive radical products also points to a possible, if inefficient, role in DNA photodamage and, possibly, in the formation of reactive oxygen intermediates.
Keywords: light driven, electron, proton, transfer, photoEPT
Abstract
The phenols 4-methylphenol, 4-methoxyphenol, and N-acetyl-tyrosine form hydrogen-bonded adducts with N-methyl-4, 4′-bipyridinium cation (MQ+) in aqueous solution as evidenced by the appearance of low-energy, low-absorptivity features in UV-visible spectra. They are assigned to the known examples of optically induced, concerted electron–proton transfer, photoEPT. The results of ultrafast transient absorption measurements on the assembly MeOPhO-H---MQ+ are consistent with concerted EPT by the instantaneous appearance of spectral features for MeOPhO·---H-MQ+ in the transient spectra at the first observation time of 0.1 ps. The transient decays to MeOPhO-H---MQ+ in 2.5 ps, accompanied by the appearance of oscillations in the decay traces with a period of ∼1 ps, consistent with a vibrational coherence and relaxation from a higher υ(N-H) vibrational level or levels on the timescale for back EPT.
Proton-coupled electron transfer (PCET) reactions, in which both electrons and protons are transferred, play an important role in redox processes in chemistry and biology with examples in water oxidation (1–4), CO2 reduction (5), mitochondrial respiration (6), and conversion of nucleotides to 2′-deoxynucleotides (7, 8). Mechanistically, PCET occurs by stepwise, electron transfer followed by proton transfer(ET-PT) (9–13) or proton transfer followed by electron transfer (PT-ET), or concerted pathways (EPT) with concerted transfer in a single step. Although more complex microscopically, EPT can offer a significant advantage in avoiding high-energy intermediates (14, 15).
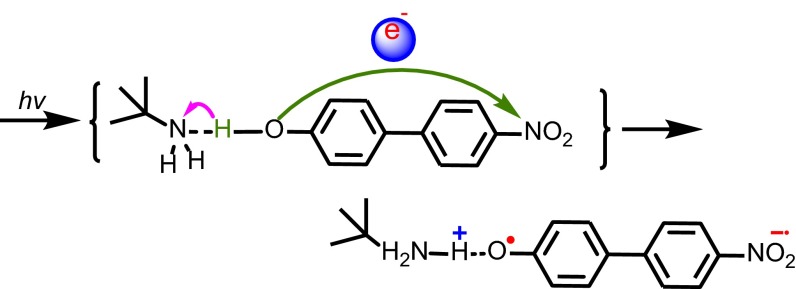
Light driven, photochemical EPT (photoEPT) has been reported (16–18), by Westlake et al. (18) for amine adducts with 4-nitro-4′-biphenylphenol, Scheme 1. In these adducts, intramolecular charge transfer (ICT) excitation is accompanied by proton transfer to an H-bonded base, Scheme 1, as shown by ultrafast and coherent Raman measurements.
Scheme 1.
Illustration of photoEPT excitation in the hydrogen-bonded adduct between p-nitrophenylphenol and t-butylamine (18). The green and pink arrows illustrate electron and proton transfer motion, respectively.
Although seemingly a breakdown of the Franck–Condon principle, the appearance of optically induced electron–proton transfer was rationalized by noting that optical excitation and associated changes in electronic structure result instantaneously in a spatially fixed proton in the vibrational force field of the electronic excited state. Subsequent theoretical analysis by Hammes-Schiffer and coworkers (19, 20) supported this conclusion and the coexistence of distinct spectroscopic states, one a conventional ICT state, with excitation followed by proton transfer (photoET-PT), and a concerted photoEPT state.
An important, if largely unrecognized, role for photoEPT could exist and play a role broadly, for example, in DNA photodamage (21, 22) or in forming reactive oxygen intermediates (ROS) (23). Nonetheless, reports of photoEPT and its role in excited state reactivity in chemistry and biology are rare (17–20, 24, 25).
In electron transfer, a significant advance came from the appearance and analysis of low-energy intervalence transfer (IT) (26) absorption bands in mixed-valence complexes (27), Eq. 1, and from an analysis by Hush (28). In appropriate limits, the Hush treatment provides quantitative relationships between absorption band energies, widths, and oscillator strengths. Analysis of IT absorption bands provides intramolecular and medium reorganization energies and electronic matrix elements arising from donor–acceptor wave function mixing:
 |
[1] |
In gaining further experimental insight into photoEPT, the direct observation of an optical transition or transitions analogous to a mixed valence IT band would be an important step forward. It would enable further characterization of the coupled electron/proton transfer process by using absorption band properties to assess barriers and the extent of electronic coupling. Given the expected relatively weak electronic coupling between donor and acceptor across a linking H bond as in Eq. 1, absorptivities for these transitions are expected to be low, making direct observation of a photoEPT transition difficult experimentally.
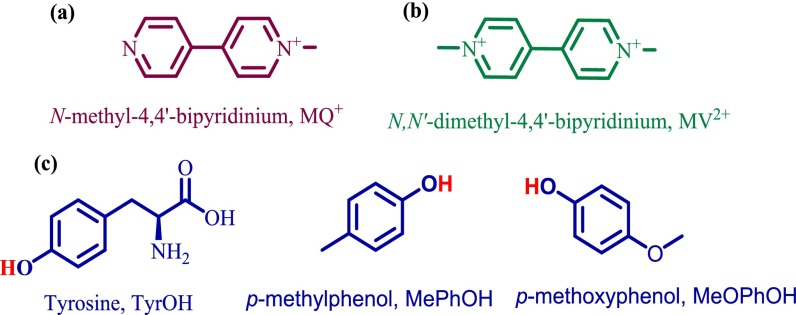
We report observation of photoEPT absorptions, here in H-bonded complexes between N-methyl-4,4′-bipyridinium cation (MQ+) and the biologically relevant donor tyrosine (TyrOH) and the phenols 4-methylphenol (p-MePhOH) and 4-methoxyphenol (p-MeOPhOH). Structures are shown in Chart 1.
Chart 1.
Structural formulas of (A) N-methyl-4,4′-bipyridinium, MQ+, (B) N,N′-dimethyl-4,4′-bipyridinium, MV2+, and (C) phenols used in this study tyrosine, p-methylphenol, and 4-methoxyphenol, MeOPhOH.
MQ+ is transparent in the visible spectrum whereas the spectrum of the reduced form of its dimethylated analog, methyl viologen (MV+•), includes a characteristic intense π → π* absorption in the visible spectrum with ε = 1.37 × 104 M−1⋅cm−1 at 590 nm (16,950 cm−1) in acetonitrile (29, 30). In the optical experiments, MQ+ was used as the proton acceptor with simultaneous electron/proton transfer signaled by the appearance of an intense absorption for MQH+• analogous to MV+•. In MQ+, the uncoordinated pyridine is both electron and proton acceptor with pKa ∼ 3.5 for MQH2+ and pKa ∼ 8.6 for the singly reduced cation, MQH+• (31, 32). Phenols were used as EPT donors in these experiments because of their enhanced acidities upon oxidation. For tyrosine, pKa decreases from 10 to −2 in the radical cation (33).
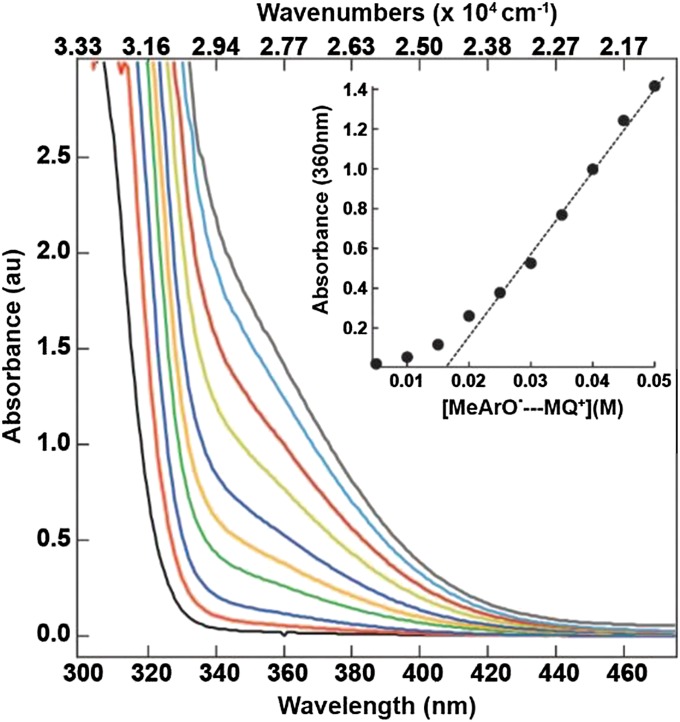
H-bonded adducts form between MQ+ and the phenols in solution as shown by the appearance of low-absorptivity, low-energy bands in low-energy UV-visible spectra. Fig. 1 shows the spectral changes that occur in aqueous solutions containing equimolar concentrations of MQ+ and p-MePhOH. The formation of the adduct is accompanied by the appearance of a low-energy shoulder at ∼375 nm (26,660 cm−1) that appears on the low-energy side of a π → π* transition of the phenol.
Fig. 1.
UV-Vis spectra of N-methyl-4,4′-bipyridinium (MQ+) + p-methylphenol (p-MePhOH) where each spectrum was obtained with equimolar MQ+ and p-MePhOH in the concentration range of 5–50 mM, in 50 mM Tris buffer, pH 8.5, ionic strength maintained at I = 0.8 M with NaCl at 23 ± 2 °C. Inset shows plot of absorbance at 360 nm as a function of the extent of adduct formation. The titration data are summarized in Table S1.
From these data, and the linear region of absorbance increase in Fig. 1, essentially complete complex formation begins at ∼30 mM N-methyl-4,4′-bipyridinium with 30 mM added phenol. Spectrophotometric analyses of complex formation, with the evaluation of equilibrium constants for association, KA, were carried out under conditions with less than 100% complex formation by the method of Curtis and Meyer (34). Based on the KA values, free energies of formation for the series of H-bonded adducts were ∼2 kcal/mol (700 cm−1) (Table 1), within the range expected for hydrogen bond interactions 1–5 kcal/mol (300–1,500 cm−1) (Supporting Information) (16, 35).
Table 1.
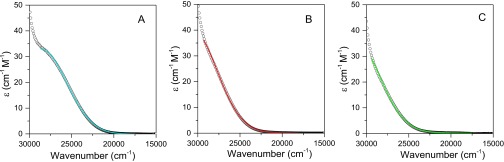
Absorption spectral profiles were analyzed by a Gaussian deconvolution procedure that provided absorption band maxima (Eop), band widths at half height (∆ῡ1/2), and integrated intensities (Fig. S1). Results for the three complexes are shown in Table 1 and spectral fits in Supporting Information. Oscillator strengths (fosc), calculated from Eq. 2, and transition moments from Eq. 3 are also listed in Table 1. Mulliken–Hush theory and Eqs. 4 and 5 were used to obtain a classical reorganizational energy (λ) and free energy of the excited state above the ground state (∆G°ES):
| [2] |
| [3] |
| [4] |
| [5] |
The electronic coupling matrix element (HDA) was calculated by using Eq. 6 with d = 7 Å, the average of limiting values for d as described by the procedure in Curtis and Meyer (34):
| [6] |
Values for the three adducts are listed in Table 1. Detailed interpretation of band properties by the classical Hush treatment may be inappropriate given the probable contribution to the absorption manifolds from the high-frequency υ(O-H)/υ(N-H) transfer mode as evidenced by the large bandwidths and calculated reorganization energies (λ ∼ 22,000 ± 1,000 cm−1) (Table 1). The reorganization energies are of similar magnitude (λ ∼ 9,000–21,000 cm−1) to a related series of intramolecular amino-derivatized, 2,4-di-tert-phenyl analogs that undergo intramolecular thermally activated EPT (36). The ∆G°′ value for the tyrosine adduct from spectral fitting of ∼1.1 eV (8,900 cm−1) is comparable to ∼1.4 eV (9,200 cm−1) estimated from E°′ values for the TyrOH+/0 and MVH2+/+ couples and known pKa values (37, 38).
Fig. S1.
The experimental CT absorption spectra (black) and the corresponding Gaussian fits. (A–C) CT band formation between 40 mM (A) p-methoxyphenol (blue), (B) p-methylphenol (red), and (C) N-acetyl-tyrosine (green) + 40 mM N-methyl-4,4′-bipyridinium in 50 mM Tris buffer, pH 8.5, ionic strength maintained at I = 0.8 M with NaCl.
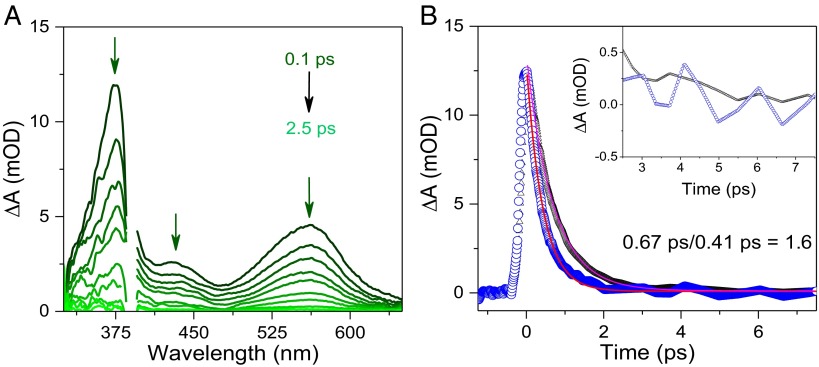
The nature of the lower-energy absorption feature for the H-bonded adducts was investigated further by ultrafast transient absorption measurements by an apparatus and data analysis described earlier (39–41) and in Supporting Information, Experimental, Ultrafast Transient Absorption Experiments. Excitation (388 nm, 250 fs FWHM) into the low-energy absorption band for the p-MeOPhO-H---MQ+ adduct, at pH 8.5 in the aqueous Tris buffer, resulted in the time-resolved transient absorption difference spectra shown in Fig. 2A. At the observation time of 250 fs, positive absorption features appear at λmax ∼360 nm, 440 nm, and 560 nm. The feature at 360 nm (27,800 cm−1) is consistent with the simultaneous appearance of the phenoxyl radical (42, 43) and HMQ+• (44), the feature at 440 nm (22,700 cm−1) to the phenoxyl radical, and the broad feature at 560 nm (17,860 cm−1) to the low-lying π → π* absorption in HMQ• analogous to the absorption at 590 nm (16,950 cm−1) MV+• in MeCN (44).
Fig. 2.
(A) Transient absorption difference spectra at different delay times from 0.10 ps to 2.5 ps following ultrafast excitation (at 388 nm, and the pump pulse energy was 25 nJ per pulse) of a 25-mM solution of p-MeOPhOH with 25 mM added MQ+ in a 50-mM Tris buffer, pH = pD = 8.5, I = 0.8 M NaCl at T = 25 °C. (B) Absorption-time decay traces at λmax ∼ 560 nm corresponding to the decay of HMQ• by back electron transfer following pulsed excitation at 388 nm with 25 mM p-MeOPhOH and 25 mM MQ+ (blue) in H2O and D2O (black). Inset shows the traces magnified from ∼2.5–7.5 ps.
The transient absorption features are consistent with excitation into the weak, low-energy absorption feature in the p-MeOPhO-H---MQ+ adduct resulting in the appearance of MeOPhO·---H-MQ+. The transient features appear within 0.1 ps and decay within 2.5 ps without significant change in band position or shape. They are consistent with Scheme 2 and photoEPT excitation leading to concerted electron–proton transfer within the H-bonded complex.
Scheme 2.
Illustration of phenol-MQ+ photoEPT and the following back reaction.
In support of this assignment, careful examination of the transient absorption decay traces in H2O in Fig. 2B provides clear evidence for a vibrational coherence (45–47). The oscillatory part of the decay from 2.5 ps to 7.5 ps is compared in H2O and D2O in Fig. 2B. The oscillations in H2O appear with a period of ∼1 ps consistent with a vibrational coherence that is sustained for picoseconds and vibrational relaxation from a higher υ(N-H) vibrational level or levels on the timescale for back electron transfer (48).
From the time-resolved, single exponential decay traces at pH = pD = 8.5, kD2O = 1.5 × 1012 s−1 for back EPT with deuteron transfer and kH2O = 2.4 × 1012 s−1 for proton transfer, a kinetic isotope effect of kH2O/kD2O = 1.6. The results from these studies are important because they demonstrate an optical process that is analogous to IT in mixed-valence molecules. From the transient experiments, excitation into the underlying transition initiates photoEPT (Scheme 2). Although related transitions may exist, they are, no doubt, typically masked by higher absorptivity transitions and appear here as low-energy absorption features in H-bonded, donor–acceptor complexes. Assignment of the optical transition is consistent with the results of ultrafast transient absorption measurements. There is evidence for vibrational coupling following photoEPT excitation.
As expected, the underlying absorptions are of relatively low absorptivity consistent with weak electronic coupling between donor and acceptor in the H-bonded adducts. Nonetheless, the existence of the transitions, and the appearance of the high-energy, radical intermediates that they produce, could play a hidden role in low-efficiency, photochemical pathways both in biology and in photochemical energy conversion processes.
Methods
Detailed spectral data and analysis are included in Supporting Information.
Experimental
Reaction Solutions.
Aqueous solutions were prepared from water purified with a MilliQ purification system (Synthesis A10) with added NaCl at 0.8 M to maintain constant ionic strength. Buffers (Tris, borate) hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich and were used as received. The concentration of buffer components was calculated based on the Henderson–Hasselbalch equation. The pKa values used in the calculations are standard values for Tris and borate (pKa = 8.1 and 9.0, respectively) in aqueous solution. Buffered solutions were adjusted to the correct pH, using HCl or NaOH with use of a Fisher Scientific Accumet AB15 pH meter. N-methyl-4,4′-bipyridinium was prepared according to the procedures described before in the literature (49). The phenols N-acetyl-tyrosine, p-methoxyphenol, and p-methylphenol were acquired from Sigma Aldrich and used as received. All phenols were made into concentrated stock solutions before the appropriate volume was transferred into the cuvette. Phenols not soluble in water were first dissolved into acetonitrile and then (typically <0.5% of total volume) transferred into the cuvette. All samples were measured on the same day of preparation.
Mixing Experiments.
For mixing experiments, buffered aqueous solutions were prepared in 0.8 M NaCl to maintain constant ionic strength. Absorbance spectra were acquired under ambient temperatures and pressures on an Agilent-8453 Diode array spectrophotometer in a 1-cm path length, 1-mL volume, quartz cell. In experiments measuring spectra of a phenol ion pair, phenol deprotonation was achieved by adding NaOH directly to the cuvette.
Ultrafast Transient Absorption Experiments.
Femtosecond transient absorption measurements were done using a pump–probe technique that has been described previously in detail (39–41). Briefly, the pump and probe pulses were derived from a Ti: Sapphire chirped pulse amplification (Clark-MXR CPA-2001) laser system that outputs at 800 mW, 775 nm, and pulse at a 1-kHz repeat rate. The 388-nm pump pulse was generated by doubling the fundamental output at 775 nm. The probe pulse was formed by continuum generation by focusing a small portion of the beam into a CaF2 window to generate a white light continuum. This beam was directed through a computer-controlled optical delay stage, passed through the sample, and coupled into a spectrometer to spectrally disperse the probe onto a high-speed 1,024-pixel complementary metal-oxide semiconductor (CMOS) detector. Transient spectra were collected on a shot-by-shot (1-kHz) basis over the range of 325–700 nm with a high signal-to-noise ratio and an instrument sensitivity of up to 0.1 mOD. The 355-nm pump pulse was created with a tunable Clark Optical Parametric Amplifier (OPA) (1,420 nm) and subsequent second (710 nm) and fourth (355 nm) harmonic generation. The data were collected at magic angle (54.7°) to avoid polarization effects.
Ground State Absorption Band Analysis
The ion-pair formation constant is defined in Eqs. S1 and S2 (34):
| [S1] |
Here, adduct is the H-bonded phenol-MQ+ assembly. In all cases, [MQ+] = [phenol], giving
| [S2] |
The data for the series of phenols, 4-methoxyphenol, 4-methylphenol, and N-acetyl-tyrosine, are summarized in Table S1.
Table S1.
Data extracted from absorption band deconvolution for the formation of adduct [PhOH---MQ+]
| 104× [MQ+] = [4-MeOArOH], M | Abs at 370 nm* | 104 × [Adduct], M, ε = 45 M−1⋅cm−1 | KA, M−1 |
| 5.00 | 0.0175 | 0.389 | 18 |
| 10.00 | 0.0810 | 1.800 | 26 |
| 15.00 | 0.1756 | 3.902 | 31 |
| 20.00 | 0.3086 | 6.858 | 39 |
| 25.00 | 0.4492 | 9.982 | 44 |
| av KA = 32 ± 10 | |||
| 5.00 | 0.0192 | 0.427 | 20.4 |
| 10.00 | 0.0533 | 1.184 | 15.2 |
| 15.00 | 0.1161 | 2.580 | 16.7 |
| 20.00 | 0.2594 | 5.765 | 28.5 |
| 25.00 | 0.3778 | 8.394 | 30.4 |
| av KA = 22 ± 6 | |||
| 5.00 | 0.0123 | 0.384 | 18.0 |
| 10.00 | 0.0370 | 1.156 | 14.8 |
| 15.00 | 0.0832 | 2.600 | 16.9 |
| 20.00 | 0.1449 | 4.528 | 18.9 |
| 25.00 | 0.2158 | 6.744 | 20.2 |
| av KA = 17 ± 2 |
All at 23 ± 2 °C in a medium of 50 mM Tris buffer, pH 8.5, with ionic strength maintained at I = 0.8 M with NaCl.
In a 1-cm path length cell.
The free energy of the adducts s was calculated using Eq. S3. For the hydrogen-bound interaction (KA ∼ 30 M−1) at pH 8.5 the energy was calculated to be ∼8 kJ/mol (2 kcal/mol), which is within the expected values for a hydrogen bond (1–5 kcal/mol):
| [S3] |
Acknowledgments
The authors thank Prof. A. B. P. Lever for valuable discussions. This research was supported by the National Science Foundation under Grant CHE-1362481. D.W.T. acknowledges research support from Natural Sciences and Engineering Research Council of Canada and Memorial University for funding a sabbatical leave. The transient absorption measurements were performed using a pump–probe transient absorption spectrometer in the instrumentation facilities of the University of North Carolina Energy Frontier Research Center: Center for Solar Fuels, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award DE-SC0001011.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611496113/-/DCSupplemental.
References
- 1.Kärkäs MD, Verho O, Johnston EV, Åkermark B. Artificial photosynthesis: Molecular systems for catalytic water oxidation. Chem Rev. 2014;114(24):11863–12001. doi: 10.1021/cr400572f. [DOI] [PubMed] [Google Scholar]
- 2.Gagliardi CJ, Vannucci AK, Concepcion JJ, Chen Z, Meyer TJ. The role of proton coupled electron transfer in water oxidation. Energy Environ Sci. 2012;5(7):7704–7717. [Google Scholar]
- 3.Tommos C, Babcock GT. Proton and hydrogen currents in photosynthetic water oxidation. Biochim Biophys Acta. 2000;1458(1):199–219. doi: 10.1016/s0005-2728(00)00069-4. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TJ, Huynh MH, Thorp HH. The possible role of proton-coupled electron transfer (PCET) in water oxidation by photosystem II. Angew Chem Int Ed Engl. 2007;46(28):5284–5304. doi: 10.1002/anie.200600917. [DOI] [PubMed] [Google Scholar]
- 5.Kang P, Chen Z, Brookhart M, Meyer TJ. Electrocatalytic reduction of carbon dioxide: Let the molecules do the work. Top Catal. 2014;58(1):30–45. [Google Scholar]
- 6.Belevich I, Verkhovsky MI, Wikström M. Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature. 2006;440(7085):829–832. doi: 10.1038/nature04619. [DOI] [PubMed] [Google Scholar]
- 7.Migliore A, Polizzi NF, Therien MJ, Beratan DN. Biochemistry and theory of proton-coupled electron transfer. Chem Rev. 2014;114(7):3381–3465. doi: 10.1021/cr4006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minnihan EC, Nocera DG, Stubbe J. Reversible, long-range radical transfer in E. coli class Ia ribonucleotide reductase. Acc Chem Res. 2013;46(11):2524–2535. doi: 10.1021/ar4000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg DR, et al. Proton-coupled electron transfer. Chem Rev. 2012;112(7):4016–4093. doi: 10.1021/cr200177j. [DOI] [PubMed] [Google Scholar]
- 10.Hammes-Schiffer S. Proton-coupled electron transfer: Classification scheme and guide to theoretical methods. Energy Environ Sci. 2012;5(7):7696–7703. [Google Scholar]
- 11.Warren JJ, Tronic TA, Mayer JM. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem Rev. 2010;110(12):6961–7001. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammes-Schiffer S. Proton-coupled electron transfer: Moving together and charging forward. J Am Chem Soc. 2015;137(28):8860–8871. doi: 10.1021/jacs.5b04087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjodin M, Styring S, Akermark B, Sun L, Hammarstrom L. The mechanism for proton-coupled electron transfer from tyrosine in a model complex and comparisons with y(z) oxidation in photosystem ii. Philos Trans R Soc Lond B Biol Sci. 2002;357(1426):1471–1479; discussion 1478–1479, 1511. doi: 10.1098/rstb.2002.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dongare P, Maji S, Hammarström L. Direct evidence of a tryptophan analogue radical formed in a concerted electron−proton transfer reaction in water. J Am Chem Soc. 2016;138(7):2194–2199. doi: 10.1021/jacs.5b08294. [DOI] [PubMed] [Google Scholar]
- 15.Huynh MH, Meyer TJ. Proton-coupled electron transfer. Chem Rev. 2007;107(11):5004–5064. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concepcion JJ, et al. Excited-state quenching by proton-coupled electron transfer. J Am Chem Soc. 2007;129(22):6968–6969. doi: 10.1021/ja069049g. [DOI] [PubMed] [Google Scholar]
- 17.Gagliardi CJ, et al. Integrating proton coupled electron transfer (PCET) and excited states. Coord Chem Rev. 2010;254(21–22):2459–2471. [Google Scholar]
- 18.Westlake BC, et al. Concerted electron-proton transfer in the optical excitation of hydrogen-bonded dyes. Proc Natl Acad Sci USA. 2011;108(21):8554–8558. doi: 10.1073/pnas.1104811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal P, Schwerdtfeger CA, Soudackov AV, Hammes-Schiffer S. Nonadiabatic dynamics of photoinduced proton-coupled electron transfer in a solvated phenol-amine complex. J Phys Chem B. 2015;119(6):2758–2768. doi: 10.1021/jp5126969. [DOI] [PubMed] [Google Scholar]
- 20.Hammes-Schiffer S. When electrons and protons get excited. Proc Natl Acad Sci USA. 2011;108(21):8531–8532. doi: 10.1073/pnas.1105806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, et al. Determining complete electron flow in the cofactor photoreduction of oxidized photolyase. Proc Natl Acad Sci USA. 2013;110(32):12966–12971. doi: 10.1073/pnas.1311073110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiagarajan V, Byrdin M, Eker APM, Müller P, Brettel K. Kinetics of cyclobutane thymine dimer splitting by DNA photolyase directly monitored in the UV. Proc Natl Acad Sci USA. 2011;108(23):9402–9407. doi: 10.1073/pnas.1101026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, et al. Ultrafast photoinduced interfacial proton coupled electron transfer from cdse quantum dots to 4,4′-bipyridine. J Am Chem Soc. 2016;138(3):884–892. doi: 10.1021/jacs.5b10354. [DOI] [PubMed] [Google Scholar]
- 25.Goyal P, Hammes-Schiffer S. Role of solvent dynamics in photoinduced proton-coupled electron transfer in a phenol–amine complex in solution. J Phys Chem Lett. 2015;6(18):3515–3520. doi: 10.1021/acs.jpclett.5b01475. [DOI] [PubMed] [Google Scholar]
- 26.Creutz C, Taube H. Direct approach to measuring the Franck-Condon barrier to electron transfer between metal ions. J Am Chem Soc. 1969;91(14):3988–3989. [Google Scholar]
- 27.Creutz C. 2007. Mixed valence complexes of d5-d6 metal centers. Progress in Inorganic Chemistry, ed Lippard SJ (Wiley, New York), Vol 30, pp 1–73.
- 28.Hush NS. 2007. Intervalence-transfer absorption. Part 2. Theoretical considerations and spectroscopic data. Progress in Inorganic Chemistry, ed Cotton FA (Wiley, New York), Vol 8, pp 391–444.
- 29.Young RC, Meyer TJ, Whitten DG. Kinetic relaxation measurement of rapid electron transfer reactions by flash photolysis. Conversion of light energy into chemical energy using the tris(2,2′-bipyridine)ruthenium(3+)-tris(2,2′-bipyridine)ruthenium(2+*) couple. J Am Chem Soc. 1975;97(16):4781–4782. [Google Scholar]
- 30.Young RC, Meyer TJ, Whitten DG. Electron transfer quenching of excited states of metal complexes. J Am Chem Soc. 1976;98(1):286–287. [Google Scholar]
- 31.Leopold KR, Haim A. Quenching of the luminescent excited state of tris(2,2′-bipyridine)ruthenium(ii) by complexes of pentaamminecobalt(iii) with pyridine, 4,4′-bipyridine, and derivatives of 4,4′-bipyridine. Inorg Chem. 1978;17(7):1753–1757. [Google Scholar]
- 32.Magnuson A, et al. Mimicking electron transfer reactions in photosystem ii: Synthesis and photochemical characterization of a ruthenium(ii) tris(bipyridyl) complex with a covalently linked tyrosine. J Am Chem Soc. 1997;119(44):10720–10725. [Google Scholar]
- 33.Hammarström L, Styring S. Coupled electron transfers in artificial photosynthesis. Philos Trans R Soc Lond B Biol Sci. 2008;363(1494):1283–1291, discussion 1291. doi: 10.1098/rstb.2007.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis JC, Meyer TJ. Outer-sphere charge transfer in mixed-metal ion pairs. Inorg Chem. 1982;21(4):1562–1571. [Google Scholar]
- 35.Nomrowski J, Wenger OS. Photoinduced PCET in ruthenium-phenol systems: Thermodynamic equivalence of uni- and bidirectional reactions. Inorg Chem. 2015;54(7):3680–3687. doi: 10.1021/acs.inorgchem.5b00318. [DOI] [PubMed] [Google Scholar]
- 36.Rhile IJ, et al. Concerted proton-electron transfer in the oxidation of hydrogen-bonded phenols. J Am Chem Soc. 2006;128(18):6075–6088. doi: 10.1021/ja054167+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tommos C, Skalicky JJ, Pilloud DL, Wand AJ, Dutton PL. De novo proteins as models of radical enzymes. Biochemistry. 1999;38(29):9495–9507. doi: 10.1021/bi990609g. [DOI] [PubMed] [Google Scholar]
- 38.Pannwitz A, Wenger OS. Proton coupled electron transfer from the excited state of a ruthenium(ii) pyridylimidazole complex. Phys Chem Chem Phys. 2016;18(16):11374–11382. doi: 10.1039/c6cp00437g. [DOI] [PubMed] [Google Scholar]
- 39.Shaw GB, Brown CL, Papanikolas JM. Investigation of interligand electron transfer in polypyridyl complexes of Os(II) using femtosecond polarization anisotropy methods: Examination of Os(bpy)32+ and Os(bpy)2(mab)2+ J Phys Chem A. 2002;106(8):1483–1495. [Google Scholar]
- 40.Shaw GB, Papanikolas JM. Triplet−triplet annihilation of excited states of polypyridyl Ru(II) complexes bound to polystyrene. J Phys Chem B. 2002;106(24):6156–6162. [Google Scholar]
- 41.Styers-Barnett DJ, et al. Exciton dynamics and biexciton formation in single-walled carbon nanotubes studied with femtosecond transient absorption spectroscopy. J Phys Chem C. 2008;112(12):4507–4516. [Google Scholar]
- 42.Soetbeer J, Dongare P, Hammarstrom L. Marcus-type driving force correlations reveal the mechanism of proton-coupled electron transfer for phenols and [Ru(bpy)3]3+ in water at low pH. Chem Sci (Camb) 2016;7(7):4607–4612. doi: 10.1039/c6sc00597g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gadosy TA, Shukla D, Johnston LJ. Generation, characterization, and deprotonation of phenol radical cations. J Phys Chem A. 1999;103(44):8834–8839. [Google Scholar]
- 44.Lomoth R, Häupl T, Johansson O, Hammarström L. Redox-switchable direction of photoinduced electron transfer in an Ru(bpy)3(2+)-viologen dyad. Chemistry. 2002;8(1):102–110. doi: 10.1002/1521-3765(20020104)8:1<102::aid-chem102>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Chenu A, Scholes GD. Coherence in energy transfer and photosynthesis. Annu Rev Phys Chem. 2015;66(1):69–96. doi: 10.1146/annurev-physchem-040214-121713. [DOI] [PubMed] [Google Scholar]
- 46.Romero E, et al. Quantum coherence in photosynthesis for efficient solar-energy conversion. Nat Phys. 2014;10(9):676–682. doi: 10.1038/nphys3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vos MH, et al. Direct observation of vibrational coherence in bacterial reaction centers using femtosecond absorption spectroscopy. Proc Natl Acad Sci USA. 1991;88(20):8885–8889. doi: 10.1073/pnas.88.20.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenmayer TJ, Buda F. Real-time simulations of photoinduced coherent charge transfer and proton-coupled electron transfer. ChemPhysChem. 2014;15(15):3258–3263. doi: 10.1002/cphc.201402444. [DOI] [PubMed] [Google Scholar]
- 49.Coe BJ, et al. Large molecular quadratic hyperpolarizabilities in donor/acceptor-substituted trans-tetraammineruthenium(ii) complexes. Inorg Chem. 1997;36(15):3284–3292. doi: 10.1021/ic961465m. [DOI] [PubMed] [Google Scholar]