Significance
Liver disease of unknown cause represents an unmet medical need. Using exome sequencing, we describe a syndrome associated with homozygous loss of Acyl CoA Oxidase 2 (ACOX2) featuring elevated transaminase levels, liver fibrosis, ataxia, and cognitive impairment. ACOX2 encodes the peroxisomal branched-chain acyl-CoA oxidase and is involved in the bile acid biosynthetic pathway. Importantly, this disorder is potentially reversible, because the bile acid synthetic pathway can be suppressed with exogenous bile acids, diminishing the production of the toxic metabolites likely causing liver and neurologic dysfunction. This study provides the means to diagnose ACOX2 deficiency in patients with chronic idiopathic liver disease and/or cryptogenic cirrhosis.
Keywords: branched-chain acyl-CoA oxidase, bile acid metabolism, peroxisomal disorder, whole-exome sequencing, idiopathic liver disease
Abstract
Acyl CoA Oxidase 2 (ACOX2) encodes branched-chain acyl-CoA oxidase, a peroxisomal enzyme believed to be involved in the metabolism of branched-chain fatty acids and bile acid intermediates. Deficiency of this enzyme has not been described previously. We report an 8-y-old male with intermittently elevated transaminase levels, liver fibrosis, mild ataxia, and cognitive impairment. Exome sequencing revealed a previously unidentified homozygous premature termination mutation (p.Y69*) in ACOX2. Immunohistochemistry confirmed the absence of ACOX2 expression in the patient’s liver, and biochemical analysis showed marked elevation of intermediate bile acids upstream of ACOX2. These findings define a potentially treatable inborn error of bile acid biosynthesis caused by ACOX2 deficiency.
Despite major advances in Mendelian genetics, the role in normal human biology and the impact of mutation of >75% of the 20,000 protein-coding genes in the human genome remain to be determined (1). One example of an unmet medical need is idiopathic liver disease, which remains a challenge in both pediatric and adult hepatology. We and others have shown the utility of whole-exome sequencing in the diagnosis of such patients (2–6). Children with unexplained liver disease who are the offspring of a consanguineous union are excellent candidates for recessive disease-causing mutations. Such homozygous mutations can now be readily identified by exome sequencing (7). Acyl CoA Oxidase 2 (ACOX2) encodes the branched-chain acyl-CoA oxidase believed to participate in the metabolism of branched-chain fatty acids and bile acid intermediates in the peroxisomes. Although genetic disorders resulting from mutations in many other genes in this pathway have been described, mutations in ACOX2 have not been attributed to a phenotype in humans or other animals. We describe a previously unrecognized syndrome resulting from recessive ACOX2 deficiency.
Results
Case Report.
An 8-y-old boy of Turkish ancestry, the offspring of a second-cousin union, was evaluated in liver clinic. He was born at full term without complications, and had no family history of liver or neurologic disease in his parents or 10-y-old sibling. At 8 mo of age, he presented with vomiting presumed to be secondary to acute gastroenteritis, and elevated transaminase levels were detected. Liver and spleen were not enlarged. Over subsequent years, transaminase levels were intermittently elevated [aspartate aminotransferase, 30–131 U/L (normal range, 10–30 U/L); alanine aminotransferase, 19–297 U/L (normal range, 6–29 U/L)], with normal gamma-glutamyl transpeptidase levels and preserved liver synthetic function, as indicated by normal albumin, bilirubin, and INR levels. Hypolipidemia, defined as total cholesterol (TC) <100 mg/dL or low-density lipoprotein (LDL) cholesterol <50 mg/dL, was also detected, with TC values of 75–96 mg/dL and LDL cholesterol values of 14–44 mg/dL. He also had vitamin D deficiency (serum level 12–17 ng/mL; normal range, 20–50 ng/mL), but normal levels of liposoluble vitamins A and E. In addition, he had an elevated steatocrit value (a semiquantitative measure of fat content in fecal samples), indicating steatorrhea and fat malabsorption. Serologies for viral hepatitis, TORCH [Toxoplasma gondii; other (coxsackievirus, chickenpox, Chlamydia, HIV, HTLV, syphilis), rubella, CMV, and HSV-2] and Epstein–Barr virus; tests for autoimmunity (IgG and anti-smooth muscle, anti-nuclear, anti–double-stranded DNA, anti-liver kidney microsomal, anti-soluble liver pancreas, anti-cytosolic, anti-tissue transglutaminase, and anti-thyroglobulin antibodies); and metabolic tests (alpha-1-antitrypsin, ceruloplasmin, and urine copper levels; urine organic acid analysis; and sweat chloride test), were all normal. Abdominal Doppler ultrasound was unremarkable.
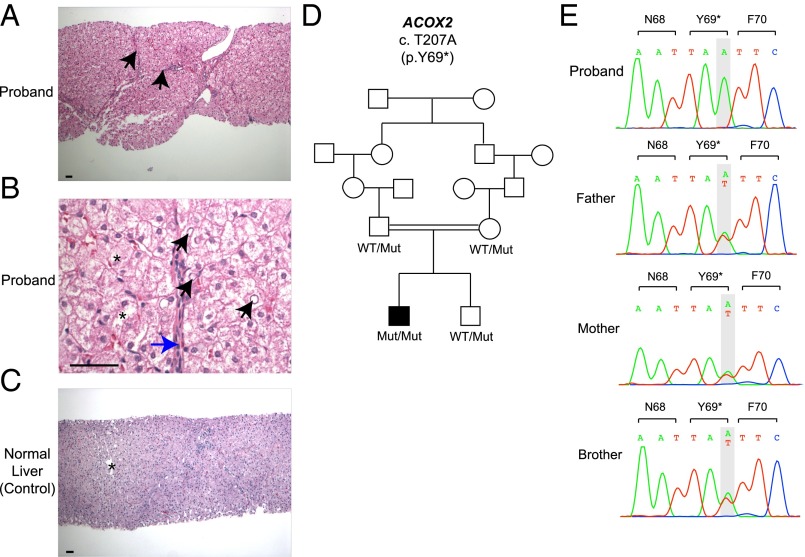
The proband underwent a liver biopsy at age 6 y. In contrast to a normal liver, his liver showed many thin fibrous septa, swollen hepatocytes, glycogenated nuclei, and focal acinar transformation, consistent with hepatocellular injury and regeneration (Fig. 1 and Fig. S1A). There was no obvious cholestasis, cholate stasis, or steatosis. The fibrous septa and portal areas contained sparse lymphocytes without any interface activity, and there was no lobular inflammatory activity or evident acidophil bodies. Trichrome stain showed thin fibrous septa and some nodularity (“incomplete septal cirrhosis”), with no features of well-established cirrhosis (Fig. S1B).
Fig. 1.
Liver histology and ACOX2 mutation in a subject with an unrecognized bile acid synthesis disorder. (A) Liver parenchyma of the proband shows many thin fibrous septa (black arrows) and some nodularity (“incomplete septal cirrhosis”). Features of well-established cirrhosis are not seen. (B) Details of the lobular parenchyma show swollen hepatocytes (asterisks), glycogenated nuclei (black arrows), and very thin incomplete fibrous septa (blue arrow). (C) Liver parenchyma from age- and sex-matched patient showing normal-appearing portal tracts and a central venule (asterisk). The lobular architecture is preserved with no fibrosis. (D) The affected proband and unaffected subjects are shown in black and white symbols, respectively. Consanguineous union is represented by a double line. ACOX2 alleles are denoted as WT or Mut (p. Y69*). (E) Sanger sequencing chromatograms of the proband, his unaffected parents, and his brother. The ACOX2 p.Y69* mutation is homozygous in the proband and heterozygous in the unaffected parents and sibling. (Scale bars: 50 μm.)
Fig. S1.
Additional histological findings in the proband’s liver. (A) Hepatocytes are pale and swollen with focal acinar transformation (black arrows). (Scale bar: 50 μm.) (B) Low-magnification of liver biopsy showing thin fibrous septa (black arrows) and some nodularity on Trichrome stain. (Scale bar: 200 μm.)
The proband’s growth had been normal, with weight ranging between the 5th and 90th percentiles, height between the 50th and 75th percentiles, and head circumference between the 25th and 50th percentiles. Specifically, his weight was 2,500 g (5th percentile) at birth and 33.8 kg (90th percentile) at age 8 y on his last clinic visit in June 2016, height was 49 cm (50th percentile) at birth and 133 cm (75th percentile) at age 8 y, and head circumference was 34 cm (25th percentile) at birth and 53 cm (50th percentile) at age 8 y.
The proband also exhibited neurologic abnormalities. Developmental milestones included social smile at 3 mo (mild delay), sitting at 6 mo, and walking at 14 mo (both within normal limits). He demonstrated mildly delayed language development, producing several single words by 2.5 y and sentences by 5 y. At age 6.5 y, his parents and elementary school teachers noted mild intellectual disability. Neurologic examination was remarkable for slurred speech, vertical gaze palsy, slight dysmetria, and mild gait ataxia. The ocular fundus examination was normal bilaterally. He had a total score of 66 on the Wechsler Intelligence Scale for Children–Revised (average range, 90–110), supporting a diagnosis of mild intellectual disability. Brain magnetic resonance imaging was reported as normal.
Homozygous Loss-of-Function Mutation in ACOX2.
The proband’s exome was sequenced to a mean depth of 60 independent reads per targeted base, with 96% of targeted bases having more than eight independent reads, providing high-confidence calling of homozygous and heterozygous variants across the exome (Table S1). Because the proband was the offspring of a second-cousin union (Fig. 1D), we sought rare alleles (allele frequency ≤ 0.01 in databases) that were likely to result in loss of function of the encoded proteins (i.e., premature termination, frameshift, and splice site variants) that were homozygous in the proband. Consistent with consanguinity, this analysis identified five rare homozygous genotypes in the proband. Four of these genotypes resulted in missense variants predicted to be tolerated variants by MetaSVM and unlikely to be pathogenic in this patient (Table S2). The other homozygous variant encoded premature termination at codon 69 (NM_003500, p.Y69*) in ACOX2, which encodes a branched-chain acyl-CoA oxidase, a peroxisomal enzyme expressed in the liver and kidney and thought to be involved in bile acid biosynthesis (8). This variant allele was absent among >100,000 alleles in the ExAC database and >1,500 alleles in persons of Turkish ancestry, and was not found in any other database examined. Moreover, no homozygous loss-of-function genotypes in this gene were found among ∼61,000 exomes in ExAC or 894 people of Turkish ancestry. Sanger sequencing confirmed the homozygous variant in the proband and showed that both of his parents and his brother were heterozygous for this variant (Fig. 1E).
Table S1.
Sequencing coverage and quality metrics for the proband’s exome
| Metric | Number or percentage |
| Mean independent reads per targeted base | 59.8 |
| Percent of bases mapping to genome | 91.14 |
| Percent of targeted bases with ≥8 independent reads | 95.9 |
| Mean error rate, % | 0.44 |
Table S2.
Rare homozygous protein-altering variants detected in the proband
| Gene symbol | Chr: position (hg19) | AA change | MetaSVM score (prediction) | NHLBI | 1000G | Turkish DB (894 subjects) | ExAC (overall) | ExAC (highest subfrequency) |
| ACOX2 | 3: 58520203 | Y69* | Truncating | 0 | 0 | 0 | 0 | 0 |
| CHDH | 3: 53851817 | A591V | −1.051 (T) | 0 | 0 | 0 | 0 | 0 |
| LRRIQ1 | 12: 85531684 | I1422M | −0.927 (T) | 0.007414 | 0.01 | 0.0006 | 0.00861 | 0.02184 (south Asian) |
| OSBPL6 | 2: 179255872 | R817W | −0.412 (T) | 0 | 0 | 0 | 0 | 0 |
| RASEF | 9: 85620405 | R347W | −0.118 (T) | 0 | 0 | 0 | 4.94E-05 | 0.0002312 (east Asian) |
None of the listed genes are OMIM-related genes. AA, amino acid; Chr, chromosome; DB, database; ExAC, Exome Aggregation Consortium database; NHLBI, National Heart, Lung, and Blood Institute Exome Sequencing Project database; 1000G, 1000 Genome database. MetaSVM scores missense variants on a scale of −2 to 3, with scores <0 predicted to be tolerated (T) and scores >0 predicted to be damaging (D).
ACOX2 Is Absent in the Proband’s Liver.
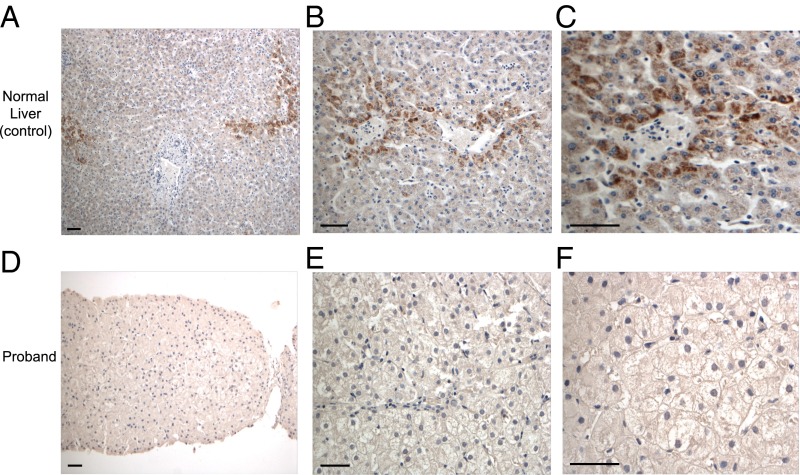
The livers of normal control subjects and the proband were stained with antibodies specific for ACOX2. Whereas normal livers showed intense granular staining in the pericentral (zone 3) hepatocytes (Fig. 2 A–C), along with faint hepatocellular cytoplasmic staining, the proband’s liver showed a complete absence of staining, consistent with the mutation resulting in absence of this protein (Fig. 2 D–F).
Fig. 2.
Absence of ACOX2 in the proband’s liver. (A–C) Immunohistochemistry for ACOX2 in normal liver shows intense granular staining in the pericentral (zone 3) hepatocytes, but faint staining in remaining hepatocytes, including periportal hepatocytes (zone 1). (D–F) Immunohistochemistry for ACOX2 in the proband’s liver biopsy, showing complete absence of staining. (Scale bars: 50 μm.)
Elevated Levels of Bile Acid Intermediates.
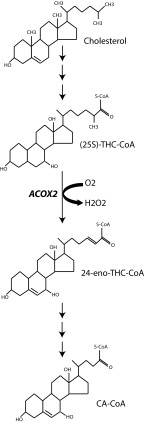
The primary bile acids, cholic acid and chenodeoxycholic acid, are derived from cholesterol in the liver by the action of at least 14 different enzymes (9). The final steps of bile acid synthesis occur in peroxisomes. ACOX2, a peroxisomal branched-chain acyl-CoA oxidase, is believed to mediate the first step in the β-oxidation of 27 carbon (C27) bile acid intermediates into 24 carbon (C24) bile acids (8, 10), resulting in the CoA esters of cholic and chenodeoxycholic acid (Fig. 3). Thus, we assessed the proband’s plasma and urine for elevated levels of C27 bile acids upstream of ACOX2 activity. Consistent with a loss of ACOX2 function, the proband’s plasma and urine had strikingly elevated levels of the C27 intermediate bile acids 3α,7α-dihydroxy-5β-cholestanoic acid (DHCA) and 3α,7α,12α-trihydroxy-5β-cholestanoic acid (THCA), principally as taurine conjugates (Table 1). For example, the proband’s plasma tauro-THCA level was 7.94 μM, 25-fold higher than the upper limit of normal (0.31 μM), and his level of urinary tauro-THCA (normally absent in urine) was 66.19 μmol/mol creatinine. He had low-normal levels of cholic acid and conjugates, as are seen in other bile acid biosynthesis disorders (11). His heterozygous parents showed no elevation of C27 intermediate bile acids. These findings indicate that homozygous loss of ACOX2 results in defective peroxisomal beta-oxidation of DHCA-CoA and THCA-CoA.
Fig. 3.
Schematic representation of the ACOX2 β-oxidation of trihydroxycholestanoyl-CoA. CA, cholic acid.
Table 1.
Biochemical data from proband’s plasma and urine
| Parameter | Proband | Mother | Father | Reference values |
| ACOX2 genotype (p.Y69*) | Mut/Mut | Wt/Mut | Wt/Mut | NA |
| Plasma | ||||
| Bile acid intermediates | ||||
| Tauro-DHCA, μM | 0.32 (↑) | 0 | 0 | 0–0.09 |
| Tauro-THCA, μM | 7.94 (↑) | 0.011 | 0.01 | 0–0.31 |
| Glyco-DHCA, μM | 0.06 (↑) | 0.01 | 0 | 0–0.01 |
| Glyco-THCA, μM | 0.24 (↑) | 0.01 | 0.02 | 0–0.19 |
| DHCA, μM | 0.262 (↑) | 0.003 | 0.004 | 0–0.006 |
| THCA, μM | 0.10 (↑) | 0.01 | 0.02 | 0–0.07 |
| Bile acids | ||||
| Glycodihydroxycholanoates, μM | 0.58 | 0.76 | 1.41 | 0.22–4.16 |
| Glycocholate, μM | 0.41 | 0.08 | 0.24 | 0.05–2.52 |
| Taurodihydroxycholanoates, μM | 0.14 | 0.04 | 0.03 | 0.03–1.43 |
| Taurocholate, μM | 0.07 | 0.003 | 0.009 | 0.001–0.61 |
| Dihydroxycholanoates, μM | 0.03 (↓) | 0.53 | 0.18 | 0.09–2.21 |
| Cholate, μM | 0.003 | 0.09 | 0.03 | 0.003–0.19 |
| Very-long-chain fatty acids | ||||
| C26:0, μM | 1.07 | NA | NA | ≤1.30 |
| C24:0, μM | 34.45 | NA | NA | ≤91.4 |
| C22:0, μM | 32.43 | NA | NA | ≤96.3 |
| C24:0/C22:0 | 1.06 | NA | NA | ≤1.39 |
| C26:0/C22:0 | 0.03 | NA | NA | ≤0.023 |
| Branched-chain fatty acids | ||||
| Phytanic acid, μM | 1.40 | NA | NA | ≤9.88 |
| Pristanic acid, μM | 0.14 | NA | NA | ≤2.98 |
| Urine | ||||
| Bile acid intermediates | ||||
| Tauro-DHCA, μmol/mol creatinine | 0.24 (↑) | 0 | 0 | 0 |
| Tauro-THCA, μmol/mol creatinine | 66.19 (↑) | 0 | 0 | 0 |
| Tauro-tetraOH-cholestanoic acids, μmol/mol creatinine | 371.1 (↑) | 0 | 0 | 0–0.77 |
| Glyco-THCA, μmol/mol creatinine | 14.11 (↑) | 0.31 | 0.39 | 0–0.78 |
| Glyco-tetraOH-cholestanoic acids, μmol/mol creatinine | 53.93 (↑) | 2.07 | 2.24 | 0–13.20 |
| THCA, μmol/mol creatinine | 4.62 (↑) | 0 | 0 | 0–0.13 |
NA, not assessed; ↑, above the reference values; ↓, below the reference values.
ACOX2 also has been thought to be involved in the degradation of long branched fatty acids (phytanic and pristanic acids) (8). Interestingly, however, the proband’s levels of branched-chain fatty acids (phytanic and pristanic acids) were both within the normal range (Table 1). As expected, there was no elevation of very-long-chain fatty acids in the plasma, consistent with their metabolism by straight-chain acyl-CoA oxidase (ACOX1).
Discussion
Our findings define a previously undescribed inborn error of bile acid synthesis in a child with intermittent elevated transaminase levels, liver fibrosis, and mild neurologic impairment, and implicate homozygous loss of ACOX2 in its pathogenesis. The evidence implicating ACOX2 is strong. The proband exhibited a homozygous loss of function mutation in ACOX2, no evidence of ACOX2 protein in the liver, and elevated C27 bile acid intermediates predicted to result from loss of ACOX2 function, along with low or low-normal levels of downstream primary bile acids. His hypocholesterolemia, fat malabsorption, and vitamin D deficiency are expected findings owing to decreased intestinal absorption of fat and lipids with reduced bile acid secretion into the gut. Of note, the proband’s heterozygous 10-y-old brother (TC, 162 mg/dL; LDL cholesterol, 85 mg/dL), mother (TC, 159 mg/dL; LDL, 78 mg/dL), and father (TC, 204 mg/dL; LDL, 97 mg/dL) had normal lipid levels, providing no evidence of a phenotype in the heterozygous state.
In addition, intermediate bile acids are believed to be hepatotoxic and neurotoxic (12, 13), providing a likely explanation for the proband’s elevated transaminase levels, liver histology abnormalities, and neurologic defects. Mild to severe hepatocellular damage and/or neurologic impairment, including ataxia and intellectual disability, is seen in other defects in bile acid synthesis (9). Alternatively, deficiency of ACOX2 enzymatic activity and its downstream metabolites, and/or an independent function of ACOX2, could contribute to the proband’s neurologic and cognitive impairment. No other variants were identified that can likely explain aspects of the proband’s clinical features. The full spectrum of clinical features attributable to loss of ACOX2 remains to be determined by the study of other patients with ACOX2 deficiency. This report should stimulate the study of other children with unexplained elevation in transaminase levels and/or neurologic features.
Other patients with reported increases in C27 bile acid intermediates without general peroxisomal defects have proven distinct from our present case. A patient with cirrhosis and liver failure at age 4 y exhibited recessive loss of ABCD3 (14), which transports branched-chain acyl CoAs into peroxisomes. Like our patient, this patient had normal levels of phytanic and pristanic acid. Other patients have been described with sensorimotor neuropathy with recessive loss of alpha-methyl-acyl-CoA racemase (AMACR) (15), the enzymatic step in bile acid synthesis before ACOX2. These patients have elevated levels of phytanic and pristanic acid.
Whereas ACOX2 has also been proposed to be involved in the oxidation of long branched fatty acids (8, 10), normal levels of phytanic and pristanic acids were measured in the proband’s plasma. In humans, phytanic and pristanic acids are dietary in origin, predominantly from dairy products, beef, and lamb (16). The proband is not a vegetarian and ingests dairy products and beef regularly, providing no support for low dietary intake. This suggests that another pathway (e.g., omega oxidation) can achieve full catabolism of these branched-chain fatty acids in children. The fact that phytanic and pristanic levels are elevated in patients without AMACR, which converts 2R pristanoyl-CoA to the S enantiomer, suggests that this alternate pathway can metabolize only S enantiomers. We infer that as a consequence of ACOX2 deficiency, C27-intermediate bile acids accumulate in peroxisomes as CoA esters and are readily converted into taurine conjugates (by bile acyl-CoA amino acid acyltransferase), and much smaller amounts are converted to glycine conjugates. This is consistent with the observation that taurine conjugates predominate in peroxisome biogenesis disorders (17).
Importantly, the clinical consequences of defects in bile acid synthesis can be mitigated using primary bile acid therapy. Oral bile acid replacement with cholic acid reduces endogenous bile acid synthesis, thereby decreasing the accumulation of toxic bile acid intermediates and correcting fat malabsorption (18, 19). This therapy also has proven effective in normalizing transaminase levels and improving neurologic findings (20). Given the proband’s low-normal levels of cholic acid and conjugates, cholic acid therapy has the potential for clinical impact. A trial with close monitoring of liver function tests and bile acid intermediates levels is planned.
At present, most peroxisomal disorders are diagnosed through the identification of probands with severe phenotypes with marked biochemical abnormalities, such as increased levels of substrates normally handled by peroxisomes (e.g., very long-chain fatty acids, pristanic acid, phytanic acid, DHCA, THCA) and decreased levels of end products of peroxisomal metabolism (cholic and chenodeoxycholic acid), followed by investigation leading to identification of the genetic defect. It is noteworthy that our patient presented with a relatively nonspecific clinical presentation, and that a primary defect in bile acid biosynthesis was not considered before the ACOX2 mutation was identified. Particularly because of the potential for mitigation of the clinical consequences of ACOX2 deficiency, this diagnosis should be considered in children with unexplained transaminase elevations and neurologic abnormalities, and raise the question of whether ACOX2 mutation also might contribute to cases of cryptogenic cirrhosis later in life.
Materials and Methods
Human Subjects.
The study protocol was approved by the Human Investigation Committees of Gazi University and Yale University. All subjects provided written informed consent.
DNA Isolation, Exome Capture, and Sequencing.
Genomic DNA of the proband, his unaffected parents, and his brother was isolated from peripheral blood leukocytes using standard procedures. Exome sequencing of the proband was performed using the Roche/Nimblegen SeqCap EZ Human Exome Library v2.0, with 74 base paired-end sequencing on the Illumina HiSeq platform as described previously (21).
Exome Analysis.
Exome sequence data were aligned to the reference human genome (build 19), and variants were called using GATK. The impact of missense variants was predicted using MetaSVM (22). Allele frequencies of identified variants were determined in the National Heart, Lung, and Blood Institute’s Exome Variant Server (4,300 European subjects and 2,203 African American subjects; last accessed March 2015), 1000 Genomes (1,094 subjects of various ethnicities), the Exome Aggregation Consortium (ExAC; 61,000 subjects of various ethnicities; January 2015 release), and a Yale database of 894 Turkish exomes, predominantly of consanguineous union.
Sanger Sequencing of Genomic DNA.
Sanger sequencing of the identified ACOX2 mutation (p.Y69*) was performed by PCR amplification of genomic DNA of the proband, his parents, and his sibling using the following primers: forward, 5′-TCTTCTAACCAACCCAGGCG-3′; reverse, 5′-CAGAAACCTCACCCAGGTCC-3′. The nomenclature of the ACOX2 variant is based on National Center for Biotechnology Information reference sequence NM_003500.
ACOX2 Liver Immunohistochemistry.
Anti-ACOX2 antibody (HPA038280; Sigma-Aldrich) at 1:200 dilution was used to perform immunohistochemistry in normal and ACOX2 mutant liver sections using standard techniques.
Mass Spectrometry.
Analysis of cholanoic acids (normal bile acids) and their taurine and glycine conjugates was performed by targeted UPLC-MS/MS essentially as described previously (23), with modifications to include the analysis of the cholestanoic acids (C27 bile acids) THCA and [27,27,27-D3]-THCA, purchased from Herman Ten Brink of the VU Medical Centre, Amsterdam, The Netherlands. UPLC retention times and mass spectrometry transitions for the C27 bile acids are shown in Table S3. Quantitation of glycine and taurine conjugates was achieved by measuring the ratio of the peak area produced by the C27 bile acids in the patient sample to the corresponding deuterated C24 bile acid standard (e.g., tauro-THCA/D5-taurocholic acid). Some compounds were identified by comparison with samples from previous patients diagnosed with peroxisomal disorders.
Table S3.
Retention times, cone voltage, collision energy, precursor, and product ions used in identification of bile acids detected in patients with peroxisomal disorders
| Bile acid | Conjugation (abbreviation) | Retention time, min | Cone voltage, V | Collision energy, V | Precursor ion, m/z | Product ion, m/z |
| Dihydroxycholestanoic, DHCA (3α,7α-diOH-5β-C27) | Tau (Tauro-DHCA) | 1.85 | 116 | 59 | 540.0 | 80.0 |
| Trihydroxycholestanoic, THCA (3α,7α,12α-triOH-5β-C27) | Tau (Tauro-THCA) | 1.91 | 116 | 59 | 556.3 | 80.0 |
| Tetrahydroxycholestanoics, hydroxy-THCA (3α,7α,12α,ξ-tetraOH-5β-C27) | Tau (Tauro-tetraOH-cholestanoics) | 1.5–1.8 | 116 | 59 | 572.0 | 80.0 |
| Dihydroxycholestanoic, DHCA (3α,7α-diOH-5β-C27) | Gly (Glyco-DHCA) | 1.85 | 88 | 31 | 490.0 | 74.0 |
| Trihydroxycholestanoic, THCA (3α,7α,12α-triOH-5β-C27) | Gly (Glyco-THCA) | 1.78 | 88 | 31 | 506.0 | 74.0 |
| Tetrahydroxycholestanoics, hydroxy-THCA (3α,7α,12α,ξ-tetraOH-5β-C27) | Gly (Glyco-tetraOH-cholestanoics) | 1.56 | 88 | 31 | 522.0 | 74.0 |
| Dihydroxycholestanoic, DHCA (3α,7α-diOH-5β-C27) | None (DHCA) | 1.94 | 86 | 10 | 433.0 | 433.0 |
| Trihydroxycholestanoic, THCA (3α,7α,12α-triOH-5β-C27) | None (THCA) | 1.86 | 86 | 10 | 449.3 | 449.3 |
| D3-THCA | None (D3-THCA) | 1.86 | 86 | 10 | 452.3 | 452.3 |
5β-C27, 5β-cholestan-26-oic acid; Tau, taurine; Gly, glycine
Acknowledgments
We thank the proband and his family for their invaluable contribution to this work; the staff of the Yale Center for Genome Analysis for exome sequencing; and Lynn Boyden, Weizhen Ji, and Samir Zaidi for helpful discussions and critical reads of the manuscript. This work was supported by the National Institutes of Health’s Center for Mendelian Genomics (Grant U54 HG006504) and in part by National Institutes of Health–Yale Liver Center Grant P30DK034989. S.V. is the recipient of an American Association for the Study of Liver Diseases Foundation Sheila Sherlock Clinical and Translational Research Award in Liver Diseases. R.P.L. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: Dr. Lifton and Dr. Valle were coauthors (neither of them first or last author) on a 40-author review of progress in Mendelian genetics published in 2015. This paper came about because they are independently funded grantees from NIH for discovery of new Mendelian loci. They have never been research collaborators.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613228113/-/DCSupplemental.
References
- 1.Chong JX, et al. Centers for Mendelian Genomics The genetic basis of mendelian phenotypes: Discoveries, challenges, and opportunities. Am J Hum Genet. 2015;97(2):199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilarinho S, et al. Individual exome analysis in diagnosis and management of paediatric liver failure of indeterminate aetiology. J Hepatol. 2014;61(5):1056–1063. doi: 10.1016/j.jhep.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilarinho S, et al. Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. J Hepatol. 2014;61(5):1178–1183. doi: 10.1016/j.jhep.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Vilarinho S, et al. Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension. Hepatology. 2016;63(6):1977–1986. doi: 10.1002/hep.28499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sambrotta M, et al. University of Washington Center for Mendelian Genomics Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46(4):326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haack TB, et al. Biallelic mutations in NBAS cause recurrent acute liver failure with onset in infancy. Am J Hum Genet. 2015;97(1):163–169. doi: 10.1016/j.ajhg.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilgüvar K, et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467(7312):207–210. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanhove GF, et al. The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J Biol Chem. 1993;268(14):10335–10344. [PubMed] [Google Scholar]
- 9.Sundaram SS, Bove KE, Lovell MA, Sokol RJ. Mechanisms of disease: Inborn errors of bile acid synthesis. Nat Clin Pract Gastroenterol Hepatol. 2008;5(8):456–468. doi: 10.1038/ncpgasthep1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart E, et al. Molecular characterization of the human peroxisomal branched-chain acyl-CoA oxidase: cDNA cloning, chromosomal assignment, tissue distribution, and evidence for the absence of the protein in Zellweger syndrome. Proc Natl Acad Sci USA. 1996;93(24):13748–13753. doi: 10.1073/pnas.93.24.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinandusse S, Houten SM. Peroxisomes and bile acid biosynthesis. Biochim Biophys Acta. 2006;1763(12):1427–1440. doi: 10.1016/j.bbamcr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Keane MH, et al. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome-deficient PEX2 Zellweger mice. Hepatology. 2007;45(4):982–997. doi: 10.1002/hep.21532. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinandusse S, Denis S, Dacremont G, Wanders RJ. Toxicity of peroxisomal C27-bile acid intermediates. Mol Genet Metab. 2009;96(3):121–128. doi: 10.1016/j.ymgme.2008.11.165. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinandusse S, et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum Mol Genet. 2015;24(2):361–370. doi: 10.1093/hmg/ddu448. [DOI] [PubMed] [Google Scholar]
- 15.Ferdinandusse S, et al. Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Genet. 2000;24(2):188–191. doi: 10.1038/72861. [DOI] [PubMed] [Google Scholar]
- 16.van den Brink DM, Wanders RJ. Phytanic acid: Production from phytol, its breakdown and role in human disease. Cell Mol Life Sci. 2006;63(15):1752–1765. doi: 10.1007/s00018-005-5463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson AM, Madigan MJ, Shortland D, Clayton PT. Rapid diagnosis of Zellweger syndrome and infantile Refsum's disease by fast atom bombardment–mass spectrometry of urine bile salts. Clin Chim Acta. 1986;161(2):221–231. doi: 10.1016/0009-8981(86)90215-9. [DOI] [PubMed] [Google Scholar]
- 18.Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34(3):593–604. doi: 10.1007/s10545-010-9259-3. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales E, et al. Oral cholic acid for hereditary defects of primary bile acid synthesis: A safe and effective long-term therapy. Gastroenterology. 2009;137(4):1310–1320 e1-3. doi: 10.1053/j.gastro.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KD, et al. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology. 1992;15(2):198–207. doi: 10.1002/hep.1840150206. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire M, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45(5):531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong C, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015;24(8):2125–2137. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzacuva F, et al. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 2016;590(11):1651–1662. doi: 10.1002/1873-3468.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]