Abstract
Due to complex cellular microenvironments of both the liver and kidney accurate modeling of transport function has remained a challenge, leaving a dire need for models that can faithfully recapitulate both the architecture and cell-cell interactions observed in vivo. The study of hepatic and renal transport function is a fundamental component of understanding the metabolic fate of drugs and xenobiotics however; there are few in vitro systems conducive for these types of studies. For both the hepatic and renal systems we provide an overview of the location and functions of the most significant phase I/II/III (transporter) enzymes then review current in vitro systems for transporter function study suitability and provide details on microphysiological systems that lead the field in these investigations. Microphysiological modeling of the liver and kidney using “organ-on-a-chip” technologies is rapidly advancing in transport function assessment and has emerged as a promising method to evaluate drug and xenobiotic metabolism. Future directions for the field are also discussed along with technical challenges encountered in complex multiple-organs-on-chips development.
Hepatic Microphysiological Systems
Basic aspects of Xenobiotic Metabolism in the Liver
The creation and maintenance of an in vitro microphysiological system that correctly mimics hepatic function to study drug and xenobiotic metabolism must include functional biotransformation enzymes and transporters. In this section, we review the primary hepatic phase I/II/II enzymes and transporters and survey hepatic in vitro systems with an emphasis on how well they have been used to study metabolism. Overall, there has been a trend for the development of more complex systems that utilize multiple cell types, physiologically relevant geometries and microfluidics to capture the complex interactions within the liver. Together, these attributes create a favorable cellular microenvironment that allows for the expression and correct cellular orientation of the transporters.
The liver is the main organ where xenobiotics and endogenous compounds are metabolized and excreted due to its physiological placement ‘downstream’ from the gastrointestinal tract, high perfusion rate, large size and high concentration of biotransformation enzymes.
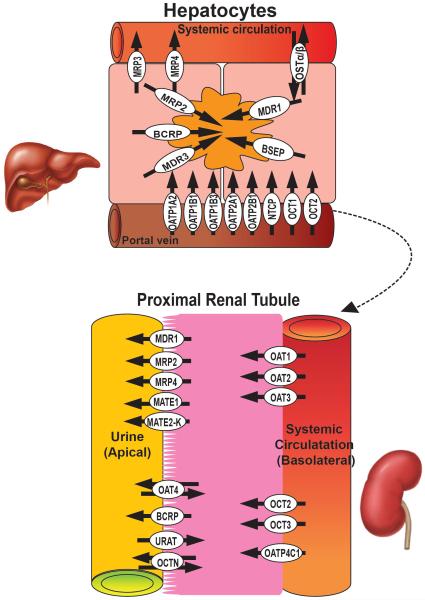
To predict a compound’s metabolic fate, including detoxification or bioactivation to a toxic metabolite, it is very critical to understand the biotransformation enzymes and transporters expressed in the liver. Since the liver receives nearly all of the blood perfusing the GI tract, it is anatomically situated to be able to potentially remove xenobiotics absorbed from the gut prior to reaching the systemic circulation– the hepatic first pass effect (Figure 1). To model a correctly functioning hepatic in vitro microphysiological system it is imperative that the biotransformation enzymes are expressed and active in order to accurately study parent compound and metabolite transporter dynamics.
Figure 1.
Graphic illustration of the relationship between subcellular localization of transporters in the liver (top) and kidney (bottom) as well as the preferential flux directionality for each individual transporter (arrows).
Biotransformation enzymes are often called drug-metabolizing enzymes. These enzymes are involved in xenobiotic (drugs and other chemicals foreign to the body) disposition via the processes of ADME (absorption, distribution, metabolism, and elimination) and are classified as either phase I (generally oxidation, reduction or hydrolysis reactions), phase II (generally conjugation or hydrolysis reactions) or phase III, (transmembrane transporters), based on their metabolic function.
Generally, phase I enzymes are responsible for catalyzing hydrolysis, reduction, and oxidation of xenobiotics. Among the phase I enzymes, cytochromes P450 (CYPs; detailed below) are the largest and most important superfamily: CYP3A4, CYP2D and CYP2C subfamilies are responsible for 50%, 25% and 20% of the biotransformation of all drugs, respectively (1). Phase II enzymes can transfer a functional group to xenobiotics- a process called conjugation, including acetylation, methylation, glutathione conjugation, sulfate conjugation, and glucuronidation.
When xenobiotics are absorbed into hepatocytes from the portal vein and hepatic artery, transporters in the sinusoidal (basolateral) membrane of hepatocytes assist xenobiotics to enter hepatocytes for later biotransformation.
The most abundant Phase I/II enzymes and transporters expressed in the human liver are listed in Supplemental Table 1. One result of ADME is to convert toxic xenobiotics into non-toxic, water-soluble compounds which can easily be eliminated - a process called detoxification or inactivation. However, in certain cases, phase I and/or phase II enzymes can transform xenobiotics into more toxic chemicals – a process called bioactivation. The concept of bioactivation has been adapted to the pharmacological idea of prodrug-where the administered parent molecule has little or no pharmacological activity, but one or more metabolites act as the major contributor to the desired pharmacological response. For example, codeine is a morphine prodrug that requires oxidative demethylation by CYP2D6 to form morphine to achieve its pharmacological activity.
Phase I enzymes of pharmacological and toxicological significance: (Casarett & Doull’s, (2))
Hydrolysis
There are multiple gene products with a wide variety of potential substrates, including: Carboxylesterase, Alkaline phosphatase (ALP), Dipeptidyl peptidase-4, Epoxide hydrolases (microsomal EH; cytosolic EH); Paraoxonase (PON1, 2, and 3)
Reduction
NAD(P)H- quinone oxidoreductases (NQO1 and NQO2), Aldo-keto reductases (AKRs), carbonyl reductase (CR), Cytochrome b5/ NADH-cytochrome b5, NADPH: P450 reductase, Aldehyde oxidase
Oxidation
Examples of enzymes involved in xenobiotic oxidation include: Aldehyde dehydrogenases (ADH1 and 2), Alcohol dehydrogenases (ALDH1), Aldehyde oxidase, Xanthine oxidase (XO), Monoamine oxidase (MAO), Peroxidase- glutathione peroxidase (GSHPx), Flavin-dependent-monooxygenase (FMO3, 4, 5) Cytochrome P450 (CYPs): are generally considered to be the most important group of oxidative enzymes involved in phase I biotransformation of xenobiotics. There are 56 different human CYP genes, but many are involved only in endogenous metabolic processes. Major human forms involved in xenobiotic biotransformation are: CYP1A1, IA2, 1B1, 2A6, 2A13, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 2F1, 2J2, 3A4, 3A5, 3A7, 4F3
Phase II enzymes: Conjugation
UDP-Glucuronosyltransferase (UGTs). There are two major UGT families involved in xenobiotic conjugation, UGT1 and UGT2. Within the UGT1 family are multiple gene variants that give rise to 10 (UGT1A1 – 1A10) distinct gene products. The UGT2 family has two sub-families, UGT2A (3 members) and UGT2B (4 members.) All UGT enzymes use uridinediphosphoglucuronic acid (UDPGA) as the cofactor.
Sulfotransferases are also multigene families of enzymes, with SULT1A, 1B, 1E, and 2A1involved in xenobiotic conjugation. All SULT enzymes use 3’ phosphoadenosine-5’ phosphosulfate (PAPS) as the cofactor. Glutathione S-transferase (GSTs) are also involved in xenobiotic conjugation, with ~15 different human genes, in 5 classes (GSTA1-5, GSTM1-5, GSTT1 and 2, GSTO1 GSTS1 and GSTZ1). Also GSTs use the tripeptide, glutathione, as a cofactor. Other important conjugation reactions include N-acetyltransferase (NATs- NAT1 and 2), with the cofactor, acetyl coenzyme A (acetyl-CoA), and several methyltransferases with different substrate specificities (O- methyltransferase, N-methyltransferase and S-methyltransferase).
Basolateral Uptake Transporters
These transporters mediate the hepatic uptake of xenobiotics and endogenous substances such as bile acid and cholesterol. These uptake transporters belong to a multi-gene family of solute carriers (SLCs). In humans, the main uptake transporters are organic anion transporting peptides (OATPs)- OATP1A2, 1B1,1B3, 2A1, 2B1; the sodium-dependent taurocholate cotransporting protein (NTCP); and the organic cation transporters (OCT) OCT1and OCT2 (3). Many of these carrier proteins can transport substrates bi-directionally, depending on the substrate concentration gradient.
OATPs are integral membrane proteins with 12 transmembrane helices and are the main drug carrier proteins supporting the sodium-independent hepatic uptake organic anions (4). Because of the prominent expression of OATPs on the basolateral membrane of hepatocytes, OATPs are responsible for a critical mechanism of chemical uptake into the liver, including a variety of substrates containing steroidal or peptide structural backbones and/or anionic or cationic chemicals. For example, OATP 1A2 is associated with the uptake of sulfobromophthalein (BSP), BQ-123, [d-Pen2,d-Pen5]-enkephalin (DPDPE), fexofenadine, levofloxacin, ouabain, and methotrexate. OATP 1B1 and -1B3 are the OATP1B isoforms expressed in human livers (5) and can transport bilirubin and its glucuronide conjugates (6). OATP1B1 not only transports various statin drugs, but also can carry thyroxine, taurocholate, and dehydroepiandrosterone sulfate (reviewed by (7)) while OATP2A1 supports the transport of prostaglandins, including PGE2 and PGF2α (8).
Chemicals and drugs that belong to small class I organic cations, including tetramethylammonium, tetraethylammonium, tetrabutylammonium, thiamine, choline, dopamine, serotonin, histamine, adrenalin, and noradrenalin, are transported in hepatocytes by OCT1 (reviewed by (3)).
NTCP predominantly transports bile salts and sulfated compounds in the sodium dependent manner in addition to thyroid hormones, estrone 3-sulfate and certain statin drugs such as rosuvastatin and pitavastatin (9-11). A recent study implicated NTCP as the receptor for hepatitis B and D viruses, showing its clinical importance (12).
Apical Efflux Transporters
These transporters are located on the apical surface of hepatocytes and pump out endogenous metabolites and xenobiotics via biliary excretion processes. These transporters belong to the ATP –binding cassette transporter (ABC) family containing ATP- binding domains that have ATPase activity to provide the energy necessary for active transport of substrates across the cell membrane, most often against a 100-1000-fold concentration gradient (reviewed by (7)). The most abundant and important transporters on the apical side of hepatocytes are the multiple resistance proteins (MRPs) - MRP2, multidrug resistance-associated proteins (MDRs) - MDR1 and 3, bile salt export pump (BSEP), and breast cancer resistance protein (BCRP/ ABCG2) (reviewed by (7)).
MRP2 is involved in the efflux of both hydrophobic uncharged molecules and water-soluble anionic compounds (reviewed by (3)). MDR1 (ABCB1, P-glycoprotein, P-gp) can transport amphipathic organic cations and neutral compounds across the canaliculus membrane to the bile. MDR1 (ABCB1) efflux transporter substrates include: glutathione, glucuronide, and sulfate conjugates; many macrolide antibiotics such as erythromycin, azithromycin, and clarithromycin; and chemotherapeutics such as tamoxifen, doxorubicin, and paclitaxel (reviewed by (3)). MDR3 (ABCB4) can transport phospholipids whereas BSEP primarily transports conjugated bile acids, including taurochenodeoxycholate, taurocholate, tauroursodeoxycholate, glycochenodeoxycholate, and glycocholate. In addition, BSEP can transport pharmaceuticals such as pravastatin (reviewed by (7)).
BCRP transports a highly diverse range of hydrophobic substrates, including chemotherapeutic agents such as mitoxantrone, methotrexate, topotecan and irinotecan. BCRP can also transport hydrophilic conjugated organic anions, particularly the sulfated conjugates with high affinity (reviewed by (13)).
Genetic defects or secondary consequences of hepatobiliary obstruction or destruction can cause cholestasis, which are often involved in impaired function or a sustained inhibition of these apical efflux transporters. Inherited mutations in the human MDR3 gene can cause progressive familial intrahepatic cholestasis type 3 (PFIC-III), a rare disease characterized by an early onset of cholestasis that leads to cirrhosis and liver failure before adulthood (14). In addition, mutations in BSEP are responsible for PFIC-type 2 patients with high serum bile acid concentrations and low biliary bile acid but normal serum γ-glutamyltranspeptidase activity and cholesterol (15).
Basolateral Efflux Transporters
Removal of endogenous and xenobiotic chemicals from hepatocytes to sinusoidal blood is mediated by transporters on the basolateral side, including MRP3, MRP4 and organic solute and steroid transporter, Ost alpha-Ost beta (OSTα/β).
MRP3 has a high affinity for glucuronide conjugates, which is involved in detoxification and excretion of polar chemicals that have undergone the process of glucuronidation, including morphine-3-glucuronide, bilirubin-glucuronide, etoposide-glucuronide, and acetaminophen-glucuronide. It is suggested that MRP3 has a defense-related function and contributes to the excretion of toxic anions, as expression is upregulated during hepatic injury such as cholestasis as it is associated with bile acid homeostasis in spite of low affinity for bile acids (reviewed in (7)).
MRP4 has a wide range of substrates, including antiviral agents (azidothymidin, adefovir, and ganciclovir), anticancer agents (methotrexate, 6-mercaptopurin, and camptothecins) and cardiovascular agents (loop diuretics, thiazides, and angiotensin II receptor antagonists), as well as endogenous chemicals (steroid hormones, prostaglandins, bile acids, and the cyclic nucleotides cAMP and cGMP). MRP4 has higher affinity for sulfate conjugates of bile acids and steroids. MRP4 is similar to MRP3, as both are upregulated in cholestasis, suggesting a protective role in preventing hepatotoxicity. Indeed, MRP4-null mice developed cholestasis after bile duct ligation, implying that MRP4 is important in bile acid homeostasis (review by (7)).
OSTα/β proteins are present as heterodimers and/or heteromultimers in the cell membrane. OSTα/β -mediated transport is bidirectional (uptake or efflux) and ATP-independent, depending on the electrochemical gradient. Although it is expressed in high levels in the liver, OSTα/β is also expressed widely in small intestine, colon, kidney, testes, ovaries and adrenal gland, the latter of which is involved in steroid and bile acid homeostasis. The evidence from a study with Ost alpha null mice demonstrated OSTα/β as a target for interrupting the enterohepatic circulation of bile acids. OSTα/β substrates include steroid hormones and endogenous compounds such as estrone sulfate and dehydroepiandrosterone sulfate, bile acids, and PGE2, as well as the cardiac glycoside digoxin (reviewed by (16)).
The study of hepatic transporter function has relied on (1) in vitro/ex vivo hepatocytes in suspension or two dimensional (2D) plated monolayer cell formats for uptake and/or accumulation efflux assays using radioactive or fluorescent probe substrates and (2) in vivo pharmacokinetics studies on mutant animals with deficient in specific transporter genes or transporter gene knockout mice (reviewed by (17)) . There are several in vitro methods used in assessing human drug metabolism and active transport of drug, including using immortalized cell lines with transient or stable overexpression of transporters. In vitro transporter assays can help to determine whether the compound is taken up at the sinusoidal surface by hepatocytes and/or whether its metabolites can be eliminated at the canalicluar membrane for biliary excretion. Uptake and inhibition assays often involve OATP1B1, -2B1 and -1B3, and biliary efflux assays may include MDR1, MRP2 and BCRP, which can be used to predict compound disposition. The BSEP inhibition assay can be applied to screen whether the compound can lead to cholestasis or hyperbilirubinemia (reviewed by (18)).
Unfortunately, preclinical in vitro cell model systems sometimes poorly predict biotransformation and elimination in humans. First, extrapolation from in vitro findings to the in vivo situation remains complex with poor in vitro-to-in vivo (IVIV) correlation. In addition, expression levels of phase I/II enzymes and transporter in transformed cell lines such as HepG2 human hepatoma cells are very low and variable (reviewed by (19)). OCT1 and OATP1B1 mRNA were abundantly expressed in human liver tissue whereas these two transporters were expressed at low levels in HepG2 cells (20). Thus, transporter expression in HepG2 did not match the tissue expression pattern. Furthermore, due to overlapping substrate specificities and lack of selectivity with currently available inhibitors, it is challenging to fully understand the role of a given transporter in the disposition of specific drugs. Even though in vivo pharmacokinetic studies can provide integrated analysis of drug’s disposition, the preclinical results from transgenic or mutant animal models sometimes fail to predict the clinical outcomes. Expression profiles of transporters in laboratory animals such as rats and mice are different from humans. For example, rodent mdr1a and mdr1b genes are correlated to the MDR1 gene in humans. However, functional studies in MDR1, mdr1a, and mdr1b expressing cells demonstrated that substrates for rodent mdr1a and mdr1b are unlikely to be substrates for human MDR1, showing species- dependency in the spectrum of drug efflux activity (21).
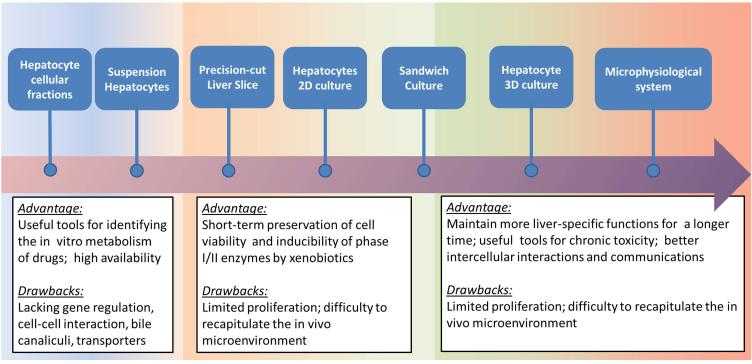
We review in vitro/ ex vivo human hepatic cell systems used in biotransformation and transporter studies from traditional assays to the most recently advanced three-dimension (3D) cultures, including microsomes, cell lines cultured in 2D, primary hepatocyte suspensions, liver slices, sandwich cultures, 3D culture systems, and MPS culture systems (Figure 2 /Table 1).
Figure 2.
Comparisons of the commonly used methodologies used to model hepatic metabolism and transport, including the pros and cons of each modality, with increasing complexity illustrated from left to right.
Table 1.
Microphysiological Technologies Used to Model the Hepatic Microenvironment
| Representative Image | Platform | Scaffold/Format | Co-culture or integrated with other organ tissues |
Validated functions of phase I/II and transporters |
Other liver-specific functions |
Methods used to assess hepatotoxicity |
|---|---|---|---|---|---|---|
|
HepatoPac Hepregen (32-33) |
Micropatterned 24- and 96-well plates |
Primary hepatocytes islands co-cultured with 3T3 fibroblasts |
Metabolite measurements:
CYP1A: Ethoxy-resorufin; CYP2A6: Coumarin; CYP2B6: Bupropion; CYP3A4: testosterone UGTs and SULTS: 7- hydroxycoumarin mRNA expression : phase I CYPs, FMO, MAO, OX, EH; phase II- UGTs, SULTs, NATs, GSTs, methyltransferases Transporters-MDR1, MRP3, OCT1, NTCP, BSEP canalicular flow by CMFDA fluorescent probe |
Albumin (ELISA) and urea (colorimetric endpoint assay) secretion |
MTT assay, ATP, and glutathione levels using Cell Titer-Glo and GSH-Glo luminescent kits (Promega) |
|
RegeneMed Liver Transwell RegenMed (35) |
24-well transwell plate | Primary human or rat hepatocytes with hepatic NPCs,including vascular and bile duct endothelial cells, Kupffer cells and hepatic stellate cells |
Phase I activity: CYP1A1, 2C9, and 3A4 by P450-Glo assays (Promega) Basolateral transporter uptake measured by isotope labeled tracer - 3H-labeled estrone-3-sulphate |
Albumin, urea, fibrinogen, transferrin production (ELISA); glycogen synthesis (isotope labeling) LPS induced IL-1β, IL- 6, IL-8, TNF-α, GM- CSF: multiplex-based ELISA |
Levels of ATP and GSH, LDH releasing |

|
3D InSight™ Microtissues InSphero (36) |
Spheroids in 96-well plate |
Primary human hepatocytes with NPCs (Kupffer and endothelial cells) |
CYPs activity/induction: CYP1A1, 2B6, 2C8,2C9,2C19,2D6 CYP3A4 and 2E1 (IHC staining) MDR1,BSEP (IHC staining) |
Albumin secretion (ELISA) IL-6 and TNF-α releasing (ELISA) |
ATP, and glutathione (GSH) levels using CellTiter-Glo and GSH-Glo luminescent kits (Promega) LDH releasing, mitochondrial activity |
|
3D print Liver Organovo |
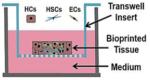
3D bioprinted liver tissues in a transwell plate |
Primary human hepatocytes with NPCs |
CYP450 enzymes 3A4 (mRNA expression levels and probe metabolite formation) |
Albumin and transferrin production Immunologic: IL-6, GM-CSF,MCP-1 LPS stimulated TNF-α, IL-1β, IL-12p70, IL-10, IL-2, IL-13, and IL-4 |
LDH releasing, GSH and ATP level Gene/protein expression profiling and histological tissue assessment |

|
HμREL hepatic cultures Hurel (38) |
mCCA biochip connected with pump mCCA biochip can integrate multiple organ tissues |
Primary human hepatocytes with NPCs |
mRNA expression and
metabolite formation: CYP450 enzymes 1A2, 3A4, 2C19, SULT, UGT Metabolic clearance: Biliary efflux assay (LC-MS/MS) BSEP canaliculi (CMFDA) |
MTT assay, ATP, and glutathione (GSH) levels using CellTiter- Glo and GSH-Glo luminescent kits (Promega), LIVE/DEAD stain (Thermo Fisher) |
|

|
LiverChip™ System CN Bio Innovations (39-41) |
Multiwell plate platform with pneumatic pump |
Primary hepatocytes co- cultures with NPCs and Kupffer cells |
Metabolite formation:
CYP450 enzymes 1A2, 2B6, 2C9, 2D6, 3A4 |
LIVE/DEAD stain (Thermo Fisher) |
|

|
CellAsic (42) | Primary human hepatocytes |
Metabolite formation: CYP450 enzymes 1A1/2, 2C9, 3A4, UGT BSEP canaliculi (CMFDA) |
LIVE/DEAD stain( fluorescent probe; Thermo Fisher) |
||

|
SQL-SAL University of Pittsburgh (43) |
Nortis chip (Nortis,
Inc.) with microfluidic flow |
Primary human hepatocytes
with cell lines derived from NPCs |
Metabolite formation: CYP450
enzymes 1A, 2C9, 3A4 IHC staining: MDR1,MRP2, BSEP, BRCP |
TNF-α releasing |
LDH leakage,
fluorescent protein ROS biosensors Stellate cell activation and migration |
|
IdMOC™ In Vitro ADMET Lab (72) |
24 or 96-well plate format integrated with multiple organ |
Primary human hepatocytes with 3T3 cells |
MTT assay, ATP, and glutathione (GSH) levels Using CellTiter-Glo and GSH-Glo luminescent kits (Promega) |
||

|
Microcompartment hollow fiber BAL (37) |
Hollow fiber | Primary human liver cells ( not pure hepatocytes) |
Urea production , albumin
synthesis , glucose secretion , and lactate production IHC: expressed cell marker of Kupffer cells) |
LDH and AST releasing |
1. Human liver subcellular fractions, microsome/supersomes, cytosol fractions, and S9 fractions
These subcellular fractions contain CYPs and UGT, or NATs, SULTS, and GSTs, are useful for xenobiotic biotransformation research. These assays are traditionally used for in vitro based prediction of metabolic clearance and drug-drug interactions. However, due to the loss of structural integrity of the cell, and the optimization of enzyme kinetic conditions by adding cofactors such as NAPDH and PAPS that are at concentrations not normally encountered, the results using these methods cannot be accurately used for transporter studies and quantitative estimations of in vivo human biotransformation.
2. Cell lines cultured in 2D
The HepG2 cell line is the most frequently used and best characterized immortalized human hepatoma cell line. However, compared to primary human hepatocytes, overall CYPs activity remains low (22). Expression profile of transporters in HepG2 cells is not highly correlated to human liver tissue so the HepG2 cell line is not a suitable model for transport assays. In general, 2D culture condition cannot provide the optimal microenvironment for cells to establish polarization; thus the use of 2D cell cultures has architectural limitations in transporter assays.
A new human liver cell line derived from a hepatocellular carcinoma – HepaRG recently drew substantial attention in the field of pharmaceutics and toxicology. Differentiated HepaRG cells expressed high levels of phase I/II enzymes, and transporters were comparable to freshly isolated human hepatocytes (22). HepaRG cells can maintain a proliferative state in undifferentiated culture medium for several weeks, and can differentiate into hepatocytes and biliary epithelial cells by adding differentiation culture medium after reaching confluence (22).
3. Primary human hepatocytes suspension and cultured in 2D
Primary human hepatocyte cultures are a preferred in vitro system for predicting in vivo drug biotransformation and clearance as they maintain critical metabolic features. After isolation by collagenase perfusion, primary human hepatocytes in suspension are viable for only a few hours but never-the-less can be used in rodent models for kinetic characterization of transporter function. However, this is generally not possible for human hepatocytes. Thus, studies with human hepatocytes rely on establishing primary cultures. Once plated in a monolayer culture, human primary hepatocytes maintain good viability for several days. However, they usually lose cell-specific functions such as albumin production and CYPs expression as both decline quickly over the first 24-48 hours of culture as the cells lose their differentiation status. Due to the scarcity of available human liver tissue and successful cryopreservation techniques, a good supply of human primary hepatocytes is now commercially available. In culture, previously cryopreserved hepatocytes can recover and maintain phase I/II enzyme activity after thawing for at least seven days (23). Individual donor variation in metabolic enzyme activity due to genetic polymorphisms and other factors can be compensated for by the mixing of hepatocytes from multiple donors to generate homogeneous enzyme activities. Hepatocytes represent the majority of the hepatic cellular mass (about 80%), while other non-parenchymal cells (NPCs), including vascular and biliary epithelial cells (i.e. cholangiocytes), Kupffer cells and hepatic stellate cells (HSC), provide key physiological functions. For example, cholangiocytes not only can contribute to bile secretion, but also can enable the absorption of ions, bile acids, amino acids, glucose, and other molecules, playing an important role in the modification of hepatic canalicular bile (reviewed by (24)). In vivo, stellate and Kupffer cells have been shown to play an important role in the hepatoxicity of some compounds, and thus the absence non-parenchymal cells in primary culture systems is a potentially serious limitation for toxicology studies.
4. Precision-cut liver slices
Precision-cut liver slices have several advantages for drug biotransformation and toxicology studies as they maintain the native liver structure with multiple cell types and zonation, and have good in vitro/in vivo correlations of drug biotransformation features. Cultured liver slices can retain phase II enzyme activity, albumin production, and gluconeogenesis for up to 20-96 hours and while regulating gene expression of the uptake transporters- NTCP and OATP and efflux transporters- BSEP, MDR1 and MRP2 (25).
In spite of the preservation of the overall hepatic architecture, drug biotransformation and intrinsic clearance rates are lower than isolated hepatocytes as necrosis can occur after 48-72 hours while CYPs activities are greatly reduce within 6-72 hours (26).
5. Sandwich culture
Sandwich cultures with primary human hepatocytes plated between two layers of extracellular matrix (collagen or Matrigel®, derived from Engelbreth-Holm- Swarm sarcoma) were developed to maintain liver-specific functions over longer culture periods. The use of extracellular matrix overlays allows for a favorable cellular attachment environment and is thought to be one of the main reasons why cells polarize in this type of culture. Hepatocyte sandwich cultures with various medium constituents have been shown to maintain albumin secretion, viability, and cuboidal-shape morphology with phase I/II and transporter expression similar to that of liver tissue (27). Biliary excretion can also be evaluated in sandwich cultures with both basal and inducible biliary enzyme activities that allow assessments of hepatobiliary disposition. Taken together, sandwich cultures can provide a robust means to evaluate hepatic compound uptake, metabolism, efflux and biliary excretion, while closely mimicking in vivo characteristics. Compared to other 2D models, sandwich cultures have significant advantages and can be considered as a bridge between 2D and 3D cultures (reviewed by (28)).
6. Transwell culture for drug efflux
Evaluation of the human hepatocyte uptake and efflux transporters MDR and MRP2 have been performed in trans-well systems with transfected cell lines, including porcine kidney epithelial cells (LLC-PK1) and Manine-Darby canine kidney epithelial cells (MDCK). Transwell culture cells are grown on a permeable membrane filter that allows for the physical separation of the apical and basolateral domains and has been used to study drug uptake and efflux transporter activity (29).
7. Co-culture systems, 3D culture systems and MPS culture systems
Traditional in vitro methods such as microsomes and suspension cultures usually have too short of a time window to perform assays, which can lead to imprecisions in the prediction of human biotransformation/clearance and toxicity. In order to improve the predictability of drug safety and efficacy in clinical development, and to have a clearer perspective of toxicity outcomes, while reducing the use of animals for toxicity studies, recent research efforts have been focusing on development of advanced in vitro models based on the applications of co-culture systems, 3D cultures, and microphysiological system (MPS) cultures that utilize microfluidic flow and often are referred as organ-on-chips or organoid culture (30).
The liver is a complex organ, consisting of hepatocytes, NPCs, and various ECM. NPCs play an important role in hepatic physiological functions as well as hepatotoxicity. Many studies have demonstrated that co-cultures of hepatocytes with NPCs can sustain liver-specific function, morphology and expression of liver-specific transcription factors via the activation of cell adhesion molecules and redistribution of cytoskeleton involved in cell-cell and cell-matrix interactions (reviewed in (31)). Several liver organoid cultures based on the application of co-culture have been reported, and some have been commercialized. These include advanced 3D culture systems based on cellular microenvironment dynamics between ECMs, micro-perfusion flow rates, and co-cultures of various cell types. The MPS model represents an interconnected set of cellular constructs designed to recapitulate the structure and function of human organs. Here, we review current advanced hepatic culture systems (Table 1):
HepatoPac® is a co-culture system of human hepatocytes with mouse fibroblasts (3T3-J2 fibroblast), commercially available through Hepregen (Medford, MA). This system consists of micro-patterned hepatocyte islands surrounded and stabilized by stromal cells in a 24-well plate format. This culture system can maintain liver-specific function for up to six weeks, including stable albumin secretion, urea synthesis, phase I/II drug biotransformation and formation of bile canaliculi with efflux transporters ((32), reviewed by (33)). Studies with the human HepatoPac® platform have demonstrated an IVIV correlation with hepatic uptake of faldaprevir by multiple transporters (including OATP1B1 and Na+-dependent transporters) and biotransformation by CYP3A4 (34).
RegeneMed 3-D Liver (San Diego, CA) is a liver tissue co-culture system used for screening hepatic ADME, using the transwell culture approach (35). NPCs are seeded in a nylon screen sandwich mesh insert with a 140 μm pore size and stabilized for a week, followed by incubation with hepatocytes to form a 3D liver tissue. Liver-specific functions, including production of albumin, fibrinogen, transferrin and urea, can be maintained up to three months, and the induction of CYP1A1, 2C9, and 3A4 activity for up to two months. Co-culture with Kupffer cells allows for study of the inflammatory response as the release of pro-inflammatory cytokines can be observed with lipopolysaccharide (LPS) exposures. Basolateral cell uptake drug transport activity using 3H-labeled estrone-3-sulphate (E3S) as the tracer has been demonstrated to occur in this system. Though images of bile canaliculi-like structures were presented, efflux transporter activity or bile excretion were not reported.
The 3D micro-tissue spheroid culture- 3D InSight™ provided by InSphero (Schieren, Switzerland) is a hanging drop co-culture system that uses gravity-forced cellular self-assembly of hepatocytes and NPCs into spheroids using a 96-well format (36). This format is well suited for high throughput applications and stable viability and liver-specific function such as persistent albumin secretion are preserved over five weeks. MDR1 and BSEP are expressed in this microtissue, evidence that these cultures exhibit cell polarization and bile canaliculi formation. Inflammation-mediated toxicity and chronic toxicity assays with acetaminophen and diclofenac have also been evaluated in this system. However, to date, there are no published data that demonstrate that this system can be applied to transporter assays.
Organovo (San Diego, CA) uses a 3D bioprinting technique to generate small-scale hepatic tissues using human primary cells in a platform called exVive3D™ Liver. This product can maintain stable viability and albumin secretion for up to four weeks and has rifampicin-inducible CYP3A4 activity, as midazolam biotransformation to 1-hydroxy-midazolam was increased by rifampicin pretreatment. As Kupffer cells are also present, this system can respond to immune stimulation (LPS) with the release of pro-inflammatory cytokines.
3D liver bioreactors designed by the Charité Universitätsmedizin Berlin are derived from bioartificial livers (BAL) used in the clinic. This hollow-fiber and perfusion based bioreactor provides a continuous mass exchange of culture media and controlled oxygenation with a scaffold for cells to maintain a physiologically relevant environment (37). This 3D bioreactor is co-cultured with hepatocytes and NPCs and can maintain albumin secretion and CYP activity (CYP1A, CYP2C9 and 3A4) for up to 2-3 weeks, as well as expression of canalicular transporters- MRP2, MDR1, and BCRP. Limitations of this system for use in pharmacokinetic and drug toxicity testing include a lack of zonation seen in liver tissues and low throughput, as only one condition can be evaluated per system.
HμREL® microdevice, a MPS platform provided by Hurel (Beverly Hills, CA), is an integrated and microfluidic system that assembles multiple units of microfluidic microscale cell culture analogs (μCCA) cultured from hepatic tissue or other organ tissues in parallel. The Hurel plastic biochips are connected to a fluid reservoir and pump system interconnected with a complex set of tubing that serves to recirculate the media. The hepatic co-culture system with NPCs forms a 2-D monolayer and maintains high viability for up to nine days, with higher expression of CYPs, SULT and UGT, and in vivo-like hepatic clearance of diclofenac, indomethacin and coumarin, compared to traditional static culture conditions (38). The formation of the bile canaliculi network was visualized by using carboxy-2′, 7′-dichlorofluorescein diacetate (CMFDA), a fluorescent substrate of BSEP. However, there was no evidence to demonstrate that this system can maintain other hepatic function such as albumin secretion, urea excretion, or transporter activity.
A multiple-well plate platform- LiverChip™ by CN Bio Innovations (Hertfordshire, UK) uses a flow system driven by a pneumatic pump at the bottom of the plate. This system was designed to recapitulate the hepatic microenvironment in terms of fluid flow, oxygen gradient and shear stress (145 μM to 50 μM at a flow of 0.25 mL/min). Compared to 2D static conditions, hepatocytes cultured in this system can maintain CYPs activity (1A2, 2B6, 2C9, 2D6, and 3A4), albumin secretion, expression of phase II-UGT enzymes and transporters (MDR2, MRP2, BCRP) for up to seven days (39). Hepatocytes co-cultured with liver sinusoidal endothelial cells (LSEC) enriched NPCs fractions can maintain high viability, CYPs activity, albumin secretion and urea excretion for up to 13 days (40). Kupffer cells incorporated in the model can release pro-inflammatory cytokines by LPS stimulation. The intrinsic clearance values of hydrocortisone and its metabolites generated in this system correlated well with human data, demonstrating that this system has great potential for high throughput use in hepatic metabolic research (41).
CellAsic (Hayward, CA) has a microfluidic liver sinusoid model with a microporous endothelial-like barrier that mimics liver sinusoids. This platform utilizes a 96-well plate format containing 32 small units that utilized s perfusion system with a flow of 10-20 nL/min and ~250 cells in each unit. Hepatocytes cultured in this system can maintain high viability for up to seven days and respond to drugs (42). Other liver-specific functions and the characterization of transporters need to be further analyzed.
Vernetti et al. developed a human, 3D, microfluidic, four-cell, sequentially layered, self-assembly liver model (SQL-SAL) based on a MPS device platform by Nortis Inc. (Woodinville, WA) (43). The current SQL-SAL uses a co-culture of primary human hepatocytes along with human endothelial (EA.hy926), immune (U937) and stellate (LX-2) cells in physiological relevant ratios that are viable and functional for at least 28 days under continuous flow. This model can maintain canaliculi structure, phase I/II activity, albumin production and urea excretion. In addition, by the integration of protein-based fluorescence biosensors, the system can be used in reporting drug –induced mechanistic toxicity (MOT) such as apoptosis and ROS for high throughput screening (reviewed by (33))
The above described 3D or liver-on-a-chip platforms generally provide suitable microenvironments for recapitulating most in vivo hepatic functions compared to traditional 2D hepatocyte cultures. However, these state-of-the-art in vitro/ex vivo technologies still have limitations in accurately predicting human hepatotoxicity and first pass drug clearance. Further development of advanced 3D hepatic culture systems needs to consider the complexity of the liver, including the co-culture ratio between hepatocytes and NPCs, the source of hepatocytes, and zonation effects. Because the liver has a wide range of diverse functions, hepatic cells show large heterogeneity and plasticity of functions. Oxygen gradients, hormones and ECM all can regulate zonal variations, which are reflected in drug biotransformation capabilities. It would be interesting if these zonation characteristics could be established and sustained on the 3D liver-on-chip systems (44).
Although these 3D models have great potential in pharmacokinetic prediction of first pass drug clearance, additional “proof of concept” studies are needed to validate IVIV correlations using selected clinical drugs. In addition, most current models are individual liver organ systems, lacking the gastrointestinal and/or renal transport/metabolism modules, which are needed to understand the whole profile of drug ADME in vivo.
Renal Nephron Microenvironment – Proximal Tubule
The status of renal in vitro microphysiological systems to study transporter function is not as well developed as the liver. As with the liver section, we review renal transporters in the proximal tubule and in vitro renal systems that have been used to study their function. Knowledge of the function of renal transporters in the proximal tubule is important for understanding the crucial role this organ plays in drug disposition. Demonstration that these transporters are functional in an in vitro renal system is critical for method validation and proper data interpretation.
The nephron is the functional unit of the kidney responsible for maintaining both the homeostasis of electrolytes as well as fluid volume (45). However, from a drug-development perspective the nephron is the primary site of renal handling involving any combination of filtration and phase III mediated-secretion/reabsorption. Structurally, the nephron is a series of segmented tubules that individually play a critical role in its overall function. The proximal tubule, found in juxtaposition to the glomerulus, has been shown to be the primary site of transport-mediated reabsorption/secretion xenobiotics. Active vectorial transport of xenobiotics are achieved given the polarized configuration of the proximal tubule epithelial cells involving both transporters found on the brush-border containing apical side (facing urine) and the basolateral side (facing systemic circulation) (Figure 1).
Basolateral Transporters
The solute carrier (SLC) transporters are the major family of multi-specific transporters that mediate the proximal tubule uptake of both xenobiotic and endogenous substrates that are circulating within the blood. SLC transporters include the Organic Anion Transporters (OAT1/2/3), Organic Cation Transporters (OCT2/3), and the Organic Anion Transporter Polypeptide (OATP4C1).
Apical Efflux Transporters
The SLC transporters are also found on the apical side of the proximal tubule and are responsible for the removal of exogenous and endogenous substrates that have accumulated within the cell via diffusion, reabsorption, and/or uptake from the circulatory side. SLC transporters found on the apical side of the proximal tubule include the Organic Anion Transporter (OAT4), Multidrug and Toxin Extrusion Protein (MATE1/2-K), Urate Anion Exchanger (URAT1), and Organic Cation/Carnitine Transporter (OCTN1/2). Additionally, another superfamily of multispecific transporters found on the apical side includes the ATP-binding cassette (ABC) transporters, which use ATP hydrolysis to drive molecules across cell membranes. The ABC transporters on the apical side include Multidrug Resistance Protein (MDR1 or Pgp), Breast Cancer Resistance Protein (BCRP), and Multidrug Resistance Associated Protein (MRP2/4).
Proximal Tubule Transport Model – Historical perspective and current status
In recent studies, there has been a vast improvement of the modeling capabilities of xenobiotic transport using models and cell types that accurately recapitulate human physiology. Traditionally, researchers have used established immortalized cell lines from animals, including canine (MDCK), opossum (OK), and porcine cells (LLC-PK1), as a cell source to optimize models (46-48). Transwell studies, using cells grown in monolayers on a permeable scaffold, are utilized to investigate the facilitated transport from one compartment to the other resembling the apical-basolateral relationship seen in vivo (49).
While established immortalized cell lines have remained as the gold standard for in vitro transport studies, there are drawbacks associated with their use, particularly the poor expression and/or absence of human-specific transporters (50). To combat this problem, investigators have used molecular techniques to transfect the established animal cell lines with specific human transporters (51). Additionally, investigators have immortalized primary renal cortex cells from human kidney (HK-2) and have shown that not only do they retain proximal tubule cell phenotypes; they also possess the functional aspect of transport and sensitivity to toxin (52). However, as demands increase to resemble the in vivo phenotype to its highest extent, there are shortcomings with the immortalized human cell lines that research groups have addressed (53). As models continue to become more advanced, there has been a widespread shift from immortalized cells to primary renal cells to more accurately represent the native in vivo microenvironment (54). Brown et al. has shown, using primary human proximal tubule cells grown on permeable filter supports, differentiated cells expressing a wide array of transporters as well as functional transport activity of OAT1/3, MRP2/4, OCT2, MDR1, and MRP2 (55).
Engineered transport modeling of the proximal tubule
The utility of bioengineered models to replicate function of the kidney proximal tubule is summarized in Table 2. These have ranged from a macro scale designed for clinical use in patients with kidney failure to microphysiological scales designed to study biochemical and toxicological processes.
Table 2.
Microphysiological Technologies Used to Model the Renal Microenvironment
| Representative Image | Platform | Scaffold/Format | Co-culture or integrated with other organ tissues |
Validated functions of phase I/II and transporters |
Other kidney-specific functions |
Methods used to assess nephrotoxicity |
|---|---|---|---|---|---|---|

|
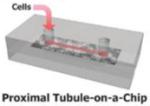
Bioartificial Renal Tubule Assist Device (RAD) (58, 59) |
Permeable synthetic hollow- fiber membrane |
MDCK, Primary Porcine, Primary human proximal tubule cells |
Phase I
25-(OH)D3-12-hydroxylase: 25-OH-D3 Transporter function OAT1/3: Para-amminohippurate |
Fluid reabsorption, Bicarbonate transport, Glucose transport, Glutathione transport and metabolism, Ammoniagenesis |
Cytokine Release |
|
Human kidney proximal tubule-on-a-chip (61) |
Porous membrane integrated within a PDMS multilayer system |
Primary human proximal tubule cells |
Transporter function
MDR1: Calcein-AM |
FITC-Albumin uptake, Alkaline phosphatase activity, Glucose transport, |
LDH release (Cisplatin Toxicity), Apoptosis via TUNEL Assay |

|
Human proximal tubule epithelial cells cultured on hollow fiber membranes (HFM) (65) |
Coated HFM | Conditionally immortalized proximal tubule epithelial cells (ciPTEC) |
Transporter function OCT2: 4-(4- (dimethylamino)styryl)-N- methylpyridinium iodide (ASP+) |
||
|
Human kidney-derived cells (hKDC) cultured on small intestinal submucosa (SIS) (66) |
SIS derived from porcine small intestine to serve as a natural scaffold |
hKDC, HK-2 cells | N/A | FITC-BSA uptake | |

|
Kidney derived micro- scaffolds (KMS) (67) |
Decellularized kidney fragments with intact ECM |
HK-2 cells | N/A | qPCR data of kidney associated genes, cytokine release |
|

|
Nortis microphysiological device (68, 69) |
Collagen type I matrix coated with collagen type IV ECM protein |
Primary human proximal tubule cells |
N/A | Cell-associated staining of Kidney Injury Molecule-1 (KIM-1) and Heme Oxygenase-1 (HO-1) upon CdCl2 exposure |
|

|
Perfused kidney-on-a-chip (70) |
Porous polyester membrane cut out from Transwell™ plates |
MDCK cells | N/A | Live/dead stain, KIM- 1 concentration, and permeability assessment in response to Gentamicin exposure |
1. Bioartificial renal tubule assist device (RAD)
Documented as one of the earliest systems to effectively model the microenvironment of the kidney, researchers developed the bioartificial renal tubule assist device (RAD) with the perspective of improving renal replacement therapies and outcomes of patients suffering from end-stage renal disease(56). The RAD is described as a perfusion bioreactor system in which cells are grown in a monolayer on a permeable synthetic hollow-fiber membrane (270 μm inner diameter, 35 μm wall thickness, 2.5 cm length). Earlier developments used MDCK cells as cell source for system validations in which functional confluence and fluid transport was demonstrated using radiolabeled inulin. Mackay et al. observed >98.9% recovery of perfused radiolabeled inulin in monolayer systems as well as a significant increase in fluid reabsorption in response to albumin-induced oncotic pressure when compared to both a baseline and ouabain-inhibited state (57). Further improvements of the RAD demonstrated its ability to reabsorb fluid, transport various solutes, glutathione transport and metabolism, ammoniagenesis, and vitamin D activation, using two different RADs varying in length, wall thickness, and diameter (smaller unit: 200 μm inner diameter, 40 μm wall thickness, 17 cm length – larger unit: 250 μm inner diameter, 70 μm wall thickness, 12 cm length). Using primary porcine cells from harvested proximal tubule segments, Humes et al. first used radiolabeled inulin leakage (< 5 - 10%) as a selection criterion for confluent monolayers to be used in other functional experiments. Like the previous study, fluid reabsorption was demonstrated using the smaller RAD unit with albumin-induced oncotic pressure in comparison to fluid reabsorption at baseline and ouabain-inhibited state. To show the transport capability of cells cultured within the RAD, a series of transport studies were demonstrated using bicarbonate, glucose, and para-aminohippurate (PAH). Bicarbonate transport was assessed using the larger RAD unit and significant inhibition was observed in the presence of specific carbonic anhydrase inhibitor, acetazolamide. Glucose reabsorption was assessed using the larger RAD unit and significant inhibition of reabsorption was achieved in a dose-dependent manner using the SGLT inhibitor, phlorizin. PAH secretion was assessed using the larger RAD unit and significant inhibition of secretion was achieved using the inhibitor, probenecid. To assess glutathione transport and metabolism, the smaller RAD unit was used and significant inhibition of glutathione removal was achieved using the specific inhibitor of γ-glutamyl transpeptidase, acivicin. Lastly, the functional aspect of the RAD was demonstrated by both the production of ammonia in response to a decrease in perfusion pH as well as the metabolic capacity to convert 25-OH vitamin D3 to its active form, 1,25-(OH)2D3 (58). Future experiments illustrated the functional consistency of the RAD, via the integration of human proximal tubule cells, in combination with a hemofiltration cartridge that resembles an efficient metabolic replacement of kidney function in both animals as well as a patient population suffering from acute renal failure (59, 60). The RAD system has not only served as a translational therapy targeted against renal failure but a foundational model when approaching recapitulation of the functional aspect of the proximal tubule microenvironment.
2. Human kidney proximal tubule-on-a-chip
Successful mimicry of the renal microenvironment is a complex undertaking given the three-dimensional architecture of the proximal tubule and the fluidic environment it is exposed to. A number of research groups have developed multi-layer systems using polydimethyl siloxane (PDMS) scaffolds in combination with a porous membrane to which cells can culture three-dimensionally. Jang et al. developed their multi-layer microfluidic device (MMD) using two compartments (one static chamber, one flow chamber) separated by a porous membrane to which rat renal tubule cells cultured and polarized on. The MMD provided a fluidic microenvironment (fluid shear-stress of 1 dyne/cm2) similar to what has been observed in the nephron which has been proposed to being a key component in tubule cell cytoskeletal reorganization and remodeling of junctional complexes (61).
Leading the organ-on-chips focus at the Wyss Institute, the Ingber laboratory has improved upon the MMD by microfabricating a PDMS microfluidic device containing both an interstitial fluidic compartment and luminal flow channel (fluid shear-stress 0.2 dyne/cm2) separated by a porous membrane coated with extracellular matrix protein, collagen type IV. Using primary human kidney proximal tubule epithelial cells cultured within the device, the fluidic proximal tubule microenvironment was demonstrated by the presence of tight junction protein, ZO-1, as well as basolateral distribution of Na/K-ATPase and cytoplasmic expression of aquaporin 1. Furthermore, acetylated tubulin staining was used to visualize primary cilia, a fluid shear stress mechanosensor important for regulation of tubular morphology. Functionally, the microfluidic device was used to evaluate albumin uptake, cellular alkaline phosphatase (ALP) activity, and transepithelial glucose transport. Jang et al. observed, in response to static cultures, a significant uptake of FITC-albumin, a significant level of ALP activity, and significant levels of glucose transported by fluidic cultures of proximal tubule epithelial cells. Additionally, the microfluidic device was used as a model of nephrotoxicity using the prototypical nephrotoxin, cisplatin (100 μM), in the absence and presence of cimetidine, which has been previously shown to suppress cisplatin-induced injury. Using lactate dehydrogenase (LDH) release and apoptosis, via Annexin V staining and a TUNEL assay, significant cisplatin-induced cellular injury was observed in static cultures compared to fluidic cultures as well as a significant decrease in injury when cimetidine was co-administered with cisplatin. Finally, to assess the transport capabilities of the microfluidic device, cellular accumulation of calcein-AM was evaluated in the presence and absence of p-glycoprotein ATP-binding cassette membrane transporter (Pgp) inhibitor, verapamil (100 μM). Static cultures showed higher accumulation of calcein-AM (presence/absence of verapamil) in comparison to fluidic cultures lending evidence to the concept of the fluidic microenvironment enhancing transporter expression and improving the phenotype of the proximal tubule cells (62, 63).
Using unique conditionally immortalizing proximal tubule epithelial cells (ciPTEC) previously characterized by their lab group, Jansen et al. functionally tested ciPTECs grown on hollow fiber membranes (HFM) using microfluidics (64). Upon maturation of ciPTECs within the HFM, collagen IV extracellular matrix (ECM) was visualized using immunocytochemistry lending evidence to renal lineage. Additionally, using FITC-inulin, transepithelial barrier function was significantly greater (and caused less FITC-inulin leakage) for cell containing HFM in comparison to empty HFM. Morphologically, the mature cultured ciPTECs displayed well-developed organelles as well as cell-surface microvilli, a typical marker of the proximal tubule epithelial cell. To assess the polarity of the ciPTECs cultured in the HFM, confocal microscopy was implored using immunocytochemistry to observe the high expression of tight junction protein, ZO-1, and correct localization of basolateral transport protein, OCT2. Furthermore, OCT2 transport activity was measured using real-time fluorescent 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide (ASP+) uptake in the absence and presence of OCT2 inhibitors, cimetidine (100 μM) and uremic toxin mix (UTmix), showing significant inhibition of OCT2-mediated uptake (65).
Additional models of the human renal microenvironment
There are a number of groups currently developing outstanding models of the renal proximal tubule model that faithfully recapitulate the microenvironment - but have yet to assess the transport capabilities. Hoppensack et al. used highly proliferative human kidney-derived cells (hKDCs) cultured in monolayers on small intestinal submucosa (SIS) to show the correct phenotype of the proximal tubule using immunohistochemical staining as well as detecting basement membrane proteins and microvilli (66). Finesilver et al. prepared kidney micro-scaffolds (KMS) and cultured HK-2 cells within the microstructures in comparison to traditionally cultured HK-2 cells. Cells grown within the KMS for 25 days showed a significant (greater than 2-fold) increase in expression of genes AQP-1, ATP1B1, LRP-2, CCL-2, SLC23A1, and SLC5A2 (except for 21-24 days). Additionally, the research group showed less cytokine release in response to stimulation for HK-2 cells grown in the KMS compared to cultures grown traditionally (67). Kelly et al. is developing a microfluidic system that uses primary renal epithelial cells cultured three-dimensionally on a collagen scaffold coated with ECM proteins. In response to cadmium chloride exposure, the microphysiological system showed sensitivity and expressed biomarkers (Heme oxygenase-1 / Kidney injury molecule-1) of injury showing the potential of the model to serve as a suitable predictor of toxicity ex vivo (68, 69). In recent light, additional groups are interested in the toxicity modeling aspect as evidenced by Kim et al. exposing fabricated microfluidic devices with MDCK cells cultured on porous membranes to gentamicin over time. In response to gentamicin exposure, the researchers observed a significant difference of injury between culture types (static/shear) of HK-2 cells as well as dosing regimens (D1 – bolus mimicking regimen, D2 – continuous infusion regimen) (70).
Future Directions
While great strides have been made in developing isolated hepatic and renal microphysiological systems the integration of the two organ systems to model complex metabolic processes is the current focus of several groups. Our group has developed an integrated liver-kidney MPS system for identifying potentially nephrotoxic liver-metabolized chemicals, by connecting a liver-on-a-chip with primary rat or human hepatocytes, to a kidney-on-a-chip device with human proximal tubule epithelial cells (PTECs) in MPS devices developed by Nortis, Inc (Woodinville, WA) (69). To test the hypothesis that first pass hepatic clearance of a nephrotoxic chemical might have significant importance in determining ultimate kidney toxicity, we utilized aristolochic acid I (AA-I), a well-known nephrotoxin and carcinogen, that undergoes extensive hepatic metabolism to form toxic metabolites. Our results provide mechanistic insights into the important role of hepatic biotransformation for the kidney-specific toxicity of AA-I toxicity. This integrated in vitro/ex vivo MPS model provides a novel approach for investigating the mechanisms that underlay pharmacokinetically and toxicologically important organ-organ interactions.
Other integrated MPS models include the HμREL® microdevice platform that assembles multiple cultures units of hepatic tissue with other organ tissues in parallel. Used in combination with a mathematical modeling approach (PK–PD modeling), this novel platform provides improved predictability for drug biotransformation, clearance, and toxicity (71). Integrated Discrete Multiple Organ Co-culture (IdMOC™) by In Vitro ADMET Laboratories (Columbia, MD) uses the “wells-in-a-well” concept, interacting multiple cell types via the overlying medium. This model can mimic multiple organs in a human body interacting via the systemic circulation (72). The ultimate goal of accurate assessment of human drug toxicity will rely on the development of in vitro platforms with multiple organs, including the immune system, with each organ represented by multiple cell types and communication among organs achieved using human plasma or equivalent. It would be interesting to see more results of selected clinical drugs treated in these integrated models for validating IVIV correlation.
There are still a number of technical challenges in multiple-organs-on-chips development that have been important research areas for this field (reviewed by (73)):
Microfluidic volume problems: The physiological relevant scaling of fluid volumes and flow rates that are associated with individual organ microphysiological systems are difficult to acquire, as delicate and reliable engineering systems are required for the connection of reservoirs, pumps, and tubing to deliver accurate flow rates to the cultured cells. Also, the determination of the physiologically relevant flow rate for hepatic cells cultured in microphysiological systems is difficult to determine given that the liver is a complex organ architecturally with varying fluid channel areas and perfusion rates that can expose hepatic cells to a wide range of shear forces. One approach to this problem would be to incorporate vascular systems within hepatic or renal chips to allow for cell controlled flow rates between the vascular system and organ-specific cells.
Universal cell culture media: Individual cell types, especially primary cells, require customized media for optimal cell culture performance. A universal culture media that can sustain multiple cell types from different organs will need to be developed to successfully co-culture cells from multiple organs with an optimal balance of nutrients, osmolality, pH, and supplements.
To address these challenges, the Defense Advanced Research Projects Administration (DARPA), National Center for Advancing Translational Sciences of the National Institutes of Health (NIH-NCATS), FDA, and the Environmental Protection Agency (EPA) have funded several groups for MPS development and organotypic culture models for predictive toxicity and pharmacokinetic study (74, 75). The European Commission is funding a Body on a Chip project to many collaborated groups between multiple European academic and industrial partners, to achieve the goal of developing a comprehensive in vitro model that allows identification of multi-organ toxicity and pharmacogenetics.
Supplementary Material
Acknowledgements
This work was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health under award UH3 TR000504, NIEHS grant 5P30ES070033 and in part under Assistance Agreement No. 83573801 awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
References
- 1.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 2.Anzenbacher P, Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cellular and molecular life sciences : CMLS. 2001;58(5-6):737–47. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faber KN, Muller M, Jansen PL. Drug transport proteins in the liver. Advanced drug delivery reviews. 2003;55(1):107–24. doi: 10.1016/s0169-409x(02)00173-4. [DOI] [PubMed] [Google Scholar]
- 4.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. British journal of pharmacology. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120(2):525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- 6.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacological reviews. 2011;63(1):157–81. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 7.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological reviews. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268(5212):866–9. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 9.Visser WE, Wong WS, van Mullem AA, Friesema EC, Geyer J, Visser TJ. Study of the transport of thyroid hormone by transporters of the SLC10 family. Molecular and cellular endocrinology. 2010;315(1-2):138–45. doi: 10.1016/j.mce.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793–806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Fujino H, Saito T, Ogawa S, Kojima J. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. The Journal of pharmacy and pharmacology. 2005;57(10):1305–11. doi: 10.1211/jpp.57.10.0009. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2014;3 doi: 10.7554/eLife.00049. [DOI] [PubMed] [Google Scholar]
- 13.Ni Z, Bikadi Z, Rosenberg MF, Mao Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2) Current drug metabolism. 2010;11(7):603–17. doi: 10.2174/138920010792927325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falguieres T, Ait-Slimane T, Housset C, Maurice M. ABCB4: Insights from pathobiology into therapy. Clinics and research in hepatology and gastroenterology. 2014;38(5):557–63. doi: 10.1016/j.clinre.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Kubitz R, Droge C, Kluge S, Stross C, Walter N, Keitel V, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clinical reviews in allergy & immunology. 2015;48(2-3):273–84. doi: 10.1007/s12016-014-8457-4. [DOI] [PubMed] [Google Scholar]
- 16.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Frontiers in bioscience. 2009;14:2829–44. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Bachmeier C, Miller DW. In vitro and in vivo models for assessing drug efflux transporter activity. Advanced drug delivery reviews. 2003;55(1):31–51. doi: 10.1016/s0169-409x(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 18.Sahi J. Use of in vitro transporter assays to understand hepatic and renal disposition of new drug candidates. Expert opinion on drug metabolism & toxicology. 2005;1(3):409–27. doi: 10.1517/17425255.1.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Brandon EF, Raap CD, Meijerman I, Beijnen JH, Schellens JH. An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicology and applied pharmacology. 2003;189(3):233–46. doi: 10.1016/s0041-008x(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 20.Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug metabolism and disposition: the biological fate of chemicals. 2007;35(8):1333–40. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki M, Neway WE, Ohe T, Chen I, Rowe JF, Hochman JH, et al. In vitro substrate identification studies for p-glycoprotein-mediated transport: species difference and predictability of in vivo results. The Journal of pharmacology and experimental therapeutics. 2001;296(3):723–35. [PubMed] [Google Scholar]
- 22.Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(6):988–94. doi: 10.1124/dmd.109.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richert L, Liguori MJ, Abadie C, Heyd B, Mantion G, Halkic N, et al. Gene expression in human hepatocytes in suspension after isolation is similar to the liver of origin, is not affected by hepatocyte cold storage and cryopreservation, but is strongly changed after hepatocyte plating. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(5):870–9. doi: 10.1124/dmd.105.007708. [DOI] [PubMed] [Google Scholar]
- 24.Tabibian JH, Masyuk AI, Masyuk TV, O'Hara SP, LaRusso NF. Physiology of cholangiocytes. Comprehensive Physiology. 2013;3(1):541–65. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elferink MG, Olinga P, van Leeuwen EM, Bauerschmidt S, Polman J, Schoonen WG, et al. Gene expression analysis of precision-cut human liver slices indicates stable expression of ADME-Tox related genes. Toxicology and applied pharmacology. 2011;253(1):57–69. doi: 10.1016/j.taap.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Martin H, Sarsat JP, Lerche-Langrand C, Housset C, Balladur P, Toutain H, et al. Morphological and biochemical integrity of human liver slices in long-term culture: effects of oxygen tension. Cell biology and toxicology. 2002;18(2):73–85. doi: 10.1023/a:1015379815897. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer O, Ohtsuki S, Kawakami H, Inoue T, Liehner S, Saito A, et al. Absolute quantification and differential expression of drug transporters, cytochrome P450 enzymes, and UDP-glucuronosyltransferases in cultured primary human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2012;40(1):93–103. doi: 10.1124/dmd.111.042275. [DOI] [PubMed] [Google Scholar]
- 28.Swift B, Pfeifer ND, Brouwer KL. Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug metabolism reviews. 2010;42(3):446–71. doi: 10.3109/03602530903491881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppler D. Uptake and efflux transporters for conjugates in human hepatocytes. Methods in enzymology. 2005;400:531–42. doi: 10.1016/S0076-6879(05)00029-7. [DOI] [PubMed] [Google Scholar]
- 30.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nature reviews Drug discovery. 2015;14(4):248–60. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Critical reviews in toxicology. 2012;42(6):501–48. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khetani SR, Kanchagar C, Ukairo O, Krzyzewski S, Moore A, Shi J, et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicological sciences : an official journal of the Society of Toxicology. 2013;132(1):107–17. doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 33.Bale SS, Vernetti L, Senutovitch N, Jindal R, Hegde M, Gough A, et al. In vitro platforms for evaluating liver toxicity. Experimental biology and medicine. 2014;239(9):1180–91. doi: 10.1177/1535370214531872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsden D, Tweedie DJ, Chan TS, Taub ME, Li Y. Bridging in vitro and in vivo metabolism and transport of faldaprevir in human using a novel cocultured human hepatocyte system, HepatoPac. Drug metabolism and disposition: the biological fate of chemicals. 2014;42(3):394–406. doi: 10.1124/dmd.113.055897. [DOI] [PubMed] [Google Scholar]
- 35.Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicology and applied pharmacology. 2013;268(1):1–16. doi: 10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Messner S, Agarkova I, Moritz W, Kelm JM. Multi-cell type human liver microtissues for hepatotoxicity testing. Archives of toxicology. 2013;87(1):209–13. doi: 10.1007/s00204-012-0968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeilinger K, Schreiter T, Darnell M, Soderdahl T, Lubberstedt M, Dillner B, et al. Scaling down of a clinical three-dimensional perfusion multicompartment hollow fiber liver bioreactor developed for extracorporeal liver support to an analytical scale device useful for hepatic pharmacological in vitro studies. Tissue engineering Part C, Methods. 2011;17(5):549–56. doi: 10.1089/ten.TEC.2010.0580. [DOI] [PubMed] [Google Scholar]
- 38.Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochemical pharmacology. 2010;79(7):1036–44. doi: 10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivares A, Salle-Lefort S, Arabeyre-Fabre C, Ngo R, Penarier G, Bremond M, et al. Morphological behaviour and metabolic capacity of cryopreserved human primary hepatocytes cultivated in a perfused multiwell device. Xenobiotica; the fate of foreign compounds in biological systems. 2015;45(1):29–44. doi: 10.3109/00498254.2014.944612. [DOI] [PubMed] [Google Scholar]
- 40.Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab on a chip. 2010;10(1):51–8. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar U, Rivera-Burgos D, Large EM, Hughes DJ, Ravindra KC, Dyer RL, et al. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug metabolism and disposition: the biological fate of chemicals. 2015;43(7):1091–9. doi: 10.1124/dmd.115.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnology and bioengineering. 2007;97(5):1340–6. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 43.Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Ying Shun T, Gough A, et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Experimental biology and medicine. 2016;241(1):101–14. doi: 10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnology and bioengineering. 2003;82(3):253–62. doi: 10.1002/bit.10569. [DOI] [PubMed] [Google Scholar]
- 45.Zhuo JL, Li XC. Proximal nephron. Compr Physiol. 2013;3(3):1079–123. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang M, Ramsey CR, Knox FG. The paracellular permeability of opossum kidney cells, a proximal tubule cell line. Kidney Int. 1999;56(6):2304–8. doi: 10.1046/j.1523-1755.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 47.Malstrom K, Stange G, Murer H. Identification of proximal tubular transport functions in the established kidney cell line, OK. Biochim Biophys Acta. 1987;902(2):269–77. doi: 10.1016/0005-2736(87)90305-1. [DOI] [PubMed] [Google Scholar]
- 48.Gstraunthaler G, Pfaller W, Kotanko P. Biochemical characterization of renal epithelial cell cultures (LLC-PK1 and MDCK) Am J Physiol. 1985;248(4):F536–44. doi: 10.1152/ajprenal.1985.248.4.F536. Pt 2. [DOI] [PubMed] [Google Scholar]
- 49.Fouda AK, Fauth C, Roch-Ramel F. Transport of organic cations by kidney epithelial cell line LLC-PK1. J Pharmacol Exp Ther. 1990;252(1):286–92. [PubMed] [Google Scholar]
- 50.Kuteykin-Teplyakov K, Luna-Tortos C, Ambroziak K, Loscher W. Differences in the expression of endogenous efflux transporters in MDR1-transfected versus wildtype cell lines affect P-glycoprotein mediated drug transport. Br J Pharmacol. 2010;160(6):1453–63. doi: 10.1111/j.1476-5381.2010.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gartzke D, Fricker G. Establishment of optimized MDCK cell lines for reliable efflux transport studies. J Pharm Sci. 2014;103(4):1298–304. doi: 10.1002/jps.23901. [DOI] [PubMed] [Google Scholar]
- 52.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney International. 1994;45(1):48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 53.Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012;464(6):601–11. doi: 10.1007/s00424-012-1163-2. [DOI] [PubMed] [Google Scholar]
- 54.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney International. 1984;25(2):383–90. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 55.Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D'Haese PC, et al. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicology and applied pharmacology. 2008;233(3):428–38. doi: 10.1016/j.taap.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Humes HD. The bioartificial renal tubule: prospects to improve supportive care in acute renal failure. Ren Fail. 1996;18(3):405–8. doi: 10.3109/08860229609052810. [DOI] [PubMed] [Google Scholar]
- 57.MacKay SM, Funke AJ, Buffington DA, Humes HD. Tissue engineering of a bioartificial renal tubule. ASAIO J. 1998;44(3):179–83. doi: 10.1097/00002480-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Humes HD, MacKay SM, Funke AJ, Buffington DA. Tissue engineering of a bioartificial renal tubule assist device: in vitro transport and metabolic characteristics. Kidney Int. 1999;55(6):2502–14. doi: 10.1046/j.1523-1755.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 59.Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP, Luderer JR, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004;66(4):1578–88. doi: 10.1111/j.1523-1755.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 60.Saito A, Sawada K, Fujimura S, Suzuki H, Hirukawa T, Tatsumi R, et al. Evaluation of bioartificial renal tubule device prepared with lifespan-extended human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2012;27(8):3091–9. doi: 10.1093/ndt/gfr755. [DOI] [PubMed] [Google Scholar]
- 61.Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10(1):36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 62.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12(12):2156–64. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 63.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integrative biology : quantitative biosciences from nano to macro. 2013;5(9):1119–29. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 64.Jansen J, Schophuizen CM, Wilmer MJ, Lahham SH, Mutsaers HA, Wetzels JF, et al. A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp Cell Res. 2014;323(1):87–99. doi: 10.1016/j.yexcr.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Jansen J, De Napoli IE, Fedecostante M, Schophuizen CM, Chevtchik NV, Wilmer MJ, et al. Human proximal tubule epithelial cells cultured on hollow fibers: living membranes that actively transport organic cations. Sci Rep. 2015;5:16702. doi: 10.1038/srep16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoppensack A, Kazanecki CC, Colter D, Gosiewska A, Schanz J, Walles H, et al. A human in vitro model that mimics the renal proximal tubule. Tissue Eng Part C Methods. 2014;20(7):599–609. doi: 10.1089/ten.tec.2013.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finesilver G, Bailly J, Kahana M, Mitrani E. Kidney derived micro-scaffolds enable HK-2 cells to develop more in-vivo like properties. Exp Cell Res. 2014;322(1):71–80. doi: 10.1016/j.yexcr.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, et al. A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J Am Soc Nephrol. 2016;27(4):1015–28. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly EJ, Wang Z, Voellinger JL, Yeung CK, Shen DD, Thummel KE, et al. Innovations in preclinical biology: ex vivo engineering of a human kidney tissue microperfusion system. Stem cell research & therapy. 2013;4(Suppl 1):S17. doi: 10.1186/scrt378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S, LesherPerez SC, Kim BC, Yamanishi C, Labuz JM, Leung B, et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8(1):015021. doi: 10.1088/1758-5090/8/1/015021. [DOI] [PubMed] [Google Scholar]
- 71.Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab on a chip. 2010;10(4):446–55. doi: 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- 72.Li AP. Evaluation of Adverse Drug Properties with Cryopreserved Human Hepatocytes and the Integrated Discrete Multiple Organ Co-culture (IdMOC(TM)) System. Toxicological research. 2015;31(2):137–49. doi: 10.5487/TR.2015.31.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wikswo JP. The relevance and potential roles of microphysiological systems in biology and medicine. Experimental biology and medicine. 2014;239(9):1061–72. doi: 10.1177/1535370214542068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersen ME, Betts K, Dragan Y, Fitzpatrick S, Goodman JL, Hartung T, et al. Developing microphysiological systems for use as regulatory tools--challenges and opportunities. Altex. 2014;31(3):364–7. doi: 10.14573/altex.1405151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutherland ML, Fabre KM, Tagle DA. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem cell research & therapy. 2013;4(Suppl 1):I1. doi: 10.1186/scrt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.