Abstract
The purpose of this study was to determine the effects of arthroscopic irrigation on cartilage superficial zone lubricin and surface friction. Arthroscopic partial meniscectomy is one of the most commonly performed orthopaedic surgeries in the United States, but rates of osteoarthritis progression following this procedure are high. The effect of arthroscopic irrigation on articular surface lubrication has not been previously considered as a contributing factor in outcomes after arthroscopy. Fourteen bovine stifle joints were randomized to receive arthroscopic irrigation (n=7) or no treatment (n=7). Full-thickness osteochondral explants from these joints underwent friction testing to measure static and dynamic coefficients of friction. Following mechanical testing, samples were fixed and stained for lubricin. Percent integrated density, a measure of the amount of lubricin in the superficial zone (0–100um depth), was determined. Static and dynamic coefficients of friction were found to be significantly greater in arthroscopy specimens compared to controls (p=0.02 and p<0.001, respectively). Percent integrated density of lubricin in the superficial zone was significantly lower in arthroscopy specimens compared to controls (p<0.001).
Keywords: Arthroscopy, lubricin, osteoarthritis, osteoarthrosis, tribosupplementation
1. Introduction
Arthroscopic partial meniscectomy is one of the most common orthopaedic surgeries in the United States (Molina et al., 2015). This is concerning, as Englund and Lohmander have reported radiographic osteoarthritis in 48% of subjects 15 to 22 years after partial meniscectomy (Englund and Lohmander, 2004) Additionally, increased rates of cartilage loss have been detected after partial meniscectomy compared to healthy controls via magnetic resonance imaging (MRI) at a mean follow up of 28.6 months (Cicuttini et al., 2002), and a retrospective review of repeat knee MRIs during a seven year interval in patients with meniscal tears found significantly greater cartilage loss in patients post-meniscectomy versus those who did not undergo meniscectomy (Cohen et al., 2012). While selection bias toward surgical intervention might result in patients with more active, symptomatic disease electing to undergo arthroscopic surgery, other factors that might accelerate cartilage loss include iatrogenic injury due to removal of functional meniscal tissue or inadvertent direct cartilage injury. Another potential mechanism accelerating cartilage loss after knee arthroscopy is the effect of arthroscopic irrigation on cartilage health, via its impact on cartilage lubrication. During arthroscopy, pressurized fluid is used to distend and lavage the joint. Current post-arthroscopy protocols do not replenish synovial fluid lubricants post-procedure. Patients are generally allowed to bear weight on the knee immediately after arthroscopic partial meniscectomy. However, this may result in mechanical damage to the cartilage surface if lubrication is impaired.
The mucinous glycoprotein lubricin (PRG4) has been recognized as an essential boundary lubricant that protects articular cartilage from damage (Jay et al., 2012; Rhee et al., 2005; Waller et al., 2013). Lubricin is found on cartilage and meniscal surfaces (Musumeci et al., 2014) within the superficial zone (Elsaid et al., 2012), and within the synovial fluid(Jay, 1992; Schmidt et al., 2007). It is expressed embryologically shortly after diarthrodial joint nucleation (Rhee et al., 2005), when joint surfaces become demarcated. Lubricin provides both anti-adhesive and lubricating activity (Chang et al., 2008) as an end-grafted brush molecular configuration (Zappone et al., 2007). Lack of lubricin gene function results in Camptodactyly Arthropathy Coxa Vara Pericarditis (CACP) Syndrome (Marcelino et al., 1999), where advanced multifocal joint degeneration develops by the second to third decades of life (Faivre et al., 2000). Lubricin null mice show an increased coefficient of friction (COF) ex vivo (Jay et al., 2007) and superficial and upper intermediate zone chondrocyte apoptosis(Waller et al., 2013) resulting in cellular loss (Karamchedu et al., 2016) in murine knee joints. Cartilage under pressure and shear stimulates lubricin expression in vitro (Grad et al., 2006; Nugent et al., 2006), which is impeded by additive IL-1α which also raised COF (Larson et al., 2016).
The present study was undertaken to determine the effects of arthroscopic irrigation on the frictional properties of the cartilage surface post-arthroscopic irrigation. Using live cartilage explants from fresh bovine stifle joints, our hypothesis was that arthroscopic irrigation of the cartilage surface would be associated with elevated static and dynamic COF and with decreased lubricin within the superficial cartilage zone compared to control samples lubricated with synovial fluid.
2. Materials and methods
Fourteen individual bovine stifle joints with intact capsules were obtained from a local abattoir. Samples for each specimen were obtained; experiments were completed, and fixed for histology within eight hours of euthanasia. For control specimens (n=7), a superior capsulotomy was performed and whole synovial fluid aspirated using a 10 ml syringe and 18-gauge needle. For arthroscopy specimens (n=7), a superior capsulotomy was performed and an arthroscopic cannula introduced into the joint. Arthroscopic irrigation was performed using 6 liters of lactated ringers (LR) solution at 55 mmHg fluid pressure. During irrigation, joints were passively ranged through flexion and extension to distribute the fluid and simulate arthroscopy. Approximately 10 ml of fluid was collected from arthroscopy joints after 3 L of total irrigation and saved for use as a lubricant during mechanical testing.
Following synovial fluid collection (Controls) or irrigation (Arthroscopy), joint capsules were opened and full thickness large (12 mm) and small (6 mm) osteochondral explant pairs were harvested from three regions in the weight-bearing portion of the medial femoral condyle: anterior, middle, and posterior (Fig. 1). Explants were harvested using custom drill bits cooled with phosphate buffered saline (PBS). Following harvest, explants were rinsed in PBS to remove debris and then incubated in the test lubricant; either synovial fluid (Controls) or LR irrigant (Arthroscopy).
Figure 1.
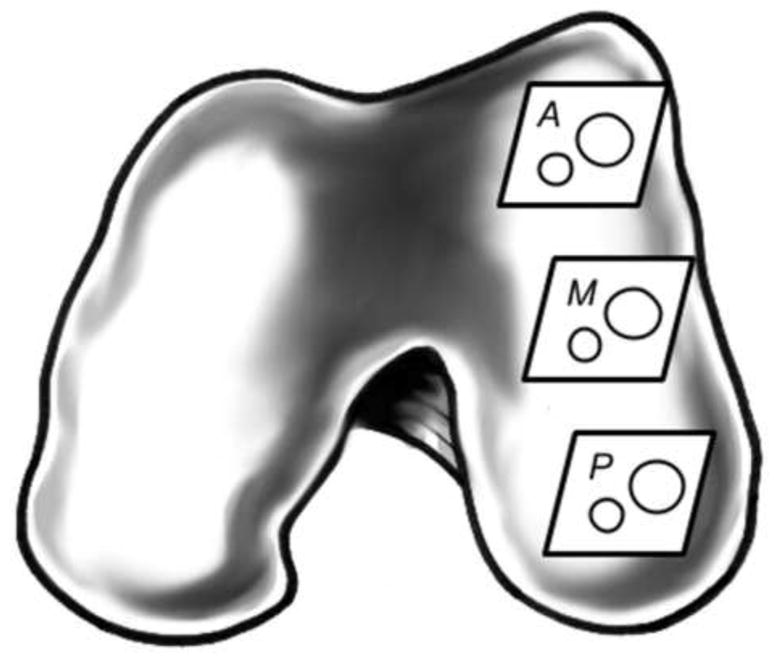
Graphic showing anterior (A), middle (M), and posterior (P) cartilage harvest sites from the medial femoral condyle of a bovine stifle joint.
Static and dynamic COF were measured for explant pairs using a Bose Electroforce 3200 Series III Material Testing System (Bose, Framingham, MA). Each explant pair was mounted in a custom fixture with the cartilage surfaces apposed. Explant surfaces were kept moist with regular applications of test lubricant. Additional lubricant was applied to cartilage surfaces just before mechanical testing was initiated. The mechanical testing protocol was based upon that described by Waller et al (Waller et al., 2012). During mechanical testing, a 12 N compressive load was applied across the explant surfaces followed by an 8 minute dwell to ensure stress relaxation and surface engagement. The large explant was then rotated relative to the small explant for 12 rotations of +/− 720° while axial torque (τ) was recorded. COFs were calculated as COF = τ/((2/3)*(r)*(load), where r = measured radius of the small explant and load is the equilibrium force following the 8 minutes of cartilage decompression (Schmidt and Sah, 2007; Waller et al., 2013). Static COF was calculated from maximal torque measured during the first 20° of rotation, while dynamic COF was calculated using average torque measured during the last 720° rotation.
Immediately following testing, the 12 mm explants were immersed in formalin for a minimum of 72 hours prior to decalcification and paraffin embedding for histology. Sections from the central contact area of each explant were stained for lubricin. Briefly, sections were heated to 60°C for 30 min, deparaffinized and hydrated in three changes xylene and serial alcohol and antigen retrieval using pepsin solution (Thermo scientific). After two PBS washes, 9g3 monoclonal antibody at 1:200 dilution was added to the slides and incubated at 4°C overnight. After washing with PBS three times, the sections were incubated with fluorescein goat anti-mouse igG (F2761, Thermo fisher scientific, USA) at 1:100 dilution for one hour at room temperature in darkness. The sections were washed five times using PBS and slip covered with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc, Burlingame, CA). Images acquired using a fluorescence microscope (Nikon, ECLIPSE90i) were imported to ImageJ (Jensen, 2013) and the mean intensity and % area occupied were calculated. Mean intensity values were corrected for background and normalized (Model and Burkhardt, 2001) using a 10% solution of standard fluorescein dye (Catalog#F1300, Thermo Fisher Scientific). Percent integrated density, defined as the corrected mean intensity times the % area occupied by lubricin in the superficial zone (0–100 μm), was reported.
Comparisons between treatments (arthroscopic versus control) and regions (anterior, middle, versus posterior) were performed using two-way analysis of variance. The model consisted of two-fixed factors, treatment (an across-subject factor) and region (a within-subjects factor) and their interaction. Dependent variables were static COF, dynamic COF, and percent integrated density of lubricin in the superficial zone.
3. Results
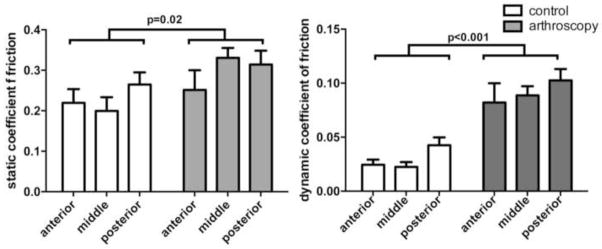
Static COF was significantly greater in arthroscopy specimens compared to controls (p=0.02; Fig. 2). The mean increase in static COF among arthroscopy specimens was 33.0% (range 14.6%–65.9% by condylar location). Static COF demonstrated no region effect for the femoral condyle (anterior, middle, posterior; p=0.32). Though the difference between arthroscopy specimens and controls was observed to be greater at mid region than the anterior and posterior regions, there was no evidence that the effect of treatment varied by region (p=0.33).
Figure 2.
Mean static and dynamic coefficients of friction for control and arthroscopically irrigated cartilage explants. Static and dynamic friction was significantly higher in the arthroscopy explants compared to controls.
Dynamic COF was significantly greater in arthroscopy specimens compared to controls (p<0.001; Fig. 2). The mean increase in the dynamic COF among arthroscopy specimens was 223.6% (range 140.6%–294.3% by condylar region). The dynamic COF demonstrated no region effect for femoral condyle location (anterior, middle, posterior) (p=0.12) and there was no evidence that the effect of treatment varied by region (p=0.91).
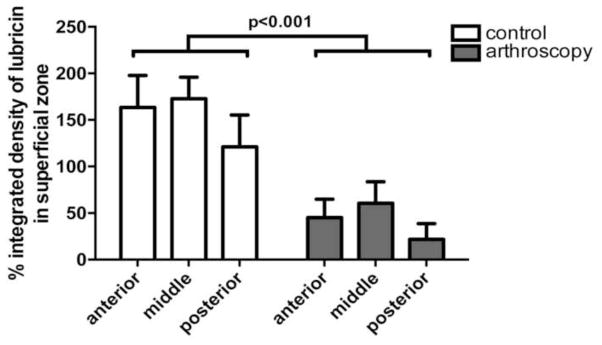
Surface and superficial zone (0–100um depth) lubricin was significantly lower in arthroscopy treated specimens compared to controls (p<0.001; Fig. 3 and Fig. 4). Mean percent integrated density of lubricin in arthroscopy treated specimens was 65.2% (range 64.7%–87.2% by condylar region) and there was no significant difference in the effect of treatment by region (p=0.93).
Figure 3.
Mean percent integrated density of lubricin in the superficial zone (0=100um) of the medial femoral condyle for control and arthroscopically irrigated cartilage explants. Surface and superficial zone lubricin was significantly lower in arthroscopy samples compared to controls.
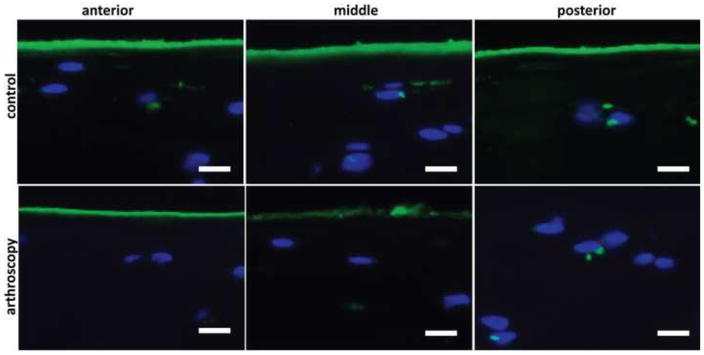
Figure 4.
Representative median images of histochemical detection of PRG4 with mAb 9G3in the medial femoral condyle cartilage by region show reduced surface lubricin in arthroscopy treated samples. Scale bars indicate 20um.
4. Discussion
The results confirm our hypothesis that arthroscopic irrigation of the bovine stifle joint depletes the cartilage surface of superficial zone lubricin resulting in significantly increased cartilage surface static and dynamic coefficients of friction post-arthroscopic irrigation compared to non-irrigated controls. We observed region effects for static and dynamic COF by medial femoral condyle region, which were not found to vary by treatment. These results suggest that removal and dilution of synovial fluid lubricants may negatively impact cartilage surface friction immediately post-arthroscopy.
In contrast to the current protocol, previous experiments investigating the effects of saline versus synovial fluid lubricants on cartilage friction and chondrocyte viability used cultured cartilage explants that had undergone multiple cycles of ultracentrifugation and rinsing with saline and culture media prior to lubricant testing (Waller et al., 2012). The present study was therefore undertaken to determine if irrigation fluid volumes and pressures during arthroscopy would be sufficient to deplete the articular surface of endogenous lubricants. Our results demonstrate that arthroscopic irrigation may likewise impair cartilage surface lubrication and decrease surface and superficial zone lubricin. Similar to our findings, Neu et al., (Neu et al., 2010) reported increased superficial zone protein (i.e., lubricin) expression in load-bearing cartilage regions and Moore and Burris, (Moore and Burris, 2015) reported location-dependent variability in cartilage material properties in the bovine stifle joint. These findings of location-dependent variability in surface lubrication and COFs have implications for experiments using osteochondral explants and suggest that explant harvest location should be controlled for between test groups.
One study limitation is that healthy bovine stifle joints may not accurately represent the pre-surgical joint conditions of patients. Synovial fluid analyses performed in the setting of synovitis (Jay et al., 2004) and anterior cruciate ligament deficiency (Elsaid et al., 2008), have demonstrated reduced lubricin levels. Injured joints undergoing arthroscopy may have greater impairments in lubrication than the fresh, uninjured joints used in this study. This consideration opens up the possibility that arthroscopic irrigation may cause even greater lubrication impairments than those reported here, and joints being treated with arthroscopy may have already sustained superficial zone cartilage damage. Another study limitation is that it represents only a “time zero” evaluation post-arthroscopic irrigation. The time course for the natural recovery of synovial fluid lubricating ability post-arthroscopy remains unknown. However, other studies are teaching us about how long a joint can withstand temporary elevated friction. Hill et al. found that restoring PRG4 gene function in a gene trap mouse at 3 weeks improved of friction and chondrocyte viability compared to restoration at 2 and 6 months after birth (Hill et al., 2015). Adding purified human synoviocyte lubricin to CACP synovial fluid and testing this as a lubricant in bovine cartilage friction experiments produced similar static and dynamic COF values as wild-type human synovial fluid (Waller et al., 2013). Larson et al. observed restored lubricin expression and lower COF in bovine cartilage explants treated with IL-1″ for 7 days, when recombinant human PRG4 was added as a lubricant in cartilage friction experiments (Larson et al., 2016).
Tribosupplementation with lubricin has previously been shown to modulate posttraumatic osteoarthritis in animal models, (Flannery et al., 2009; Teeple et al., 2011; Waller et al., 2012) suggesting that supplemental articular lubrication may provide an opportunity to modify osteoarthritis progression post-arthroscopic partial meniscectomy. Further in vivo work is required to better characterize the effects of arthroscopic irrigation on articular surface lubrication and the biologic response to this intervention.
Acknowledgments
This project was supported by grants from the National Institutes of Health P20-GM104937 (COBRE Bioengineering Core); NIAMS grants R01-AR050180, R42-AR057276; NIH T32-AR055885-01; and Department of Defense grant PR110746. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest:
GDJ has co-authored and was granted a United States patent (# 6743774) for the therapeutic use of lubricin in joints. GDJ receives support from a National Institutes of Health funded Phase II STTR (R42-AR057276) in these clinical translation efforts. ET has performed contract work under the above-mentioned Phase II STTR, which is separate from the contents and analysis of this manuscript. This manuscript neither materially nor financially affects Dr. Jay’s patent relating to rhPRG4 nor does it impact or include Dr. Teeple’s previously performed contract work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183–1193. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–1956. [PubMed] [Google Scholar]
- Cohen SB, Short CP, O’Hagan T, Wu HT, Morrison WB, Zoga AC. The effect of meniscal tears on cartilage loss of the knee: findings on serial MRIs. Phys Sportsmed. 2012;40:66–76. doi: 10.3810/psm.2012.09.1983. [DOI] [PubMed] [Google Scholar]
- Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid KA, Zhang L, Waller K, Tofte J, Teeple E, Fleming BC, Jay GD. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012;20:940–948. doi: 10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- Faivre L, Prieur AM, Le Merrer M, Hayem F, Penet C, Woo P, Hofer M, Dagoneau N, Sermet I, Munnich A, Cormier-Daire V. Clinical variability and genetic homogeneity of the camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Am J Med Genet. 2000;95:233–236. doi: 10.1002/1096-8628(20001127)95:3<233::aid-ajmg9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, Morris EA, Glasson SS. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- Grad S, Lee CR, Wimmer MA, Alini M. Chondrocyte gene expression under applied surface motion. Biorheol. 2006;43:259–269. [PubMed] [Google Scholar]
- Hill A, Waller KA, Cui Y, Allen JM, Smits P, Zhang LX, Ayturk UM, Hann S, Lessard SG, Zurakowski D, Warman ML, Jay GD. Lubricin restoration in a mouse model of congenital deficiency. Arthritis & rheumatology. 2015;67:3070–3081. doi: 10.1002/art.39276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connective tissue research. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- Jay GD, Elsaid KA, Kelly KA, Anderson SC, Zhang L, Teeple E, Waller K, Fleming BC. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64:1162–1171. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, Chichester CO. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–564. [PubMed] [Google Scholar]
- Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, Elsaid K, Kim KS, Cui Y, Warman ML. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 2013;296:378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]
- Karamchedu NP, Tofte JN, Waller KA, Zhang LX, Patel TK, Jay GD. Superficial zone cellularity is deficient in mice lacking lubricin: a stereoscopic analysis. Arthritis Res Ther. 2016;18:64. doi: 10.1186/s13075-016-0967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson KM, Zhang LX, Elsaid KA, Schmidt TA, Fleming BC, Badger GJ, Jay GD. Restoration of Endogenous Proteoglycan 4 Expression and Reduction of Friction Chondroprotection by Recombinant Human Proteoglycan 4 in IL-1″ Stimulated Bovine Cartilage Explants. J Orthop Res 2016 July 13, DOI: 10.1002/jor.23367 (Epub ahead of print) [PMID: 27411036] Journal of Orthopardic Research. 2016 doi: 10.1002/jor.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, Sood R, Makalowska I, Baxevanis A, Johnstone B, Laxer RM, Zemel L, Kim CA, Herd JK, Ihle J, Williams C, Johnson M, Raman V, Alonso LG, Brunoni D, Gerstein A, Papadopoulos N, Bahabri SA, Trent JM, Warman ML. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- Model MA, Burkhardt JK. A standard for calibration and shading correction of a fluorescence microscope. Cytometry. 2001;44:309–316. doi: 10.1002/1097-0320(20010801)44:4<309::aid-cyto1122>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473:1574–1581. doi: 10.1007/s11999-014-3597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AC, Burris DL. Tribological and material properties for cartilage of and throughout the bovine stifle: support for the altered joint kinematics hypothesis of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23:161–169. doi: 10.1016/j.joca.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Trovato FM, Loreto C, Leonardi R, Szychlinska MA, Castorina S, Mobasheri A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: a morphological, immunohistochemical and biochemical study. Acta Histochem. 2014;116:965–972. doi: 10.1016/j.acthis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680–2687. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54:1888–1896. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, Carpten JD. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, Bliss TF, Fleming BC. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39:164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013;110:5852–5857. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KA, Zhang LX, Fleming BC, Jay GD. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and hylan G-F 20. J Rheumatol. 2012;39:1473–1480. doi: 10.3899/jrheum.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92:1693–1708. doi: 10.1529/biophysj.106.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]