Abstract
Epilepsy is among the most common brain network disorders, and it is associated with substantial morbidity and increased mortality. While focal epilepsy was traditionally thought of as a regional brain disorder, growing evidence has demonstrated widespread network alterations in this disorder which extend beyond the epileptogenic zone from which seizures originate. The goal of this review is to summarize recent investigations examining functional and structural connectivity alterations in focal epilepsy, including neuroimaging and electrophysiology studies utilizing model-based or data-driven analysis methods. A significant subset of studies in both mesial temporal lobe epilepsy and focal neocortical epilepsy have demonstrated patterns of increased connectivity related to the epileptogenic zone, coupled with decreased connectivity in widespread distal networks. Connectivity patterns appear to be related to the duration and severity of disease, suggesting progressive connectivity reorganization in the setting of recurrent seizures over time. Global resting-state connectivity disturbances in focal epilepsy have been linked to neurocognitive problems, including memory and language disturbances. While it is possible that increased connectivity in a particular brain region may enhance the propensity for seizure generation, it is not clear if global reductions in connectivity represent the damaging consequences of recurrent seizures, or an adaptive mechanism to prevent seizure propagation away from the epileptogenic zone. Overall, studying the connectome in focal epilepsy is a critical endeavor which may lead to improved strategies for epileptogenic zone localization, surgical outcome prediction, and a better understanding of the neuropsychological implications of recurrent seizures.
Keywords: brain networks, epilepsy, fMRI, functional connectivity, neuroimaging
Introduction
To understand neural circuit dysfunction in neurological disorders, one must appreciate not only discrete brain structures in isolation, but also the connections between these regions. In the modern view of brain networks, both healthy neurophysiological processes and neurological disorders can be studied in the context of the human connectome.1 Significant attention and funding has recently been allocated to large national and international programs – including the Human Connectome Project, the BRAIN Initiative, and the European Human Brain Project – aimed at mapping and modulating neural circuits in the normal brain and various disease states.1; 2 Among neurological disorders, epilepsy provides one of the clearest examples of the potentially devastating effects of neural circuit dysfunction.
Epilepsy is among the most common neurological disorders, affecting nearly 1% of the population and leading to substantial morbidity and mortality.3 Growing evidence suggests that the pathophysiological underpinnings of seizure generation may involve both aberrant structural integrity in certain brain regions as well as abnormal connections between these areas, resulting in large-scale network instability.5–7 The primary goals of investigating these brain network disturbances in human epilepsy include: 1) an improved characterization of the circuits involved in seizure generation and propagation, 2) a better understanding of the deleterious effects of recurrent seizures on brain function, including devastating cognitive and neuropsychological effects, and 3) the development of novel surgical therapies which specifically interrupt seizure-generating networks. These goals are consistent with the National Institute for Neurological Disorders and Stroke (NINDS) Benchmarks for Epilepsy Research, which call for a better understanding of the causes of epilepsy, as well as improved treatment strategies for seizures and their adverse effects across the lifespan.8
The number of studies examining brain connectivity in epilepsy has rapidly grown in recent years, as reflected by a steep yearly increase in relevant citations in the U.S. National Library of Medicine (“PubMed”) database (Fig. 1). A database search with the terms “epilepsy connectivity” including manuscripts from 1995 to 2015 returns 910 citations, with nearly 50% published within the last three of those years (Fig. 1A). In comparison, only 12% of the 158,000 citations for “epilepsy” alone were published during the last three years (Fig. 1B). Given the relative novelty of this field, and the diversity of analysis methods utilized, the goal of this review is to summarize recent literature examining functional and structural connectivity alterations in epilepsy, with a particular focus on surgically-remediable focal epilepsy. After briefly reviewing common modalities and methods utilized in recent connectomic studies, this review will discuss connectivity patterns associated with the EZ and distant from it, and relate these patterns to disease and outcome characteristics.
Figure 1. Rapid recent increase in published academic works addressing connectivity in epilepsy.
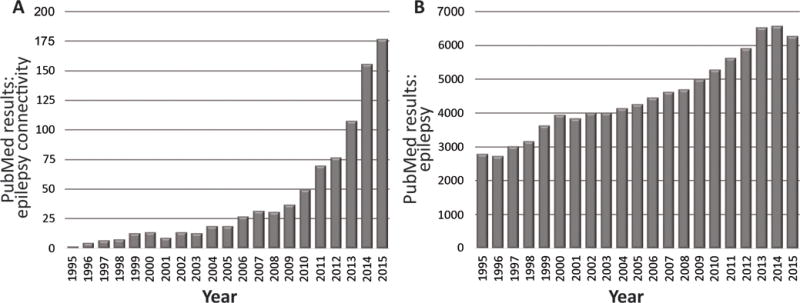
A) Utilizing search terms “epilepsy connectivity” to search the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) for manuscripts between 1995 to 2015 reveals a rapid increase in manuscript hits in recent years, with 48% of all manuscripts published in the last three years (2013–2015). B) By comparison, the increase in results returned for the search term “epilepsy” alone is notably less steep, with only 12% of all manuscripts published in the last three years.
Connectivity analysis modalities and methods
Connectivity analysis techniques in epilepsy are often categorized as either anatomic or functional. Anatomic connectivity studies typical examine axonal density (i.e, the neural highways) between separate brain regions as a measure of connection strength. Most commonly, anatomic connectivity studies utilize diffusion tensor imaging (DTI) from structural magnetic resonance imaging (MRI) recordings.9 DTI relies on the best estimate of water diffusion along white matter pathways, and the quality of images is dependent on several factors, including the resolution and quality of structural images.10 Conversely, functional connectivity analysis utilizes statistical techniques to examine the inter-dependency of signals from disparate brain regions and estimate communication between these areas (i.e., the traffic on the neural highways).11; 12 Some functional connectivity methods also infer the directionality of signals within these connections to measure effective connectivity (i.e., the direction of the traffic).13
Several techniques have been used to study functional connectivity in focal epilepsy. Table 1 summarizes the advantages and disadvantages of common technical modalities used in these investigations. In human epilepsy patients, while electrocorticography (ECoG) is the gold standard modality to most directly measure neural signals, ECoG is highly invasive and signals available for analysis are limited to the select areas of brain covered with electrodes.14 Noninvasive “whole-brain” modalities for functional connectivity analysis include functional MRI (fMRI), magnetoencephalography (MEG), and scalp electroencephalography (EEG). FMRI is markedly more available than MEG, although fMRI connectivity analysis is based on slow hemodynamic signals which indirectly reflect neural activity.15 MEG data more closely reflects neural signals, but source localization is challenging with deep structures.16 Although scalp EEG is also widely available and noninvasive, connectivity analyses are limited by low spatial resolution and signal degradation by the scalp and skull.17
Table 1.
Common modalities used to study functional connectivity in focal epilepsy
| A) Modality | Advantages | Disadvantages |
|---|---|---|
| MEG | noninvasive; whole-brain coverage; high spatial resolution; high temporal resolution | source reconstruction challenging from deep structures; ictal recordings very challenging; not widely available |
| fMRI | noninvasive; whole-brain coverage; reliable signals from deep structures; relatively high spatial resolution; widely available | low temporal resolution; indirect measurement of neuronal activity; ictal recordings very challenging |
| ECoG | high spatial & temporal resolution; most direct measurement of neuronal activity; reliable ictal recordings | highly invasive; limited to brain area covered with electrodes; no data from normal controls |
| Scalp EEG | noninvasive; high temporal resolution; widely available and inexpensive | low spatial resolution; signal degradation by skull and scalp |
ECoG: electrocorticography; EEG: electroencephalography; fMRI: functional magnetic resonance imaging; MEG: magnetoencephalography
Analysis techniques in functional connectivity studies are often categorized as either model-based or data-driven. Table 2 summarizes some of the most common model-based and data-driven methods utilized. Model-based methods are based on prior knowledge, and thus require a specific hypothesis for testing.13 These include measurements of coherence, phase synchrony, and cross-correlation analysis. Measurements using model-based methods often originate from a seed region. Conversely, data-driven methods are exploratory and are thus not dependent on a prior hypothesis, but are more prone to uncovering spurious relationships.18
Table 2.
Common analysis methods to study functional connectivity in focal epilepsy
| A) Model-based methods | Summary | Considerations |
|---|---|---|
| coherence | measures the degree of linear dependency of signals between brain regions by examining similar frequency components | requires selection of a specific frequency band, risking overlooking dependencies in other bands; frequency-specific analysis may be more likely to uncover true, not spurious, neural connections |
| cross-correlation analysis | measures degree of similarity of signals amongst brain regions as a function of the time lag between these signals | does not require frequency band selection; correlation measurements at zero-time lag are more likely to detect artifact or shared source connections |
| phase synchrony | compares only the phase of neural oscillations between brain regions to estimate cross-talk | time resolved, with better temporal resolution than coherence; only sensitive to signal phase and not amplitude |
| model-based methods, overall | non-exploratory, often seed-based methods built upon prior hypotheses | less likely to uncover spurious relationships than data-driven methods, but necessitates prior knowledge for specific hypothesis testing |
| B) Data-driven methods | Summary | Considerations |
|
| ||
| principle component analysis (PCA) | uses orthogonal transformation to convert possibly correlated neural signals into a set of uncorrelated signals (principal components) in order to uncover network patterns | may reveal novel network patterns and interdependencies; requires selection of regions of interest given otherwise unmanageable amount of data; may be preprocessing step for ICA for further analysis |
| independent component analysis (ICA) | identifies a linear combination of components (brain region signals) like PCA, but is restricted to those that are statistically independent | can identify statically independent neural sources (unlike PCA), but statistical dependence between neural signals would degrade results; can be used to further interrogate components identified in PCA |
| clustering analysis | identifies groups of brain regions with similar neural signals (clusters) based on the time courses of those signals | useful for novel identification of neural networks and included brain regions; the number of clusters must be chosen, and this may affect connectivity results |
| data-driven methods, overall | exploratory, non-seed-based analysis methods free of prior hypotheses | may uncover novel networks given lack of prior knowledge, but more likely to uncover spurious relationships |
Finally, it is important to consider the conditions surrounding data collection. For instance, while most functional connectivity studies in epilepsy have examined the “resting” state (awake in the absence of a task), with data collapsed over a certain period of time, task-based or sequential dynamic measurements of connectivity can also be performed.19 However, the majority of functional connectivity studies in epilepsy have examined resting-state functional connectivity, and the remainder of this review will be limited to that scope.
Regional EZ connectivity patterns
Human neuroimaging and electrophysiology studies have allowed insight into lateralized and regional connectivity patterns in focal epilepsy, using various analysis techniques. Goals of these investigations include improved EZ localization strategies for surgical intervention, and prediction of post-operative outcome after epilepsy surgery. Several fMRI reports have demonstrated asymmetric connectivity reorganization in focal epilepsy which may be influenced by a unilateral EZ. For instance, Morgan and colleagues examined measures of both static and dynamic resting-state connectivity in MTLE patients using model-based fMRI techniques.20 Relatively soon after disease onset, patients showed disruption of cross-hemispheric networks and increased static functional connectivity ipsilateral to the EZ. Over time, ipsilateral static connectivity diminished, while dynamic connectivity measures demonstrated progressive functional independence of the seizure propagation network. Negishi et al. also examined laterality in focal epilepsy using resting-state fMRI, utilizing an activation cluster which overlapped with the region of resection.21 As depicted in Figure 2, the authors observed that connectivity laterality patterns significantly predicted seizure outcome after epilepsy surgery, as patients who achieved seizure freedom exhibited more lateralized connectivity than those with persistent seizures. In another fMRI report by this group, differences between intrahemispheric and interhemispheric connectivity revealed localized connectivity abnormalities that often overlapped with a region producing interictal spikes on ECoG, presumably approximating the EZ.22 Others have demonstrated the ability to predict laterality of the epileptogenic hemisphere with fMRI using a linear model based on random forest and a support vector machine.23 Together, these neuroimaging reports suggest lateralized connectivity patterns in patients with focal epilepsy that are related to the hemisphere of seizure onset.
Figure 2. Functional MRI connectivity laterality as a predictor of surgical outcome in epilepsy.
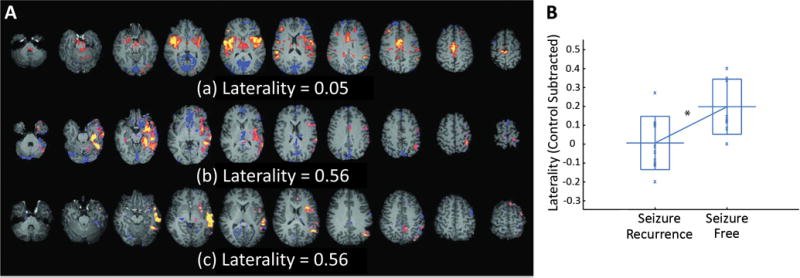
A) Examples of spike-correlated fMRI-seeded functional connectivity maps with low (a), medium (b), and high (c) laterality indices. The laterality values displayed are before laterality values of the controls are subtracted. The distinct lateralization of the functional connectivity can be clearly recognized in (b) and (c). B) Control-subtracted laterality index of the spike-correlated fMRI seeded functional connectivity. Patients in the non–seizure-free group had significantly lower laterality indices than patients in the seizure-free group. The long horizontal bar shows the average, the rectangle shows the range between the average plus and minus the standard deviation, and the “x” shows a data point. *p<0.05. fMRI: functional magnetic resonance imaging. Modified with permission from Negishi et al., 2011.21
One early resting-state fMRI study to specifically examine regional functional connectivity at the EZ in focal epilepsy was reported by Liao et al.24 The investigators used data-driven graph theory analysis to compare correlation matrices between MTLE patients and controls and observed enhanced connectivity within the ipsilateral mesial temporal structures of patients compared to controls. In another fMRI investigation examining blood oxygen level dependent (BOLD) signal fluctuations between specific seed regions in MTLE, Haneef and colleagues reported enhanced connectivity in the hippocampus and other limbic regions, including insula and thalamus, in patients compared to controls.25 Other fMRI studies in MTLE have also noted increased regional connectivity related to ipsilateral limbic structures,26 and DTI analysis has suggested enhanced anatomic connectivity between the hippocampus and thalamus in MTLE.27 Increased hippocampal connectivity in MTLE has been specifically related to duration of epilepsy, suggesting a gradual process that may evolve over the course of the disease. In a resting-state fMRI study using a modified temporal clustering algorithm, Morgan and others observed a progressive increase in connection strength between bilateral hippocampi in MTLE over time (Fig. 3A,B), and suggested that the contralateral hippocampus may begin to drive the epileptogenic hippocampus after several years of epilepsy (Fig. 3C).28 Overall, these fMRI studies in MTLE suggest patterns of increased connectivity amongst limbic structures involved in seizure generation and propagation.
Figure 3. Relationship between cross-hippocampal connectivity and Granger causality laterality with duration of illness in MTLE.
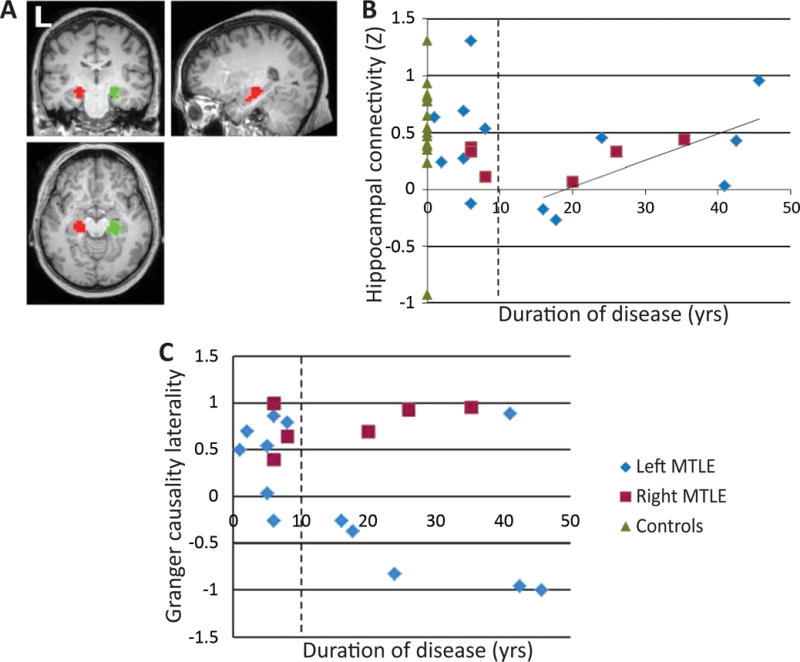
A) Location of the left hippocampus (red) and right hippocampus (green) functional ROIs determined using 2dTCA. B) Relationship between hippocampal connectivity and duration of disease across patients with left and right MTLE using functional ROIs, showing a linear increase in hippocampal connectivity with increasing duration of disease after 10 years. C) Granger causality laterality versus duration of disease. Positive laterality indicates left hippocampus influences right. Negative laterality indicates right hippocampus influences left. 2dTCA: modified temporal clustering algorithm; MTLE: mesial temporal lobe epilepsy; ROI: region of interest. Modified with permission from Morgan et al., 2011.28
Several investigations have examined regional connectivity in FNE. Luo and others performed seed-based resting-state fMRI analysis in frontal lobe epilepsy patients, and noted elevated connectivity related to a frontal lobe seed producing interictal spikes, but diminished connectivity between the seed and distal networks.29 Recently, Pedersen et al. calculated regional homogeneity and weighted degree centrality as measures of fMRI connectivity in both FNE and MTLE patients, and reported increased regional connectivity in the insula, piriform cortex, and thalamus, but diminished connectivity in prefrontal and lateral temporal cortices.30 Using resting-state MEG-based analysis, Wu and colleagues observed regions of elevated imaginary coherence at neocortical areas which produce spike activity in FNE patients, suggesting increased functional connectivity at the EZ.31 These studies demonstrate that increased connectivity related to the EZ may be seen in FNE as well as MTLE.
ECoG studies have also provided insight into connectivity related to the EZ in focal epilepsy. Zaveri and others observed elevated interictal coherence amongst electrodes at the EZ and up to several centimeters away in six patients with focal epilepsy.32 Using power spectral density analysis, Bettus et al. reported increased signal interdependencies in resting ECoG data from MTLE patients, suggesting enhanced connectivity amongst mesial temporal structures.33 Also, examination of slow oscillatory ECoG signals in FNE has suggested increased connectivity within seizure propagation networks, although functional isolation of the EZ may be seen at high frequencies prior to seizure onset.34; 35 Higher synchronization likelihood and normalized clustering index observed in mesial temporal ECoG recordings from patients with MTLE but not FNE also supports a focal increase in connectivity at the seizure onset zone.36 One study examining bilateral hippocampal field potential correlations from depth electrodes reported more favorable seizure outcomes in MTLE patients possessing more highly connected heterogenous networks versus weakly connected homogenous networks.37 These studies provide further evidence of regional connectivity elevations at the EZ in focal epilepsy, suggested by direct electrographic recordings rather than slow fMRI signal fluctuations.
While animal studies of connectivity in epilepsy have been rare, one group examined coherence using intracranial recordings from rats experiencing recurrent flurothyl-induced limbic seizures.38 Animal with seizures demonstrated increased functional connectivity between the dorsal and ventral hippocampus and prefrontal cortex compared to control animals. However, treatment with bumetanide resulted not only in a normalized seizure threshold and behavioral stabilization, but also a normalization of connectivity patterns. This suggests that connectivity perturbations may be prevented by preventing seizures in an animal model of focal epilepsy.
While most of the aforementioned studies suggest that regional EZ connectivity is increased in focal epilepsy, some fMRI studies have included dissimilar observations. Pittau and colleauges performed connectivity analysis seeding bilateral hippocampi and amygdalae in MTLE patients and found depressed connectivity between these structures and default mode regions, as well between bilateral mesial temporal structures.39 Doucet et al. also seeded the hippocampus of individuals with left or right MTLE, and noted decreased connectivity with thalamus, angular gyrus, posterior cingulum and medial frontal cortex compared to controls.40 Other investigations using data-driven ICA have also reported decreases in functional41 or anatomic connectivity42 between the mesial temporal lobe and other regions, including the default mode network.
In summary, most studies have suggested regional connectivity elevations at the EZ in focal epilepsy, although some investigators have reported decreased EZ connectivity. Reasons for these discrepancies are not fully understood, but studies do differ in the analysis methods utilized and in how the EZ is defined. While the EZ is characterized by electrographic or neuroimaging findings in most reports, seizure freedom after resection of a specific region may also help confirm EZ localization. For instance, in a recent MEG study examining regional imaginary coherence in MTLE and FNE patients, some patients showed increased resting-state connectivity at the region of resection, while others displayed focal decreases.43 Individuals with higher connectivity at the resection area were significantly more likely to become seizure free after surgery than those with lower regional connectivity, suggesting that the true seizure generators exhibit increased connectivity compared to non-epileptogenic regions of interest, and that the pre-operative definition of the true EZ may not always be accurate. Ultimately, regional connectivity patterns observed in these studies may aid in the identification of biomarkers to assist EZ localization and surgical outcome prediction.
Global connectivity alterations
Neuroimaging, electrophysiology, and behavioral studies suggest that neural dysfunction in focal epilepsy is not restricted to the EZ, but can affect widespread networks throughout the brain. This premise has been supported by recent reports demonstrating global connectivity alterations in MTLE and FNE, including network reorganization distal from the EZ. Luo et al. used group ICA to examine several discrete resting state networks in MTLE and FNE patient fMRI recordings.44 Diminished functional connectivity was noted in patients compared to controls in default mode regions, frontoparietal association cortices, and primary sensorimotor networks. Connectivity decreases were more prominent in FNE compared to MTLE in most of these networks. Other investigators have also found particularly pronounced global connectivity disturbances in pediatric patients with frontal lobe epilepsy. Widjaja and colleagues performed DTI analysis in children with nonlesional frontal lobe epilepsy, observing reduced structural connectivity in patients versus controls reflected by diminished network strength, clustering coefficient, path length, and global efficiency.45 This global network dysfunction may be related to widespread thinning of the neocortex also noted in this patient population.46 Furthermore, diminished functional connectivity within the frontal lobe has been shown in children with frontal lobe epilepsy, as well as reductions in connection strength between the frontal lobe and distant neocortical and subcortical regions.47 These investigations demonstrate widespread impairments of connectivity that extend well beyond the EZ in focal epilepsy.
In assessing resting-state connectivity disturbances, it is important to consider the default mode network, which is comprised of regions shown to be active at rest, including the precuneus/posterior cingulate, angular/temporoparietal area, and medial prefrontal cortex.48 Several fMRI studies have demonstrated reduced functional connectivity amongst default mode regions in MTLE patients compared to non-epileptic individuals,39; 49–51 although increases in connectivity have also been described immediately preceding epileptiform activity.52 Often in the same study, diminished connectivity in the default mode network and association cortices are contrasted with increased connectivity associated with EZ networks.24; 25; 30; 43 For instance, Nedic et al. examined resting-state fMRI recordings in MTLE patients using the autocorrelation function – an entropic measure of regulation and feedback – and observed long-range decreases but local temporal lobe increases in network efficiency in these individuals.53 Overall, these fMRI studies suggest diminished connectivity amongst default mode regions in focal epilepsy.
Global decreases in connectivity have also been found in MEG-based analysis of imaginary coherence in a recent study of MTLE and FNE patients.43 As shown in Figure 4, diminished connectivity appeared most prominent in the orbital frontal cortex, frontal and parietotemporal association cortices, and subcortically in the basal forebrain and anterior thalamus. On multivariate analysis, the only factors significantly predicting larger decreases in global connectivity were longer duration of illness and higher seizure frequency. Examining both functional and volumetric MRI measures, Morgan et al. noted progressively diminished connectivity in the temporal lobe, precuneus, and cingulate – as well hippocampal and palidal volume loss – over time in MTLE patients.54 Furthermore, using graph theory analysis, Haneef and colleagues noted global decreases in resting fMRI connectivity in MTLE, along with a progressive reduction in connectivity diversity over time.55 Several other investigations have also demonstrated positive relationships between disease severity and/or duration with intrinsic connectivity aberrations in various networks of both MTLE20; 56–58 and FNE59; 60 patients. Although directionality cannot be definitively established from these correlational studies, the progressive nature of connectivity alterations seen in focal epilepsy suggests that brain network perturbations likely develop over time with recurrent seizures, and are less likely to represent inherent network properties which predispose the brain to developing epilepsy.
Figure 4. Widespread decreases in neocortical functional connectivity in focal epilepsy patients.
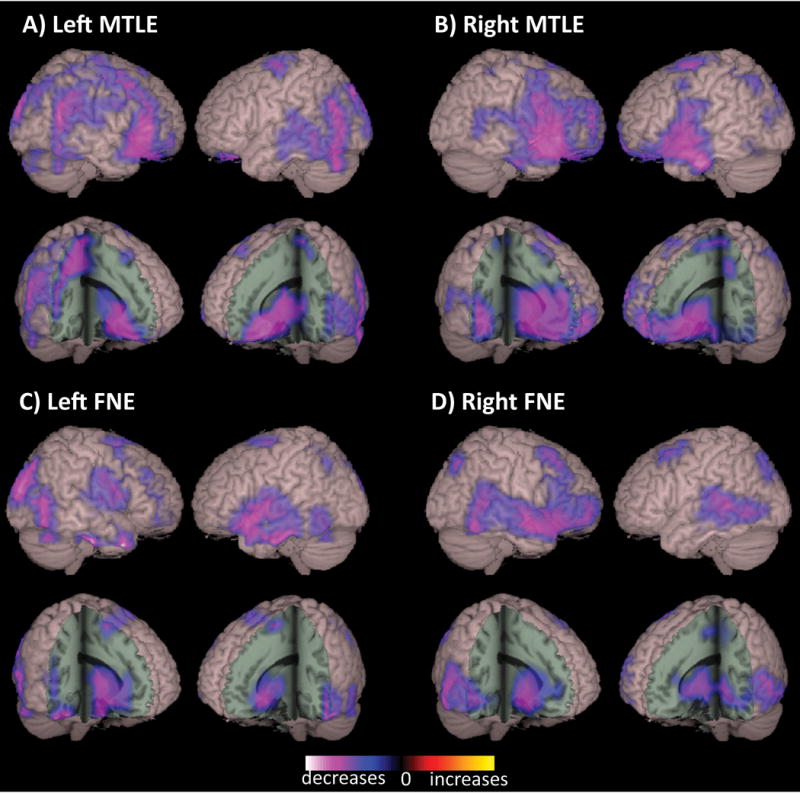
Compared to control subjects, patients with left MTLE (A), right MTLE (B), left FNE (C), and right FNE (D) demonstrated decreased functional connectivity in widespread neocortical regions, including fronto-parietal and posterior temporal association cortex, peri-sylvian neocortex, orbital frontal cortex, as well as decreased subcortical connectivity in basal forebrain and anterior thalamus. Connectivity maps represent t-tests (threshold p < 0.01, FDR-corrected) of alpha-band imaginary coherence in patients with left MTLE (N = 18), right MTLE (N = 12), left FNE (N = 17) or right FNE (N = 14) compared to controls, overlaid on a 3D-rendered template brain. FDR: false discovery rate; FNE: focal neocortical epilepsy; MTLE: mesial temporal lobe epilepsy; RSFC: resting-state functional connectivity. Modified with permission from Englot et al, 2015.43
Another important consideration in examining global connectivity in epilepsy is the potential impact of interictal discharges on connectivity patterns recorded during the “resting” state. Very few studies have examined connectivity with relation to interictal spikes, although Lopes et al. did describe decreased default mode network ipsilateral to the EZ using fMRI-based graph theory analysis in 6 MTLE patients.52 Furthermore, Ibrahim et al. reported an increase in default mode network clustering and a decrease in characteristic path length both preceding and following interictal discharges in 26 children with focal epilepsy, in a study incorporating both fMRI and MEG.61 Given these preliminary findings, it will be important to consider and further examine the potential influence of interictal discharges on measurements of connectivity.
Neurocognitive correlates of connectivity patterns
While numerous studies clearly suggest widespread connectivity disturbances in focal epilepsy patients, what are the potential clinical implications of these changes? Previous investigations have related both regional and global connectivity patterns to various neurocognitive parameters, including memory, language, and executive function.
In one study, Voets and colleagues examined resting-state fMRI recordings in MTLE patients, and found that aberrant seed-based hippocampal-cortical connections were associated with poor performance on a scene encoding memory task.62 Holmes et al. examined connectivity seeded from the left hippocampus in left MTLE patients, observing that connectivity strength with the right precuneus and inferior parietal clusters correlated with verbal memory retention scores.63 This suggests the potential for contralateral compensatory functional in left-sided disease. In another seed-based resting-state fMRI study, Doucet and others found that decreased connectivity between the left non-epileptogenic mesial temporal structures and the medial frontal cortex was positively correlated with delayed recall scores non a non-verbal memory test in right MTLE patients, further demonstrating that adaptive changes may help preserve memory function in unilateral MTLE.40 In another report, this group related fMRI connectivity to both memory and language outcomes after temporal lobectomy, finding that integration patterns in the left inferior frontal region were most closely related to post-operative expressive language function, while connectivity patterns in the healthy hippocampus predicted working memory outcomes.64
Dinkelacker et al. used fMRI and DTI to relate both functional and anatomical connectivity patterns, respectively, to executive function measures in MTLE patients.27 The investigators observed that the number of connections between the diseased hippocampus and thalamus was negatively correlated with performance on a variety of executive function tasks, suggesting a pathologic pattern of limbic remodeling, perhaps along seizure propagation pathways. Impaired executive function and other neuropsychological parameters have also been related to connectivity disturbances in children with frontal lobe epilepsy in two resting-state fMRI studies by another group.47; 65 Diminished connectivity within the frontal lobe and between frontal and other cortical regions was correlated with impaired performance in a visual searching task and other measures of global cognitive function in these investigations. Interestingly, one MEG study in adults noted that reduced functional connectivity in the frontal lobes is most closely tied to duration and severity of epilepsy in both FNE and MTLE, which may contribute to progressive neurocognitive problems seen in these patient populations over time.43 Overall, these reports suggest a clear relationship between altered connectivity patterns and neurocognitive impairments in focal epilepsy, which may reflect the effects of recurrent seizures on involved brain networks.
Possible mechanistic underpinnings of connectivity disturbances
While results vary between studies, a significant portion of the literature suggests that focal epilepsy is associated with regional connectivity increases associated with the EZ, paired with diminished connectivity in widespread distal networks, including neocortical regions important for arousal and cognition. Given that the magnitude of connectivity disturbances mirrors duration of epilepsy or seizure frequency in several investigations,20; 28; 43; 54–60 it is likely that these network changes are related to overall seizure burden over time. However, if seizures in focal epilepsy are by definition confined to a specific brain region, how might recurrent partial seizures result in global network changes?
While the mechanistic underpinnings of interictal global connectivity disturbances in focal epilepsy are poorly understood, several human and animal studies have examined the ictal effects of partial seizures on widespread distal brain networks. Overall, these investigations do support ictal “disconnection” of the EZ from distal cortical networks during focal seizures. ECoG recordings from MTLE patients have shown that while fast poly-spike seizure during focal seizures typically remains confined to the temporal lobe, large-amplitude slow activity in the frontoparietal neocortices during ictal loss of consciousness suggests impaired cortical function.66; 67 Similarly, ictal single-photon emission computed tomography (SPECT) studies have demonstrated elevated temporal lobe cerebral blood flow during consciousness-impairing focal seizures, but reduced cerebral blood flow in the frontoparietal cortices, further signifying neocortical impairment.68 Studies in rodent models have also demonstrated widespread network dysfunction during focal limbic seizures, resulting in decreased neuronal activity, cerebral blood flow, and fMRI signals in bilateral neocortices that are associated with ictal behavioral impairment.69 This suppressed neocortical activity during focal seizures in rodents is starkly contrasted with cortical excitation during secondarily-generalized seizures, which is instead associated with increases in neuronal activity, cerebral blood flow, and fMRI signals.69 Furthermore, ictal neocortical inhibition during focal seizures is associated with aberrant activity in subcortical structures important for cortical activation, such as the thalamus and brainstem reticular activation system,70; 71 and activation of thalamic and brainstem reticular targets during seizures may mitigate this cortical suppression.72; 73
These human and animal studies support the “network inhibition hypothesis” of neocortical deactivation during focal consciousness-impairing seizures, and it is possible that recurrent network inhibition during seizures leads to long-term connectivity reorganization. As summarized in Figure 5, a small consciousness-sparing focal seizure (or simple-partial seizure, using previous terminology) in MTLE may remain confined to the mesial temporal lobe and not impair global cerebral function (Fig. 5A). However, if seizure activity propagates beyond the mesial temporal lobe (Fig. 5B), this may produce aberrant activity in subcortical structures important for neocortical activation (Fig. 5C). Impairment of normal subcortical activating signals may then lead to cortical deactivation, such as that seen during a consciousness-impairing focal seizure (or complex-partial seizure) (Fig. 5D). However, if seizure activity overwhelms the network and spreads broadly to the neocortex, a secondarily-generalized tonic-clonic seizure results (Fig. 5E).
Figure 5. The network inhibition hypothesis of neocortical depression during focal consciousness-impairing seizures.
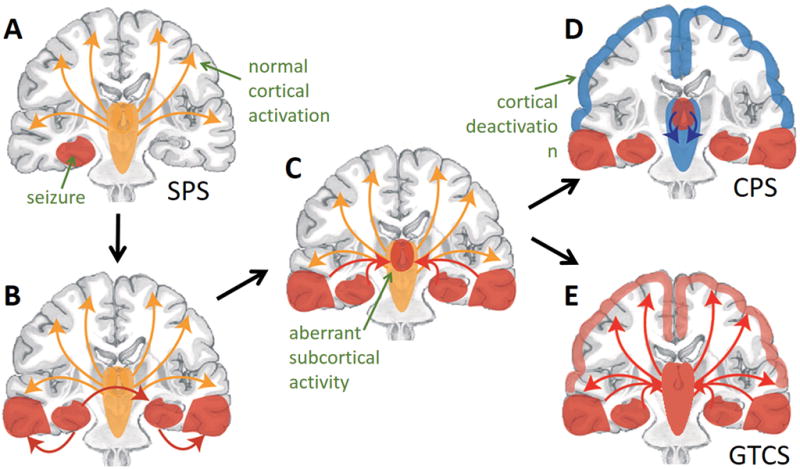
A) In this model, small simple partial seizures (SPS) do not interfere with normal cortical activation, and do not impair consciousness. B,C) However, seizure activity often propagates out of the mesial temporal lobe (B) and begins to involve subcortical activating structures (C). D) Aberrant subcortical activity may lead to diminished cortical activation and thus impairment of consciousness during complex-partial seizures (CPS). E) However, if seizure activity propagates further to widespread neocortical regions, this may lead to a secondarily-generalized tonic-clonic seizures (GTCS, which is also associated with impaired consciousness. Modified with permission from Englot and Blumenfeld, 2009.78
Over time, ictal network inhibition may result in progressive reorganization of interictal subcortical-cortical connectivity in focal epilepsy. Some interictal resting-state studies have indeed demonstrated reductions in thalamocortical connectivity in MTLE,74; 75 and one fMRI investigation which parcellated specific thalamic nuclei suggested that connectivity patterns in the anterior thalamus and pulvinar are most closely tied to duration of epilepsy.76 Furthermore, resting-state thalamocortical connectivity reorganization has been quantitatively related to impairments in long-term memory or alertness.74; 77 While the mechanistic underpinnings of altered subcortical-cortical connectivity are not yet understood, it is conceivable that this phenomenon may represent an evolutionarily adaptive mechanism to limit distal seizure propagation and generalization, or simply damage to neural pathways caused by recurrent seizures.
Summary
Numerous investigations in recent years have studied brain connectivity to better understand network disturbances in focal epilepsy. These have made it clear that while focal epilepsy was traditionally thought of as a regional brain disorder, it actually involves widespread network alterations which extend beyond the EZ. Several studies have related connectivity changes to duration of epilepsy, suggesting that network reorganization results from recurrent seizures, and it is less likely to represent an inherent factor which predisposes the brain to developing epilepsy.
While there is variability among findings across the literature, a significant subset of neuroimaging and electrophysiology studies in MTLE and FNE suggest increased connectivity related to the EZ, coupled with decreased connectivity in widespread networks distal to the EZ. This interictal neocortical deactivation may result over time from ictal network inhibition caused by recurrent focal seizures. Global resting-state connectivity disturbances in focal epilepsy have been linked to neurocognitive problems, including memory and language disturbances. While it is possible that connectivity enhancement in a particular brain region may enhance the propensity for seizure generation, it is not clear if global reductions in connectivity represent the damaging consequences of recurrent seizures, or an adaptive mechanism to prevent seizure spread out of the EZ.
Overall, studying the connectome is focal epilepsy is an important endeavor which may lead to improved strategies for EZ localization, surgical outcome prediction, and a better understanding of the neuropsychological implications of this disorder. Progress has already been made towards each of these goals, but this field remains quite young, and challenges remain. Inconsistent results across studies may be influenced by the heterogeneity of this disease, and by the diversity of anti-epileptic medications taken by patients, of which the impact on brain connectivity remains unknown. Furthermore, it is likely that differences in study modality and analysis methods between investigations contributes to varied findings.
Future directions
In future studies, it will be useful to apply several modalities and connectivity analysis protocols to the same patients under the same conditions, to better elucidate the implications of methodology in influencing connectivity results in focal epilepsy. For instance, comparing fMRI, MEG, and ECoG-based measurements of connectivity in the same patients may not only help clarify discrepancies in previous studies utilizing only one technique, but will also help elucidate the similarities and differences between connectivity measures from these various modalities.
It will also be important to clarify the role of anti-epileptic medications in influencing connectivity patterns, given that nearly all of the patients in the studies summarized here were taking anticonvulsants at the time of the investigation. As anti-epileptic drugs are often weaned or held during hospital admissions for long-term seizure monitoring, this time period may present a valuable opportunity for serial connectivity measurements on and off of medication.
Finally, while several studies have demonstrated progressive connectivity alterations in epilepsy, it will be important to re-examine patients who have become seizure free after successful epilepsy surgery, to determine whether connectivity perturbations in epilepsy are reversible. In patients who experience neurocognitive improvement (or decline) after epilepsy surgery, it will also be valuable to examine the connectivity correlates of these changes. Ultimately, it is hoped that a better understanding of regional and global network properties in focal epilepsy will lead to improved treatment strategies for both seizures and neurocognitive sequelae in this devastating disorder.
Key Bullet Points.
Brain connectivity studies in focal epilepsy have demonstrated widespread network alterations which extend beyond the epileptogenic zone.
It is important to consider study modality and analysis methods when interpreting and comparing connectivity studies in epilepsy.
Many studies suggest connectivity increases related to the epileptogenic zone with widespread decreases in distal brain networks.
Altered connectivity patterns may be related to neurocognitive deficits, and may aid surgical planning and outcome prediction in epilepsy.
Acknowledgments
This work was supported in part by NIH K99 NS097618 (DJE) and NIH R01 NS075270 (VLM).
Footnotes
Disclosures of Conflicts of Interest
The authors have no conflicts of interest to disclose.
Ethical publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Sporns O. The human connectome: origins and challenges. Neuroimage. 2013;80:53–61. doi: 10.1016/j.neuroimage.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Weiss PS. President Obama announces the BRAIN Initiative. ACS Nano. 2013;7:2873–2874. doi: 10.1021/nn401796f. [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300:2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 4.Hughlings-Jackson J. Notes on the physiology and pathology of the nervous system. Med Times Gazette. 1868:696. [Google Scholar]
- 5.Jiruska P, de Curtis M, Jefferys JG, et al. Synchronization and Desynchronization in Epilepsy: Controversies and Hypotheses. J Physiol. 2013 doi: 10.1113/jphysiol.2012.239590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel J, Jr, Thompson PM, Stern JM, et al. Connectomics and epilepsy. Curr Opin Neurol. 2013;26:186–194. doi: 10.1097/WCO.0b013e32835ee5b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittau F, Megevand P, Sheybani L, et al. Mapping epileptic activity: sources or networks for the clinicians? Front Neurol. 2014;5:218. doi: 10.3389/fneur.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NINDS. NINDS Benchmarks for Epilepsy Research, 2014. 2014 Available at: http://www.ninds.nih.gov/research/epilepsyweb/2014benchmarks.htm.
- 9.Liu M, Chen Z, Beaulieu C, et al. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia. 2014;55:674–682. doi: 10.1111/epi.12581. [DOI] [PubMed] [Google Scholar]
- 10.Chang HC, Sundman M, Petit L, et al. Human brain diffusion tensor imaging at submillimeter isotropic resolution on a 3Tesla clinical MRI scanner. Neuroimage. 2015;118:667–675. doi: 10.1016/j.neuroimage.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constable RT, Scheinost D, Finn ES, et al. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Front Neurol. 2013;4:39. doi: 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 13.Li K, Guo L, Nie J, et al. Review of methods for functional brain connectivity detection using fMRI. Comput Med Imaging Graph. 2009;33:131–139. doi: 10.1016/j.compmedimag.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolston JD, Ouyang D, Englot DJ, et al. National trends and complication rates for invasive extraoperative electrocorticography in the USA. J Clin Neurosci. 2015;22:823–827. doi: 10.1016/j.jocn.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centeno M, Carmichael DW. Network Connectivity in Epilepsy: Resting State fMRI and EEG-fMRI Contributions. Front Neurol. 2014;5:93. doi: 10.3389/fneur.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalal SS, Guggisberg AG, Edwards E, et al. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englot DJ, Nagarajan SS, Wang DD, et al. The sensitivity and significance of lateralized interictal slow activity on magnetoencephalography in focal epilepsy. Epilepsy Res. 2016;121:21–28. doi: 10.1016/j.eplepsyres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan VL, Li Y, Abou-Khalil B, et al. Development of 2dTCA for the detection of irregular, transient BOLD activity. Hum Brain Mapp. 2008;29:57–69. doi: 10.1002/hbm.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan VL, Abou-Khalil B, Rogers BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect. 2015;5:35–44. doi: 10.1089/brain.2014.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negishi M, Martuzzi R, Novotny EJ, et al. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52:1733–1740. doi: 10.1111/j.1528-1167.2011.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HW, Arora J, Papademetris X, et al. Altered functional connectivity in seizure onset zones revealed by fMRI intrinsic connectivity. Neurology. 2014;83:2269–2277. doi: 10.1212/WNL.0000000000001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Choupan J, Reutens D, et al. Lateralization of Temporal Lobe Epilepsy Based on Resting-State Functional Magnetic Resonance Imaging and Machine Learning. Front Neurol. 2015;6:184. doi: 10.3389/fneur.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao W, Zhang Z, Pan Z, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haneef Z, Lenartowicz A, Yeh HJ, et al. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maccotta L, He BJ, Snyder AZ, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013;2:862–872. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinkelacker V, Valabregue R, Thivard L, et al. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: Enhanced connectivity per hippocampal voxel. Epilepsia. 2015;56:1217–1226. doi: 10.1111/epi.13051. [DOI] [PubMed] [Google Scholar]
- 28.Morgan VL, Rogers BP, Sonmezturk HH, et al. Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia. 2011;52:1741–1749. doi: 10.1111/j.1528-1167.2011.03196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo C, An D, Yao D, et al. Patient-specific connectivity pattern of epileptic network in frontal lobe epilepsy. Neuroimage Clin. 2014;4:668–675. doi: 10.1016/j.nicl.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen M, Curwood EK, Vaughan DN, et al. Abnormal Brain Areas Common to the Focal Epilepsies: Multivariate Pattern Analysis of fMRI. Brain Connect. 2016 doi: 10.1089/brain.2015.0367. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Ge S, Zhang R, et al. Neuromagnetic coherence of epileptic activity: an MEG study. Seizure. 2014;23:417–423. doi: 10.1016/j.seizure.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Zaveri HP, Pincus SM, Goncharova, et al. Localization-related epilepsy exhibits significant connectivity away from the seizure-onset area. Neuroreport. 2009;20:891–895. doi: 10.1097/WNR.0b013e32832c78e0. [DOI] [PubMed] [Google Scholar]
- 33.Bettus G, Wendling F, Guye M, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Bandt SK, Bundy DT, Hawasli AH, et al. The role of resting state networks in focal neocortical seizures. PLoS One. 2014;9:e107401. doi: 10.1371/journal.pone.0107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim GM, Anderson R, Akiyama T, et al. Neocortical pathological high-frequency oscillations are associated with frequency-dependent alterations in functional network topology. J Neurophysiol. 2013;110:2475–2483. doi: 10.1152/jn.00034.2013. [DOI] [PubMed] [Google Scholar]
- 36.Bartolomei F, Bettus G, Stam CJ, et al. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin Neurophysiol. 2013;124:2345–2353. doi: 10.1016/j.clinph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Antony AR, Alexopoulos AV, Gonzalez-Martinez JA, et al. Functional connectivity estimated from intracranial EEG predicts surgical outcome in intractable temporal lobe epilepsy. PLoS One. 2013;8:e77916. doi: 10.1371/journal.pone.0077916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes GL, Tian C, Hernan AE, et al. Alterations in sociability and functional brain connectivity caused by early-life seizures are prevented by bumetanide. Neurobiol Dis. 2015;77:204–219. doi: 10.1016/j.nbd.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittau F, Grova C, Moeller F, et al. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. 2012;53:1013–1023. doi: 10.1111/j.1528-1167.2012.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doucet G, Osipowicz K, Sharan A, et al. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2013;34:2202–2216. doi: 10.1002/hbm.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Lu G, Zhong Y, et al. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 2010;1323:152–160. doi: 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 42.Voets NL, Beckmann CF, Cole DM, et al. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- 43.Englot DJ, Hinkley LB, Kort NS, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015;138:2249–2262. doi: 10.1093/brain/awv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo C, Qiu C, Guo Z, et al. Disrupted functional brain connectivity in partial epilepsy: a resting-state fMRI study. PLoS One. 2011;7:e28196. doi: 10.1371/journal.pone.0028196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widjaja E, Zamyadi M, Raybaud C, et al. Disrupted Global and Regional Structural Networks and Subnetworks in Children with Localization-Related Epilepsy. AJNR Am J Neuroradiol. 2015;36:1362–1368. doi: 10.3174/ajnr.A4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widjaja E, Mahmoodabadi SZ, Snead OC, 3rd, et al. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52:1685–1691. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- 47.Braakman HM, Vaessen MJ, Jansen JF, et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia. 2013;54:446–454. doi: 10.1111/epi.12044. [DOI] [PubMed] [Google Scholar]
- 48.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haneef Z, Lenartowicz A, Yeh HJ, et al. Network analysis of the default mode network using functional connectivity MRI in Temporal Lobe Epilepsy. J Vis Exp. 2014:e51442. doi: 10.3791/51442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haneef Z, Lenartowicz A, Yeh HJ, et al. Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav. 2012;25:350–357. doi: 10.1016/j.yebeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucukboyaci NE, Kemmotsu N, Cheng CE, et al. Functional connectivity of the hippocampus in temporal lobe epilepsy: feasibility of a task-regressed seed-based approach. Brain Connect. 2013;3:464–474. doi: 10.1089/brain.2013.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes R, Moeller F, Besson P, et al. Study on the Relationships between Intrinsic Functional Connectivity of the Default Mode Network and Transient Epileptic Activity. Front Neurol. 2014;5:201. doi: 10.3389/fneur.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nedic S, Stufflebeam SM, Rondinoni C, et al. Using network dynamic fMRI for detection of epileptogenic foci. BMC Neurol. 2015;15:262. doi: 10.1186/s12883-015-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan VL, Conrad BN, Abou-Khalil B, et al. Increasing structural atrophy and functional isolation of the temporal lobe with duration of disease in temporal lobe epilepsy. Epilepsy Res. 2015;110:171–178. doi: 10.1016/j.eplepsyres.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haneef Z, Chiang S, Yeh HJ, et al. Functional connectivity homogeneity correlates with duration of temporal lobe epilepsy. Epilepsy Behav. 2015;46:227–233. doi: 10.1016/j.yebeh.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiang S, Stern JM, Engel J, Jr, et al. Structural-functional coupling changes in temporal lobe epilepsy. Brain Res. 2015;1616:45–57. doi: 10.1016/j.brainres.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Fan W, Yang J, et al. Asymmetry in cross-hippocampal connectivity in unilateral mesial temporal lobe epilepsy. Epilepsy Res. 2015;118:14–21. doi: 10.1016/j.eplepsyres.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Madhavan D, Heinrichs-Graham E, Wilson TW. Whole-brain functional connectivity increases with extended duration of focal epileptiform activity. Neurosci Lett. 2013;542:26–29. doi: 10.1016/j.neulet.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 59.Cao X, Qian Z, Xu Q, et al. Altered intrinsic connectivity networks in frontal lobe epilepsy: a resting-state fMRI study. Comput Math Methods Med. 2014;2014:864979. doi: 10.1155/2014/864979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Dellen E, Douw L, Hillebrand A, et al. Epilepsy surgery outcome and functional network alterations in longitudinal MEG: a minimum spanning tree analysis. Neuroimage. 2014;86:354–363. doi: 10.1016/j.neuroimage.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim GM, Cassel D, Morgan BR, et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. 2014;137:2690–2702. doi: 10.1093/brain/awu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voets NL, Zamboni G, Stokes MG, et al. Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci. 2014;34:4920–4928. doi: 10.1523/JNEUROSCI.4281-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holmes M, Folley BS, Sonmezturk HH, et al. Resting state functional connectivity of the hippocampus associated with neurocognitive function in left temporal lobe epilepsy. Hum Brain Mapp. 2014;35:735–744. doi: 10.1002/hbm.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doucet GE, Rider R, Taylor N, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56:517–526. doi: 10.1111/epi.12936. [DOI] [PubMed] [Google Scholar]
- 65.Vaessen MJ, Braakman HM, Heerink JS, et al. Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cereb Cortex. 2013;23:1997–2006. doi: 10.1093/cercor/bhs186. [DOI] [PubMed] [Google Scholar]
- 66.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 68.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 69.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Englot DJ, Modi B, Mishra AM, et al. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furman M, Zhan Q, McCafferty C, et al. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: Effects on cortical physiology. Epilepsia. 2015;56:e198–202. doi: 10.1111/epi.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen XM, Huang DH, Chen ZR, et al. Temporal lobe epilepsy: decreased thalamic resting-state functional connectivity and their relationships with alertness performance. Epilepsy Behav. 2015;44:47–54. doi: 10.1016/j.yebeh.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 75.He X, Doucet GE, Sperling M, et al. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia. 2015 doi: 10.1111/epi.13085. [DOI] [PubMed] [Google Scholar]
- 76.Morgan VL, Rogers BP, Abou-Khalil B. Segmentation of the Thalamus Based on BOLD Frequencies Affected in Temporal Lobe Epilepsy. Epilepsia. 2015;56:1819–1827. doi: 10.1111/epi.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voets NL, Menke RA, Jbabdi S, et al. Thalamo-Cortical Disruption Contributes to Short-Term Memory Deficits in Patients with Medial Temporal Lobe Damage. Cereb Cortex. 2015;25:4584–4595. doi: 10.1093/cercor/bhv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res. 2009;177:147–170. doi: 10.1016/S0079-6123(09)17711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


