Abstract
PURPOSE
To examine the range of practice in laboratory testing utilization among a subset of uveitis specialists using a scenario-based survey.
DESIGN
Cross-sectional survey.
METHODS
A web-based survey consisting of 13 patient scenarios was presented to the Executive Committee and Trustees of the American Uveitis Society. The participants were allowed to choose preferred testing in a free-form manner. The patterns of test utilization were studied and the cost of the testing was calculated based on Noridian Medicare reimbursal rates for Seattle, Washington.
RESULTS
Nearly all providers recommended some testing for all scenarios. Forty-five different tests, including laboratory investigations and imaging and diagnostic procedures, were ordered. The mean number of tests ordered per scenario per provider was 5.47 ± 2.71. There was limited consensus among providers in test selection, with most tests in each scenario ordered by fewer than half of the providers. Average cost of testing per scenario per provider was $282.80, with 4 imaging tests (fluorescein angiography, magnetic resonance imaging, chest radiograph, and chest computed tomography) together contributing 59.9% of the total testing costs.
CONCLUSIONS
Uveitis specialists have a high rate of laboratory testing utilization in their evaluation of new patients. There is substantial variability in the evaluations obtained between providers. Imaging tests account for the majority of evaluation cost. The low agreement on specific testing plans suggests need for evidence-based practice guidelines for the evaluation of uveitis patients.
Uveitic conditions are commonly encountered in ophthalmology practice, with estimated incidence in large epidemiologic studies in the United States of approximately 50 per 100 000 person-years.1 This suggests approximately 150 000 incident cases in the United States each year. Determination of the etiology of uveitic disease typically entails laboratory testing. This testing is essential to ensure that treatable infectious diseases are identified and appropriately treated; to identify possible comorbid systemic disease associations that should be evaluated and potentially treated; and to provide prognostic information for the patient and physician. Retrospective studies have suggested that a definitive etiology (either infectious or associated with a systemic condition) is found overall in 26%–40% of patients in a tertiary referral setting.2,3
A detailed history, review of systems, and accurate physical examination are essential in guiding the appropriate diagnostic tests in the evaluation of uveitis. There is no “standard laboratory workup” for uveitis, and unfocused ordering of diagnostic tests can be difficult to interpret and costly.4,5 Over-testing may lead to improper treatment as well, owing to false-positive results.6,7 Clinicians ordering laboratory testing are primarily interested in the positive predictive value (the probability that the patient has a given condition, given a positive test) and the negative predictive value (the probability that the patient does not have disease, given a negative test). These values can be calculated using Bayesian statistics for a given test if the sensitivity and specificity of the test are known, as well as the prevalence of the condition in the tested population.7 However, the result of a particular test must be interpreted with caution, given that the predictive parameters of each test can vary in different settings.8
As cost containment becomes more prevalent in medical practice, scrutiny of laboratory testing practices is increasing.9 At present, there are no global practice guidelines for laboratory testing in uveitis. The purpose of the present study was to examine the range of practice in laboratory testing utilization among a subset of uveitis specialists and determine its cost implications.
METHODS
A web-based survey was presented to the Executive Committee and Trustees of the American Uveitis Society. The University of Washington Institutional Review Board approval was waived for this research, and the study was in adherence to the Declaration of Helsinki. The survey included 13 hypothetical clinical case-based scenarios (Supplementary Table; Supplemental Material available at AJO.com). The scenarios were designed to be representative of real-life diagnostic challenges. For none of the cases was an exclusive diagnosis strongly suggested by the history and findings. For each scenario, participants were asked to list the investigations that they would order. A comprehensive list of laboratory investigations, imaging modalities, and diagnostic procedures were included as a guide, but the participants were allowed to specify any testing they desired. The survey also included 5 additional questions regarding the practice pattern of the responding physicians.
The descriptive statistics and all analyses were performed with R (www.r-project.org). The cost of the testing was calculated based on 2013 Noridian Medicare reimbursement rates for Seattle, Washington.
RESULTS
Twelve of 14 members of American Uveitis Society executive committee and trustees responded to the survey. One physician reported not having a majority uveitis practice (seeing fewer than 50 new uveitis patients a year) and was excluded from the analysis. The mean number of years since completion of fellowship was 17.16 (range 8–32). Fifty-percent were hospital based and 50% outpatient office based. Of the respondents, 45.45% saw approximately 101–250 new uveitis patients annually, while 27.27% each saw either between 51 and 100 or greater than 250. Demographics of respondents are shown in Table 1.
TABLE 1.
Practice Patterns of Survey Participants
| Provider ID | # of New Uveitis Patients Annually | Primary Practice Sitea | Consideration of the Patient’s Insurance Status | Years in Practice | Majority Uveitis Practice |
|---|---|---|---|---|---|
| 1 | 101–250 | Outpatient | Weakly | 24 | True |
| 2 | 51–100 | Hospital | Weakly | 8 | True |
| 3 | 101–250 | Hospital | Moderately strongly | 11 | True |
| 4 | 101–250 | Outpatient | Not at all | 8.75 | True |
| 5 | >250 | Outpatient | Moderately strongly | 32 | True |
| 6 | 101–250 | Hospital | Moderately strongly | 8 | True |
| 7 | >250 | Hospital | Moderately strongly | 20 | True |
| 8 | 51–100 | Outpatient | Not at all | 25 | True |
| 9 | 51–100 | Hospital | Moderately strongly | 8 | True |
| 10 | 101–250 | Outpatient | Moderately weakly | 29 | True |
| 11 | >250 | Hospital | Moderately weakly | 15 | True |
Outpatient: outpatient, office-based; hospital: hospital-based.
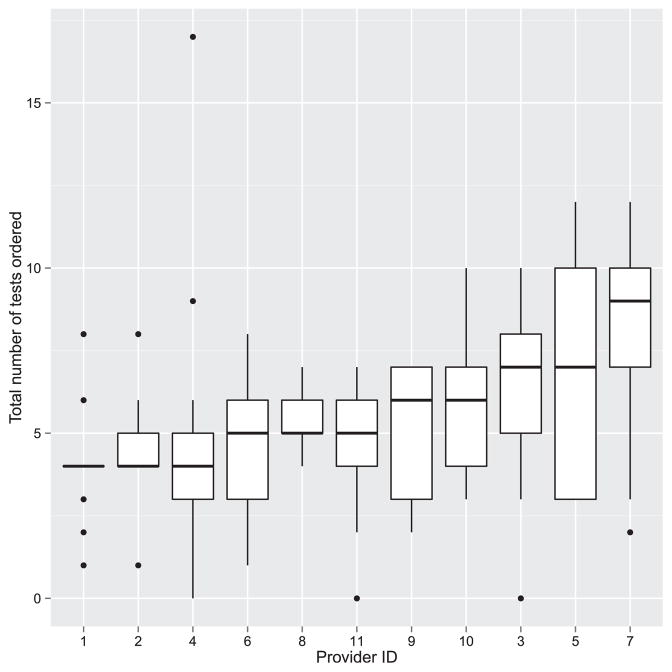
The details of the specific tests ordered by each physician are summarized in Table 2. A total of 782 investigations were ordered by the 11 providers for the 13 scenarios. Forty-five different tests, including laboratory tests and imaging and diagnostic procedures, were ordered. The mean number of tests ordered per case scenario per provider was 5.47 ± 2.71; the median was 5.0 (Figure 1). In aggregate, the highest number of tests was ordered for Scenario 5 (46-year-old man with prior Bacillus Calmette-Guérin [BCG] vaccine presenting with positive purified protein derivative [PPD] test presenting with bilateral perivenous sheathing, unilateral neovascularization and vitreous hemorrhage, 83 tests, 7.54 tests per provider) and the lowest for Scenario 12 (27-year-old woman with unilateral acute anterior uveitis in the setting of known ulcerative colitis, 31 tests, 2.82 tests per provider). The highest test utilizer ordered 105 tests (average of 8.18 tests/scenario), while the lowest ordered 54 tests (average 4.15/scenario). In only 3 instances (2.1% of opportunities) did any provider decline to order laboratory testing (Scenarios 12 and 13).
TABLE 2.
Specific Tests Ordered per Scenario by All Responders (N = 11)
| Scenario | Tests Ordered | Average Number of Tests |
|---|---|---|
| 1 | ACE (3), CBC (1), CT chest (1), CXR (8), HLA-B27 (11), Lyme (2), Lysozyme (1), OCT (1), PPD (2), RPR (5), Syphilis ab (10) | 4.09 |
| 2 | A1C (1), ACE (4), ANA (1), B2 microglobulin (4), Bartonella (1), CBC (8), CMP (5), CXR (10), Creatinine (4), ESR (4), FA (4), Fundus photo (1), HIV (1), Lyme (2), Lysozyme (1), MRI brain (1), OCT (3), PPD (2), QuantiFERON (1), RF (1), RPR (5), Syphilis ab (10), UA (6) | 7.27 |
| 3 | ACE (5), ANA (2), CBC (6), CMP (5), CT chest (3), CXR (9), ESR (2), FA (7), ICG (1), Lyme (2), Lysozyme (2), MRI brain (1), OCT (6), PPD (4), QuantiFERON (1), RF (1), RPR (4), Skin biopsy (1), Syphilis ab (11), UA (1), Viral PCR (1) | 6.82 |
| 4 | ACE (5), CBC (2), CMP (2), CT chest (2), CXR (9), ESR (1), FA (5), Fundus photo (1), HLA-A29 (1), HTLV (1), Lyme (4), Lysozyme (1), MRI brain (5), OCT (8), PPD (3), RPR (4), Syphilis ab (11) | 5.91 |
| 5 | ACE (4), ANA (2), ANCA (1), Bartonella (2), CBC (4), CMP (3), CRP (2), CT chest (4), CXR (8), ESR (3), FA (9), Fundus photo (1), HIV (1), HLA-B51 (1), HTLV (1), Lupus ab (1), Lyme (3), Lysozyme (2), OCT (1), PPD (1), QuantiFERON (8), RF (2), RPR (4), Syphilis ab (10), Toxoplasmosis ab (1), West Nile (1), anti-CCP (1), anti-RNP (1), anti-SS (1) | 7.55 |
| 6 | ACE (1), ANA (10), CBC (5), CMP (2), CXR (4), ESR (1), HLA-B27 (2), Lyme (2), Lysozyme (1), OCT (5), PPD (1), RF (2), RPR (1), Syphilis ab (3) | 3.64 |
| 7 | Bartonella (3), CBC (6), CMP (2), CXR (4), Creatinine (1), Fundus photo (2), HIV (1), ICG (1), Liver panel (1), Lyme (1), PPD (4), QuantiFERON (1), RPR (1), Syphilis ab (8), Toxocara ab (1), Toxoplasmosis ab (10) | 4.27 |
| 8 | ACE (2), ANA (1), ANCA (1), CBC (5), CMP (4), CXR (6), ESR (1), FA (2), Fundus photo (2), HIV (3), HLA-B27 (1), HLA-B51 (1), Lyme (2), MRI brain (1), PPD (3), RPR (6), Syphilis ab (10), Toxoplasmosis ab (4), UA (2), Viral PCR (8) | 5.91 |
| 9 | ANA (6), ANCA (11), CBC (6), CMP (4), CRP (4), CXR (6), Creatinine (2), ESR (7), Lyme (2), PPD (2), RF (7), RPR (1), Syphilis ab (8), UA (5), anti-CCP (5) | 6.91 |
| 10 | ACE (3), CBC (7), CMP (4), CT chest (2), CXR (9), ESR (1), FA (5), Fundus photo (1), HTLV (1), Lyme (4), Lysozyme (1), MRI brain (4), OCT (3), PPD (4), RPR (4), Syphilis ab (10), UA (1), Viral PCR (1) | 5.91 |
| 11 | ACE (4), CBC (5), CMP (4), CT chest (2), CXR (6), Creatinine (2), ERG (2), FA (7), Fundus photo (2), GVF (1), HLA-A29 (9), HVF (2), Hepatitis panel (1), ICG (3), Liver panel (1), Lyme (2), Lysozyme (1), OCT (6), RPR (5), Syphilis ab (9), UA (1) | 6.82 |
| 12 | ACE (1), CBC (2), CMP (1), CXR (5), ESR (2), HLA-B27 (8), Lyme (1), PPD (1), RPR (4), Syphilis ab (6) | 2.82 |
| 13 | ACE (2), CXR (7), HLA-B27 (10), Lyme (2), Lysozyme (1), PPD (1), RPR (4), Syphilis ab (8) | 3.18 |
A1C = Hemoglobin A1C; ACE = angiotensin-converting enzyme; ANA = antinuclear ab; ANCA = antineutrophil cytoplasmic ab; anti-CCP = anti–cyclic citrullinated peptide ab; anti-RNP = anti-ribonucleoprotein ab; anti-SS = anti–Sjogren syndrome ab; CBC = complete blood count; CT = computed tomography; CXR = chest radiograph; ESR = erythrocyte sedimentation rate; FA = fluorescein angiography; HTLV = human T-cell lymphotropic virus; ICG = indocyanine green angiography; Lupus ab = lupus anticoagulant; MRI = magnetic resonance imaging; OCT = optical coherence tomography; PCR = polymerase chain reaction; PPD = purified protein derivative test; RF = rheumatoid factor; RPR = test including rapid plasma reagin and venereal disease research laboratory test; Syphilis ab = test including fluorescent treponemal antibody (ab), microhemagglutination assay, and treponema pallidum particle agglutination; Toxocara ab = toxocara IgM or IgG; Toxoplasmosis ab = toxomplasmosis IgM or IgG; UA = urinalysis with microbiology.
FIGURE 1.
Distribution of the total number of ordered tests per scenario by each provider.
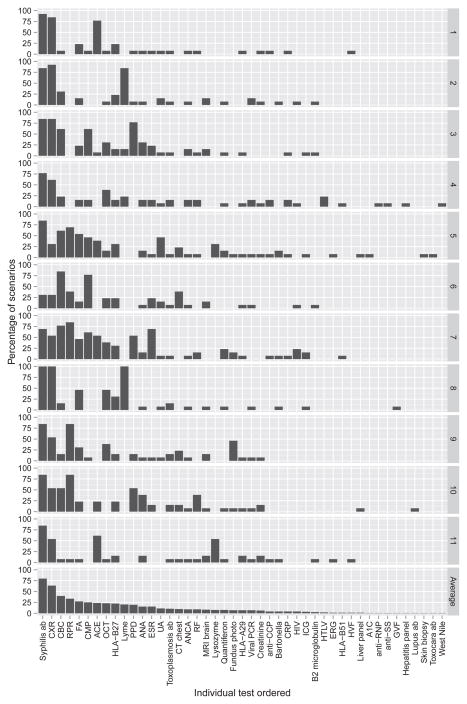
The total number of times any test could be ordered was 143 (13 scenarios multiplied by 11 respondents). The frequency of each specific test being ordered in this study, the cost per test, and the total cost of the ordered tests are presented in Table 3. The most commonly ordered tests were treponemal antibody tests (ie, fluorescent treponemal antibody - absorption test, treponema pallidum particle agglutination, or microhemagglutination assay [114 of 143 possible orders, 79.72%]), followed by chest radiography (91, 63.63%), complete blood count (57, 39.86%), non–treponemal tests (ie, rapid plasma reagin or venereal disease research laboratory test; 48, 33.57%), purified protein derivative/QuantiFERON (39, 27.27%), fluorescein angiogram (39, 27.27%), and angiotensin-converting enzyme (34, 23.78%). Remarkably, there was almost no consensus on evaluation between providers (Figure 2). Only for 1 test (antineutrophil cytoplasmic antibody) for 1 scenario (Scenario 9, unilateral scleritis) was there universal agreement. Most laboratory tests were ordered by less than half of the participants (Figure 2).
TABLE 3.
Details of the Frequency of the Ordered Tests and the Associated Costs of All 13 Clinical Scenarios from 11 Uveitis Providers
| Diagnostic Test | Number of Orders | Cost per Order ($) | Total Cost ($) |
|---|---|---|---|
| Tests With No Diagnostic Value | |||
| CBC | 57 | 8.9 | 507.3 |
| CMP | 36 | 14.5 | 522 |
| Creatinine | 9 | 7 | 63 |
| Hgb A1C | 1 | 13.3 | 13.3 |
| Liver panel | 2 | 11.2 | 22.4 |
| Hepatitis panel | 1 | 20.1 | 20.1 |
| ESR | 22 | 3.7 | 81.4 |
| CRP | 6 | 7.1 | 42.6 |
| Ocular Tests | |||
| Fundus photo | 10 | 69.2 | 692 |
| FA | 39 | 199.2 | 7768.8 |
| ICG | 5 | 199.2 | 996 |
| OCT | 33 | 56.5 | 1864.5 |
| HVF | 2 | 75.1 | 150.2 |
| GVF | 1 | 50.5 | 50.5 |
| ERG | 2 | 121.9 | 243.8 |
| Viral PCR | 10 | 196 | 1960 |
| Non–Ocular Tests | |||
| ACE | 34 | 20.1 | 683.4 |
| Lysozyme | 11 | 25.8 | 283.8 |
| ANA | 22 | 16.6 | 365.2 |
| ANCA | 13 | 17.8 | 231.4 |
| RF | 13 | 7.8 | 101.4 |
| anti-CCP | 6 | 17.8 | 106.8 |
| anti-RNP | 1 | 24.7 | 24.7 |
| anti-SS | 1 | 49.3 | 49.3 |
| HLA-B27 | 32 | 37.7 | 1206.4 |
| HLA-A29 | 10 | 33.1 | 331 |
| HLA-B51 | 2 | 81.9 | 163.8 |
| Syphilis ab | 114 | 18.2 | 2074.8 |
| RPR | 48 | 6.1 | 292.8 |
| HIV | 6 | 33.1 | 198.6 |
| HTLV | 3 | 11.5 | 34.5 |
| Bartonella | 6 | 48.2 | 289.2 |
| Lupus ab | 1 | 11.7 | 11.7 |
| Lyme ab | 29 | 23.4 | 678.6 |
| Toxocara ab | 1 | 17.9 | 17.9 |
| Toxoplasmosis ab | 15 | 19.8 | 297 |
| West Nile | 1 | 58.9 | 58.9 |
| PPD | 28 | 6 | 168 |
| QuantiFERON | 11 | 103 | 1133 |
| UA with micro | 16 | 4.4 | 70.4 |
| Urine B2 | 4 | 20 | 80 |
| Skin biopsy | 1 | 0 | 0 |
| MRI brain | 12 | 539.7 | 6476.4 |
| CT chest | 14 | 307 | 4298 |
| Chest XR | 91 | 62.8 | 5714.8 |
| Total | 782 | 2677.7 | 40 439.7 |
| Per provider | 3676.34 | ||
| Per scenario per provider | 282.80 | ||
ACE = angiotensin-converting enzyme; ANA = antinuclear ab; ANCA = antineutrophil cytoplasmic ab; anti-CCP = anti–cyclic citrullinated peptide ab; anti-RNP = anti-ribonucleoprotein ab; anti-SS = anti-Sjogren syndrome ab; CBC = complete blood count; CMP = complete metabolic panel; CRP = C-reactive protein; CT = computed tomography; ERG = electroretinogram; ESR = erythrocyte sedimentation rate; FA = fluorescein angiography; GVF = Goldmann visual field; Hgb = hemoglobin; HIV = human immunodeficiency virus; HTLV = human T-cell lymphotropic virus; HVF = Humphrey visual field; ICG = indocyanine green angiography; Lupus ab = lupus anticoagulant; MRI = magnetic resonance imaging; OCT = optical coherence tomography; PCR = polymerase chain reaction; PPD = purified protein derivative skin test; RF = rheumatoid factor; RPR = test including rapid plasma reagin and venereal disease research laboratory test; Syphilis ab = test including fluorescent treponemal antibody absorption test, microhemagglutination assay, and treponema pallidum particle agglutination; Toxocara ab = toxocara IgM or IgG; Toxoplasmosis ab = toxoplasmosis IgM or IgG; UA with micro = urinalysis with microbiology; Urine B2 = urine beta2 microglobulin; XR = radiography.
FIGURE 2.
Frequency of test orders across all scenarios by provider. Bottom row shows average percentage of cases in which an individual test was ordered by all 11 providers. Provider numbers (1–11) are designated on right y-axis. Test abbreviations: Syphilis ab = fluorescent treponemal antibody (ab) absorption test, microhemagglutination assay, treponema pallidum particle agglutination; CXR = chest radiograph; CBC = complete blood count; RPR = rapid plasma reagin, venereal disease research laboratory test; FA = fluorescein angiography; CMP = complete metabolic panel; ACE = angiotensin-converting enzyme; OCT = ocular coherence tomography; PPD = purified protein derivative skin test; ANA = antinuclear antibody; ESR = erythrocyte sedimentation rate; UA = urinalysis; ANCA = antineutrophil cytoplasmic ab; RF = rheumatoid factor; anti-CCP, cyclic citrullinated peptide ab; CRP = C-reactive protein; ICG = indocyanine green angiography; HTLV = human T-cell lymphotropic virus; ERG = electroretinogram; HVF = Humphrey visual field; A1C = hemoglobin A1C; anti-RNP = anti-ribonucleoprotein ab; antiSS = anti–Sjögren syndrome ab; GVF = Goldmann visual field.
The mean cost per scenario per provider was $282.80 (range $0 to $1145.50). The scenario that incurred the highest average cost was Scenario 4 ($544.14) and the lowest was Scenario 12 ($76.22). The testing that had the highest impact on cost were fluorescein angiography (19.21% of total testing cost), magnetic resonance imaging (16.01%), chest radiograph (14.13%), and chest computed tomography (10.63%), which together contributed to 59.99% of the total testing costs.
Participants were asked whether the patient’s insurance status would be in consideration when formulating a diagnostic evaluation. Five respondents (45.45%) reported that they would consider it moderately strongly and 2 (18.18%) reported that they would consider it moderately weakly. Two (18.18%) of each reported that they would consider it weakly or not at all.
A linear regression model for predicting the number of tests ordered was created using the following as predictors: number of patients seen, years since training, attitude toward insurance status, and type of practice (hospital or outpatient). None of these predictors was significant, although there was a trend for individuals seeing a high number of patients to order more testing.
DISCUSSION
This study surveyed the range of Laboratory testing utilization among a subset of uveitis specialists. Survey respondents generally agreed on the need for laboratory testing in most of the scenarios presented (which were typical presentations to a tertiary uveitis practice); however, there was minimal agreement on the specific laboratory tests appropriate for any particular scenario.
As would be expected, utilization of laboratory testing varied with clinical scenario. The scenario that generated the least amount of testing was the only case in which the patient already had a diagnosis of a systemic inflammatory condition, ulcerative colitis. For this patient, who presented with unilateral anterior chamber and vitreous inflammation, 2 survey participants chose to order no testing. In contrast, the scenario that generated the most number of tests was the fifth case, in which a 46-year-old man with positive PPD in the setting of prior BCG vaccination presented with perivenous sheathing in both eyes and vitreous hemorrhage in 1 eye. Interestingly, the diagnosis of juvenile idiopathic arthritis (JIA) was a highly likely diagnosis for Case 6. Even though the presence of the antinuclear antibodies (ANA) is one of the main diagnostic criteria for JIA,10,11 only 83% of the uveitis specialist participants chose to test for ANA in this scenario.
The significant variability in the utilization persisted when considering just ophthalmologic testing (ie, optical coherence tomography [OCT], fluorescein angiography [FA], indocyanine green angiography, fundus photography, Humphrey visual field, Goldmann visual field, viral polymerase chain reaction). FA and OCT were the most frequently ordered ophthalmologic tests; utilization of these tests by provider varied from 7.69% to 53.85% and from 0 to 46.15% of cases, respectively. Thus, it appears that consensus as to optimal testing remains low even when considering only ophthalmic imaging modalities.
There are several possible explanations for the substantial diversity in the evaluation of these scenarios among well-established uveitis specialists. First, participants’ decisions may depend on the extent of clinical practice or previous experience with similar patients. However, our study did not find any statistically significant correlation between the years in practice and the number of tests ordered. Second, the decision tree may be in part dictated by either the geographic location or the referral base of the practice, leading to different pretest probabilities. For example, Provider 9 tested for Lyme disease in 100% of cases while half of all providers never ordered the Lyme test (Figure 2). Given the geographic variability in prevalence of disease, one would expect this type of variation. However, taken together these results suggest that the absence of firm guidelines for laboratory testing in uveitis has resulted in marked variation in individual practice patterns.
A similar cross-sectional survey (comprising 5 anterior uveitis scenarios) was conducted among practicing ophthalmologists, fellows, and residents in Canada.12 Similar to the current results, a wide range of tests were chosen by the 498 respondents and many of the tests were found to be of low diagnostic yield by the authors. Unlike our study, the Canadian study comprised only anterior uveitis scenarios, surveyed trainee and general ophthalmologists, and did not have “no testing” as an option for selection.
There are relatively few data on the yield of laboratory testing in uveitis. Interestingly, Rodriguez et al reviewed 1237 patients with uveitis, and found that only 17% of patients had a definitive diagnosis made on initial presentation but the diagnosis was eventually confirmed (57%) or strongly suspected (8%) in 805 patients (65%). In 85% of those who had a confirmed diagnosis, it was made during the longitudinal follow-up based on repeated clinical and laboratory evaluations.3
One may argue that screening is cost-effective if the detected condition is easily treatable, resulting in significant improvement of patients’ visual or health outcome, even if the pretest probability is low. This is likely why direct treponemal testing was the most commonly ordered test, given that the failure to detect this condition could lead to serious systemic and visual complications. However, indiscriminate clinical diagnostic testing substantially increases health-care costs. There is increasing pressure in promoting cost-effective health care and increasing scrutiny from payers. Our study showed that on average, the total costs of diagnostic investigation were higher than professional evaluation and management costs for new patients. As health-care costs become increasingly attributed to attending physician practices, this may result in uveitis specialists being identified as “high utilizers.” The consensus among the current expert group that significant laboratory testing is warranted in most scenarios of presenting uveitis suggests that uveitis practitioners need to be benchmarked against fellow uveitis specialists in order to determine utilization standards.
Rosenbaum and Wernick have advocated the application of Bayes’ theorem to the evaluation of uveitis patients and the selective testing based on individual history and examination findings.13,14 However, many physicians may entertain a broad approach in their investigation to avoid missing certain diagnoses. In some cases, this may be owing to “defensive testing,” to avoid any liability risks for clinical errors and misdiagnoses, or owing to inherent belief that earlier diagnoses or detection of any diseases are beneficial to patients.15
There are several limitations in this study. The results are based on the survey of a small subset of uveitis subspecialists. Among the participants, there was a significant variation in the number of patients they see; therefore their experience as uveitis specialists may explain some of the practice variations. The clinical scenarios are likely not as informative as actual patient encounters. However, the current results do suggest that a significant variability in testing among all uveitis specialists likely exists, as has been shown previously with general ophthalmologists.12 A larger study of uveitis specialists may provide further insights into understanding the source of discrepancy in test selection.
Uveitis specialists have high utilization of laboratory testing in evaluation of new patients and there is substantial variability. The significant difference in initial evaluation of uveitic patients suggests lack of an evidence-based diagnostic approach. Significant attention should be focused on appropriate, evidence-based laboratory utilization. A consensus expert panel recommendation may be appropriate.
Acknowledgments
FUNDING/SUPPORT: Unrestricted grant from research to prevent blindness (New York, NY); NEI K23EY024921, P30-EY001730 (Bethesda, MD).
The authors thank Sue Rath, COT (University of Washington, Seattle) for assistance with calculation of reimbursements.
Footnotes
Financial disclosures: The following authors have no financial disclosures: Cecilia S. Lee, Sandeep Randhawa, Aaron Y. Lee, Deborah L. Lam, and Russell N. Van Gelder. All authors attest that they meet the current ICMJE criteria for authorship.
Supplemental Material available at AJO.com.
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A, Buitenhuis HJ, Meenken C, et al. Uveitis and systemic disease. Br J Ophthalmol. 1992;76(3):137–141. doi: 10.1136/bjo.76.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114(5):593–599. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 4.Augsburger JJ. Unnecessary clinical tests in ophthalmology. Trans Am Ophthalmol Soc. 2005;103:143–146. discussion146–147. [PMC free article] [PubMed] [Google Scholar]
- 5.Becker MD, Rosenbaum JT. Essential laboratory tests in uveitis. Dev Ophthalmol. 1999;31:92–108. doi: 10.1159/000060759. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum JT, Wernick R. Selection and interpretation of laboratory tests for patients with uveitis. Int Ophthalmol Clin. 1990;30(4):238–243. doi: 10.1097/00004397-199030040-00002. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder RN. Focal Points: Diagnostic Approach to Uveitis. Vol. 16. San Francisco: American Academy of Ophthalmology; 2013. pp. 2–16. [Google Scholar]
- 8.Moons KG, van Es GA, Deckers JW, et al. Limitations of sensitivity, specificity, likelihood ratio, and Bayes’ theorem in assessing diagnostic probabilities: a clinical example. Epidemiology. 1997;8(1):12–17. doi: 10.1097/00001648-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal-Barby JS. “Choosing wisely” to reduce low-value care: a conceptual and ethical analysis. J Med Philos. 2013;38(5):559–580. doi: 10.1093/jmp/jht042. [DOI] [PubMed] [Google Scholar]
- 10.Moorthy RS, Rao PK, Read RW, et al., editors. Intraocular Inflammation and Uveitis. Basic Science and Clinical Science Course. Singapore: American Academy of Ophthalmology; 2011. Non-infectious (autoimmune) ocular inflammatory disease; pp. 128–131. [Google Scholar]
- 11.Rosenberg AM. Uveitis associated with juvenile idiopathic arthritis: envisioning the future. J Rheumatol. 2002;29(11):2253–2255. [PubMed] [Google Scholar]
- 12.Forooghian F, Gupta R, Wong DT, et al. Anterior uveitis investigation by Canadian ophthalmologists: insights from the Canadian National Uveitis Survey. Can J Ophthalmol. 2006;41(5):576–583. doi: 10.1016/S0008-4182(06)80026-8. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108(9):1291–1293. doi: 10.1001/archopht.1990.01070110107034. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum JT. An algorithm for the systemic evaluation of patients with uveitis: guidelines for the consultant. Semin Arthritis Rheum. 1990;19(4):248–257. doi: 10.1016/0049-0172(90)90004-y. [DOI] [PubMed] [Google Scholar]
- 15.DeKay ML, Asch DA. Is the defensive use of diagnostic tests good for patients, or bad? Med Decis Making. 1998;18(1):19–28. doi: 10.1177/0272989X9801800105. [DOI] [PubMed] [Google Scholar]