Abstract
Soluble epoxide hydrolase (sEH) converts epoxyeicosatrienoic acids that are endothelium-derived hyperpolarizing factors into less active dihydroxyeicosatrienoic acids. Previously, we reported a decrease in adenosine A1 receptor (A1AR) protein levels in sEH knockout (sEH−/−) and an increase in sEH and A1AR protein levels in A2AAR−/− mice. Additionally, KATP channels are involved in adenosine receptor (AR)-dependent vascular relaxation. Thus, we hypothesize that a potential relationship may exist among sEH overexpression, A1AR up-regulation, inactivation of KATP channels and increased in vascular tone. We performed DMT myograph muscle tension measurements and western blot analysis in isolated mouse mesenteric arteries (MAs) from wild type (WT) and endothelial over-expression of sEH (Tie2-sEH Tr) mice. Our data revealed that NECA (a non-selective adenosine receptors agonist)-induced relaxation was significantly reduced in Tie2-sEH Tr mice, and CCPA (A1AR agonist)-induced contraction was increased in Tie2-sEH Tr mice. A1AR-dependent contraction in Tie2-sEH Tr mice was significantly attenuated by pharmacological inhibition of CYP4A (HET0016, 10 μM), PKCα (GO6976, 1 μM), and ERK1/2 (PD58059, 1 μM). Our western blot analysis revealed significantly higher basal protein expression of CYP4A, A1AR, and reduced p-ERK in MAs of Tie2-sEH Tr mice. Notably, pinacidil (KATP channel opener)-induced relaxation was also significantly reduced in MAs of Tie2-sEH Tr mice. Furthermore, KATP channel-dependent relaxation in MAs was enhanced by inhibition of PKCα and ERK1/2 in WT but not Tie2-sEH Tr mice. In conclusion, our data suggests that over-expression of sEH enhances A1AR-dependent contraction and reduces KATP channel-dependent relaxation in MAs. These results suggest a possible interaction between sEH, A1AR, and KATP channels in regulating vascular tone.
Keywords: sEH, A1AR, KATP channel, mesenteric artery, contraction, relaxation
Introduction
Soluble epoxide hydrolase (sEH) is an enzyme found in the cytosol and peroxisomes of variety of tissues that regulates the conversion of epoxyeicosatrienoic acids (EETs) to their corresponding less-active diols, dihydroxyeicosatrienoic acids [1-3]. EETs are produced by the metabolism of arachidonic acid (substrate) through CYP-epoxygenases, which are known to play an important role in protection against ischemic injury, and act as vasodilators in arteries from the brain, intestines, skeletal muscle, heart, and aorta [4-10]. In arteries, sEH is highly expressed in endothelial cells and present lesser in smooth muscle cells [7,11,12]. Genetic deletion and inhibition of sEH protected against myocardial ischemia-reperfusion injury, improved vascular function, and decreased blood pressure in various disease models [13-16]. Endothelial overexpression of sEH impaired vasodilation response in the mouse cerebral artery [17]; however, the effect of sEH overexpression in mesenteric arteries has not been done yet.
Adenosine regulates vascular function by acting on four different adenosine receptors (ARs). Stimulation of A1AR or A3AR results in vascular contraction, while stimulation of A2AAR or A2BAR produces vascular relaxation [18]. We previously reported enhanced adenosine receptors (AR)-dependent vascular relaxation in the aorta of sEH knockout (−/−) mice [19]. This increase in relaxation was attributed to enhanced A2AAR and decreased A1AR protein expression [19]. In A2AAR−/− mouse aorta, we found higher expression of A1AR [20] and sEH [21]. Moreover, in mouse aorta of A2AAR−/−, sEH inhibition significantly reduced A1AR-induced vascular contraction [21]. These findings strongly indicate a relationship between sEH and ARs, especially A1AR, which plays an important role in the regulation of vascular tone.
A1AR-mediated vascular contraction occurs through the activation of protein kinase C-α (PKCα) leading to p42/p44 MAPK (ERK1/2) phosphorylation in coronary artery smooth muscle cells [22, 23]. We reported that A1AR activation resulted in contraction via cytochrome P450 epoxygenase type 4A (CYP4A), PKCα, and ERK1/2 in mouse aorta [24, 25]. Moreover, in A2AAR−/− mouse aorta, contraction occurred through CYP4A and ERK1/2 via upregulation of A1AR and PKCα [20]. We recently showed, in mouse mesenteric arteries (MAs), that, after acute angiotensin II stimulation, A1AR-dependent vascular contraction involved CYP4A and ERK1/2 [26]. Thus, it is likely that sEH modulates A1AR-dependent vascular contraction, which involves CYP4A, PKCα, and ERK1/2.
ATP-sensitive potassium (KATP) channels regulate the membrane potential of smooth muscle, thereby playing a major role in vascular function [27]. KATP channel phosphorylation by PKC negatively regulates channel function, affecting contractility in arteries [28, 29]. Interestingly, A1AR-dependent aortic contraction was dependent upon the activation of PKCα [20, 24, 25]. In A2AAR−/− mouse aorta, higher A1AR expression [20] and reduced KATP channel-dependent vascular relaxation were observed [29]. In mouse MAs, KATP channels were involved in A2BAR-dependent vascular relaxation [26]. Notably, in wild type (WT) mice, sEH inhibition resulted in enhanced A2AAR-dependent aortic relaxation [21]. Thus, sEH may regulate KATP channel-dependent vascular relaxation, which involves A1AR, CYP4A, ERK1/2, and PKCα.
In the present study, we investigated the role of sEH in the regulation of MA vascular tone. In mouse MAs, vascular contraction is A1AR-dependent, while A2BAR is responsible for relaxation [26, 30]. Interestingly, A2AAR has no functional role [26, 30], while A2BAR-dependent vascular relaxation involves KATP channels [26]. Similar to findings from A2AR−/− mouse aorta [20], MAs from A2BAR−/− mice showed higher A1AR expression [30]. Thus, we hypothesized that over-expression of sEH in Tie2-sEH Tr mice, enhances A1AR-dependent vascular contraction and reduce KATP channel-dependent vascular relaxation in MAs. We found reduced KATP channel-dependent relaxation, and enhanced A1AR-dependent contraction involving CYP4A, PKCα, and ERK1/2.
Material and methods
Animals
Tie2-sEH Tr mice were developed by Schlaeger and coworkers [31]. Human sEH cDNA (NM001979) was amplified with primers (Fwd:5′-TAAGCTTGGCTGCAGACCCGCCGCCATGACG-3′, Rev:5′ TAGCGGCCGCTCTACATCTTTGAGACCACC-3′) and subcloned downstream of the murine Tie2 promoter and upstream of the Tie2 full enhancer sequences [31]. Tie2-sEH Tr mice were provided by Dr. Darryl Zeldin from the National Institute of Environmental Health Sciences/National Institutes of Health (NIH) and were on a pure C57BL/6 genetic background. Immuno-histochemitry analysis of Tie2sEH Tr mouse brains for human sEH revealed higher expression of sEH in vascular endothelium of blood vessels [17]. All animal care and experimentation protocols were approved and carried out in accordance with the West Virginia University Institutional Animal Care and Use Committee and were in accordance with the principles and guidelines of the NIH's Guide for the Care and Use of Laboratory Animals. Both male and female mice (14 –18 wk old) in equal ratio were used for isolation of MAs in our study.
Materials
50-(N-ethylcarboxamido)adenosine (NECA), 2-chloro-N cyclopentyladenosine (CCPA), phenylephrine (PE), acetyl choline (ACh), PD98059, and all other chemicals were purchased from Sigma-Aldrich. HET0016 and GO6976 were purchased from Sigma-Aldrich and Cayman chemicals, respectively. Antibodies CYP4A sc-271983, pERK sc-7383, ERK1 sc-93, sEH sc-166961, and actin sc-47778 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. PKCα was purchased from BD Transduction Labs, San Diego, CA. A1AR antibody was purchased from Sigma-Aldrich
Muscle tension measurement
A DMT (Danish Myo Technology A/S, Aarhus, Denmark) myograph system was used to perform muscle tension measurements as described previously [26]. After anesthetizing the mice with pentobarbital sodium (100 mg/kg, intraperitoneally), the mouse mesentery with intestines was carefully excised and placed in cold oxygenated and (5% CO2 and 95% O2) modified Krebs–Henseleit physiological salt solution (PSS, in mM: NaCl 120, NaHCO3 25, KCl 4.7, KH2PO4 1.2, CaCl2 1.8, MgSO4 1.2, glucose 15, and EDTA 0.05, pH 7.4). First order MA rings (3–5 mm long, 75–150 μm in diameter) were isolated and mounted on the DMT myograph with continuous oxygenation at 37°C. A preload of 300 mg was given to the MAs and equilibrated for 90 minutes. The viability of the arteries and the presence of endothelium was tested using KCl (50 mM) and ACh (1 μM), respectively. Concentration response curves were obtained in MAs precontracted with PE (1 μM). The muscle tension generated in each concentration response curve was plotted and normalized to the corresponding % contraction to the basal tension obtained using PE (1 μM). Intact endothelium was present in all of our muscle tension measurement experiments.
Pharmacological inhibitors were used to study the role of CYP4A (HET0016 10 μM) and ERK1/2 (PD98059 1μM) [26]. The role of PKCα was studied by using GO6976 (1 μM) [25]. MAs were preincubated with or without these inhibitors (10 minutes), and concentration responses curves to NECA, CCPA, and pinacidil were obtained.
Western blotting
Western blot analysis was performed as described previously [26]. MAs isolated from WT and Tie2-sEH Tr mice were homogenized with 125 μL of RIPA lysis and extraction buffer mixed with 1 mM of Na3VO4, 1 mg/mL of leupeptin and 1mM NaF), vortexed, and centrifuged for 10 minutes at 10,000 rpm at 4°C. Protein was estimated by Lowry's method using Bio-Rad Laboratories protein assay concentrate. Samples (2–5 μg of total protein) were loaded into slab gels (10% acrylamide; 1.5 mm thick), separated by sodium dodecyl sulfate polycracrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Hybond-ECL). Membranes were blocked with 5% nonfat dry milk for 45 minutes and incubated overnight with primary antibodies. BSA (5%) was used to block the membrane when evaluating pERK expression. All antibody dilutions were 1:1000. All membranes were developed using enhanced chemiluminescence (Amersham BioSciences) and X-ray film. Densitometry analysis was performed using Multi Guage V3.0 software. The data obtained were normalized to actin and presented as the normalized ratio (% change) of target protein to WT protein expression.
Statistical analysis
All data are presented as mean ± standard error. Data in concentration response curves (CRC) was analyzed between groups at the same concentrations. Comparisons between two groups were made using Student's t-test. ANOVA, followed by Tukey's test, was used to compare significance between three or more groups. Two-way ANOVA was used to compare the statistical relationship between concentration response curves. Values at p < 0.05 were considered statistically significant. In all data, “n” represents the number of animals used to perform experiments.
Results
Enhanced vascular contraction was present in MAs from Tie2-sEH Tr mice
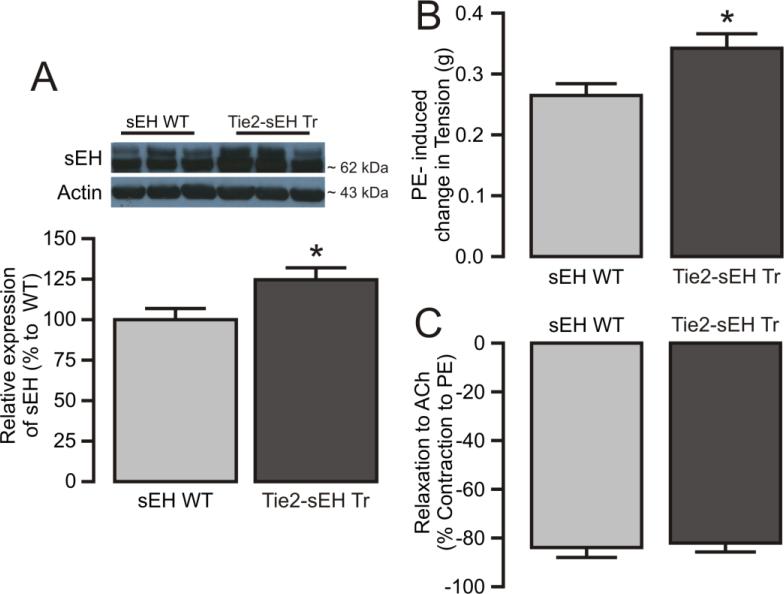
MAs isolated from WT and Tie2-sEH Tr mice were used to study vascular tone regulation by sEH. Higher sEH protein expression was noted in MAs from Tie2-sEH Tr mice (Figure 1A). Muscle tension measurement studies showed significantly higher contraction to KCl (0.322 ± 0.015 vs. 0.274 ± 0.014 g, p<0.05, data not shown) and PE (0.343 ± 0.024 vs. 0.265 ± 0.019 g, Figure 1B) in MAs from Tie2-sEH Tr mice compared to WT mice, respectively. Notably, acetylcholine-induced relaxation was comparable between MAs from WT and Tie2-sEH Tr mice (Figure 1C) suggesting that the endothelial function was intact in our in vitro studies. Thus, over-expression of endothelial sEH altered the contractile responses in MAs.
Figure 1. Characterization of vascular responses in MAs from WT and Tie2-sEH Tr mice.
(A) The top panel shows representative western blot expression of sEH in MAs from sEH WT (8 samples) and Tie2-sEH Tr mice (8 samples). The bottom panel graph shows quantification of sEH expression in sEH WT and Tie2-sEH Tr mice MAs (n=8, p<0.05). (B) PE (1 μM)-induced vascular contractions were significantly higher in Tie2-sEH Tr mice. (C) ACh (1 μM)-induced relaxation of MAs was comparable in sEH WT and Tie2-sEH Tr mice (n = 6, WT and n = 7, Tie2-sEH Tr). * indicates p < 0.05 compared to sEH WT.
Enhanced A1AR-dependent vascular contraction in MAs isolated from Tie2-sEH Tr mice
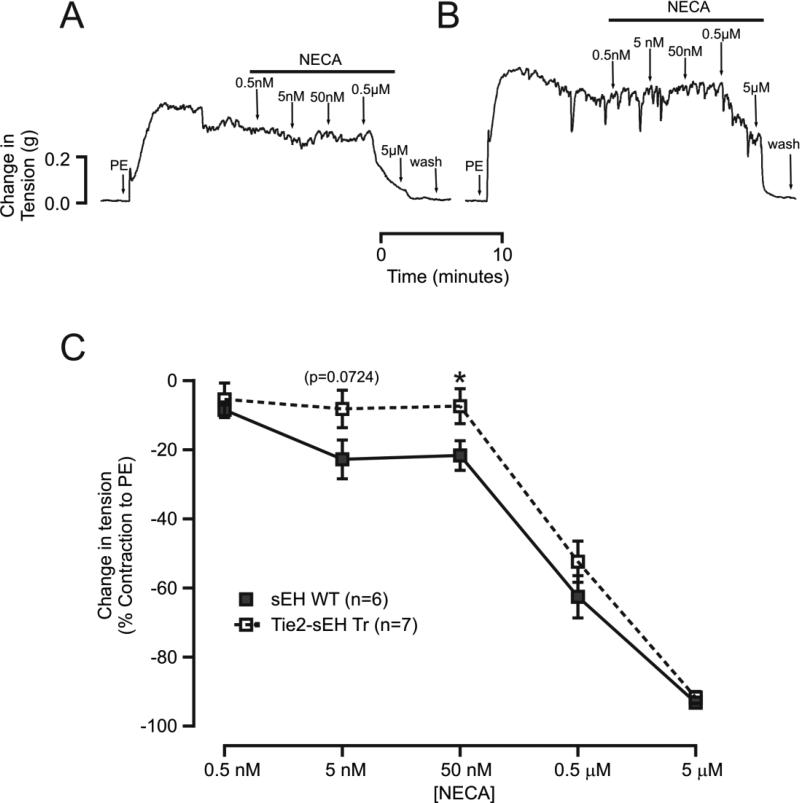
There is strong evidence suggesting that sEH modulates A1AR-dependent signaling mechanisms, thereby regulating vascular tone; thus, we investigated alterations in AR-dependent signaling mechanisms in Tie2-sEH Tr mice. Muscle tension studies revealed that a non-selective adenosine agonist (NECA)-induced vascular relaxation was significantly inhibited in Tie2-sEH Tr mice compared to WT mice (Figure 2A, B). The muscle tensions generated in response to NECA (5 nM, 50 nM and 0.5 μM) in WT were (in %) −22.81 ± 5.614, −21.70 ± 4.290 and −62.58 ± 6.130, respectively (Figure 2C). In Tie2-sEH Tr mice, muscle tensions were −8.213 ± 5.413 (p=0.724), −7.443 ± 5.049 (p<0.05) and −52.41 ± 5.970 (ns), respectively (Figure 2C).
Figure 2. NECA-induced vascular relaxation was reduced in Tie2-sEH Tr mice.
In the top panel, (A) and (B) shows representative traces of concentration-dependent muscle tension generated in response to NECA in sEH WT and Tie2-sEH Tr mice, respectively. In the bottom panel, (C) the graph summarizes the quantification of responses to increasing concentrations of NECA (* indicates p < 0.05 compared to sEH WT).
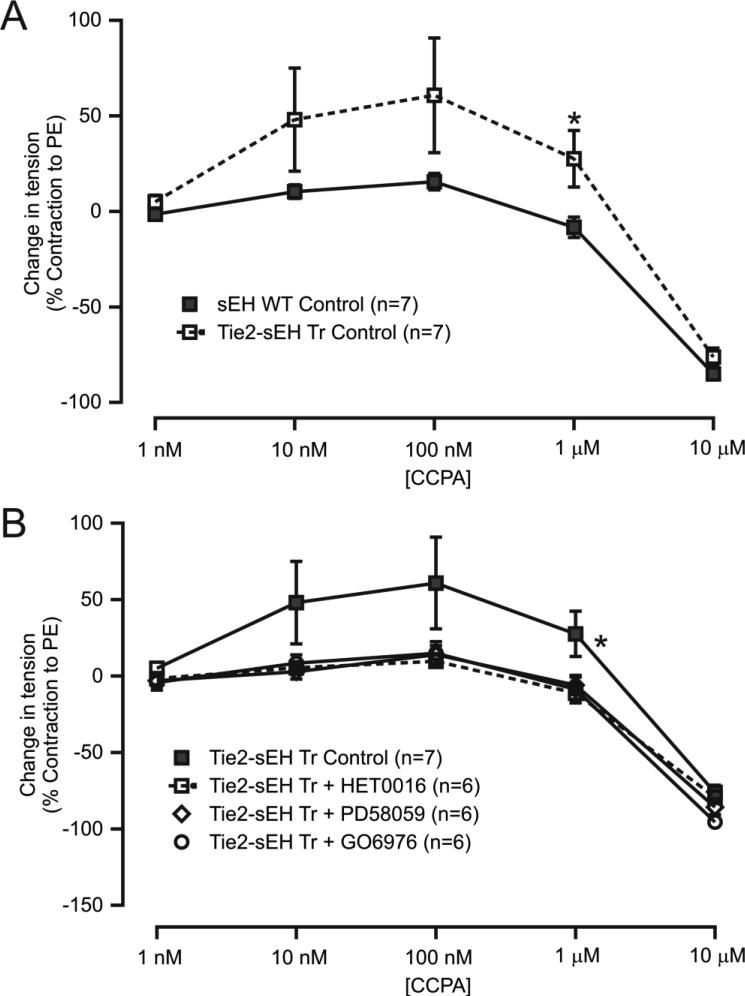
The selective A1AR agonist CCPA was used to create concentration response curves in MAs. Data showed enhanced CCPA-induced contraction in MAs from Tie2-sEH Tr mice (Figure 3A). In addition, enhanced contraction to CCPA was significantly reduced after pre-treatment of MAs with pharmacological inhibitors, namely HET0016 (CYP4A), 10 μM; PD58059 (ERK1/2), 1 μM; and GO6976 (PKCα), 1 μM (Figure 3B) for 10 minutes. This suggests that sEH enhanced A1AR-dependent vascular contraction, which are dependent on CYP4A, ERK1/2, and PKCα in MAs.
Figure 3. CCPA-induced vascular contractions were higher in Tie2-sEH Tr mice.
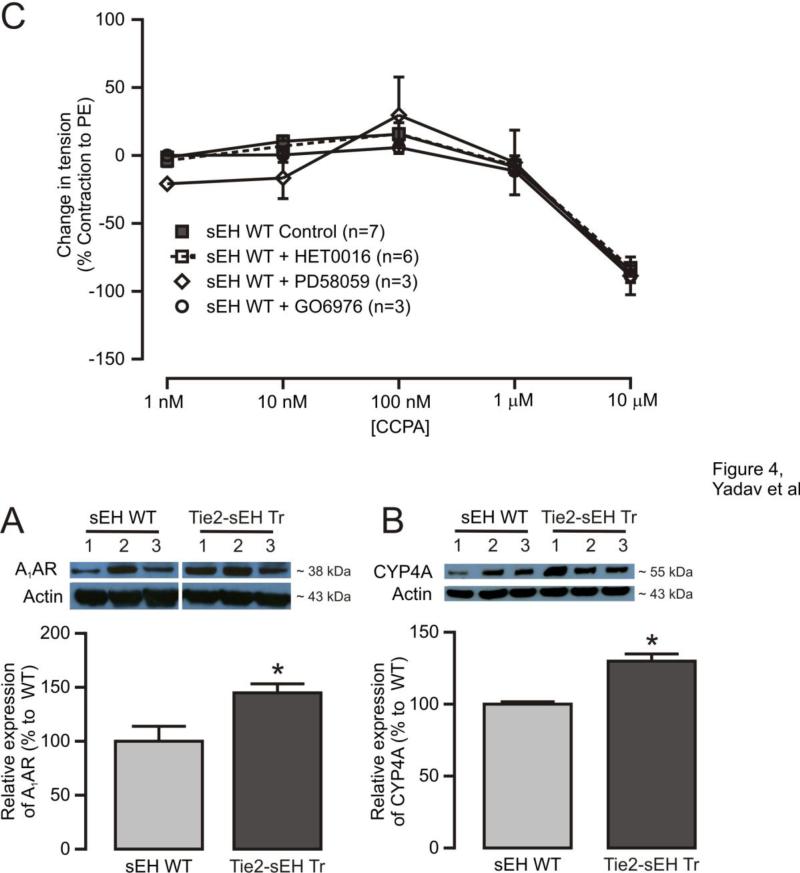
(A) CCPA-induced concentration response curves showed higher contractions in Tie2-sEH Tr mice. * indicates p < 0.05 compared to sEH WT. (B) The higher contractions observed in Tie2-sEH Tr mice were inhibited by preincubation of MAs with HET0016 (10 μM), PD58059 (1 μM), or GO6976 (1 μM) for 10 minutes. (*p < 0.05 treated Tie2-sEH Tr vs. non-treated Tie2-sEH Tr mice). (C) Effect of HET0016 (10 μM), PD58059 (1 μM), or GO6976 (1 μM) preincubation for 10 minutes in MAs of sEH WT mice.
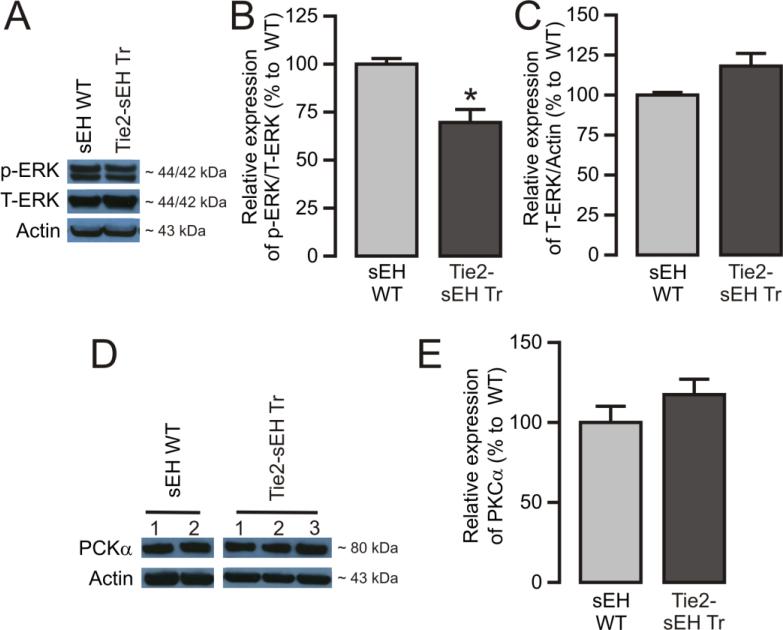
Increased levels of A1AR, CYP4A, and reduced pERK, but not PKCα, were present in MAs from Tie2-sEH Tr mice
To determine the molecular players involved in altered A1AR-dependent vascular responses in Tie2-sEH Tr mice, protein expression of A1AR, CYP4A, pERK, and PKCα was measured by western blot. Our data revealed significant upregulation of A1AR and CYP4A in MAs from Tie2-sEH Tr mice (Figure 4A and 4B). Furthermore, significantly reduced levels of basal pERK (Figure 5A and 5B) with relatively higher levels ERK1/2 (Figure 5C, p=0.0537) were present in MAs from Tie2-sEH Tr mice. Notably, PKCα expression was similar between WT and Tie2-sEH Tr mice (Figure 5D and 5E). Thus, indicating the involvement of A1AR, CYP4A and basal p-ERK/t-ERK ratio in altered responses in Tie2-sEH Tr mice.
Figure 4. Higher expression of A1AR and CYP4A in MAs from Tie2-sEH Tr mice.
The top panels present A1AR (A) and CYP4A (B) expression in MAs from 7 (WT) and 8 (Tie2-sEH Tr) mice. The bottom panels summarizes the quantification of data obtained from 7 (WT) and 8 (Tie2-sEH Tr) different mice in each group. * indicates p < 0.05 compared to sEH WT.
Figure 5. Western blot analysis of ERK1/2 and PKCα in MAs isolated from WT and Tie2-sEH Tr mice.
(A) Representative blot and (B) and (C) summarizes the quantification of expression of pERK1/2 and total ERK1/2, respectively, in mouse MAs (n =7 sEH WT and 8 Tie2-sEH Tr). (D) Representative blot and (E) summarizes the quantification of PKCα expression in MAs obtained from 8 different mice. * indicates p < 0.05 compared to sEH WT.
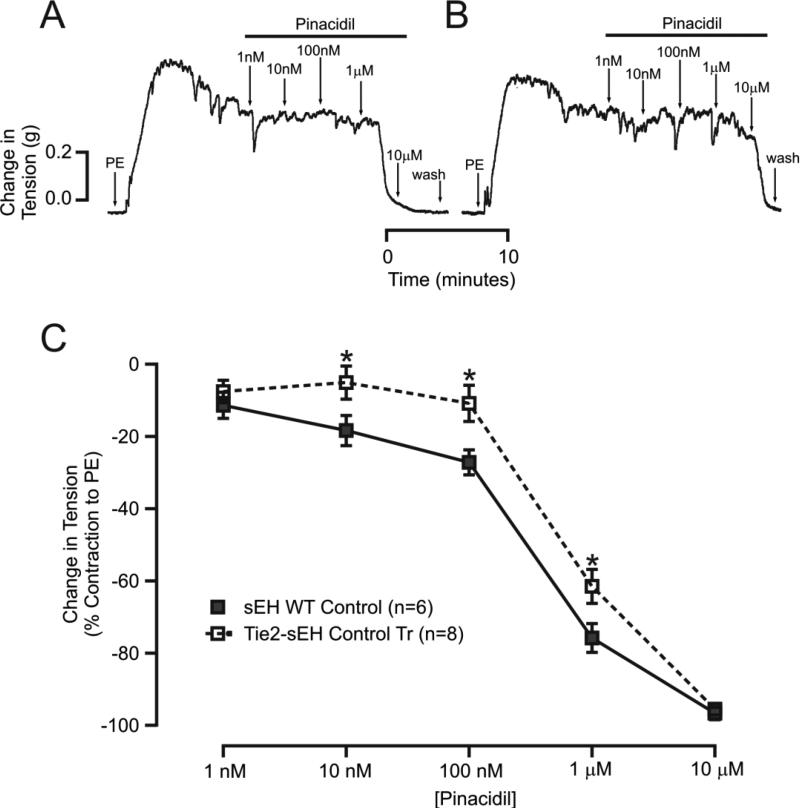
KATP channel-dependent vascular relaxation was reduced in MAs from Tie2-sEH Tr mice
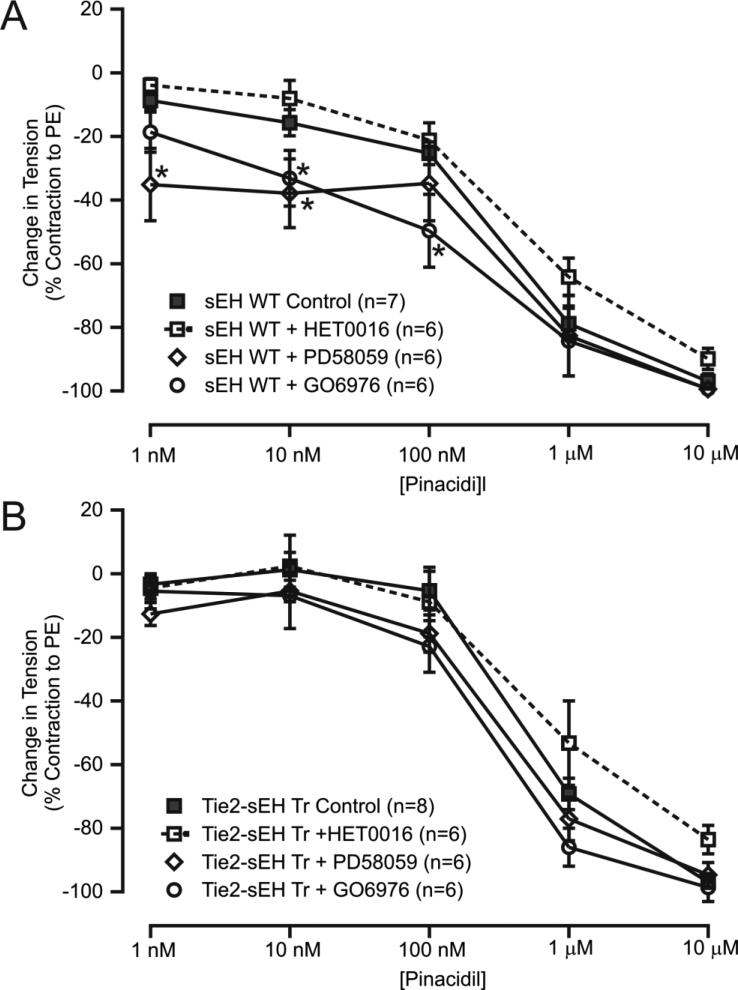
Pinacidil (a KATP channel opener)-induced concentration response curves were performed to investigate the involvement of sEH in KATP channel-dependent vascular relaxation. Pinacidil-induced relaxation was significantly reduced in MAs from Tie2-sEH Tr mice compared to WT (Figure 6 A-C). The muscle tensions generated in response to pinacidil (10 nM, 100 nM, and 1 μM) in WT were (in %) −18.40 ± 4.184, −27.21 ± 3.455, and −75.82 ± 3.992 mg, respectively. In Tie2-sEH Tr mice, the muscle tensions were −5.151 ± 4.575, −10.90 ± 5.005, and −61.54 ± 4.696 mg, respectively. Mechanisms regulating KATP channel-dependent vascular relaxation were further investigated by pre-incubating MAs with various pharmacological inhibitors for 10 minutes. In WT mice but not in Tie-sEH Tr mice, GO6976 (1 μM) and PD58059 (1 μM) significantly enhanced pinacidil-induced relaxation; HET0016 (10 μM) had no effect in either of the phenotypes of mice (Figure 7A and 7B). We found no significant effect of PD58059, HET0016 and GO6976 on the PE-induced contractions (data not shown). Together, these data suggest that sEH overexpression modulated vascular tone by inhibiting KATP channel-dependent vascular relaxation in MAs.
Figure 6. Reduced pinacidil-induced vascular relaxation in MAs from Tie2-sEH Tr mice.
In the top panel, (A) and (B) representative traces of concentration-dependent muscle tension generated in response to pinacidil in sEH WT and Tie2-sEH Tr mice, respectively. In the bottom panel, (C) summarizes the quantification of vascular relaxation to pinacidil. * indicates p < 0.05 compared to sEH WT.
Figure 7. Effect of CYP4A, PKCα, and ERK1/2 inhibition on pinacidil-induced vascular relaxation in MAs.
The graph summarizes the quantification of pinacidil concentration responses in MAs from WT (A) and Tie2-sEH Tr mice (B) pretreated with HET0016 (10 μM), PD58059 (1 μM), or GO6976 (1 μM). * indicates p < 0.05 compared to sEH WT control.
Discussion
This study found that sEH modulated AR and KATP channel-dependent vascular responses in MAs. Specifically, in MAs from Tie2-sEH Tr mice: (a) NECA-induced vascular relaxation was reduced; (b) CCPA-induced vascular contraction was enhanced; (c) CYP4A, ERK1/2, and PKCα were involved in higher CCPA contraction; (d) elevated expression of A1AR, CYP4A, and reduced pERK1/2 was present; and (e) in WT mice but not in Tie-sEH Tr mice, GO6976 (1 μM) and PD58059 (1 μM) significantly enhanced pinacidil-induced relaxation; HET0016 (10 μM) had no effect in either of the phenotypes of mice. Therefore, pinacidil-induced enhanced relaxation depends on the inhibition of PKCα and ERK1/2 activities in WT MAs but not in Tie2-sEH Tr mice.
Various studies revealed that a decrease in A1AR levels closely followed a decrease in sEH expression, and vice versa in sEH−/− and A2AAR−/− mouse aortas [19, 21]. Additionally, sEH inhibition reduced A1AR-dependent aortic contractions in A2AAR−/− mice [21]. These findings strongly suggested a potential role for sEH in regulating A1AR-dependent vascular function. Studies showed vascular effects due to the absence or inhibition of sEH, but how sEH over-expression affects AR-dependent vascular function is unclear. Since, overexpression of sEH is expected to reduce the EETs levels, and the reduction of EETs levels are believed to be responsible for enhanced vascular contraction against A1 AR agonist. Also, sEH polymorphism in human population has been shown to affect arachidonic acid metabolism which are associated with cardiovascular diseases [3]. On the other hand, ω-hydroxylases (CYP4A11, CYP4F2) metabolize arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) which has vasoconstriction effects. Genetic polymorphisms cause either lower or higher activity of these enzymes (CYP4A family) are generally associated with higher risk of hypertension or hypotension [3]. Therefore, more research is needed to confirm this association and to better understand the pathophysiologic mechanisms using mouse model of vascular endothelial overexpression of sEH (Tie2-sEH Tr).
Therefore, we sought to investigate the alterations in AR-dependent vascular responses due to endothelial sEH over-expression by performing various functional and expression studies in isolated MAs obtained from Tie2-sEH Tr mice. Notably, in MAs, A1AR mediated contraction while A2BAR regulated relaxation to AR stimulation [26, 30]. We confirmed higher expression of sEH in Tie2-sEH Tr mice (Figure 1A). Endothelial specific overexpression sEH was reported in blood vessels of brain in Tie2-sEH Tr mice [17]. Our data further revealed enhanced contraction to (Figure 1) in Tie2-sEH Tr mice. This increased trend of t-ERK expression (Figure 5C) in Tie2-sEH Tr mice may have possible link, such as in other studies, phosphorylation activates ERK1/2, which subsequently inhibits myosin light chain phosphatase, contributing to calcium sensitization and enhanced contraction [32, 33]. Thus, endothelial over-expression of sEH in MAs demonstrated enhanced vascular contractile responses to PE in Tie2-sEH Tr compared to WT mice.
We further investigated the modulation of AR-dependent vascular responses in Tie2-sEH Tr mice. As suggested by previous studies [19, 21] concerning the interaction between A1AR and sEH in mouse aorta, our data indicated reduced NECA-induced vascular dilation (Figure 2) and enhanced vascular contractions to CCPA (Figure 3A) in MAs from Tie2-sEH Tr mice, revealing the involvement of enhanced A1AR-dependent vascular contraction in MAs of sEH over-expressed mice. Previous work in MAs suggested that activation of A1AR could inhibit A2BAR-dependent vascular relaxation. A1AR-dependent contraction involved CYP4A and ERK1/2 [26]. We also found that CYP4A, PKCα, and ERK1/2 were involved in A1AR-dependent contractile responses in mouse aorta [20, 24, 25]. The present study found that higher contractile responses to an A1AR-agonist (CCPA) involved CYP4A, ERK1/2, and PKCα (Figure 3B). In support of our functional data (Figures 2 and 3), we found higher expression of A1AR (Figure 4A) and CYP4A (Figure 4B) in MAs from Tie2-sEH Tr mice. Studies in coronary artery smooth muscle showed that activation of A1AR was linked to activation of phospholipase C, PKC-α, and increased pERK1/2 signaling [22]. Notably, ERK1/2 is activated through tyrosine phosphorylation [34]. Contrary, in the present study, we found low basal p-ERK/t-ERK ratio (Figure 5B) in Tie2-sEH Tr compared to WT mice with increased trend of total ERK1/2 in Tie2-sEH Tr compared to WT mice (Figure 5C). Low ratio of basal p-ERK/t-ERK could be possibly complimentary in maintaining basal vascular tone in Tie2-sEH Tr compared to WT mice. While in response to stimulus such as CCPA, could be potentiating the contractile responses. In addition, no increase in the level of PKCα (Figure 5E) was observed. It was reported that PKC phosphorylated ERK1/2 in PBDu treated cerebral artery smooth muscle cells [35]. Further investigation is needed to fully elucidate this claim. Overall, our data revealed that overexpression of sEH enhanced A1AR-dependent contraction. Also, our data showed that higher expression of A1AR, CYP4A, and reduced p-ERK/T-ERK ratio.
KATP channels are major regulators of vascular function [27]. We previously reported that KATP channels were involved in A2AAR-stimulated mouse aortic relaxation [29]. Also, in mouse MAs, KATP channels played a role in A2BAR-dependent vascular relaxation such that inhibition of KATP channel produced contraction of MAs in response to NECA, through activation of A1AR [26]. Thus, KATP channels are important signaling components that mediate AR-dependent vascular relaxation. Considering the previous findings that sEH modulated AR expression and function [19, 21] and our current findings, we speculated that sEH may inhibit KATP channel-dependent relaxation, which may further promote AR-dependent contractile responses. As expected, our data revealed that KATP channel-induced vascular relaxation was reduced in Tie2-sEH Tr mice MAs (Figure 6). Furthermore, our data in WT mice revealed that KATP channel-dependent dilation was enhanced by pharmacological inhibition of ERK1/2 and PKCα (Figure 7A). This suggested that ERK1/2 and PKCα play roles in the regulation of KATP channels. In accordance with our findings, studies in pial arteries of piglets showed that hypoxic/ischemic injury activated ERK1/2, which in turn inhibited KATP channel-dependent vasodilation [36]. To the contrary, studies in sarcolemmal KATP channel-transfected human embryonic kidney 293 cells and ventricular cardiomyocytes isolated from rabbit showed that nitric oxide donors activated ERK1/2, which facilitated KATP channel opening, thereby ensuring protection against ischemic or hypoxic injury [37]. These findings and our results indicate that ERK1/2 may function differentially, depending on the tissue and species involved.
Our finding of PKCα's involvement in KATP channel regulation (Figure 7A) was supported by the fact that PKC phosphorylates KATP channels, inhibiting their activation [28]. In addition, studies in rat MAs revealed that KATP channels were blocked by increased intracellular calcium but were unaffected by PKC inhibition, which contributed to UTP-induced contraction [38]. This study used PKC inhibitory peptides while our study used the pharmacological inhibitor GO6976. In a study by Panhwar et al., no reduction in ET-1-induced contraction of MAs was observed [38], whereas PKC was involved in ET-1-induced contraction in porcine coronary artery smooth muscle cells [39]. This suggests that species variation in PKC action could not be ruled out. Our data further revealed no involvement of CYP4A in basal KATP channel function (Figure 7A). Overall, our data suggested the involvement of PKCα and ERK1/2, but not CYP4A, in the regulation of basal KATP channel function in WT MAs. We further investigated the mechanisms of KATP channel-dependent relaxation of MAs in Tie2-sEH Tr mice. Our data revealed that KATP channel-dependent MAs relaxation is independent p-ERK1/2, PKCα and CYP4A in MAs from Tie2-sEH Tr mice (Figure 7B). The absence of any effects of pharmacological inhibition of PKCα and ERK1/2 in Tie2-sEH Tr MAs may possibly be due to higher levels of ERK1/2. Nevertheless, our data showed that sEH overexpression results in the inhibition of KATP channel-dependent relaxation, which may further help contractile responses.
Conclusions
Our data showed that higher endothelial expression of sEH positively regulated vascular contractions in MAs. We found CCPA enhanced A1AR-dependent and CYP4A, PKCα, and ERK1/2-mediated contraction in MAs. Furthermore, our data showed that sEH over-expression negatively regulated KATP channel function. Enhanced A1AR-dependent contraction and reduced KATP channel-dependent relaxation together potentiate overall contraction in sEH overexpressing mice MAs. Similarly, endothelial sEH overexpressing mice, the vasodilation was impaired in the mouse cerebral artery [17]. It was previously shown that genetic deletion and inhibition of sEH was protective in myocardial ischemia-reperfusion injury in vivo [13]. Moreover sEH inhibition decreased renal injury in hypertensive obese rats by improving vascular function [14]. sEH inhibition decreased blood pressure in angiotensin II–induced hypertension mice [15]. Therefore, sEH is an important target, and elucidating its mechanisms is imperative in cardiovascular pathophysiology. In this context, our study becomes immensely significant by suggesting mechanisms through which sEH regulates vascular tone.
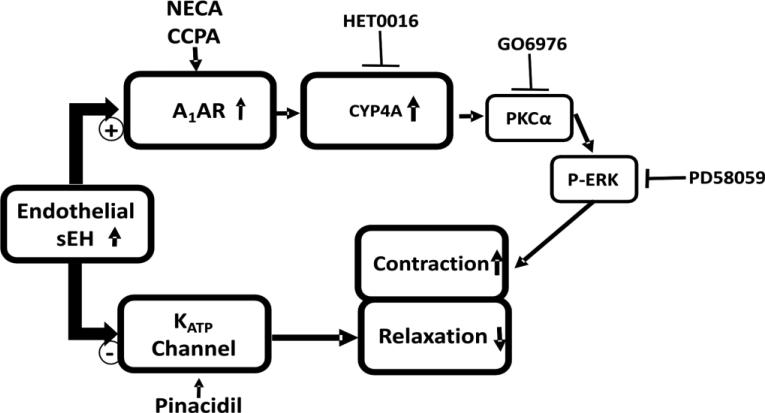
Figure 8. Effect of vascular overexpression of sEH (Tie2-sEH Tr mice) on vascular response in MAs.
The schematic diagram represents the endothelial overexpression of sEH induces A1AR-dependent vascular contraction with the possible involvement of CYP4A, PKCα and ERK1/2 in the downstream. Whereas, endothelial overexpression of sEH reduces the vascular relaxation through KATP channels.
Acknowledgement
This study was supported by HL-114559 (to Mohammed Nayeem, PhD.) and z01-ES025034 (Darryl Zeldin, M.D.). The authors would like to thank Jamal Mustafa, PhD. for allowing Vishal Yadav, PhD. (Postdoctoral Fellow in his lab) to work in this project. The authors appreciate the help provided by Sherry Xie for western blot experim
References
- 1.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Meijer J, Guengerich FP. Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme. Biochem. 1982;21:5769–76. doi: 10.1021/bi00266a007. [DOI] [PubMed] [Google Scholar]
- 3.Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Therapeutics. 2010;125:446–63. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 5.Proctor KG, Falck JR, Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987;60:50–9. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 7.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–30. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007;28:448–52. doi: 10.1016/j.tips.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev. 2014;66:1106–40. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 10.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol. 2008;295:H2068–78. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem. 2006;54:329–35. doi: 10.1369/jhc.5A6808.2005. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Huo L, He J, Ding W, Su H, Tian D, Welch C, Hammock BD, Ai D, Zhu Y. Soluble epoxide hydrolase is involved in the development of atherosclerosis and arterial neointima formation by regulating smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2015;309:H1894–903. doi: 10.1152/ajpheart.00289.2015. [DOI] [PubMed] [Google Scholar]
- 13.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2128–34. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imig JD, Walsh KA, Hye Khan MA, Nagasawa T, Cherian-Shaw M, Shaw SM, Hammock BD. Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med. 2012;237:1402–12. doi: 10.1258/ebm.2012.012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II- induced hypertension. Hypertension. 2005;45:759–65. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 16.Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol. 2014;306:H1146–53. doi: 10.1152/ajpheart.00920.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Davis CM, Edin ML, Lee CR, Zeldin DC, Alkayed NJ. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PloS one. 2013;8:e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets, Nature reviews. Drug discovery. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayeem MA, Pradhan I, Mustafa SJ, Morisseau C, Falck JR, Zeldin DC. Adenosine A2A receptor modulates vascular response in soluble epoxide hydrolase- null mice through CYP-epoxygenases and PPARgamma. Am J Physiol Regul Integr Comp Physiol. 2013;304:R23–R32. doi: 10.1152/ajpregu.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C, Mustafa SJ. Role of omega-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2A-receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R400–R408. doi: 10.1152/ajpregu.00481.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan I, Ledent C, Mustafa SJ, Morisseau C, Nayeem MA. High salt diet modulates vascular response in AAR and A AR mice: role of sEH, PPARgamma, and K channels. Mol Cell Biochem. 2015;404:87–96. doi: 10.1007/s11010-015-2368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H1032–H9. doi: 10.1152/ajpheart.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411–6. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kunduri SS, Dick GM, Nayeem MA, Mustafa SJ. Adenosine A receptor signaling inhibits BK channels through a PKCalpha-dependent mechanism in mouse aortic smooth muscle. Physiol Rep. 2013;1:e00037. doi: 10.1002/phy2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-alpha, and ERK1/2. J Cardiovasc Pharmacol. 2013;62:78–83. doi: 10.1097/FJC.0b013e3182919591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav VR, Nayeem MA, Tilley SL, Mustafa SJ. Angiotensin II stimulation alters vasomotor response to adenosine in mouse mesenteric artery: role for A1 and A2B adenosine receptors. Br J Pharmacol. 2015;172:4959–69. doi: 10.1111/bph.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11:1915–40. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- 28.Aziz Q, Thomas AM, Khambra T, Tinker A. Regulation of the ATP-sensitive potassium channel subunit, Kir6.2, by a Ca2+-dependent protein kinase C. J Biol Chem. 2012;287:6196–207. doi: 10.1074/jbc.M111.243923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Mustafa SJ. CYP- epoxygenases contribute to A2A receptor-mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1003–10. doi: 10.1152/ajpregu.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ. Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol. 2013;61:70–6. doi: 10.1097/FJC.0b013e318278575e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. PNAS. 1997;94:3058–63. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihara E, Yu Q, Chappellaz M, MacDonald JA. ERK and p38MAPK pathways regulate myosin light chain phosphatase and contribute to Ca2+ sensitization of intestinal smooth muscle contraction. Neurogastroenterol Motil. 2015;27:135–46. doi: 10.1111/nmo.12491. [DOI] [PubMed] [Google Scholar]
- 33.Ok SH, Kwon SC, Yeol Han J, Yu J, Shin IW, Lee HK, Chung YK, Choi MJ, Sohn JT. Mepivacaine-induced contraction involves increased calcium sensitization mediated via Rho kinase and protein kinase C in endothelium-denuded rat aorta. Eur J Pharmacol. 2014;723:185–93. doi: 10.1016/j.ejphar.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–3. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Zhang L, Longo LD. PKC-induced ERK1/2 interactions and downstream effectors in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2005;289:R164–71. doi: 10.1152/ajpregu.00847.2004. [DOI] [PubMed] [Google Scholar]
- 36.Armstead WM, Riley J, Cines DB, Higazi AA. tPA contributes to impairment of ATP and Ca sensitive K channel mediated cerebrovasodilation after hypoxia/ischemia through upregulation of ERK MAPK. Brain Res. 2011;1376:88–93. doi: 10.1016/j.brainres.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DM, Chai Y, Erickson JR, Brown JH, Bers DM, Lin YF. Intracellular signalling mechanism responsible for modulation of sarcolemmal ATP-sensitive potassium channels by nitric oxide in ventricular cardiomyocytes. J Physiol. 2014;592:971–90. doi: 10.1113/jphysiol.2013.264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panhwar F, Rainbow RD, Jackson R, Davies NW. Ca2+ dependent but PKC independent signalling mediates UTP induced contraction of rat mesenteric arteries. J Smooth Muscle Res. 2015;51:58–69. doi: 10.1540/jsmr.51.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cain AE, Tanner DM, Khalil RA. Endothelin-1--induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension. 2002;39:543–9. doi: 10.1161/hy0202.103129. [DOI] [PubMed] [Google Scholar]