Abstract
Problem
Immune cells within the endometrium at implantation are thought to play an important role in implantation, although their exact role is not well understood.
Method of Study
A co-culture system of rhesus monkey embryos and maternal immune cells was established. Blastocysts obtained by in vitro fertilization were co-cultured with peripheral blood cells or decidual macrophages. Culture media were collected to assess secretions. Embryo growth was monitored and trophoblasts were evaluated for proliferation, apoptosis and differentiation.
Results
Embryonic trophoblast outgrowths were visible within 6 days of culture, and the area of embryo outgrowth was reduced when blastocysts were cultured with peripheral-derived or decidual macrophages. Trophoblast proliferation was not significantly affected with macrophage co-culture while chorionic gonadotropin secretion was increased. Trophoblast expression of CDH 11 and GJA1 was increased, suggesting that macrophages accelerate differentiation of peri-implantation trophoblasts.
Conclusions
These results indicate an important role of macrophages in placentation and pregnancy success.
Keywords: decidua, embryo, implantation, macrophage, trophoblast
Introduction
About 30 to 60% of human pregnancies fail to progress after embryo attachment; however, the actual rate of early pregnancy loss is difficult to determine as many pregnancies are lost before a woman is aware that she is pregnant1. Suboptimal interaction of embryonic trophectoderm with the uterine epithelium or dysregulated differentiation into trophoblast cells that invade the uterus and form the placenta, are possible causes of early pregnancy loss2. Even with a successful implantation, abnormal placentation is thought to contribute to later pregnancy complications including preeclampsia or intrauterine growth restriction3.
In humans and non-human primates, trophoblast cells follow villous and extravillous paths of differentiation to form the placenta. Cytotrophoblasts proliferate to form the villi of the placenta, and differentiate and fuse to form a multinucleated syncytiotrophoblast layer that covers the villus4. The syncytiotrophoblast layer provides nutrient exchange between the mother and fetus5 and secretes hormones including chorionic gonadotropin (CG) and progesterone, as well as growth factors and cytokines6. Extravillous cytotrophoblasts in contact with uterine tissue separate from the villous basement membrane to form a cell column from which extravillous trophoblasts penetrate the decidua, invading and remodeling the decidual spiral arteries4, as well as migrating into the decidual stroma. During these processes, trophoblasts interact with an abundant population of leukocytes within the endometrium, which predominately consists of NK cells and macrophages, as well as lower numbers of T lymphocytes. Maternal leukocytes actively traffic to the endometrium during the luteal phase and the interaction between peripheral blood mononuclear cells (PBMCs) and trophoblasts at implantation could have a role in directing or modulating the invasion of placental cells7.
Peripheral immune cells respond to the establishment of pregnancy. For example, PBMCs of pregnant women secrete different levels of cytokines and chemokines than those from non-pregnant women8–10. Additionally, PBMCs from pregnant women stimulate progesterone production by luteal cells11. Differences in cytokine and chemokine secretion between non-pregnant and pregnant women are thought to result from PBMC exposure to pregnancy hormones8. Interaction of the endometrial leukocyte infiltrate with trophoblasts before returning to the circulating blood may also play a role, since it has been shown that immune cells can induce differentiation of the corpus luteum prior to CG secretion and cytokines IL-4 and IL-10 can induce progesterone production12.
Decidual immune cells may also influence trophoblast invasion. During early human pregnancy, NK cells make up 30–40% of the total cells in the decidua, and macrophages account for 10–15% of the total cells13. NK cells and macrophages are also observed at high density in the rhesus monkey endometrium and represent more than one third of total decidual cells14. During implantation, decidual macrophages appear to migrate to the implantation site, congregating around blood vessels and invading trophoblasts14, 15. The macrophages may act to phagocytose debris and apoptotic cells associated with trophoblast invasion, or produce cytokines such as TNF or TGFB1 to inhibit invading trophoblast cells, thus preventing over-invasion of the placenta into the myometrium14, 16. Although the precise function of decidual macrophages is not clear, their close contact with trophoblasts suggests that they contribute to successful implantation and establishment of pregnancy.
Precisely how invading trophoblast cells are influenced by PBMCs or decidual macrophages within the luteal phase endometrium, or how invading trophoblast cells influence infiltrating and resident immune cells, remains difficult to explore in human implantation. The rhesus monkey has similar embryonic and placental development to human pregnancy17. Therefore, the present study aimed to develop an in vitro rhesus monkey model of implantation to examine specific immune cell responses to the embryo. Overall, significant differences in trophoblast growth and differentiation were observed when blastocysts were cultured with macrophages, suggesting a role for macrophages not previously recognized during implantation.
Materials and Methods
Animals
Rhesus monkeys (Macaca mulatta) were from the colony maintained at the Wisconsin National Primate Research Center. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin Graduate School Animal Care and Use Committee. A total of 15 monkeys were used for oocyte collections. Additionally, decidua samples were collected from 5 monkeys at approximately day 36 of gestation.
In vitro fertilization
Rhesus monkey embryos were produced essentially as described by Wolfgang et al.18. Briefly, oocytes were obtained from the donor after twice daily treatment with 30 IU recombinant human follicle stimulating hormone (FSH) for 7 days, followed by a single injection of 1000 IU recombinant human chorionic gonadotropin (hCG). Oocytes were retrieved from visible follicles by laparoscopic surgery and matured in vitro until polar body extrusion. For in vitro fertilization, semen from males of known fertility was collected by electroejaculation and incubated at 37°C for 1 hr to allow for separation of the coagulum. Sperm were then washed in TALP-Hepes media, and incubated with 1mM of both caffeine and dibutytryl cyclic adenosine monophosphate for 30 minutes. Sperm was added to oocytes 4 hrs after polar body extrusion and gametes were co-incubated for 24 hrs, and embryos were assessed for the presence of pronuclei and second polar body extrusion. Embryos were then transferred to G1.2 5% FCS medium (IVF Science Scandinavia, Gothenburg, Sweden) and cultured until the 8-cell stage, then placed into G2.2 2% FCS medium (IVF Science Scandinavia). Embryos were then cultured to the blastocyst stage (8 to 9 days). Blastocysts that did not spontaneously hatch from the zona pellucida by day 9 underwent assisted hatching with pronase (Sigma Aldrich, St. Louis, MO).
Peripheral blood immune cell isolation
Peripheral blood was obtained from the oocyte donor on the day of oocyte retrieval for leukocyte isolation and macrophage differentiation. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll gradient centrifugation. Monocytes were obtained from these cells by adhesion on plastic for 24 hours. The monocytes were then washed twice with RPMI and cultured for five days in RPMI supplemented with 1% human serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM sodium pyruvate, 1% non-essential amino acids, 5 x 10−5 M 2-mercaptoethanol, 20 ng/mL M-CSF, and 10 ng/mL IL-1β19. Additional peripheral blood was obtained from the oocyte donor the day prior to co-culture and PBMCs were isolated from whole blood by a Ficoll gradient centrifugation. NK cells were labeled with nonhuman primate anti-CD16 microbeads (Miltenyi Biotec, Auburn, CA) and sorted by MACS on an LS column (Miltenyi Biotec). Monocytes were separated by adhesion for 2 hours.
Decidual macrophage cell isolation
Rhesus monkey decidual macrophages were isolated from decidua on Days 36 or 37 of pregnancy (equivalent to approximately week 8 in human pregnancy) from 5 separate monkeys, by enzymatic digestion with DNAse and collagenase IV (Sigma) and density gradient centrifugation as previously described20. Briefly, following tissue dispersion, cells were suspended in 30 mL RPMI-1640 (Gibco, Grand Island, NY) then slowly layered over 15 mL of Ficoll-Paque (GE Healthcare, Piscataway, NJ), and centrifuged at 800 x g for 30 minutes at room temperature. Mononuclear cells were collected, washed in RPMI-1640, labeled with nonhuman primate anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA) and sorted by MACS on an LS column (Miltenyi Biotec). The resulting CD14-positive cells were cryopreserved and stored frozen (−80C) until used.
Co-culture design
To physically stabilize an in vitro immune cell endometrial environment at implantation, immune cells were mixed into Matrigel (BD Biosciences, San Jose, CA) where 50 µl was plated per well of a 96-well plate and allowed to solidify at 37C. To assess interactions between peripheral immune cells and embryos, each well contained either all PBMCs, macrophages, monocytes, NK cells, NK cells + monocytes, NK cells + macrophages, or no immune cells, designated as the control. Wells with macrophages, monocytes, or NK cells were plated with 100,000 total cells, whereas those with a combination of cells were plated with 100,000 cells of each cell type for a total of 200,000 cells per well. The interaction between decidual macrophages and embryos was defined by adding 100,000 decidual macrophages to Matrigel. In both experiments, a day 9 hatched blastocyst was placed on the surface of the solidified Matrigel within a single well and allowed to grow into the Matrigel for up to 10 days. All co-cultures were incubated at 37°C in a humidified atmosphere of 5.5% CO2 and 5.5% O2. Media were collected on days 2, 6, and 10 for cytokine and hormone analysis.
During these experiments leukocytes and embryos were cultured in Buffalo Rat Liver (BRL) cell-conditioned media. Irradiated BRL cells were cultured in DMEM (50%) and CMRL (50%; Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). Conditioned media were collected daily and frozen for subsequent analysis. On day 10 of co-culture, cells were removed from the Matrigel with BD Cell Recovery Solution (BD Biosciences), followed by digestion of the trophoblast outgrowths with 0.15% dispase for 45 min (Gibco, Grand Island, NY) and then 0.5% trypsin for 15 min (Sigma, St. Louis, MO) to dissociate the trophoblasts.
Secreted Chorionic Gonadotropin Analysis
Chorionic gonadotropin (CG) concentrations in medium samples were determined by a Mouse Interstitial Cell Testosterone (MICT) bioassay 21.
Chemokine, cytokine and growth factor analysis in culture media
Culture media were collected on days 2, 6, and 10 after culture initiation. The levels of G-CSF (CSF3), GM-CSF (CSF2), MCP-1 (CCL2), MCP-3 (CCL7), MIP-1α (CCL3), MIP-1β (CCL4), IFNα, TNF, VEGFA, IL-1RA, IL-1β, IL-6, IL-8 (CXCL8), IL-10, IL-12, IL-15, IL-18, and IP-10 were determined using a Non-Human Primate Cytokine Milliplex Map Kit (Millipore, Billerica, MA) or Procarta Cytokine Assay Kit (Affymetrix, Santa Clara, CA) and analyzed with a Luminex 200 instrument. Assays to evaluate matrix metalloproteinases (MMP) 2, 7 and 9 were performed using an MMP Bead-Based Multiplex Assay (Millipore, MA).
Flow cytometric analysis
Cells were incubated with saturating concentrations of monoclonal antibodies for 25 minutes at 4°C in PBS supplemented with 2% fetal bovine serum (FBS) (Atlanta Biologicals), washed, and incubated with secondary antibodies, if necessary, for 25 minutes. Following monocyte differentiation into macrophages, cells were assessed for macrophage-specific markers with these antibodies: anti-CD36-FITC and anti-CD209-FITC (DC-SIGN), anti-CD86 and anti-CD163-PE, anti-HLA-DR-PerCP-Cy5.5, anti-CD14-APC and anti-CD45-APC (BD Biosciences, Bedford, MA), and anti-MARCO-APC (Thermo Scientific, Rockford, IL). For intracellular antigen labeling, cells were fixed and permeabilized with fixation and permeabilization solution (BD Biosciences). Antibodies included anti-cytokeratin-FITC, anti-caspase-3-PE and anti-Ki67-PerCp-Cy 5.5 (BD Biosciences). Decidual macrophage-cocultured trophoblasts were additionally labeled with an anti-apotosis marker using anti-BCL 2-PerCp-Cy 5.5 (BD Biosciences). To evaluate the differentiation state of trophoblasts following decidual macrophage co-culture with embryos, cells were labeled with APC-conjugated anti-CD45 (BD Biosciences, Bedford, MA), anti-Connexin 43 with PE-conjugated anti-rabbit IgG as the secondary antibody (BD Biosciences), and anti-Cadherin 11 (R&D Systems) with APC-conjugated anti-mouse IgG secondary antibody (BD Biosciences). All antibodies used were developed to human antigens and have been previously shown to recognize the appropriate leukocyte subsets in rhesus monkeys14, 19, 20. Isotype-matched nonspecific antibodies at the same concentration were used as controls. Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences) with Cell Quest software (BD Biosciences). The data were analyzed using Flow Jo software (Tree Star, Ashland, Oregon). Trophoblasts were distinguished from leukocytes based on cytokeratin (trophoblast) and CD45 (leukocyte) staining.
Statistics
Blastocysts were randomly assigned to treatment groups using a random numbers table. 1-way ANOVA or Student’s t-test were performed to define statistically significant differences using GraphPad Prism software (GraphPad Software, La Jolla, CA). Differences were considered significant at P < 0.05.
Results
Embryo outgrowth in response to peripheral blood immune cell co-culture
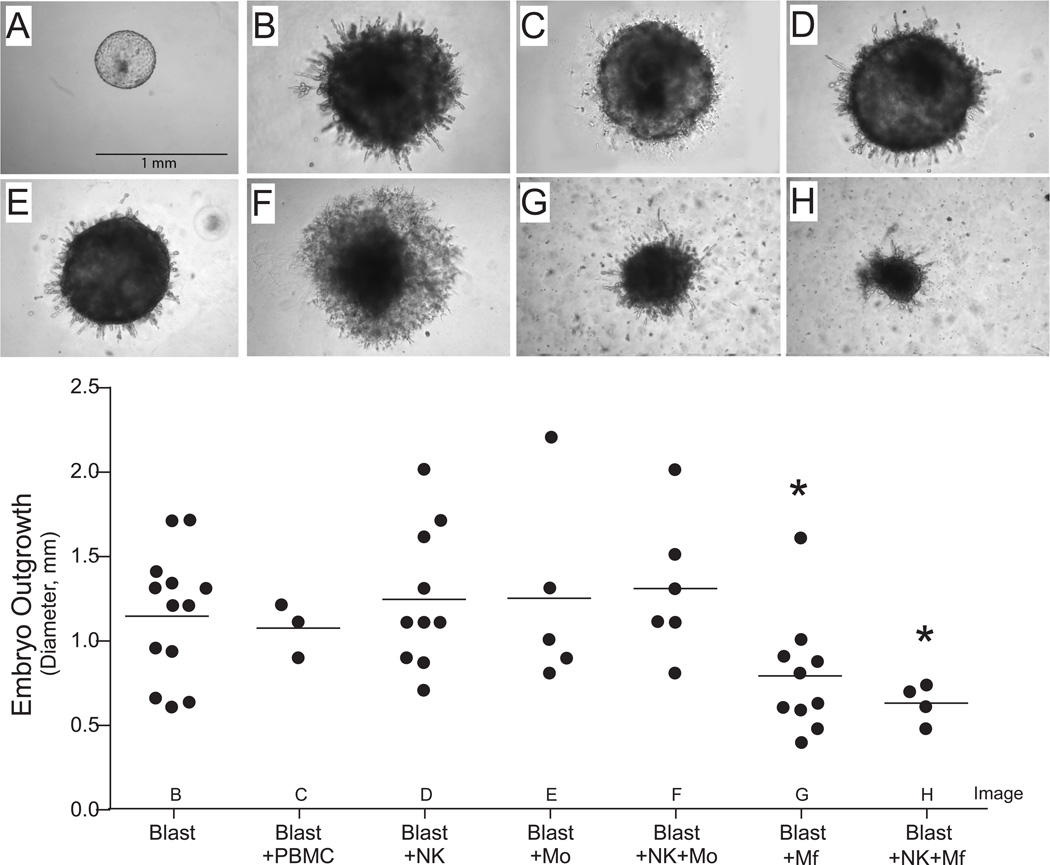
At the time of implantation, trophoblast cells arising from the trophectoderm of the blastocyst proliferate and invade into the uterine lining. In vitro, pilot studies demonstrated that blastocysts plated on an extracellular matrix coated culture surface, such as Matrigel, grow and invade into the matrix. Therefore, embryo outgrowth was evaluated in response to total PBMCs, macrophages, monocytes, NK cells, NK cells + macrophages, or NK cells + monocytes. On day 9 of embryonic development, hatched blastocysts were plated on Matrigel in the presence or absence of these leukocyte populations. Trophoblast outgrowths were visible between day 3–6 of culture and growth was monitored for 10 days. After 10 days of co-culture, embryos were imaged and the outgrowth diameter was determined (Figure 1). Co-culture with peripheral derived macrophages, regardless of the presence of NK cells, significantly reduced the diameter of embryonic outgrowth in comparison to control embryos, whereas the diameter was similar between control blastocysts and those cultured with NK cells, monocytes, and NK cells + monocytes (Figure 1).
Figure 1.
Cultured rhesus embryos (top panel): A. hatched blastocyst on day 0 of co-culture and the following are representative embryos on day 10 of co-culture with B. control, C. PBMCs, D. NK cells, E. monocytes, F. NK cells + monocytes, G. macrophages, and H. NK cells + macrophages. Outgrowth diameter (mm) was measured (lower graph) on day 10 of co-culture blastocysts cultured alone or with either PBMCs, NK cells, monocytes (Mo), NK cells + Mo, macrophages (Mφ) or NK cells + Mφ. Each data point presents a single embryo. Asterisk denotes P < 0.05, compared to Blasts alone.
Chorionic gonadotropin secretion from embryos co-cultured with peripheral blood immune cells
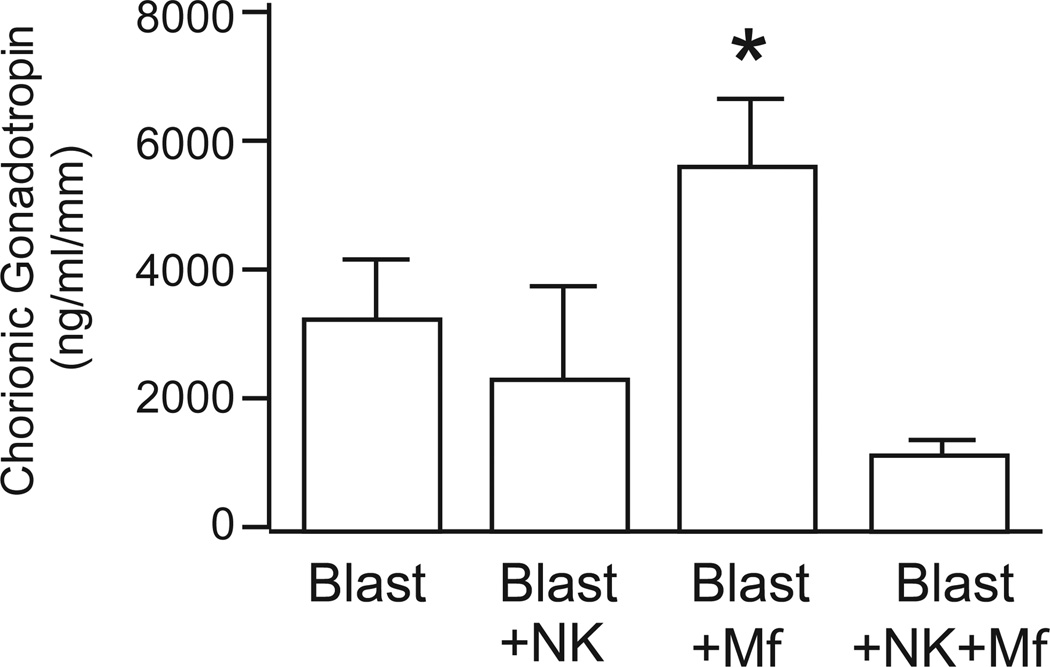
Chorionic gonadotropin (CG) is secreted by trophoblasts of the invading blastocyst. The level of CG produced by trophoblasts co-cultured with peripheral blood immune cells was determined in media collected on days 2, 6, and 10. CG was barely detectable on day 2, but was readily detectable by day 6 and day 10. Figure 2 illustrates CG levels in day 10 culture medium, normalized to embryo size. Embryos cultured with peripheral derived macrophages secreted significantly higher CG into the culture medium in comparison with control or NK cell co-cultures. In contrast, the level of secreted CG from embryos cultured with peripheral NK cells, monocytes, and NK cells + monocytes was not significantly different from control embryos.
Figure 2.
Chorionic gonadotropin (CG) secretion within day 10 culture medium, normalized by embryo diameter (mm). Blastocysts were cultured alone as a control (blast, n=7) or in the presences of NK cells (n=4), Macrophages (Mφ, n=9) or NK cells + Mφ (n=4). Data is represented as the mean ± SE, where the asterisk denotes P < 0.05 compared to control medium.
Cytokine, chemokine and growth factor secretion in peripheral blood immune cell co-cultures
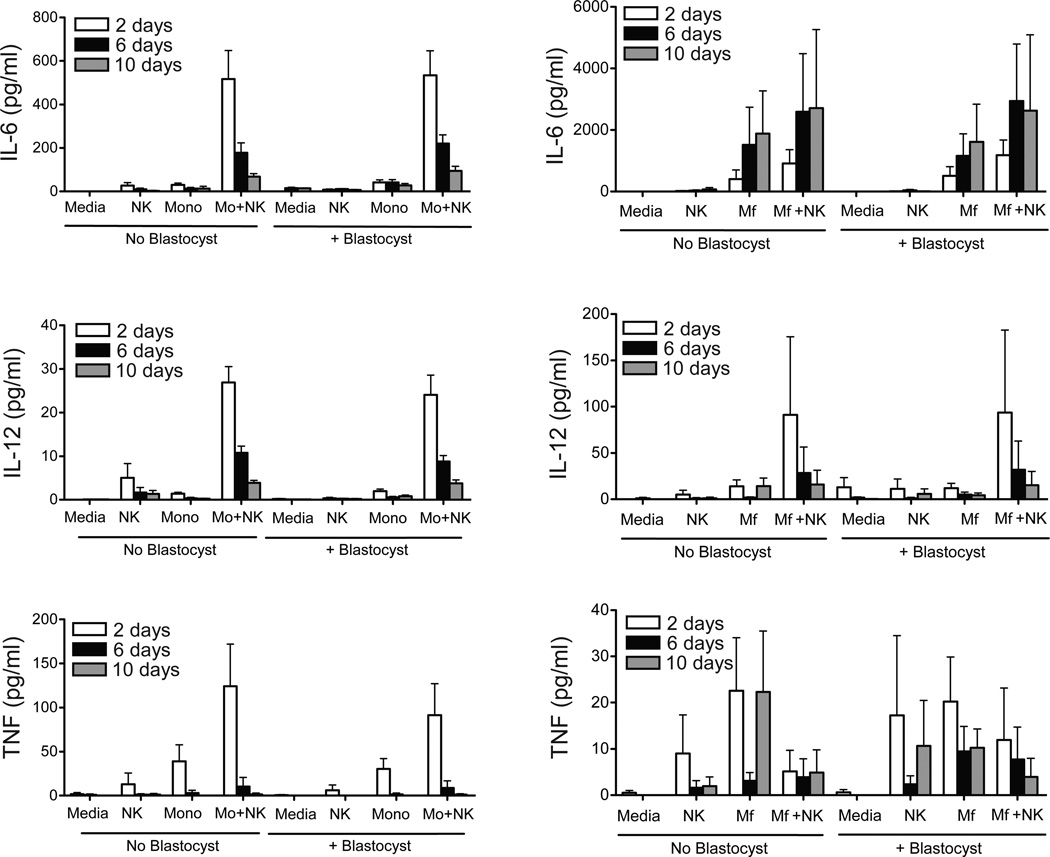
To determine whether the cytokine profile secreted by immune cells in culture is altered by the presence of the embryo, media were collected on days 2, 6, and 10 of co-culture and analyzed for a range of cytokines, chemokines, and growth factors. Supplemental Table 1 provides the average cytokine levels in media on day 2 of culture. IL-6, IL-8, IL-12, G-CSF, GM-CSF, MCP-1, MIP-1α, MIP-1β, and TNF were consistently detectable, while IL-1β, IL-1Rα, IL-15, IL-18 and IFN1γ were below detectable levels. Overall, the secretion level of cytokines (Figure 3), chemokines (Supplemental Figure 1) or growth factors (Supplemental Figure 2) was not significantly different between wells containing only peripheral immune cells in comparison to those co-cultured with a blastocyst. Increases in the secretion of all cytokines were noted in combined NK cell + monocyte co-culture versus NK cells or monocytes cultured alone, indicating significant interactions between these cell types (Figure 3). Similarly, increased secretion of cytokines were observed for NK cell + macrophage co-cultures. Although the data suggests NK cells suppressed TNF secretion by macrophages (Figure 3), the difference compared to control cultures was not statistically significant due to variability between experiments.
Figure 3.
IL-6, IL-12 (p40), and TNF concentrations in the media from leukocyte-blastocyst co-cultures on days 2, 6, and 10. The left panel represents levels within media for control wells with medium only (n=7), NK cells (n=8), monocytes (Mo, n=8), NK cells + monocytes (n=7) and in the right panels represent the levels within media for control wells with medium only (n=7), NK cells (n=8), macrophages (Mφ, n=8), or NK cells + Mφ (n=7). Data is represented as the mean ± SE. There were essentially no detectable levels of cytokines in media cultured in the absence of PBMCs.
Viability of PBMCs was assessed on day 10 of culture by recovering the PBMCs followed by staining with trypan blue. Staining revealed a lower survival rate for NK cells and monocytes, which may contribute to the lack of response to blastocysts in these cultures. However, macrophages had higher survival on day 10 of culture, and thus subsequent experiments focused on macrophage + blastocyst co-cultures.
Assessment of peripheral blood derived macrophage surface marker phenotype
To determine if peripheral blood monocyte-derived macrophages were phenotypically altered when exposed to an implanting embryo, macrophages were recovered from Matrigel co-culture on day 10 for analysis of surface markers by flow cytometry. The markers, DC-SIGN, HLA-DR, and CD86, were evaluated as they have been shown to be differentially expressed between peripheral derived macrophages versus decidual macrophages22. Additionally, CD36, CD163, and MARCO were evaluated to determine if the phenotype of the macrophages shifted towards an M2 phenotype. CD40 and CD11c were also evaluated but were not detectable. No changes were noted between macrophages cultured alone or in co-culture with an implanting blastocyst (Supplemental Figure 3).
Characterization of trophoblasts by flow cytometric analysis following peripheral immune cell co-culture
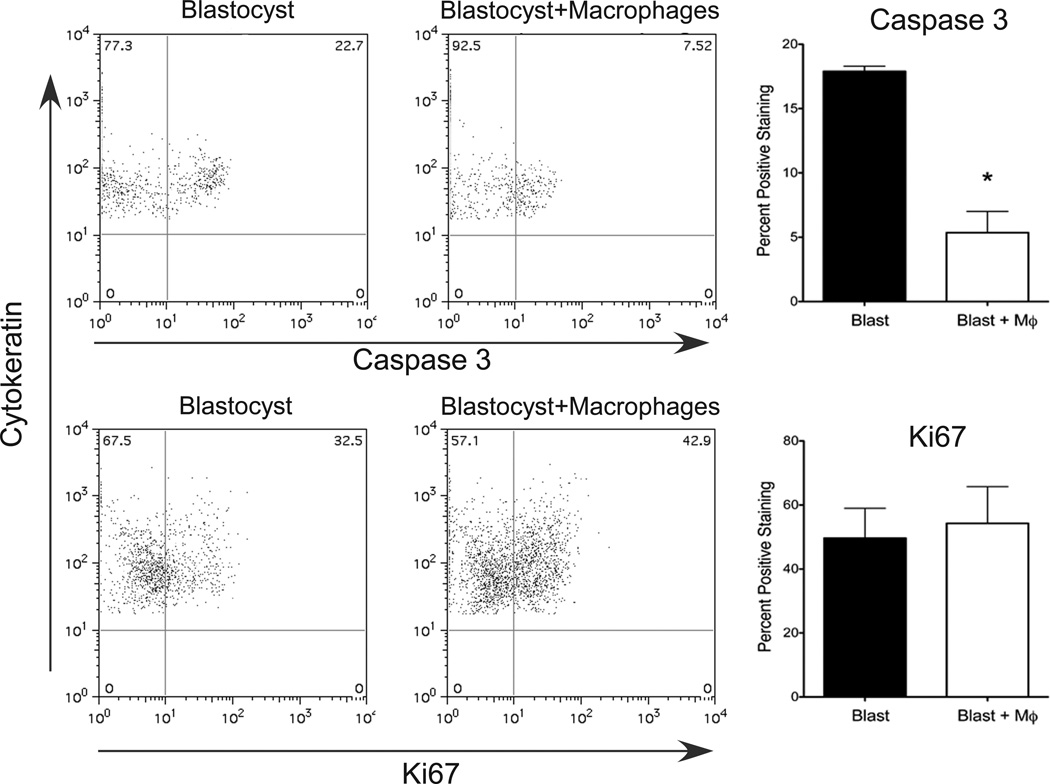
To determine if apoptosis or proliferation of the trophoblast cells were altered by peripheral derived macrophage co-culture, we analyzed cytokeratin-positive trophoblasts from enzymatically dispersed cultures for expression of Caspase-3 (apoptosis) and Ki67 (a proliferation marker). There was a significant decrease in the percent of trophoblast cells that expressed Caspase-3 when cultured with macrophages while no change was noted in Ki67 expression (Figure 4). These data suggest that peripheral-derived macrophages promote trophoblast survival.
Figure 4.
Flow cytometric analysis of trophoblast Caspase-3 (top panels) and Ki67 (bottom panels) expression in control blastocysts and blastocysts cultured with macrophages on day 10 of co-culture, n=5–8. Asterisk denotes P < 0.05.
Embryo outgrowth in response to decidual macrophage co-culture
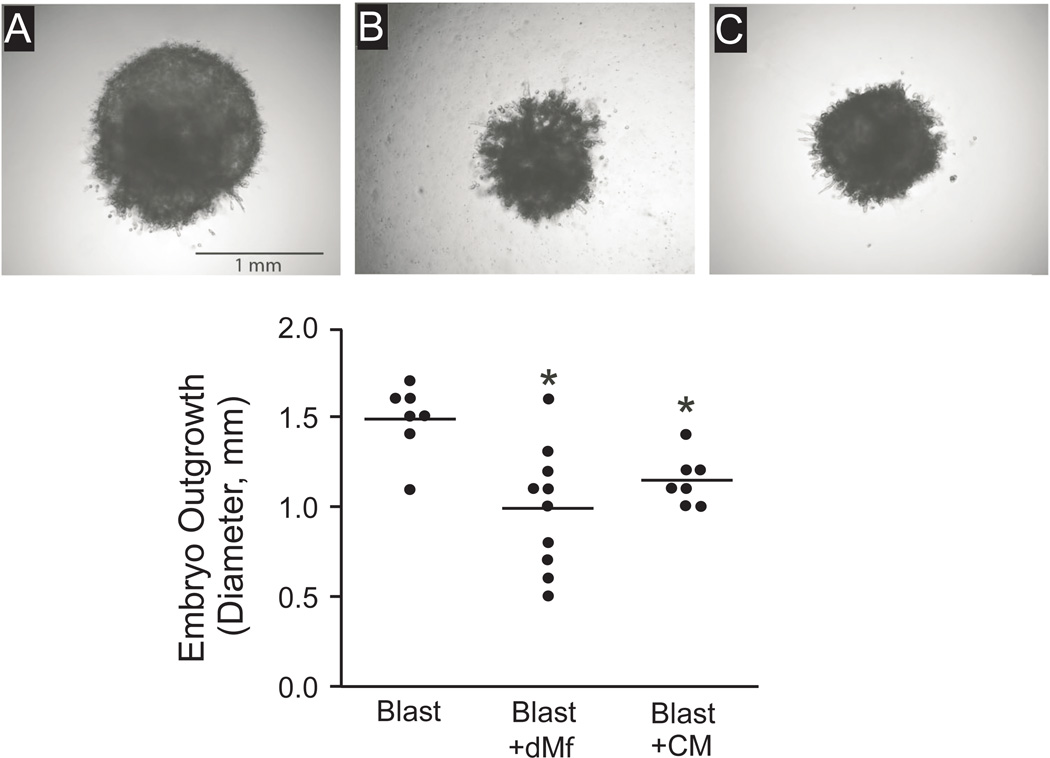
We then defined the impact of decidual macrophages on trophoblasts following co-culture. Decidual macrophages were mixed in Matrigel and the blastocysts were plated onto the surface, whereas control blastocysts were cultured on Matrigel without macrophages. On day 10 of co-culture, embryos were imaged and the diameter was evaluated. Trophoblast outgrowth was significantly decreased when the blastocysts were cultured with decidual macrophages (Figure 5).
Figure 5.
Effect of decidual macrophage (dMϕ) co-culture or decidual macrophage conditioned media (CM) on blastocyst outgrowths on day 10 of co-culture. Photomicrographs (top panel) represent embryos co-cultured (A) as a blastocyst alone, (B) with dMϕ, and (C) with dMϕCM. The graph displays embryo outgrowth diameter (mm) following culture alone or with dMφ or CM. Each data point represents one embryo, thus n= 7–10. Asterisk denotes P < 0.05, compared to control blastocysts cultured alone.
To assess whether macrophages secrete factors that alter trophoblast outgrowth, conditioned medium was collected from wells containing only macrophages in Matrigel after 2 days of culture and was used to supplement the medium for embryos cultured on Matrigel containing no macrophages. Control embryos received medium from wells containing Matrigel with no additional cells. Overall, trophoblast outgrowth from blastocysts cultured in macrophage-conditioned medium responded similarly to direct blastocyst co-culture with macrophages. The diameter of embryonic outgrowth with conditioned medium treatment was not different from embryos co-cultured with decidual macrophages, but both were significantly reduced from the control group (Figure 5).
Chorionic Gonadotropin secretion from embryos co-cultured with decidual macrophages
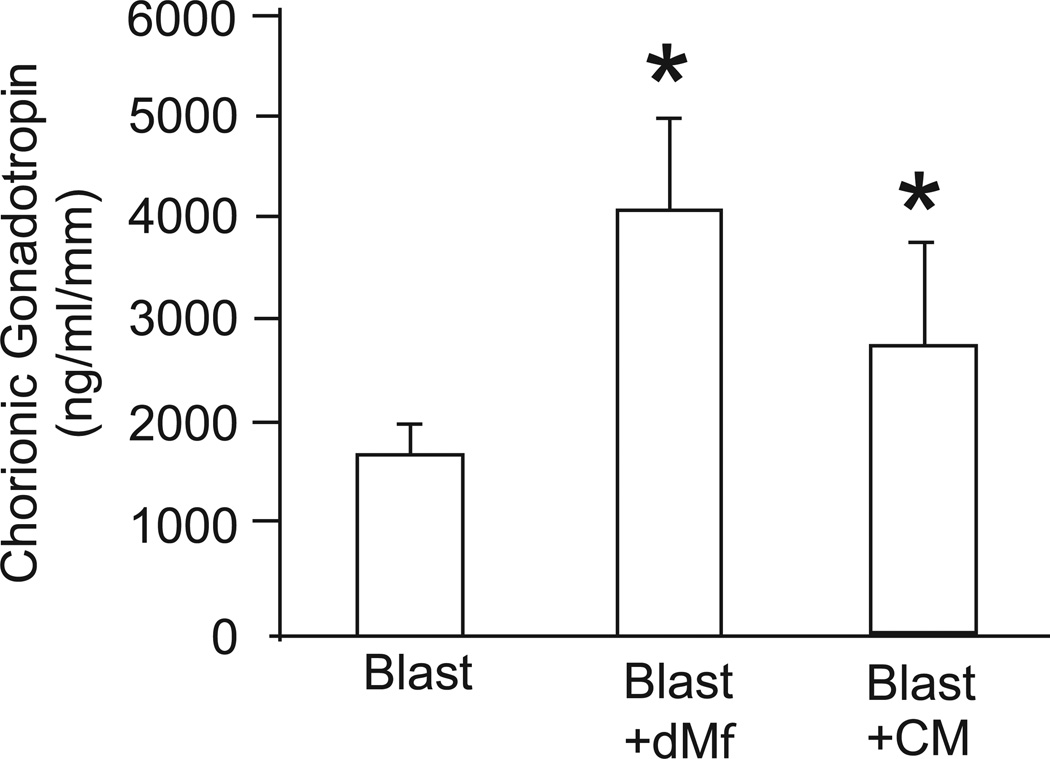
As with peripheral immune cell experiments, the level of secreted CG into the medium was evaluated for decidual macrophage and conditioned media co-cultures. The level of secreted CG on day 2 was low to barely detectable, but was detectable on days 6 and 10. CG levels in day 10 media were normalized to trophoblast outgrowth size. Secretion of CG was significantly increased when blastocysts were cultured in the presence of decidual macrophages and decidual macrophage-conditioned media in comparison to control blastocysts (Figure 6).
Figure 6.
Chorionic gonadotropin (CG) secretion within day 10 culture medium, normalized by embryo diameter (mm). Blastocysts were cultured alone as control (blast) or in the presence of decidual macrophages (dMϕ), or decidual macrophage-conditioned media (CM). Data is represented as the mean ± SE, where the asterisk denotes P < 0.05 compared to control medium.
Cytokine, chemokine and growth factor secretion in decidual macrophage cell co-cultures
The local cytokine profile of the uterus could affect the function of both resident macrophages and invading trophoblast cells. To determine if the presence of the expanding trophoblasts alters the profile of cytokines produced by macrophages in culture, media were collected on days 2, 6, and 10 and analyzed for IL-6, IL-8 IL-10, IL-12, GM-CSF, G-CSF, MCP-1, MIP-1α, MIP-1β, MCP-2, IP-10, and TNF secretion (Supplementary Figure 4). IL6 was the only cytokine detectable in media from the control (blastocyst only), and was not significantly different from levels when the blastocyst was co-cultured with macrophages, although the levels appeared to be additive on day 2 and day 6. IL-6, IL-8, MCP-1, and MIP-1α were detectable in the media from macrophage and macrophage conditioned medium groups. While IL-8 and MIP-1α decreased during cultures, MCP-1 levels were maintained through 10 days of culture. No differences in secretion from macrophages cultured alone or co-cultured with the blastocyst were noted. All other cytokines (IL-10, IL-12, GM-CSF, G-CSF, MIP-1β, MCP3, IP-10, and TNF) were below detectable levels in all experimental groups.
Matrix metalloproteinase (MMP) secretion
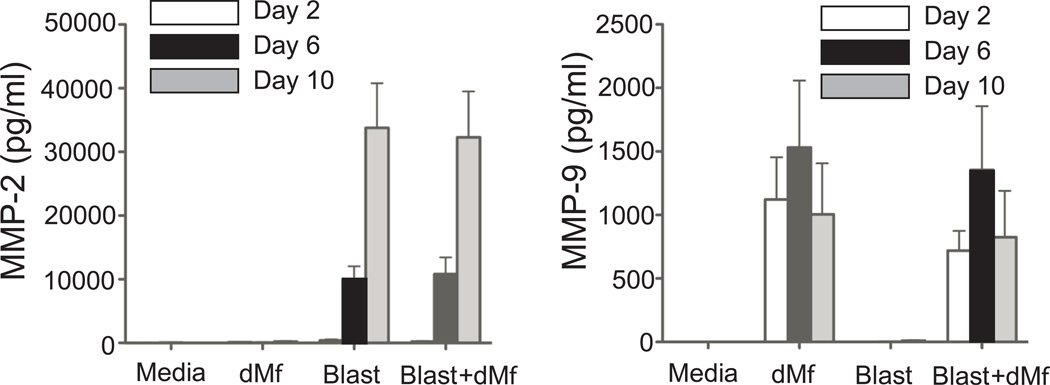
MMP- 2, 7, and 9 are secreted at the maternal-fetal interface and considered important for trophoblast invasion23. We measured secretion in co-culture to determine if decidual macrophages limit trophoblast invasion by altering MMP expression (Figure 7). In rhesus embryo cultures, MMP-2 was secreted by the blastocyst in increasing amounts during the culture period, while MMP-9 was secreted by macrophages. MMP-7 was below the detectable limits of the assay. No differences were noted for MMP-2 or 9 in co-cultures, compared to individually cultured blastocysts or decidual macrophages.
Figure 7.
Matrix metalloproteinases (MMP-2 and MMP-9) levels in media from control wells (Media, n=10), decidual macrophages (dMϕ, n=14), blastocyst alone (Blast, n=9), and blastocyst co-cultured with decidual macrophages (Blast + dMϕ, n=6) on days 2, 6, and 10. Data is represented as the mean ± SE
Flow cytometric analysis of trophoblast cells following decidual macrophage co-culture
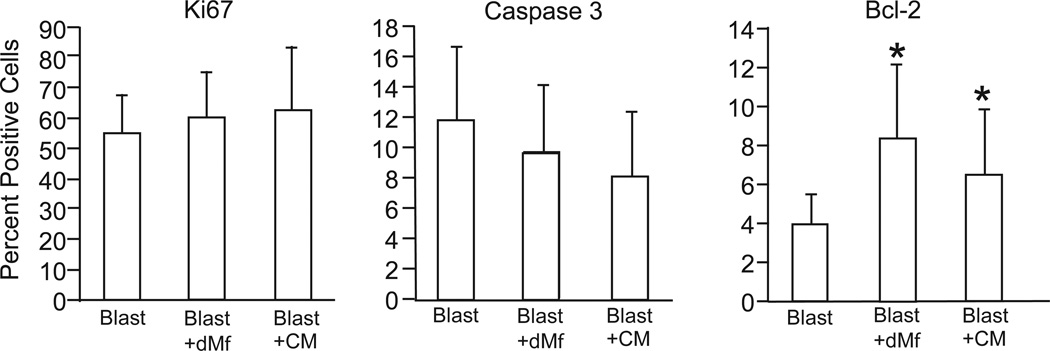
To determine whether apoptosis or proliferation contributed to reduced outgrowth with decidual macrophage co-culture, macrophages were recovered from the Matrigel and analyzed by flow cytometry. Cytokeratin-positive trophoblast cells were assessed for the expression of caspase-3 and Ki67 in the presence and absence of decidual macrophages. As demonstrated in Figure 8a, no cells co-expressed cytokeratin (a trophoblast marker) and CD45 (a macrophage marker), confirming that it was appropriate to evaluate cytokeratin-positive cells as trophoblasts. No differences were noted in Ki67 expression on trophoblasts from control blastocysts or blastocysts co-cultured with macrophages (Figure 8). The expression of caspase-3 was reduced in trophoblasts from blastocysts cultured with decidual macrophages in comparison to control blastocysts, although the difference was not statistically significant (Figure 8). Consistent with these data, anti-apoptotic Bcl-2 expression was significantly increased in blastocyst-macrophage co-culture (Figure 8). Levels of caspase-3 and Ki67 in trophoblasts from blastocysts cultured in macrophage-conditioned medium were not significantly different from blastocysts co-cultured with decidual macrophages (Figure 8). Similarly, analysis of trophoblast outgrowths from blastocysts cultured in macrophage conditioned medium showed a significant increase in the expression of Bcl-2 (Figure 8).
Figure 8.
Flow cytometric analysis of the expression of Ki67, Caspase-3 and Bcl-2 in cultured trophoblasts (cytokeratin positive) in the presence of media, decidual macrophages (dMϕ) or conditioned media (CM); n= 5–8. Data is represented as the mean ± SD, where the asterisk denotes P < 0.05 compared to blastocysts alone.
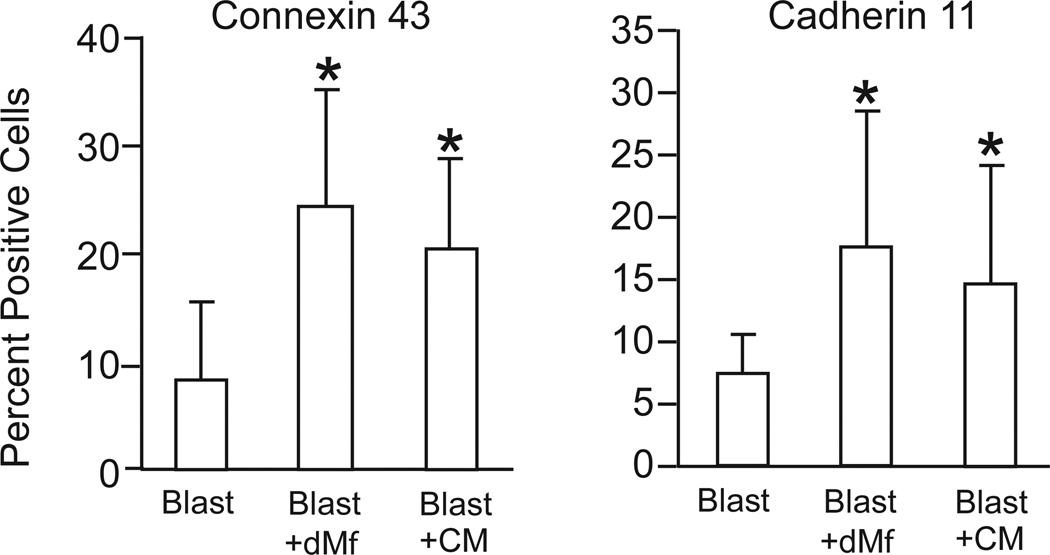
To further evaluate the possible effect of macrophages on trophoblast differentiation, the expression of Connexin43 (GJA1, a gap junction protein involved in the process of fusion of syncytiotrophoblasts), and Cadherin 11 (CDH11, found at increased levels in syncytiotrophoblasts) was determined by flow cytometry. Significantly higher levels of expression of Connexin43 and Cadherin11 were associated with trophoblasts from blastocysts co-cultured with decidual macrophages versus control blastocysts (Figure 9), suggesting these cells were undergoing accelerated differentiation. Both Connexin43 and Cadherin11 expression in trophoblasts was also significantly elevated by decidual macrophage-conditioned medium (Figure 9).
Figure 9.
Flow cytometric analysis of the surface expression of Connexin43 and Cadherin11 on trophoblasts (cytokeratin positive) cultured as blastocysts alone (Blast, n=8), blastocysts cultured in the presence of decidual macrophages (Blast + dMϕ) or in the presence of conditioned media (Blast + CM); n=4–5. Data is represented as the mean ± SD, where the asterisk denotes P < 0.05 compared to blastocysts alone.
Discussion
The present study utilized an in vitro implantation model to define the interaction between maternal immune cells and embryonic trophoblast cells. Both maternal peripheral immune cells and decidual macrophages were co-cultured with blastocysts near the periimplantation stage of development, to define the interaction between each immune cell type and trophoblasts. Consistently, co-culture of macrophages and trophoblasts resulted in reduced trophoblast outgrowth from the implanting blastocyst. Interestingly, the overall level of trophoblast proliferation was unchanged while the level of apoptosis, based on Caspase-3 expression, was decreased with trophoblast co-culture with peripheral derived macrophages and was unchanged for decidual macrophage co-culture. These data suggest that macrophages promote trophoblast survival, and illustrate the feasibility of in vitro studies to evaluate maternal immune cell effects on implantation stage non-human primate embryos.
Despite the reduction in trophoblast outgrowth, hormone levels in the culture media were higher when the blastocyst was co-cultured with peripheral derived or decidual macrophages. It is unclear which macrophage derived factor(s) are responsible for the effects on trophoblast growth and differentiation, and hormone secretion. Macrophages can influence the implanting embryo through secretion of various cytokines. The factors, TNF, TGFβ, and IGF-BP1 act to inhibit trophoblast growth, however, they also increase apoptosis. In comparison, GM-CSF, G-CSF, EGF, and CG have been shown to stimulate trophoblasts and induce differentiation to syncytium, in which only EGF has been shown to inhibit apoptosis 24. The cytokines measured in the present study are not likely to be the primary source underlying changes in trophoblast outgrowth in response to co-culture with peripheral-derived macrophages nor decidual macrophages. The highest levels of GM-CSF, G-CSF, TNF, MIP-1α, and MIP-1β were found in NK cell + monocyte co-cultures and levels of IL-6, IL-8, IL-12, and MCP-1 were similar in a greater proportion of the NK cell + monocyte and NK cell + macrophage co-cultures. This suggests that other cytokines or direct cell-cell interactions are involved in the change in trophoblast outgrowth observed when blastocysts are cultured with peripheral derived macrophages or decidual macrophages. Interestingly, the macrophage-induced increase in CG secretion was not observed when the blastocysts were cultured with peripheral NK cells + macrophages. It is possible that the dynamic interplay between secreted cytokines of NK cells and macrophages could explain this observation, although further analysis of NK cell-macrophage interactions is needed.
During implantation, the trophectoderm of the blastocyst gives rise to both villous and extravillous trophoblast compartments of the placenta. Villous cytotrophoblasts proliferate with placental growth and differentiate and fuse to form a multinucleated syncytiotrophoblast layer overlying the cytotrophoblasts. Villous cytotrophoblast fusion is carefully orchestrated, progressing through sequential events, including plasma membrane phosphatidylserine flip; increased activation of Caspase-8; expression of HERV-W (syncytin); expression of gap junction connexins, especially Connexin43; and differential expression of cadherins, from E-cadherin (CDH1) in cytotrophoblasts to Cadherin11 in syncytiotrophoblasts25, 26. Because the growth of embryonic trophoblasts in the Matrigel environment precluded morphological identification of syncytium formation, we used flow cytometric analyses of several of these markers of syncytiotrophoblast differentiation to determine whether an effect of the decidual macrophages may be to accelerate syncytialization. Expression of both Connexin43 and Cadherin11 in addition to Bcl-2 were increased on trophoblasts co-cultured with decidual macrophages, which supports the premise that macrophages support and promote trophoblast differentiation.
Connexin43 is expressed in cytotrophoblasts prior to fusion and supports communication between cytotrophoblasts and syncytiotrophoblasts27. Microarray analysis of primary rhesus syncytiotrophoblast cultures confirmed a significant increase in Connexin43 mRNA during differentiation (Golos et al., unpublished). Furthermore, Connexin43 is required for proper trophoblast fusion and differentiation as shown by the use of Connexin43 antisense RNA and a gap junction uncoupler which prevented proper fusion of primary trophoblasts cultured in vitro28, 29. Connexin43 has also been implicated in recurrent pregnancy loss based on decreased protein and RNA expression in placentas from patients with multiple spontaneous abortions30, and other studies which conclude blocking Connexin43 function with mefloquine induces pregnancy loss in humans31, 31–33.
Cadherins are membrane proteins important for cell adhesion, and multiple cadherins are expressed in the placenta. E-cadherin is expressed by villous cytotrophoblasts prior to fusion, but during the process of fusion CDH1 expression decreases while Cadherin 11 expression increases. Cadherin11 expression is required for fusion and formation of syncytiotrophoblasts and is differentially expressed on syncytiotrophoblasts34, 35.
In addition to Connexin43 and Cadherin11, syncytiotrophoblasts also differentially express BCL2. Bcl-2 is anti-apoptotic in a broad range of cell types, and in normal placentas throughout gestation, BCL2 is expressed in the cytoplasm of syncytiotrophoblasts, but not other trophoblast or placental cell types36–38. Taken together, the increase in hormone secretion, Bcl-2, Connexin43, and Cadherin11 expression noted in trophoblasts from blastocyst-macrophage co-cultures suggest the latter may be promoting the fusion and differentiation of trophoblasts in this culture system.
To gain insight into whether these effects were mediated through direct cell-cell contact, we treated blastocysts with macrophage-conditioned medium. Overall, trophoblast outgrowths from blastocysts cultured in macrophage-conditioned medium responded quite similarly to trophoblast outgrowths from blastocysts co-cultured with decidual macrophages, suggesting macrophages are secreting a factor(s) into the medium to influence the implanting trophoblasts. Conditioned medium stimulated embryonic CG secretion, and inhibited trophoblast outgrowth spreading. Bcl-2 and Connexin43 expression in trophoblasts cultured in macrophage-conditioned medium were also significantly different from trophoblasts from control embryos, but not from trophoblasts co-cultured with macrophages. The less pronounced expression of Cadherin11 on trophoblasts cultured with macrophage-conditioned medium versus trophoblasts co-cultured directly with macrophages may be due to relative instability of the active factor(s), or possibly due to the Matrigel microenvironment. Secreted factors would likely be found at higher levels near the macrophages in the Matrigel (or may be retained or bound by the Matrigel itself) and blastocysts cultured directly with decidual macrophages may be exposed to relatively higher concentration of secreted factors than that contained in the macrophage-conditioned medium. Interestingly, these results show that macrophages are secreting factors into the conditioned medium that influenced the implanting trophoblasts, even though trophoblasts were not present. It is possible that macrophages isolated from the decidua are programmed during pregnancy to continue to secrete the active factors and thus trophoblasts do not directly promote their expression. Further studies will be needed to determine the biochemical nature and ultimately the identity of the factor or factors involved.
These results may represent an important role of macrophages in establishing a successful pregnancy. Trophoblast differentiation and fusion in the formation of syncytia are crucial in proper placental formation and maintenance of pregnancy. In addition to reduced expression of Connexin43 in patients with recurrent pregnancy loss30, decreased cell fusion and a disorganized syncytium were seen in placentas from pregnancies with intrauterine growth restriction39. Conversely, an imbalanced increase in trophoblast differentiation to syncytiotrophoblasts could result in fewer cells available in the cell columns and the extravillous lineage to proliferate and invade into the uterus. Macrophage induction of altered differentiation dynamics in implantation may result in reduced trophoblast invasion and suboptimal placental development, which could lead to poor spiral artery remodeling as has been seen in placentas of patients with preeclampsia40, 41.
Although our studies demonstrate a direct effect of decidual macrophages on embryonic trophoblast function, a proper immune cell balance at the maternal-fetal interface is likely to be necessary for appropriate placentation. Part of this balance includes the presence of NK cells. Considerable attention has been given to the role of uterine NK cells in early pregnancy13, 42, 43. Thus, it was somewhat surprising that peripheral NK cells had no discernable effect on trophoblast outgrowth, even though many cytokines noted in macrophage cultures were also present in the media of NK cell cultures. Human decidual NK cells have previously been reported to promote extravillous trophoblast migration in in vitro and ex vivo cell migration studies44. It could be that macrophages function to promote trophoblast differentiation in the villi, while NK cells promote invasion of the extravillous trophoblast lineage. Decidual NK cells were not available for the current studies, however recent work has shown interaction between human decidual macrophages and NK cells45, and further studies on the interaction of decidual leukocyte subsets will be of significant interest.
Conclusions
Overall, this study establishes a platform to study non-human primate implantation in vitro in which interactions between maternal immune cells and embryos can be evaluated. Co-culture of maternal immune cells with embryos revealed that from both peripheral blood monocytes and the early pregnancy decidua, modulate trophoblast growth and function in terms of hormone secretion and differentiation. Further studies are needed with our in vitro non-human primate model of implantation to explore what factor(s) specific to macrophages directly affect trophoblasts.
Supplementary Material
Acknowledgments
We extend our appreciation to the Animal Care Staff at the Wisconsin National Primate Research Center for doing the blood draws, the members of the Scientific Protocol Implementation team for hormone injections, oocyte retrievals, and semen collection, Kim Weisgrau for assistance with the FACSCalibur, the UW-Madison HLA/Molecular Diagnostics Lab for the use of the Luminex machine, and UW-Madison Hospital and Clinics. We are thankful for funding support by NIH grants HD053925, AI076734, HD38843, AI100156, and RR021876, and ARRA grant HD053925-02S1, to T.G.G. and P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. A.E.R. was supported in part by NIH grant T32 HD041921. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
References
- 1.Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertility and sterility. 2008;89:1781–1786. doi: 10.1016/j.fertnstert.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Merviel P, Lourdel E, Cabry R, Boulard V, Brzakowski M, Demailly P, Brasseur F, Copin H, Devaux A. Physiopathology of human embryonic implantation: clinical incidences. Folia Histochem Cytobiol. 2009;47:S25–S34. doi: 10.2478/v10042-009-0058-3. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–522. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 4.Guibourdenche J, Fournier T, Malassine A, Evain-Brion D. Development and hormonal functions of the human placenta. Folia Histochem Cytobiol. 2009;47:S35–S40. doi: 10.2478/v10042-009-0110-3. [DOI] [PubMed] [Google Scholar]
- 5.Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- 6.Musicki B, Pepe GJ, Albrecht ED. Functional differentiation of placental syncytiotrophoblasts during baboon pregnancy: developmental expression of chorionic somatomammotropin messenger ribonucleic acid and protein levels. J Clin Endocrinol Metab. 1997;82:4105–4110. doi: 10.1210/jcem.82.12.4453. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama T, Fujiwara H, Maeda M, Inoue T, Yoshioka S, Mori T, Fujii S. Human peripheral blood mononuclear cells (PBMC) in early pregnancy promote embryo invasion in vitro: HCG enhances the effects of PBMC. Hum Reprod. 2002;17:207–212. doi: 10.1093/humrep/17.1.207. [DOI] [PubMed] [Google Scholar]
- 8.Denison FC, Kelly RW, Calder AA. Differential secretion of chemokines from peripheral blood in pregnant compared with non-pregnant women. J Reprod Immunol. 1997;34:225–240. doi: 10.1016/s0165-0378(97)00046-6. [DOI] [PubMed] [Google Scholar]
- 9.Dennison E, Fall C, Cooper C, Barker D. Prenatal factors influencing long-term outcome. Horm Res. 1997;48(Suppl 1):25–29. doi: 10.1159/000191262. [DOI] [PubMed] [Google Scholar]
- 10.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47:91–97. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 11.Hashii K, Fujiwara H, Yoshioka S, Kataoka N, Yamada S, Hirano T, Mori T, Fujii S, Maeda M. Peripheral blood mononuclear cells stimulate progesterone production by luteal cells derived from pregnant and non-pregnant women: possible involvement of interleukin-4 and interleukin-10 in corpus luteum function and differentiation. Hum Reprod. 1998;13:2738–2744. doi: 10.1093/humrep/13.10.2738. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara H. Do circulating blood cells contribute to maternal tissue remodeling and embryo-maternal cross-talk around the implantation period? Mol Hum Reprod. 2009;15:335–343. doi: 10.1093/molehr/gap027. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol. 2000;11:127–137. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 14.Slukvin II, Breburda EE, Golos TG. Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta. 2004;25:297–307. doi: 10.1016/j.placenta.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. American journal of reproductive immunology (New York, NY : 1989) 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolf DP. The non-human primate oocyte and embryo as a model for women, or is it vice versa? Theriogenology. 2008;69:31–36. doi: 10.1016/j.theriogenology.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Wolfgang MJ, Eisele SG, Knowles L, Browne MA, Schotzko ML, Golos TG. Pregnancy and live birth from nonsurgical transfer of in vivo- and in vitro-produced blastocysts in the rhesus monkey. J Med Primatol. 2001;30:148–155. doi: 10.1111/j.1600-0684.2001.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 19.Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Generation of macrophages from peripheral blood monocytes in the rhesus monkey. J Immunol Methods. 2009;351:36–40. doi: 10.1016/j.jim.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golos TG, Bondarenko GI, Breburda EE, Dambaeva SV, Durning M, Slukvin II. Immune and trophoblast cells at the rhesus monkey maternal-fetal interface. Methods in molecular medicine. 2006;122:93–108. doi: 10.1385/1-59259-989-3:93. [DOI] [PubMed] [Google Scholar]
- 21.Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- 22.Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Modulation of Cytokine and Chemokine Secretions in Rhesus Monkey Trophoblast Co-Culture With Decidual but not Peripheral Blood Monocyte-Derived Macrophages. Am J Reprod Immunol. 2011;66:115–127. doi: 10.1111/j.1600-0897.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and −9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39:179–195. doi: 10.1016/s0165-0378(98)00021-7. [DOI] [PubMed] [Google Scholar]
- 25.Knerr I, Huppertz B, Weigel C, Dotsch J, Wich C, Schild RL, Beckmann MW, Rascher W. Endogenous retroviral syncytin: compilation of experimental research on syncytin and its possible role in normal and disturbed human placentogenesis. Mol Hum Reprod. 2004;10:581–588. doi: 10.1093/molehr/gah070. [DOI] [PubMed] [Google Scholar]
- 26.Handwerger S. New insights into the regulation of human cytotrophoblast cell differentiation. Mol Cell Endocrinol. 2010;323:94–104. doi: 10.1016/j.mce.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malassine A, Cronier L. Involvement of gap junctions in placental functions and development. Biochim Biophys Acta. 2005;1719:117–124. doi: 10.1016/j.bbamem.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Frendo JL, Cronier L, Bertin G, Guibourdenche J, Vidaud M, Evain-Brion D, Malassine A. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J Cell Sci. 2003;116:3413–3421. doi: 10.1242/jcs.00648. [DOI] [PubMed] [Google Scholar]
- 29.Cronier L, Frendo JL, Defamie N, Pidoux G, Bertin G, Guibourdenche J, Pointis G, Malassine A. Requirement of gap junctional intercellular communication for human villous trophoblast differentiation. Biology of reproduction. 2003;69:1472–1480. doi: 10.1095/biolreprod.103.016360. [DOI] [PubMed] [Google Scholar]
- 30.Nair RR, Jain M, Singh K. Reduced expression of gap junction gene connexin 43 in recurrent early pregnancy loss patients. Placenta. 2011;32:619–621. doi: 10.1016/j.placenta.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Smoak BL, Writer JV, Keep LW, Cowan J, Chantelois JL. The effects of inadvertent exposure of mefloquine chemoprophylaxis on pregnancy outcomes and infants of US Army servicewomen. J Infect Dis. 1997;176:831–833. doi: 10.1086/517315. [DOI] [PubMed] [Google Scholar]
- 32.Nevin RL. Mefloquine blockade of connexin 43 (Cx43) and risk of pregnancy loss. Placenta. 2011 doi: 10.1016/j.placenta.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Nevin RL. Mefloquine blockade of connexin 43 (Cx43) and risk of pregnancy loss. Placenta. 2011;32:712. doi: 10.1016/j.placenta.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Getsios S, MacCalman CD. Cadherin-11 modulates the terminal differentiation and fusion of human trophoblastic cells in vitro. Dev Biol. 2003;257:41–54. doi: 10.1016/s0012-1606(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 35.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31:747–755. doi: 10.1016/j.placenta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez J, Samoto T, Maruo T. Changes in proliferative potential, apoptosis and Bcl-2 protein expression in cytotrophoblasts and syncytiotrophoblast in human placenta over the course of pregnancy. Endocr J. 2000;47:317–327. doi: 10.1507/endocrj.47.317. [DOI] [PubMed] [Google Scholar]
- 37.Soni S, Rath G, Prasad CP, Salhan S, Saxena S, Jain AK. Apoptosis and Bcl-2 Protein Expression in Human Placenta over the Course of Normal Pregnancy. Anat Histol Embryol. 2010 doi: 10.1111/j.1439-0264.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 38.Soni S, Rath G, Prasad CP, Salhan S, Saxena S, Jain AK. Apoptosis and Bcl-2 protein expression in human placenta over the course of normal pregnancy. Anat Histol Embryol. 2010;39:426–431. doi: 10.1111/j.1439-0264.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 39.Ruebner M, Strissel PL, Langbein M, Fahlbusch F, Wachter DL, Faschingbauer F, Beckmann MW, Strick R. Impaired cell fusion and differentiation in placentae from patients with intrauterine growth restriction correlate with reduced levels of HERV envelope genes. J Mol Med (Berl) 2010;88:1143–1156. doi: 10.1007/s00109-010-0656-8. [DOI] [PubMed] [Google Scholar]
- 40.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 41.Redman CW, Sargent IL. Immunology of pre-eclampsia. American journal of reproductive immunology (New York, NY : 1989) 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 42.Lash GE, Bulmer JN. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J Reprod Immunol. 2011;88:156–164. doi: 10.1016/j.jri.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Hunt JS. Immunologically relevant cells in the uterus. Biol Reprod. 1994;50:461–466. doi: 10.1095/biolreprod50.3.461. [DOI] [PubMed] [Google Scholar]
- 44.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 45.Co EC, Gormley M, Kapidzic M, Rosen DB, Scott MA, Stolp HA, McMaster M, Lanier LL, Barcena A, Fisher SJ. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol Reprod. 2013;88:155. doi: 10.1095/biolreprod.112.099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.