Abstract
Cells of the osteoblast lineage provide critical support for B lymphopoiesis in the bone marrow (BM). Parathyroid hormone (PTH) signaling in osteoblastic cells through its receptor (PPR) is an important regulator of hematopoietic stem cells; however, its role in regulation of B lymphopoiesis is not clear. Here we demonstrate that deletion of PPR in osteoprogenitors results in a significant loss of trabecular and cortical bone. PPR signaling in osteoprogenitors, but not mature osteoblasts or osteocytes, is critical for B cell precursor differentiation via IL-7 production. Interestingly, despite a severe reduction in B cell progenitors in BM, mature B lymphocytes were increased 3.5-fold in the BM of mice lacking PPR in osteoprogenitors. This retention of mature IgD+ B cells in the BM was associated with increased expression of vascular cell adhesion molecule 1 (VCAM1) by PPR-deficient osteoprogenitors, and treatment with VCAM1 neutralizing antibody increased mobilization of B lymphocytes from mutant BM. Our results demonstrate that PPR signaling in early osteoblasts is necessary for B cell differentiation via IL-7 secretion, and for B lymphocyte mobilization via VCAM1.
Keywords: Bone, Osteoprogenitors, B Cells, PTH receptor, VCAM1
INTRODUCTION
B cell development is tightly regulated and guided by interactions with niche-forming bone marrow (BM) stromal cells that drive B cell precursors from one niche to another(1,2). Paracrine factors from the stromal cells such as C-X-C motif chemokine 12 (CXCL12), stem cell factor (SCF), interleukin-7 (IL-7), and receptor activator of nuclear factor κB ligand (RANKL) are critical regulators of B cell development(2,3). Moreover the active migration of B cell precursors is a fundamental process for B lymphocyte differentiation and maturation, and is finely regulated by cell-cell interactions through integrins and cell adhesion molecules found on the cell membranes of B cells and niche forming stromal cells(4–6). In addition, circulating hormones also highly influence B cell development, as in the case of potent inhibition of B lymphopoiesis by estrogens(7,8).
Among the niche-forming stromal cells, cells of the osteoblast lineage have been shown to be important regulators of hematopoiesis(9,10). Cells of the osteoblast lineage include morphologically and functionally distinct cell types including osteoprogenitors, mature osteoblasts, and osteocytes. Numerous lines of evidence point to cells of the osteoblast lineage as major regulators of B lymphopoiesis. Conditional deletion of osteoblasts in vivo leads to a progressive failure of hematopoiesis beginning with an early defect in B lymphopoiesis and erythropoiesis(11). Induced osteocyte-deficiency in adult mice also leads to marked decrease in common lymphoid progenitors and subsequent B cell development(12). In vitro, osteoblastic cell cultures are able to sustain B cell commitment and subsequent differentiation to all stages of B lymphopoiesis starting from primitive HSCs(13). However, the cellular mechanisms for the support of B lymphopoiesis by cells of the osteoblast lineage remain largely unknown.
Parathyroid hormone (PTH) is a versatile hormone that has wide and varied effects on the skeleton, kidney, and immune system(14–16). PTH is a potent modulator of hematopoiesis via its actions on niche forming stromal cells. Removal of parathyroid glands in rats reduced overall bone marrow cellularity with the most profound decrease in lymphoid and erythroid subpopulations, suggesting that PTH may be a major regulator of lymphoid cell proliferation(17). On the other hand, continuously elevated levels of PTH are associated with impaired antibody production response of B cells(18,19). Many of the regulatory effects of PTH on hematopoiesis are mediated via PPR signaling in cells of the osteoblast lineage. For example, constitutive activation of PPR signaling in osteoblasts leads to significant expansion of hematopoietic stem and progenitor cells(20).
Increasing evidence suggests that PPR signaling components are also important regulators of B lymphopoiesis. In vitro osteoblast support of B lymphopoiesis was further augmented by PTH treatment(13) suggesting that the PTH signaling in osteoblastic cells may be a major regulator of B lymphopoiesis. Mice lacking Gsα, the stimulatory G protein subunit downstream of G protein-coupled receptors (GPCRs) including PPR, in osteoprogenitors (Osx-GsαKO mice) exhibit a dramatically hypoplastic spleen and a specific block in the transition from Prepro B to Pro B cell precursors during B lymphocyte development(21). In contrast, deletion of Gsα in mineral-embedded osteocytes did not affect B lymphocytes(22) suggesting that the defective B lymphopoiesis seen in mice with induced osteocyte deficiency(12) is most likely independent of PTH signaling.
We therefore hypothesized that PPR signaling in specific stage(s) of osteoblastic cell differentiation is a critical component of the niche regulation of B lymphopoiesis. To test this hypothesis, we generated and examined B lymphopoiesis in mice lacking PPR in osteoprogenitors (Osx-PPRKO), mature osteoblasts (OC-PPRKO), and osteocytes (DMP1-PPRKO). Osx-PPRKO mice developed severe osteopenia and exhibited a specific block in B cell precursor differentiation. By contrast, the OC-PPRKO and DMP1-PPRKO mice did not reveal any effects on B lymphopoiesis. Despite a significant reduction in B cell precursors in BM and severe lymphopenia in peripheral blood, Osx-PPRKO mice display an increased retention of mature B lymphocytes in BM that is due at least in part to overexpression of VCAM1 in Osx+ osteoprogenitors. Taken together, our study demonstrates that PPR signaling in osteoprogenitors but not maturing osteoblasts or osteocytes is essential for regulating B lymphopoiesis and B cell mobilization in BM.
MATERIALS AND METHODS
Animals
Mice lacking PPR in osteoprogenitors were generating by mating PPRfl/fl (23) mice with transgenic mice in which Cre recombinase is driven by the Osterix promoter(24). Deletion of PPR in mature osteoblasts and osteocytes was obtained by mating PPRfl/fl mice with mice expressing Cre recombinase driven by Osteocalcin (OC) and DMP1 promoters respectively(22,25). PPRfl/fl (wild-type, WT) littermates were used as controls for all the experiments. Because the presence of Osx-driven Cre recombinase transgene results in mild runting, experiments were also repeated with Osx:Cre-PPR+/+ and PPR+/+ mice as controls. There was no difference in phenotypes between PPRfl/fl and PPR+/+ mice, therefore where applicable we have presented data from PPRfl/fl and Osx:Cre-PPR+/+ mice as controls. Genotyping was performed on genomic DNA obtained from tail biopsies as previously described(21,26). All animals were housed in the Center for Comparative Medicine at the Massachusetts General Hospital and the Comparative Medicine Pavilion in Stanford University, and all procedures were approved by the MGH Subcommittee on Research Animal Care or the Stanford Administrative Panel on Laboratory Animal Care.
Skeletal Analysis
Skeletal DXA and µCT analysis was performed as described in Supplementary methods.
Bone chip cell culture
Hind limbs were harvested from 3-week old Osx-Cre:PPRfl/fl and Osx-Cre:PPR+/+ mice. After soft tissue dissection and BM removal by centrifugation(27), bones were minced into small pieces and washed at least 3 times in serum-free αMEM medium. Bone chips were then digested in serum-free αMEM medium containing 2 mg/ml Collagenase Type II (Worthington) for 2 hours at 37°C and subsequently washed again at least 3 times to remove all the cells in suspension. The resulting bone chips were resuspended in αMEM (GIBCO) medium supplemented with 10% heat inactivated fetal bovine serum (FBS) (GIBCO), 50 µg/ml ascorbic acid (Sigma) and antibiotics (GIBCO) and plated in a 10 cm dish. After 16–18 days in culture, cells were trypsinized and FACS-sorted as described in Supplementary Methods.
Flow cytometry analysis and sorting
Flow cytometric analysis was performed on bone marrow, spleen and blood while fluorescence-activated cell sorting (FACS) was performed on bone chip cell cultures using specific cell-surface fluorochrome-tagged antibodies as described in Supplementary Methods.
Gene expression analysis
Total mRNA from Osx-GFP+ sorted cells and freshly isolated marrow depleted long bones was extracted and qPCR was performed as described in Supplementary Methods.
Immunohistochemistry
Frozen section immunohistochemistry on long bones from KO and WT littermates was performed as described in Supplementary Methods.
In vivo treatments
Recombinant murine IL7 (mIL7) (R&D Systems) was reconstituted in 1× PBS and injected intraperitoneally at a dose of 0.2 mg/kg/day in Osx-PPRKO, WT and Osx-PPR+/+ mice daily from day 14 till day 21. To block VCAM-1 in vivo, Osx-PPRKO mice and WT littermates were injected intraperitoneally with 200 µg of purified antibody to mouse VCAM-1 (Biolegend, clone 429) or isotype-matched rat IgG1 at day 20. Mice were sacrificed after 24 hours and BM was collected for B cell analyses. For determination of B cell retention in BM in vivo, 5×106 splenocytes from WT donors were labeled with the PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich) for cell tracking and injected into the marrow cavity by an intra-tibia injection. Osx-PPRKO and WT littermate mice were sacrificed after 48 hours and BM from the injected tibia and spleen were collected and analyzed by flow cytometry.
Protein Array
RayBio Mouse cytokine antibody arrays (G series 2000) were purchased from RayBiotech (Norcross, GA). Bone marrow supernatants from 3 week old control and Osx-PPRKO mice were collected, pooled to obtain 10µl for each sample, diluted 10-fold with PBS and then analyzed on the protein array according to the manufacturer's instructions.
Statistics
All values are expressed as mean ± SEM. Statistical analyses were performed using two-tailed Student’s t-test and one-way or two-way analysis of variance (ANOVA) followed by appropriate post hoc tests for multiple comparisons. Differences were considered significant for P<0.05. When the asterisk symbol is presented alone, the KO group was significantly different from both control groups; when the asterisk is presented with a bar, only the groups denoted by the bar were significantly different. Analyses were performed using GraphPad Prism 6.
RESULTS
Severe osteopenia in mice lacking PPR in osteoprogenitors
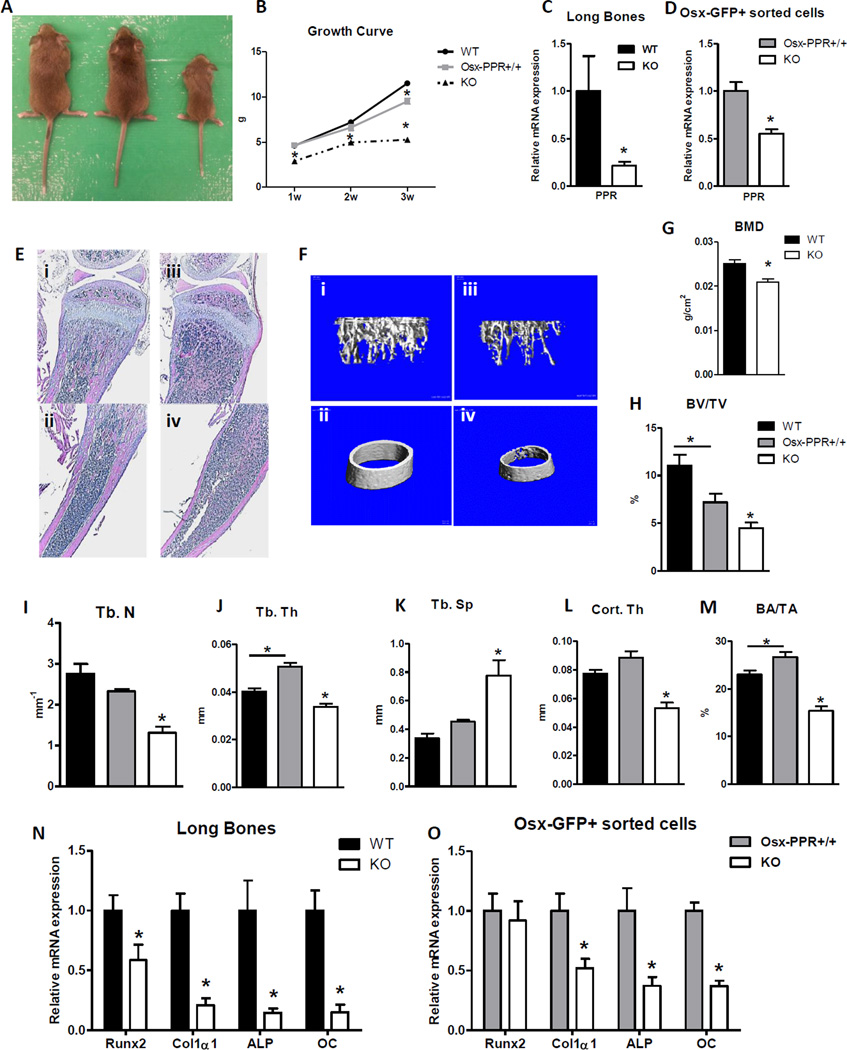
To examine the role of PPR signaling in the osteoblast lineage in regulating B lymphopoiesis, we conditionally ablated PPR in osteoprogenitors (Osx-PPRKO mice)(24). Because previous studies have shown that the presence of Osx:Cre transgene itself results in a transient decrease in body weight that normalizes by 12 weeks of age(28), in the majority of experiments we included the Osx-PPR+/+ mice along with WT littermates as controls. Osx-PPRKO mice showed growth retardation as early as 1 week of age, with more than 45% and 54% decrease in body weight by 3 weeks of age when compared to Osx-PPR+/+ and WT mice respectively (Fig. 1A, B). Osx-PPRKO mice do not survive past 1 month of age; therefore all analyses were carried out prior to postnatal day 21 (P21). PPR mRNA expression was decreased by 78.7% in BM-depleted long bones (Fig. 1C) and by 44.8% in Osx-GFP+ FACS-sorted cells (Fig. 1D), indicating successful deletion of PPR in osteoblast lineage cells.
Fig.1. Osx-PPR KO mice are osteopenic.
(A) Gross appearance at 3 weeks of age and (B) growth curve of WT, Osx-PPR+/+ and Osx-PPRKO (KO) mice. (C) Relative PPR mRNA expression in BM depleted long bones from WT (black) and KO (white) mice; n=5 WT, n=6 KO; *, p<0.05. (D) Relative PPR mRNA expression in FACS-sorted Osx-GFP+ cells from bone chip cultures of Osx-PPR+/+ (grey) and KO (white) mice; n=7 Osx-PPR+/+, n=6 KO; *, p<0.05. (E) Representative hematoxylin and eosin stained sections of proximal tibial metaphyses (upper panels; i, iii) and diaphyses (lower panels; ii, iv) of WT (i,ii) and KO mice (iii, iv). (F) Representative µCT images showing 3-D rendering of trabecular (upper panels; i, iii) and cortical bone (lower panels; ii, iv) microarchitecture of distal and midshaft femora of WT (i, ii) and KO mice (iii, iv). (G) Bone mineral density in femurs of 3 week-old KO compared to WT littermates; n=5 WT, n=6 KO; *, p<0.05. (H–K) Quantitative µCT assessment showing trabecular bone fraction (BV/TV) (H), trabecular numbers (Tb.N) (I), trabecular thickness (Tb.Th) (J), and trabecular spacing (Tb.Sp) (K) in WT, Osx-PPR+/+ and KO mice. (L–M) Quantitative µCT assessment showing cortical thickness (Cort. Th) (L) and cortical bone area fraction (BA/TA) (M) in WT, Osx-PPR+/+ and KO mice; n=5 WT, n=6 Osx-PPR+/+, n=5 KO; *, p<0.05. (N–O) Expression of osteoblast specific mRNAs including Runx2, collagen type I (Col1α1), alkaline phosphate (ALP), and osteocalcin in BM depleted long bones (N) and in Osx-GFP+ sorted osteoprogenitors (O) from 3 week-old WT and KO and Osx-Cre:PPR+/+ and KO mice, respectively; n=9 WT, n=6 KO; and n=7 Osx-Cre:PPR+/+, n=6 KO; *, p<0.05.
Histological examination showed a substantial decrease in trabecular and cortical bone (Fig. 1E). Micro-computed tomography (µCT) analysis (Fig. 1F) of distal femurs and dual-energy X-ray absorptiometry (DXA) analysis of skeletal bone mineral density (BMD) revealed a significant decrease in BMD (Fig. 1G) and a dramatic reduction in trabecular bone volume fraction (BV/TV) (Fig. 1H), trabecular number (Tb.N) (Fig. 1I), and trabecular thickness (Tb.Th) (Fig. 1J), and a significant increase in trabecular spacing (Tb.Sp) (Fig. 1K) in Osx-PPRKO mice. µCT of midshaft femurs showed a marked decrease in cortical shell thickness (Cort.Th) (Fig. 1L) and cortical bone area fraction (BA/TA) (Fig. 1M) in 3 week old Osx-PPRKO mice. Examination of osteoblast-specific gene expression in the long bones revealed a significant decrease in mRNA levels of the master osteoblast transcription factor Runx2, early markers type I collagen (Col1α1) and alkaline phosphatase (ALP), and the late marker osteocalcin (OC) in Osx-PPRKO mice (Fig. 1N). Furthermore, FACS-sorted Osx-GFP+ osteoprogenitors from OsxPPR+/+ and KO mice revealed similarly decreased expression of Col1α1, ALP, and OC genes in the Osx-GFP+ cells from Osx-PPRKO mice (Fig. 1O). These data suggest that the severe osteopenia in the Osx-PPRKO mice is mostly due to decreased osteoblast numbers and activity.
Progressive impairment of B lymphopoiesis in mice lacking PPR in osteoprogenitors is not due to increased apoptosis
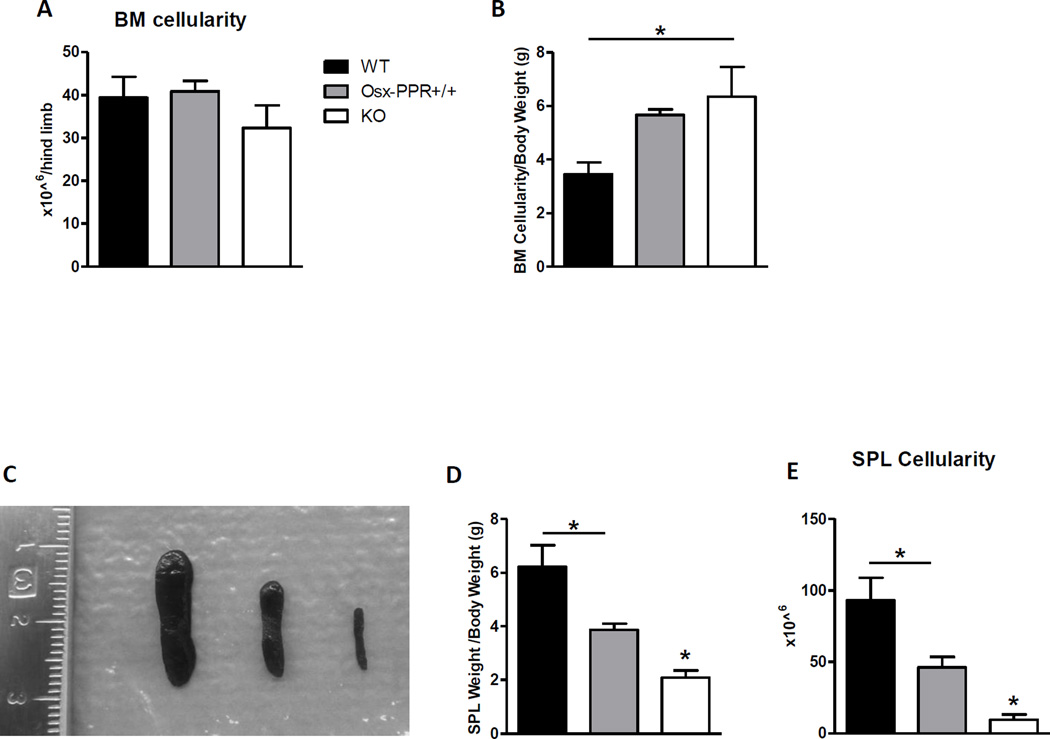
Because Gsα-dependent signaling downstream of PPR in osteoblasts regulates B lymphopoiesis, we hypothesized that Osx-PPRKO mice would also have defective B lymphopoiesis. BM cellularity is decreased at 2 weeks (data not shown) but not by 3 weeks of age (Fig. 2A). However, because Osx-PPRKO mice are significantly smaller than controls (Fig. 1B), we also normalized BM cellularity to body weight. When normalized for decreased body weight, by 3 weeks of age a relative increase in cellularity was observed in the BM of Osx-PPRKO animals as compared to WT but not Osx-PPR+/+ controls (Fig. 2B). The spleens of 3-week old Osx-PPRKO mice were extremely hypoplastic with a 2 to 3-fold decrease in the ratios of spleen weight to body weight when compared to Osx-PPR+/+ or WT controls, respectively (Fig. 2C, D), accompanied by a 5.1 to 10-fold decrease in splenocyte cellularity (Fig. 2E).
Fig.2. Osx-PPRKO mice show progressive peripheral lymphopenia.
(A) BM cellularity in the WT, Osx-PPR+/+ and KO mice at 3 weeks (3w) of age. (B) BM cellularity normalized to the body weight at 3 weeks of age. (C) Gross appearance of spleen of WT (left) Osx-PPR+/+ (middle) and KO (right), (D) spleen weight normalized to the body weight, and (E) spleen cellularity in the WT, Osx-PPR+/+ and KO mice. N≥10 WT, n=10 Osx-PPR+/+, n≥11 KO; *, p<0.05.
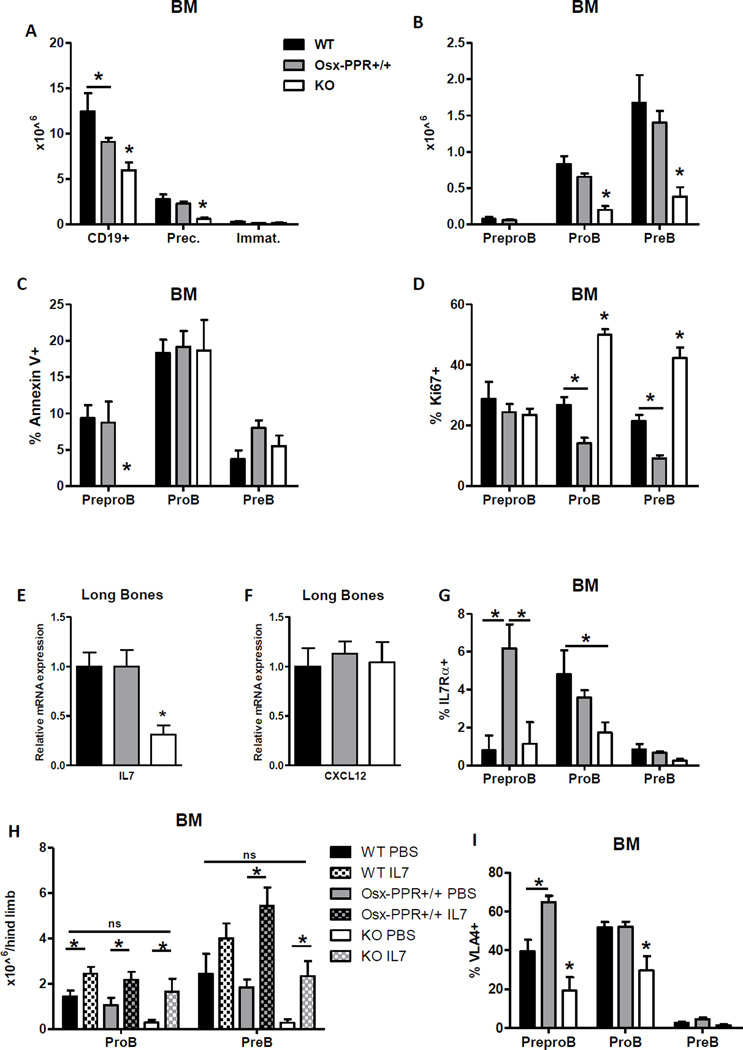
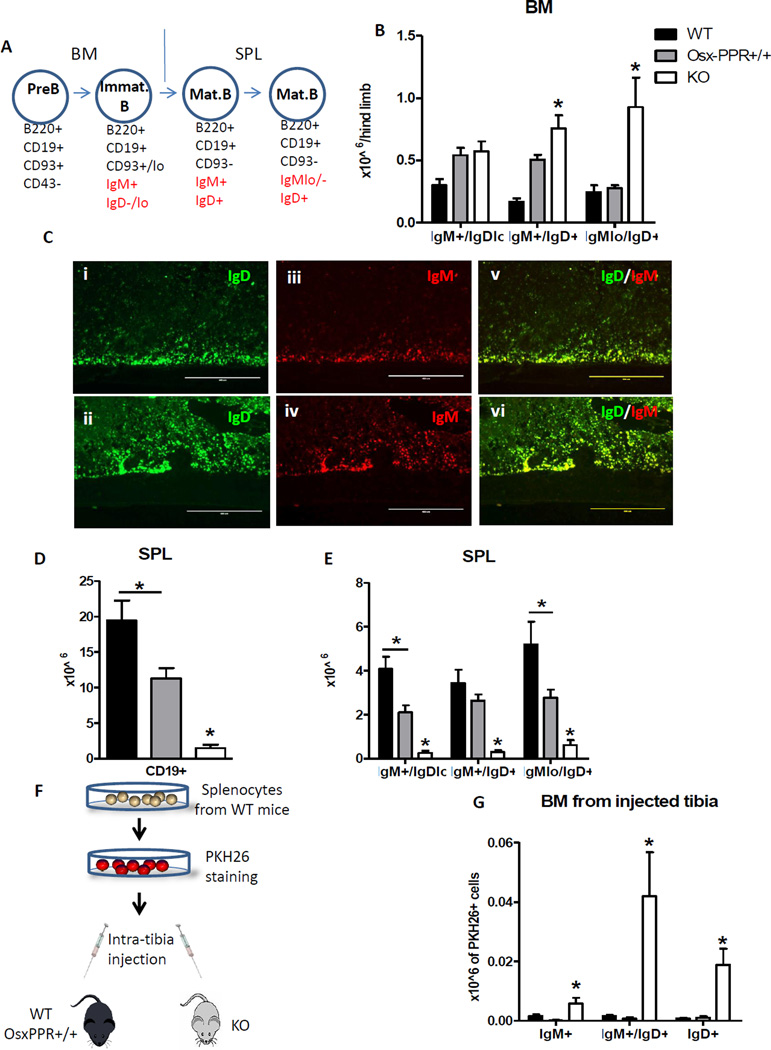
To examine B lymphocyte development in the BM we performed flow cytometry following Hardy’s B cell development surface marker definitions(29,30). Total B220+ CD19+ B cells were significantly decreased in Osx-PPRKO mice at 3 weeks of age, and the B cell precursor fraction (B220+ CD93+ IgM−) was significantly reduced while no changes were found in the immature fraction (B220+ CD93+ IgM+ IgD−) (Fig. 3A). To investigate B cell precursors in greater detail, we performed flow cytometric analysis to distinguish Prepro B, Pro B and Pre B subpopulations within the B cell precursor fraction (B220+ CD93+ IgM−). No difference was found in numbers of Prepro B cells (B220+ CD19− CD93+ CD43+) in BM of Osx-PPRKO mice relative to controls (Fig. 3B). However, as we previously reported in Osx-GsαKO mice(21), a specific block in the transition from Prepro B to Pro B cells was found in Osx-PPRKO mice with a significant reduction in Pro B (B220+ CD19+ CD93+ CD43+) and Pre B (B220+ CD19+ CD93+ CD43−) cell subsets (Fig. 3B). Importantly, the reduction in Pro B and Pre B cell subsets in Osx-PPRKO BM was not due to either increased apoptosis or reduced proliferation as apoptosis was normal (Fig. 3C) and proliferation was instead significantly increased (Fig. 3D) in these subsets.
Fig.3. Deletion of PPR in osteoprogenitors impairs Prepro B cell to Pro B cell transition with decreased IL-7 expression.
(A) Absolute numbers of B cell populations in the BM of 3-week old WT, Osx-PPR+/+ and Osx-PPRKO (KO) mice. Total B cells (B220+ CD19+), B cell precursors (Prec) (B220+ CD93+ IgM−) and immature B cells (Immat) (B220+ CD93+ IgM+). (B) Absolute numbers of Prepro B (B220+ CD19− CD93+ CD43+), Pro B (B220+ CD19+ CD93+ CD43+), and Pre B (B220+ CD19+ CD93+ CD43−) cell populations in 3 week-old WT, Osx-PPR+/+ and KO mice. (C) Frequency of Annexin V+ apoptotic B cells within the PreproB, ProB and PreB cell subsets in 3 week-old WT, Osx-PPR+/+ and KO mice. (D) Frequency of Ki67+ proliferating PreproB, ProB and PreB cell fractions in 3 week-old WT, Osx-PPR+/+ and KO mice. (E–F) Relative mRNA levels of IL-7 (E) and CXCL12 (F) in long bones of Osx-PPRKO mice and control groups. (G) Flow cytometric analysis of IL-7Rα expression in B cell precursors. (H) Absolute numbers of Prepro B, Pro B and Pre B cells after IL7 or PBS in vivo treatment in Osx-PPRKO mice and WT littermates at 3 weeks of age. N=6 WT, n=5 Osx-PPR+/+, n=6 KO; *, p<0.05. (I) Flow cytometric analysis of VLA-4 expression in B cell precursors. N≥4 WT, n≥9 Osx-PPR+/+, n≥5 KO; *, p<0.05.
Expression of IL-7 but not CXCL12 is reduced with loss of PPR in osteoprogenitors
The progression to the Pro B stage is highly regulated by the cytokine IL-7, a potent growth-stimulating factor for lymphocyte precursors, which is produced by stromal cells in BM(1,13). The specific block in the transition from Prepro B to Pro B cells in Osx-GsαKO mice was associated with decreased expression of IL-7 by osteoprogenitors, while CXCL12 levels were unchanged(21). Similarly, BM-depleted long bones of Osx-PPRKO mice showed a 69% reduction of IL-7 mRNA levels when compared to control littermates, while no difference was detected for CXCL12 mRNA (Fig. 3E, F). Interestingly, IL-7 receptor α (IL-7Rα) expression was significantly decreased in BM Pro B cell subsets of Osx-PPRKO mice (Fig. 3G). To confirm that decreased IL-7 contributes to the loss of Pro B and Pre B cells from Osx-PPRKO mice, we provided exogenous IL-7 in the form of daily injection of recombinant murine IL7 from day 14 until day 21. IL-7 but not PBS significantly increased the levels of Pro B and Pre B cells in Osx-PPRKO mice, restoring Pro B and Pre B cell numbers to levels seen in Osx-PPR+/+ and WT controls (Fig. 3H). Furthermore, expression of integrin VLA4, which mediates B cell precursor retention within supportive niches through adhesion to VCAM1 on stromal cells(1,5), was significantly reduced on the surface of both Prepro B and Pro B cells (Fig. 3I). Therefore Osx-PPRKO mice recapitulate the block in the progression from Prepro B to Pro B cell precursors as described in Osx-GsαKO mice(21). These findings indicate that PPR is a major GPCR upstream of Gsα involved in the regulation of B lymphopoiesis by osteoprogenitors, with this interaction mediated at least in part by IL-7/IL-7Rα signaling at the Pro B stage.
B cell development depends on PPR signaling in osteoprogenitors but not in mature osteoblasts or osteocytes
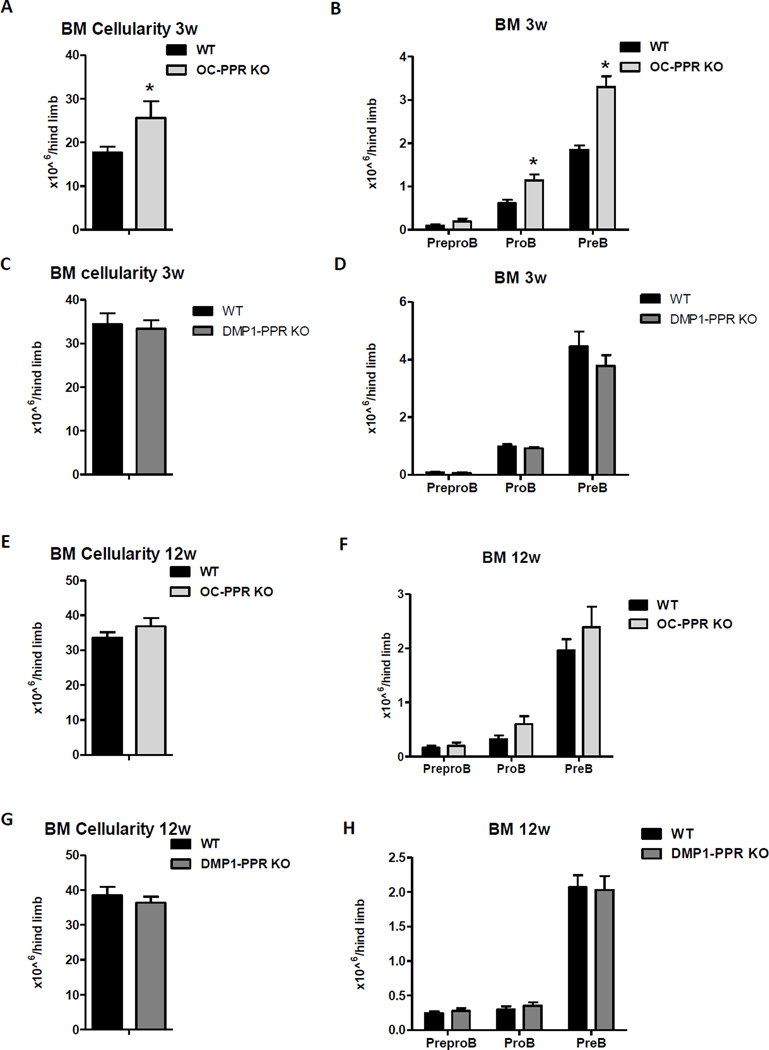
The osteoblast lineage consists of distinct cell types from osteoprogenitors to mature osteoblasts to osteocytes, and deletion of PPR in osteoprogenitors leads to the absence of PPR in more differentiated mature osteoblasts and osteocytes. Recent findings have demonstrated that osteoblast lineage cells in different maturational stages provide distinct stromal cell niches in the BM and regulate specific progenitor populations(31,32). To determine the individual contribution of specific osteoblast lineage stages to PPR-mediated regulation of B cell development, we examined B lymphopoiesis in mice lacking PPR in progressively differentiated osteocalcin-expressing mature osteoblasts (OC-PPRKO) and in Dmp1-expressing osteocytes (DMP1-PPRKO). In contrast to Osx-PPRKO mice, at 3 weeks of age OC-PPRKO mice showed increase in BM cellularity and B cell precursors compared to WT mice (Fig. 4A–B) while no difference was found in DMP1-PPR KO mice (Fig. 4C–D). OC-PPRKO mice have been reported to have decreased trabecular bone volume but increased cortical thickness by 4 weeks of age(33) while DMP1-PPR KO mice only develop increased bone mass in adulthood(22,34). We therefore next analyzed B lymphocyte development in BM of both OC-PPRKO and DMP1-PPRKO mice at 12 weeks of age. BM cellularity and B cell precursor numbers in OC-PPRKO had returned to control levels by 12 weeks (Fig. 4E–F). 12-week old DMP1-PPR KO mice showed no impairment in either BM cellularity or in B cell development; the absolute counts of Prepro B, Pro B and Pre B cell were normal (Fig. 4G–H). Therefore, expression of PPR in osteoprogenitors, but not in mature osteoblasts and osteocytes, is essential for B cell development.
Fig.4. B cell development is normal in mice lacking PPR in mature osteoblasts and osteocytes.
(A) BM cellularity of 3 week-old mice lacking PPR in mature osteoblasts and osteocytes (OC-PPRKO); n=5 WT, n=3 KO; *, p<0.05. (B) Absolute counts of Prepro B, Pro B, and Pre B populations in 3 week-old OC-PPRKO compared to WT littermates; n=5 WT, n=3 KO; *, p<0.05. (C) BM cellularity of 3 week-old mice lacking PPR in osteocytes (DMP1-PPRKO); n=6 WT, n=8 KO; *, p<0.05. (D) Absolute counts of Prepro B, Pro B, and Pre B populations in 3-week old DMP1-PPRKO compared to WT littermates; n=6 WT, n=8 KO; *, p<0.05. (E) BM cellularity of 12 week-old OC-PPRKO and WT littermates. (F) Absolute counts of Prepro B, Pro B, and Pre B populations in 12 week-old OC-PPRKO compared to WT littermates. (G) BM cellularity of 12 week-old mice lacking PPR in osteocytes (DMP1-PPRKO) and WT littermates. (H) Absolute counts of Prepro B, Pro B, and Pre B cells in 12 week-old DMP1-PPRKO compared to respective WT littermates. N≥8 WT, n≥13 KO; *, p<0.05.
Loss of PPR signaling in osteoprogenitors impairs B lymphocyte maturation and egress from BM
Pre B cell precursors give rise to IgM+ immature/naïve B cells that enter the circulation to reach the secondary lymphoid organs such as spleen, where maturation is marked by decreasing IgM and increasing IgD expression. Although B lymphocytes leave the BM before IgD upregulation, a small number of recirculating IgD+ B lymphocytes are normally found in BM(35). Interestingly, when we extended our FACS analysis to the later stages of B lymphocyte differentiation in BM (Fig. 5A) the absolute numbers of both IgM+ IgD+ and IgMlo IgD+ mature B lymphocytes were significantly increased in BM of Osx-PPRKO at 3 weeks of age (Fig. 5B). The increase of IgD+ mature B cells in BM of Osx-PPRKO mice was confirmed by immunohistochemistry of frozen tibia sections (Fig. 5C). However, total CD19+ B cells were dramatically and significantly decreased in spleen of Osx-PPRKO mice compared to WT and Osx-PPR+/+ controls (Fig. 5D), and absolute counts of IgM+ IgDlo, IgM+ IgD+ and IgMlo IgD+ maturing B cells were all significantly lower in spleen of Osx-PPRKO mice (Fig. 5E). Ki-67 staining revealed that the increase in BM IgD+ mature B cells is not due to an increase in proliferation in these subsets (Fig. S1A). In the spleens of Osx-PPRKO mice there is a slight but non-significant decrease of B cell progenitor proliferation (Fig. S1B). Taken together, these data indicate that no extramedullary B lymphopoiesis takes place in the spleen; thus the increased number of maturing B lymphocytes in BM of Osx-PPRKO mice is caused by abnormal BM retention.
Fig.5. Osx-PPRKO mice have impaired B lymphocyte maturation and egress from BM.
(A) Schematic representation of B cell maturation. The last step of B cell differentiation in BM is represented by immature IgM+ IgD−/lo B lymphocytes. Subsequently, the immature B cells migrate to the spleen where they become mature B lymphocytes through the upregulation of IgD (IgM+ IgD+) and downregulation of IgM (IgMlo/− IgD+). (B) FACS analysis of IgM+ IgD+ and IgMlo IgD+ mature B cells in BM of 3-week old Osx-PPRKO mice compared to Osx-PPR+/+ mice and WT littermates; n=4 WT, n=10 Osx-PPR+/+, n=5 KO; *, p<0.05. (C) Fluorescent immunohistochemistry showing IgD and IgM staining in diaphyses of 3-week old Osx-PPRKO mice (lower panels) and control mice (upper panels); (i–ii) IgD staining 10×, (iii–iv) IgM staining 10× and (v–vi) merged images of IgD and IgM stainings. Absolute numbers of (D) total B cells and (E) maturing B cells in spleen n≥4 WT, n≥8 Osx-PPR+/+, n≥5 KO; *, p<0.05. (F) Schematic representation of the splenocyte labeling with PKH26 and intra-tibia injection. Numbers of PKH26+ IgD+ B cells in the (G) BM of Osx-PPRKO compared to control mice after intra-tibia injection of PKH26 labeled splenocytes. N=9 WT, n=4 Osx-PPR+/+, n=7 KO;*, p<0.05.
To further confirm the impaired egress of IgD+ B lymphocytes from the BM of Osx-PPRKO mice, we injected 5 × 106 splenocytes labeled with PKH26 fluorescent-dye into the tibial BM cavity of 19 day old Osx-PPRKO and control mice (Fig. 5F). After 48 hours, analysis of PKH26+ cells in tibia of recipient mice revealed that BM of Osx-PPRKO mice had 3.6-fold increased numbers in IgM+ IgDlo, and 26- and 27-fold increased numbers in IgM+ IgD+ and IgMlo IgD+ PKH26+ populations, respectively when compared to both control groups (Fig. 5G). Taken together, these data demonstrate increased retention and impaired mobilization of mature IgD+ B cells in the BM of Osx-PPRKO mice.
Increased retention of IgD+ cells in BM is associated with increased VCAM1 in osteoprogenitors and LPAM1 in IgD+ B cells
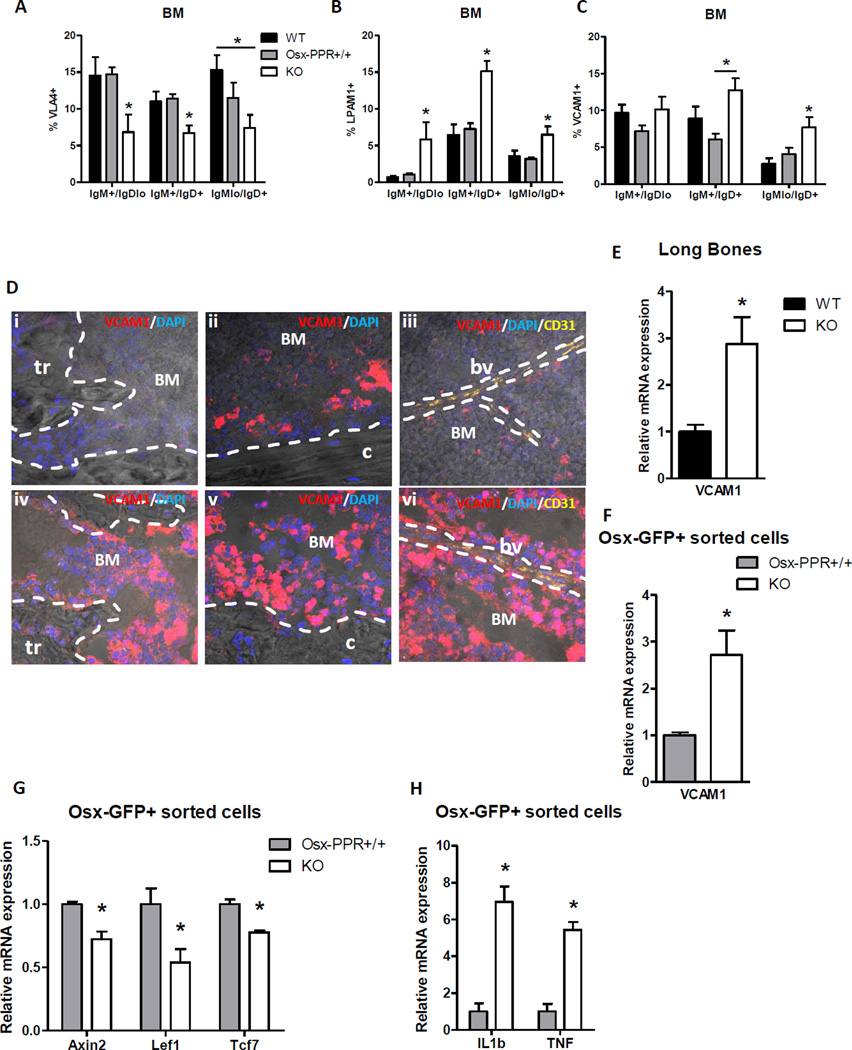
Several studies have demonstrated the importance of integrins and adhesion molecules within the BM for B lymphocyte migration. Integrin α4, which heterodimerizes with β1 or β7 subunits to form VLA4 or LPAM1, respectively, is essential for early B lymphopoiesis in BM(4), and conditional deletion of their ligand VCAM1 in Tie2-cre and Mx-Cre expressing cells leads to a selective decrease in immature B cell numbers in the BM and increase of B220+ cells in peripheral blood(36–38). Signaling through the CXCL12/CXCR4 axis has also been shown to promote VLA4/VCAM1-mediated adhesion of B cell progenitors in BM parenchyma; however, this sustained adhesion decreases as B cell maturation progresses(1,5). Other studies have shown increased B cell egress from BM in response to CXCR4 ablation or blocking. However, we found a significant reduction of CXCR4 expression in mature IgMlo IgD+ B lymphocytes from BM of Osx-PPRKO mice compared to WT littermates (Fig. S2) suggesting that the increased mature B cell retention in Osx-PPRKO mice is likely not mediated by CXCR4. The expression of VLA4 (α4β1), the main VCAM1 receptor, was significantly decreased in IgM+ IgDlo, IgM+ IgD+ and IgMlo IgD+ B lymphocytes from the BM cells of Osx-PPRKO mice (Fig. 6A). However, the expression of another VCAM1 receptor, α4β7 integrin or LPAM1, was significantly higher in IgM+ IgDlo, IgM+ IgD+ and IgMlo IgD+ B lymphocytes from BM of Osx-PPRKO mice (Fig. 6B). Interestingly, the expression of VCAM1 was also increased significantly in maturing B cells from the BM of Osx-PPRKO mice (Fig. 6C). Immunohistochemistry for VCAM1 on frozen sections revealed markedly increased VCAM1 staining in the BM of Osx-PPRKO mice compared to WT mice (Fig. 6D). These cells likely comprise both hematopoietic and stromal cells. We therefore examined total bone and Osx-GFP+ sorted cells for expression of VCAM1, and observed a 2.9-fold increase in the levels of VCAM1 mRNA in Osx-PPRKO long bones and a 2.7-fold increase in Osx-GFP+ sorted cells (Fig. 6E–F). In vitro studies have demonstrated that canonical Wnt pathway signaling suppresses VCAM1 expression on marrow stromal cells(39); in accordance with these findings mRNA levels of Wnt target genes Axin 2, Lef1 and Tcf7 were significantly reduced in osteoprogenitors of Osx-PPRKO mice compared to Osx-PPR+/+ osteoprogenitors (Fig. 6G). Expression of VCAM1 is also upregulated in response to inflammation(40). Interestingly, the gene expression of IL1β and TNF, central cytokine players in the regulation of immune and inflammatory responses, were significantly increased in Osx-GFP+ sorted cells from Osx-PPRKO mice (Fig. 6H). These findings collectively suggest that the disruption of PPR in osteoprogenitors is associated with reduced canonical Wnt signaling and increased proinflammatory cytokine production leading to an overexpression of VCAM1 on bone cells and B lymphocytes.
Fig.6. Deletion of PPR in osteoprogenitors increases the expression of VCAM1 in osterix+ cells and mature B cells.
(A–C) Expression by flow cytometric analysis of VLA-4 (A), LPAM1 (B), and VCAM1 (C) in maturing B cells in Osx-PPRKO mice compared to control groups at 3 weeks of age; n=9 WT, n=8 Osx-PPR+/+, n=8 KO; *, p<0.05. (D) VCAM1 immunohistochemistry on Osx-PPRKO (lower panel) and WT (upper panel) frozen bone sections. Representative images for (i,iv) trabecular bone (tr), (ii,v) cortical bone (c) and (iii,vi) BM showing a blood vessel (bv) stained with CD31. (E) Quantitative analysis of VCAM1 gene expression in BM depleted long bones and (F) Osx-GFP+ sorted osteoprogenitors; n≥7 WT, n=6 KO; *, p<0.05. (G) Relative mRNA levels of Axin2, Lef1 and Tcf7 in Osx-GFP+ sorted cells of Osx-PPRKO mice and controls; n=3 pools WT, n=3 pools KO; *, p<0.05. (H) Quantitative analysis of IL1β and TNF gene expression in Osx-GFP+ sorted osteoprogenitors; n=3 pools WT, n=3 pools KO; *, p<0.05.
Increased VCAM1 in osteoblasts and LPAM1 in IgD+ B cells mediate the retention of mature B cells in BM in Osx-PPRKO mice
To determine whether the impaired retention of mature B cells in the BM of Osx-PPRKO mice is caused by LPAM1 and VCAM1 overexpression, we performed in vivo neutralization of VCAM1 using an VCAM1 blocking antibody. Osx-PPRKO and WT mice were injected intraperitoneally with 200 µg of anti-VCAM1 antibody and sacrificed after 24 hours. Flow cytometric analysis of BM of mice treated with anti-VCAM1 antibody revealed a 50% reduction of IgD+ B cell frequencies in both Osx-PPRKO and control littermates when compared to isotype control antibody-treated animals. Interestingly, after a single treatment the frequencies of all three mature B cell subsets (IgM+ IgDlo, IgM+ IgD+ and IgMlo IgD+) in Osx-PPRKO mice normalized to the levels of the isotype treated WT littermates (Fig. 7A). We examined expression of ICAM1 and MadCAM1, other cell adhesion molecules of the immunoglobulin superfamily similar in structure to VCAM1, and found increased ICAM1 mRNA levels in Osx-GFP+ cells and increased levels of soluble MadCAM1 in BM supernatant of Osx-PPRKO mice (Fig. 7B–C). Taken together, these data suggest that LPAM1/VCAM1 signaling may mediate the retention of mature B cells in the BM of Osx-PPRKO mice and that ICAM1 and MadCAM1, similar in structure to VCAM1, may reinforce the process interacting with the same integrins present on mature B lymphocytes.
Fig.7. VCAM1 overexpression is responsible for mature B cell retention in BM but other cell adhesion molecules, such as ICAM1 and MadCAM1, are significantly increased in mice lacking PPR in osteoprogenitors.
(A) Reduced frequencies of maturing B cells in the BM of Osx-PPRKO and WT mice after in vivo blocking of VCAM1 with the neutralizing antibody; n=8 ISOT. WT, n=7 ISOT. KO, n=8 VCAM1 WT, n=8 VCAM1 KO; *, p<0.05. (B) Relative mRNA levels of ICAM1 in Osx-GFP+ sorted cells of Osx-PPRKO mice and controls; n=3 pools WT, n=3 pools KO; *, p<0.05. (C) Median fluorescence intensity of soluble MadCAM in BM supernatant of 3 week old Osx-PPRKO mice and controls; n=4 pools WT, n=4 pools KO; *, p<0.05.
DISCUSSION
In adults B lymphocyte development occurs continuously in BM where non-lymphoid BM stromal cells provide direct cell-cell and paracrine signaling support within the niche to guide the precise development of B cells. In the present study, we show that loss of PPR signaling in osteoprogenitors leads to a profound osteopenia with dramatic reductions in trabecular and cortical bone volume. Furthermore, deletion of PPR in osteoprogenitors, but not in mature osteoblasts and osteocytes, results in marked impairment of B lymphopoiesis in BM.
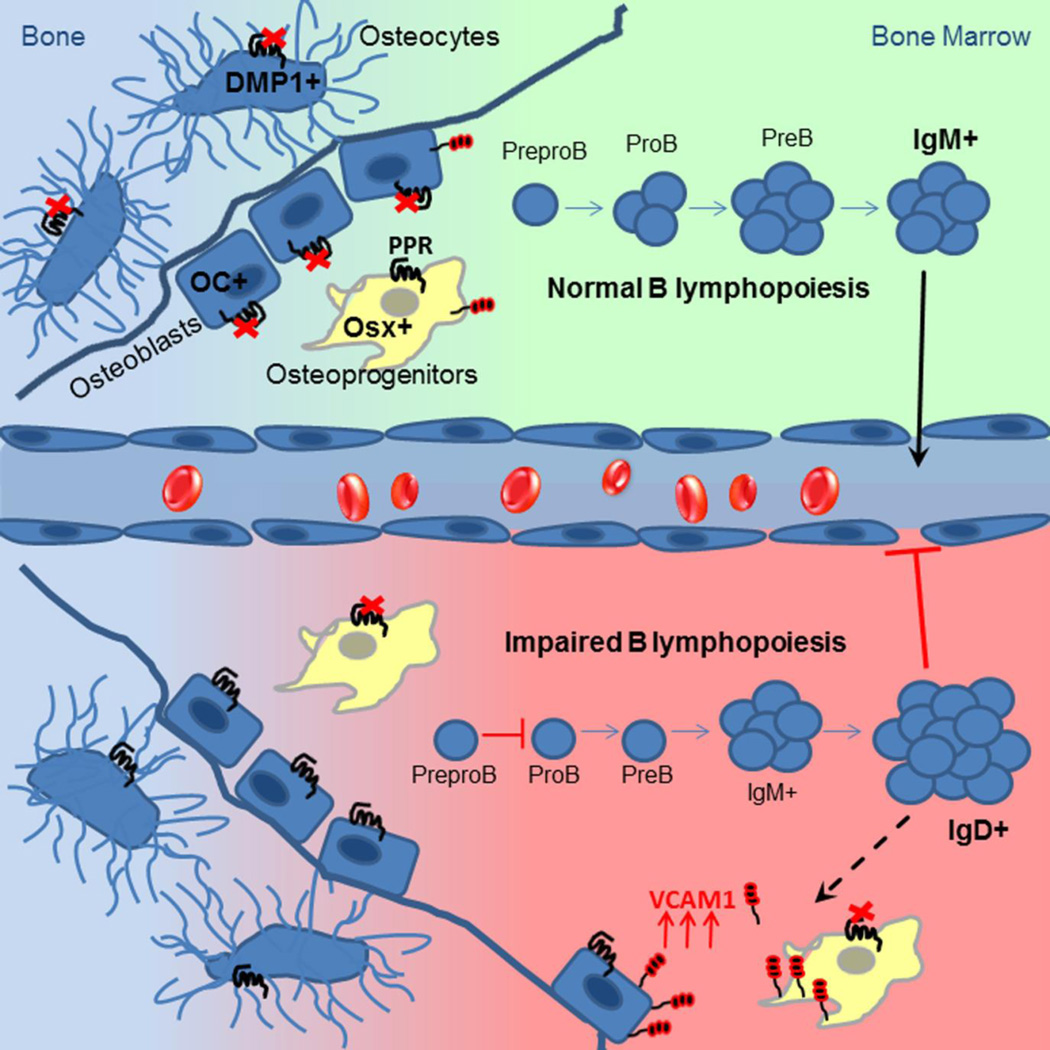
We find that PPR-deficient osteoprogenitors affect B lymphopoiesis in at least two distinct stages of development: reduced differentiation of early B cell precursors (Pro B and Pre B stages) and, unexpectedly, increased accumulation of mature B cells in BM (Fig. 8). Mice lacking Gsα in osteoprogenitors (Osx-GsαKO) also display reduced numbers of Pro B and Pre B cells in BM due to decreased expression of IL-7 by osteoprogenitor cells(21); however because Osx-GsαKO mice die even earlier, mainly before the 2nd week of life, a BM retention phenotype has not been assessed in those mice. Several GPCRs in addition to PPR signal through Gsα. Because Osx-PPRKO mice can largely recapitulate the B cell precursor development defect of Osx-GsαKO mice, we conclude that the PPR signaling is a key upstream GPCR that mediates the regulation of B cell differentiation by Gsα signaling in osteoprogenitors. Taken together, these data show that PPR signaling in osteoprogenitors is an essential cellular signaling pathway for B cell development within the supportive niche offered by osteoprogenitors.
Fig.8. Model of B lymphopoiesis regulation by osteoblastic cells.
Deletion of PPR in mature osteoblasts or osteocytes does not alter B lymphopoiesis at any level of B cell development. On the contrary, ablation of PPR in osteoprogenitors affects B lymphopoiesis in the early stage of B cell differentiation as well as in the late stage of the maturation process due to increased retention of mature B cells in BM caused by VCAM1 overexpression in osteoblastic cells.
The mechanism by which osteoprogenitors support B lymphopoiesis is of great interest. As with Osx-GsαKO mice, in Osx-PPRKO mice we find reduced expression of IL-7 in marrow-depleted bones, and in vivo injection of recombinant IL7 significantly restores the levels of Pro B and Pre B cells in Osx-PPRKO mice. Notably, while CXCL12 has also been reported to play a significant role in support of B lymphocyte differentiation, we did not find any changes in CXCL12 expression levels in marrow depleted long bones from Osx-PPRKO mice, and no defects in Prepro B cells. Therefore while CXCL12 expression by cultured osteoblasts can be augmented by PTH treatment, deletion of PPR in osteoprogenitors in vivo does not apparently affect CXCL12 expression or its effects on Prepro B cells.
These studies are consistent with others highlighting the importance of osteoprogenitors to the B lymphocyte niche. While ablation of Gsα in osteoprogenitors leads to impaired early B lymphopoiesis(21), the absence of Gsα in Dmp1-expressing osteocytes yields no B cell phenotype(22). Likewise, deletion of PPR in osteocytes has no effect on more differentiated B220+ IgM+ B cells(22). Here we have examined B cell precursor populations more carefully in mice lacking PPR in either osteoblasts or osteocytes, and found no evidence of impaired B cell precursor differentiation. Taken together, these studies further confirm that osteoprogenitors are a critical regulator of B lymphopoiesis through PPR/Gsα signaling. This does not, however, rule out that other osteoblast lineage cells may also influence B lymphopoiesis through other signaling pathways. For instance, Greenbaum et al. have reported that deletion of CXCL12 in Osx+ osteoprogenitors but not in osteocalcin-expressing osteoblasts leads to loss of common lymphoid progenitors (CLPs) and Prepro B cells(32). In addition, osteocytes may indirectly regulate B lymphopoiesis; Sato et al. recently demonstrated that ablation of osteocytes in vivo leads to impaired B cell precursor differentiation, likely through depletion of B lymphoid-supporting stromal cells(12). Moreover, very recent studies reported that Osterix promoter is not restricted to osteolineage cells during embryonic and early postnatal life, but can be found more broadly expressed in some bone marrow stromal and perivascular cells; thus, it is not possible to exclude the importance of the effects of PPR deletion on these other cell types(41,42).
We report here the novel finding that ablation of PPR in osteoprogenitors unexpectedly results in increased numbers of IgD+ B lymphocytes in the BM most likely due to aberrant IgD expression in immature B cells accompanied by enhanced retention of these cells. B cell proliferation analysis in BM revealed that B cell precursors of Osx-PPRKO mice are more proliferative when compared to their littermate controls; however, although no difference was found in apoptosis, the numbers of Pro and Pre B cells were significantly lower. On the other hand, the spleen of Osx-PPRKO mice showed no extramedullary B lymphopoiesis. Interactions between adhesion molecules and integrins are necessary for B cell migration. We further find that the abnormal retention of naïve IgD+ B lymphocytes in the BM is mediated at least in part by increased VCAM1. VCAM1 has been shown to be integral to support of B lymphocyte differentiation on osteoblasts in vitro (13). VCAM1 expression has been identified in osteoblast lineage cells(13,43) and endothelial cells(44,45). While the absence of VCAM1 does not affect B lymphopoiesis, it impairs retention of immature B cells and homing of mature B cells in BM. Specifically, VCAM1-deficient mice showed loss of immature IgM+ IgD− B cells from BM and their accumulation in peripheral blood and a significant decrease of IgD+ mature recirculating B cells in BM(37). Conditional deletion of VCAM1 in endothelial cells with Tie2-Cre leads to mild leukocytosis in peripheral blood, with elevation of IgM+ IgDlo immature B cells and accompanied by a reduction of IgM+ IgDlo and IgD+ B cells in the BM(36). Little is known about a role for PPR in the regulation of VCAM1 expression, but in cementoblasts overexpressing JunB, decreased expression of PPR is also inversely correlated with increased expression of VCAM1(46). Another in vitro study reported that canonical Wnt signaling pathway negatively regulates VCAM1 expression on marrow stromal cells(39). Interestingly, deletion of PPR in osteocytes was reported to inhibit Wnt-β-catenin signaling pathway(26) and we also have previously shown that ablation of Gsα in osteoprogenitors results in reduced canonical Wnt signaling(47). In accordance with these last findings, we found a significant downregulation of Wnt target genes such as Axin2, Lef1 and Tcf7 in Osx-PPRKO osteoprogenitors. Moreover, upregulation of VCAM-1 in endothelial cells is mediated by pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) (40) and the expression of both genes are significantly increased in Osx-GFP+ sorted cells from Osx-PPRKO mice as compared to Osx-PPR+/+ mice. Further studies to investigate the link between PPR-mediated signaling and expression of VCAM1 in osteoblasts are now underway. Treatment of the Osx-PPRKO mice with VCAM1 antibody significantly decreased the accumulation of mature B cells in the BM, indicating that the PPR signaling in osteoprogenitors also influences B cell mobilization at least in part by regulating the expression of the adhesion molecule VCAM1.
More broadly, understanding the interactions between the osteoblast lineage and hematopoietic lineages has significant clinical relevance. Several studies have highlighted the role of the microenvironment, and osteoblasts in particular, in the pathogenesis of myeloproliferative syndrome, myelodysplasia and leukemias(48–52). B lymphoid malignancies are also known to target the skeleton; for example, multiple myeloma involves well-characterized interactions between malignant plasma cells, osteoclasts, and osteoblasts. In particular, interaction of myeloma cells with stromal cells is also mediated by VCAM-1(53,54). Conversely, production of normal hematopoietic cells might one day be enhanced by treatments directed at the osteoblast lineage. In a proof of concept study, we have demonstrated that recombinant PTH (teriparatide) can significant increase circulating HSCs in postmenopausal women with osteoporosis(55). Therefore understanding the mechanisms by which B lymphocytes and their malignant counterparts home to and engraft in the bone microenvironment may enable novel therapies targeted at the normal and malignant hematopoietic cell niches.
Supplementary Material
Acknowledgments
The authors thank Dr. Andrew McMahon for providing Osx1:GFP-Cre mice, Dr. Tatsuya Kobayashi for providing PPRfl/fl mice; the MGH Center for Comparative Medicine and Stanford University Veterinary Services Center staff for care of the animals; the Harvard Stem Cell Institute flow cytometry core and the Stanford Shared FACS Facility; and the Ragon Institute imaging core. This work was supported by the Harvard Stem Cell Institute (J.Y.W.) and National Institutes of Health grants OD008466 to J.Y.W. and DK079161 and AR060211 to P.D.P.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
AUTHOR CONTRIBUTION
CP and JYW initiated the study, designed and interpreted the experiments; CP and KF performed experiments; VS and RC provided technical help; CP and JYW wrote the manuscript; PDP, KF and VS critically revised the manuscript.
The authors declare no conflict of interests.
REFERENCES
- 1.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 3.Singbrant S, Russell MR, Jovic T, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117(21):5631–5642. doi: 10.1182/blood-2010-11-320564. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 5.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. The Journal of experimental medicine. 2003;197(4):461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. The EMBO journal. 2006;25(4):889–899. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci U S A. 1994;91(12):5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuzawa T, Miyaura C, Onoe Y, et al. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94(3):1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panaroni C, Wu JY. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int. 2013;93(3):261–268. doi: 10.1007/s00223-013-9753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Asada N, Kawano Y, et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell metabolism. 2013;18(5):749–758. doi: 10.1016/j.cmet.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 14.Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187(3):311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- 15.Stojceva-Taneva O, Fadda GZ, Smogorzewski M, Massry SG. Parathyroid hormone increases cytosolic calcium of thymocytes. Nephron. 1993;64(4):592–599. doi: 10.1159/000187406. [DOI] [PubMed] [Google Scholar]
- 16.Bro S, Olgaard K. Effects of excess PTH on nonclassical target organs. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997;30(5):606–620. doi: 10.1016/s0272-6386(97)90484-4. [DOI] [PubMed] [Google Scholar]
- 17.Rixon RH, Whitfield JF. Hypoplasia of the bone marrow in rats following removal of the parathyroid glands. Journal of cellular physiology. 1972;79(3):343–352. doi: 10.1002/jcp.1040790304. [DOI] [PubMed] [Google Scholar]
- 18.Gaciong Z, Alexiewicz JM, Massry SG. Impaired in vivo antibody production in CRF rats: role of secondary hyperparathyroidism. Kidney Int. 1991;40(5):862–867. doi: 10.1038/ki.1991.286. [DOI] [PubMed] [Google Scholar]
- 19.Gaciong Z, Alexiewicz JM, Linker-Israeli M, Shulman IA, Pitts TO, Massry SG. Inhibition of immunoglobulin production by parathyroid hormone. Implications in chronic renal failure. Kidney Int. 1991;40(1):96–106. doi: 10.1038/ki.1991.186. [DOI] [PubMed] [Google Scholar]
- 20.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 21.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulzele K, Krause DS, Panaroni C, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121(6):930–939. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Soegiarto DW, Yang Y, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115(7):1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 26.Powell WF, Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 28.Davey RA, Clarke MV, Sastra S, et al. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res. 2012;21(4):885–893. doi: 10.1007/s11248-011-9581-z. [DOI] [PubMed] [Google Scholar]
- 29.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. The Journal of experimental medicine. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy RR, Hayakawa K. B cell development pathways. Annual review of immunology. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 31.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu T, Xian L, Crane J, et al. PTH Receptor Signaling in Osteoblasts Regulates Endochondral Vascularization in Maintenance of Postnatal Growth Plate. J Bone Miner Res. 2015;30(2):309–317. doi: 10.1002/jbmr.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saini V, Marengi DA, Barry KJ, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmond DG. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 36.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. The Journal of experimental medicine. 2001;193(6):741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. The Journal of experimental medicine. 2001;193(6):755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Experimental hematology. 2007;35(4):565–571. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Experimental hematology. 2009;37(1):19–30. doi: 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PloS one. 2014;9(1):e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Developmental cell. 2014;29(3):340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung Y, Wang J, Havens A, et al. Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine. 2005;32(3–4):155–162. doi: 10.1016/j.cyto.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annual review of immunology. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 45.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 46.Berry JE, Pettway GJ, Cordell KG, Jin T, Datta NS, McCauley LK. JunB as a potential mediator of PTHrP actions: new gene targets Ephrin B1 and VCAM-1. Oral diseases. 2008;14(8):713–726. doi: 10.1111/j.1601-0825.2008.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu JY, Aarnisalo P, Bastepe M, et al. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121(9):3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell stem cell. 2013;13(3):285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michigami T, Shimizu N, Williams PJ, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96(5):1953–1960. [PubMed] [Google Scholar]
- 54.Mori Y, Shimizu N, Dallas M, et al. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104(7):2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 55.Yu EW, Kumbhani R, Siwila-Sackman E, et al. Teriparatide (PTH 1–34) treatment increases peripheral hematopoietic stem cells in postmenopausal women. J Bone Miner Res. 2014;29(6):1380–1386. doi: 10.1002/jbmr.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.