Abstract
The neurocognitive function and quality of life of 58 Korean survivors of childhood medulloblastoma were assessed after surgery, cranial radiation and chemotherapy. All patients were evaluated with a battery of neurocognitive function tests and the Pediatric Functional Assessment of Cancer Therapy-Brain Tumor Survivors, which consists of self-report questionnaires on quality of life. The mean full-scale intelligence quotient (IQ), verbal IQ, and performance IQ scores were 90.2, 97.1, and 84.16, respectively. The mean memory quotient (MQ) score was 86.78, which was within 1 standard deviation of the average score of 100. Processing speed, attention, and executive function showed mild to moderate deficits. Intelligence, memory, executive function, visuospatial function, and simple motor function were significantly lower in the patients diagnosed before 8 years of age compared with those diagnosed after 8. The cognitive deficits in the patients diagnosed at younger ages might be related to earlier exposure to craniospinal irradiation and chemotherapy. The patient and parent proxy evaluations of attention, fine motor function, and quality of life did not differ. We found significant neurocognitive changes in a wide range of neurocognitive functional domains in Korean survivors of childhood medulloblastoma. Long-term follow-up studies of survivors of childhood medulloblastoma beginning at the time of their first diagnosis are required to better understand the deficits exhibited by survivors of childhood medulloblastoma, so that intervention strategies and treatment refinements that reduce the long-term neurocognitive decline can be developed.
Keywords: Medulloblastoma, Survivors, Cognition, Quality of Life, Korea
INTRODUCTION
Half of all pediatric brain tumors are located in the posterior fossa, and medulloblastomas account for about 50% of posterior fossa tumors (1). Due to the risk of local disease recurrence or central nervous system (CNS) dissemination, patients with these tumors receive aggressive treatments, which include maximal surgical resections followed by craniospinal irradiation and chemotherapy.
Because of the recent therapeutic advancements and improved survival rates of these patients, there is a growing need for evaluations of their neurocognitive outcome, which is an important contributor to the quality of life (QOL) of these children. Previous studies have shown that a young age at diagnosis, surgical complications, and extended CNS irradiation are the principal risk factors for impaired neuropsychological outcome and a decreased QOL in survivors of medulloblastoma (2).
Although many studies of the neurocognitive outcomes of survivors of brain tumors have been conducted, most have enrolled primarily pediatric patients with heterogeneous brain tumors and few patients with medulloblastomas (3). Ronning et al. (4) assessed the cognitive functions of patients with both benign and malignant posterior fossa tumors, and they included only 11 patients with medulloblastomas. Similarly, a recent study by Maddrey et al. (5) of 10-year survivors of childhood brain tumors included only 16 patients with medulloblastomas.
Moreover, most cross-sectional studies of survivors of medulloblastomas have only evaluated intellectual functioning and academic achievements, and they have not examined delayed recall or working memory, which are known to be mediated by frontal lobe structures and which are important for executive functioning (6). For example, Reeves et al. (7) only evaluated visual attention tasks and auditory verbal memory tasks, which therefore prevented comprehensive evaluations of attention and memory processes.
Another limitation of the previous studies is that the health-related quality of life (HRQOL) of the survivors of brain tumors was assessed with QOL assessment tools that were not specific to brain tumors. Maddrey et al. (5) used the Ferrans and Powers QOL Index, which is not a brain tumor-specific tool but rather an assessment tool of general well-being. Although the survival rate of children with CNS tumors now approaches 70%, the HRQOL has not been investigated properly in this population (8).
In Korea, the survival rate of patients with childhood medulloblastoma is comparable to that of developed countries (9), and there is an increasing need to assess the long-term neurocognitive function and QOL of Korean survivors of childhood medulloblastoma. This study aimed to assess the neurocognitive function and QOL of Korean survivors of childhood medulloblastoma who underwent surgery, craniospinal radiation, and chemotherapy.
MATERIALS AND METHODS
Patients and treatment
This study was a multicenter study that was conducted in 4 institutions by the Korean Society of Pediatric Neuro-Oncology (KSPNO-O082 protocol). Pediatric patients who were diagnosed with medulloblastoma and who survived without disease were included. Patients with psychiatric disorders or those who underwent hematopoietic stem cell transplantation were excluded. Patients were enrolled from January 2009 to December 2011.
All patients underwent surgery, radiotherapy, and/or chemotherapy, and these treatments involved various protocols. The medical records were reviewed to determine each patient's diagnosis, treatments, including surgical procedures, radiotherapy, and chemotherapy regimens, and long-term complications.
Neuropsychological tests and assessment tools
Neuropsychological testing was scheduled and performed by authorized personnel. Interviews of each patient’s health status, social functioning, and health habits were done by the same research nurse practitioner. The neurocognitive and QOL assessments of each patient were performed in a single session that lasted approximately 3 hours by a clinical neuropsychologist (Table 1).
Table 1. Neuropsychological tests and assessment tools.
| Classification | Test | References |
|---|---|---|
| Neurocognitive function test | ||
| Intelligence | ||
| < 15 yr | Korean Wechsler Intelligence Scale for Children-III (KWISC-III) | (10) |
| ≥ 16 yr | Korean Wechsler Adult Intelligence Scale (KWAIS) | |
| Attention | ||
| Verbal attention | Digit span forward (a Wechsler subtest) | (11) |
| Visual attention | Color trail making test 1 (CTT 1) | (12,13) |
| Memory | Rey-Kim Memory Test (RKMT) | (14) |
| Verbal memory | K-Auditory verbal learning test (KAVLT) | |
| Nonverbal memory | K-Complex figure test (KCFT) | (15) |
| Executive function | ||
| Verbal tests | Controlled oral word association (COWA) | (16) |
| Digit span backward | (10,17) | |
| Visual test | Color trails test 2 (CTT 2) | (11,13) |
| Motor Function | ||
| Fine motor | Grooved pegboard test | (16,18) |
| Simple motor | Grip strength test | |
| Health-Related Quality of Life | Pediatric functional assessment of cancer therapy-brain tumor survivors (Peds-FACT-BrS) | (20,21,30) |
Intelligence
Patients under the age of 15 years were assessed by the Korean Wechsler Intelligence Scale for Children-III (KWISC-III), and patients above 16 years were assessed by the Korean Wechsler Adult Intelligence Scale (KWAIS) (10). Three intelligence quotients (IQ) (full-scale IQ [FSIQ], verbal scale IQ [VIQ], and performance scale IQ [PIQ]) were calculated, and average IQs were defined by scores between 85 and 115.
Verbal attention
The digit span score was used to measure attention efficiency. The digit span forward, which is a subtest of the Wechsler Intelligence Scale, required the patients to immediately repeat progressively longer series of numbers that ranged from 3 to 9 digits in length that were read aloud by the examiner. If series of the same length were incorrectly repeated in 2 trials, the task was discontinued. The scores ranged from 0 to 14, and higher scores indicated better performances (11).
Visual attention
The Color Trail Test 1 (CTT 1) is widely thought to measure sustained attention and detect cognitive impairment (12,13). The CTT 1 required the subject to connect a set of circles that were numbered 1 through 25 in sequence as rapidly as possible. The score was determined by the amount of time required to complete the task, and higher scores indicated poor performance.
Memory
The Rey-Kim Memory Test (RKMT) was used to assess verbal memory performance with the K-Auditory Verbal Learning Test (KAVLT), which is the Korean version of the Rey Verbal Learning Test (14), and nonverbal memory performance with the K-Complex Figure Test (KCFT), which is the Korean version of the Rey Complex Figure Test (14). The KAVLT required the serial learning of a list of 15 unrelated words over 5 consecutive trials, with immediate recall required after each trial. After a delay of 20 minutes, the patient was again required to recall 15 words. Following completion of the delayed recall, the patient was asked to recall 15 of the 60 words in the original list that was read by the examiner. In the KCFT, which is essentially identical to the standard version of the Rey Complex Figure Test (15), the patient was required to copy the figure as accurately as possible without a time limit. An immediate recall trial was subsequently administered. After a delay of 20 minutes, the patient was again required to recall the figure. The RKMT yielded scores for the KAVLT and KCFT in each trial, and these resulted in normalized standard scores with a normative mean of 10 and a standard deviation of 3.3.
Verbal executive function tests
The Controlled Oral Word Association (COWA) required the patients to generate as many different words as possible within 60 seconds (16). First, in the Letter Fluency Test, the children were required to name as many words as possible that started with the Korean letters of K (ㄱ), S (ㅅ), and O (ㅇ). The scores were calculated by summing all of the acceptable words produced for the 3 letters. Second, for the Category Fluency Test, the patients were asked to name as many items as possible in the semantic categories of animals and supermarket. The dependent variables were the number of correct responses in each category.
Another subtest, the Digit Span Backward, which is thought to assess working memory, was conducted. It required the patients to repeat a series of numbers that ranged in length from 2 to 8 digits in reverse sequence immediately after they were read aloud by the examiner (10,17). This subtest was discontinued and scored with the same procedures as those used in the Digit Span Forward subtest. Higher scores indicated better performances.
Visual executive function tests
The Color Trails Test 2 (CTT 2) was used to measure cognitive flexibility (11,13). Like the CTT 1, this test required the patient to connect a set of circles that were numbered 1 through 25 in sequence as rapidly as possible, but, in the CTT 2, the numbered circles needed to be connected in sequence while alternating between the pink and yellow colors. Both trials were scored for the frequency of errors.
Motor function
Fine motor dexterity was assessed with the Grooved Pegboard Test, which required the patients to place grooved pegs into the matching holes as quickly as possible with their dominant or nondominant hands (16,18). Simple motor function was measured with the grip strength of their dominant and nondominant hands.
Health related quality of life
The Pediatric Functional Assessment of Cancer Therapy-Brain Tumor Survivors (PedsFACT-BrS) consists of both patient-reported and parent-reported forms. The same procedures were used on all of the scales as those used in the Functional Assessment of Chronic Illness Therapy measurement system (19).
The PedsFACT-BrS version 2 consists of 4 sets of developmentally appropriate generic core scales and brain tumor survivor-specific (BSS) modules that measure the HRQOLs of individual children or adolescents aged 7–12 or 13–18, respectively. Both self-report and parent proxy-report versions of the questionnaires are available, and they both use a 5-point Likert scale upon which the patients or parents rate “how true each of the following statements have been for you during the past 4 weeks” (0, not at all; 1, a little bit; 2, somewhat; 3, quite a bit; and 4, very much) (20,21)
The Korean PedsFACT-BrS version used for patients under the age of 12 consists of the following 34 items: 21 generic concerns (physical well-being [PWB, 7 items], emotional well-being [EWB, 10 items], and social family well-being [SFWB, 5 items]) and BSS concerns (12 items). The Korean PedsFACT-BrS version used for patients between 13 and 18 years of age consists of the following 37 items: 21 generic concerns (PWB [7 items], EWB [13 items], and SFWB [5 items]) and BSS concerns (12 items).
Statistical analyses
All of the patient data were analyzed. The patient data were divided into groups according to initial risk, radiation dose, age at diagnosis, and time after diagnosis and compared. The scores reflecting the neurocognitive function of each individual were converted into standard scores according to the age-related means and standard deviations from test standardization norms. In both the Wechsler Intelligence Test and RKMT, the standard scores had a mean of 100 and a standard deviation of 15. We calculated the mean values and standard deviations of each group. One-sample t-tests were administered to test the hypothesis that there would be deficits in the scaled scores from the subtests of the K-WAIS, K-WISC-III, and RKMT, which were compared with a mean of 10. Because there were no Korean norms for the COWA test and motor function tests (both the grooved pegboard and grip strength test), we analyzed the raw scores of these tests. The t-tests were used to analyze the parametric data, while Mann-Whitney U-tests were used to analyze the nonparametric data. All of the statistical analyses were performed with SPSS for Windows, version 18 (IBM Corporation, Armonk, NY, USA).
Ethics statement
This protocol was approved by the institutional review boards of Seoul National University Hospital (H-0812-049-266) and each hospital in which the study was performed. Informed consents were obtained from all of the patients or their parents or guardians.
RESULTS
Characteristics of the patients and their parents
Fifty-eight patients were evaluated during the study period. Their basic clinical characteristics are summarized in Table 2. Their mean age at diagnosis was 8 years (range, 1–22 years). Fourteen patients were over 10 years old at the time of diagnosis. Most of the patients underwent a combination of treatments involving surgery, radiotherapy, and/or chemotherapy. Detailed data on the radiotherapy were available for 47 patients: 44 patients underwent craniospinal irradiation, 1 patient underwent additional posterior fossa irradiation, and 2 patients underwent additional irradiation of the tumor bed. The 44 patients who underwent craniospinal irradiation received the following doses between 2,160 and 3,960 cGy: 2,160 cGy (n = 1), 2,340 cGy (n = 22), 2,520 cGy (n = 2), 2,700 cGy (n = 1), 3,060 cGy (n = 9), 3,240 cGy (n = 2), 3,600 cGy (n = 6), or 3,960 cGy (n = 1). Eighteen patients received radiation doses over 3,000 cGy, and 7 patients received doses over 3,600 cGy. Among those who received doses over 3,000 cGy, 8 patients were under 8 years old and 10 were over 10 years old at the time of irradiation.
Table 2. Characteristics of the patients (n = 58).
| Characteristics | Patients (n = 58) | |
|---|---|---|
| Mean (range) | No. (%) | |
| Gender | ||
| Male | 36 (62.1) | |
| Female | 22 (37.9) | |
| Age at diagnosis | 8 (1–22) | |
| < 3 yr | 4 (6.9) | |
| 3–7 yr | 25 (43.1) | |
| 8–10 yr | 15 (25.9) | |
| > 10 yr | 14 (24.1) | |
| Age at neurocognitive function testing | 13.7 (6–26) | |
| 6–10 yr | 12 (20.7) | |
| 10–15 yr | 29 (50.0) | |
| 15–20 yr | 9 (15.5) | |
| 20–25 yr | 7 (12.1) | |
| > 25 yr | 1 (1.7) | |
| Time after diagnosis | ||
| < 3 yr | 13 (22.4) | |
| 3–7 yr | 20 (34.5) | |
| 8–10 yr | 14 (24.1) | |
| > 10 yr | 11 (19.0) | |
| Duration of education | 7 (1–15) | |
| Educational status | ||
| ≤ high school | 50 (86.2) | |
| ≥ college graduate | 8 (13.8) | |
The chemotherapy regimens varied, and most of the patients were classified as average risk. Thus, the KSPNO-M-051 for average-risk medulloblastoma (22) was the most common regimen used in 11 patients, and 8 drugs in 1 day in 6 patients (23), POG 9031 in 6 patients (24), and CCG A9961 in 4 patients (25).
The mean age of the patients at the time of the neurocognitive function tests was 13.7 years (range, 6–26 years). The time after diagnosis was less than 3 years in 13 patients (22.4%) and more than 10 years in 11 patients (19.0%). The educational statuses of the patients at the time of the tests were below high school in most (n = 50, 86.2%). In this study, we also collected data on the parents, including 5 fathers, 43 mothers, and 10 mother-father pairs. The mean age of the fathers was 47.3 years, and that of the mothers was 43.9 years. The mean duration of education was 13.7 years in the fathers and 12.9 years in the mothers. Most caregivers were currently married (86.8%), and 91.2% of the parents had educations that exceeded the high school level.
Neuropsychological tests and assessments for intelligence
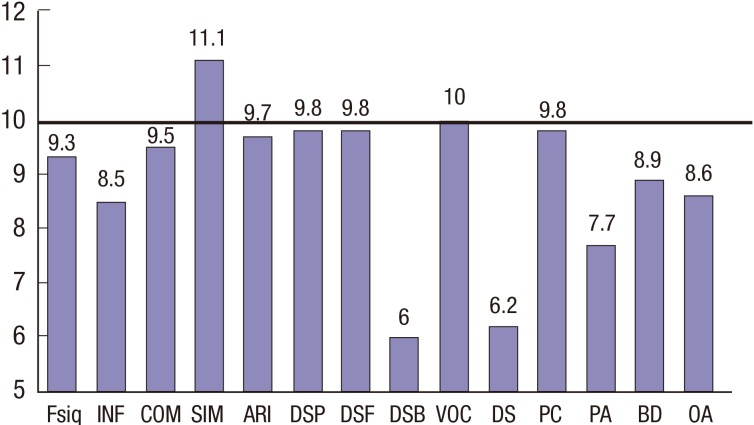
The data on the mean FSIQ, VIQ, and PIQ are summarized in Table 3. The mean estimated FSIQ score for the patients in this study was 90.2 (range, 50–129), which was within 1 standard deviation of the average IQ score of 100. Six patients had standard deviations above 1 for their average FSIQ score of 115. Eleven patients (19.0%) had average FSIQ values below 85, 7 patients (12.1%) had average VIQ values below 85, and 17 patients (29.3%) had average PIQ values below 85. The mean values of all of the KWISC-III and KWAIS subtests are shown in Fig. 1. These data revealed values that were lower than normal on the nonverbal performance subtests of block design (T = −2.26, P < 0.05), object assembly (T = −2.02, P < 0.05), picture arrangement (T = −5.51, P < 0.001), and, especially, digit symbol (T = −8.38, P < 0.001). For verbal function, the scores for information (T = −3.62, P < 0.001) were lower than normal, and the digit span backward scores for visual executive function (T = 10.54, P < 0.001) were significantly lower than borderline (mean score = 6.00).
Table 3. Summary of intelligence quotient (IQ) and memory quotient scores in all patients (n = 58).
| Scores | Mean (SD) | Minimum | Maximum | IQ less than 1 SD (IQ < 85) No. (%) |
|---|---|---|---|---|
| Full-scale IQ | 90.20 (19.97) | 50 | 129 | 11 (19.0) |
| Verbal IQ | 97.10 (18.67) | 50 | 130 | 7 (12.1) |
| Performance IQ | 84.16 (20.96) | 50 | 131 | 17 (29.3) |
| Memory quotient | 86.78 (15.67) | 45 | 127 | 23 (39.7) |
SD = standard deviation.
Fig. 1.
Mean scores of the KWISC-III and KWAIS subtests of all patients (n = 58). The bold horizontal line represents the normal value.
KWISC-III = Korean Wechsler Intelligence Scale for Children-III, KWAIS = Korean Wechsler Adult Intelligence Scale, FSIQ = full-scale intelligence quotient, INF = information, COM = comprehension, SIM = similarities, ARI = arithmetic, DSP = digit span, DSF = digit span forward, DSB = digit span backward, VOC = vocabulary, DS = digit symbol, PC = picture completion, PA = picture arrangement, BD = block design, OA = object assembly.
We compared the scores on the intelligence subtests between the patients under 8 years old at diagnosis (Patient Group 1) and patients 8 years or older at diagnosis (Patient Group 2) (Table 4). The group 1 patients showed significantly lower scores than the group 2 patients on all of the tests, except for the digit span test. In particular, the arithmetic and picture arrangement scores were considerably lower in the patients who were diagnosed under the age of 8 years.
Table 4. Means and standard deviations of the patient scores according to age at diagnosis.
| Variables | Age at diagnosis | T valueP value | |
|---|---|---|---|
| < 8 yr (n = 29) | ≥ 8 yr (n = 29) | ||
| Mean (SD) | Mean (SD) | ||
| Intelligence | |||
| FSIQ | 83.31 (18.48) | 96.55 (20.21) | −2.42* |
| VIQ | 91.41 (18.48) | 102.79 (17.33) | −2.44* |
| PIQ | 77.72 (16.03) | 90.59 (23.48) | −2.63* |
| Information | 7.52 (3.33) | 9.41 (2.81) | −2.32* |
| Comprehension | 8.48 (3.25) | 10.59 (3.29) | −2.42* |
| Similarities | 11.30 (3.90) | 12.24 (2.87) | −2.38* |
| Arithmetic | 7.79 (3.18) | 11.52 (3.37) | −4.33‡ |
| Digit span | 9.39 (4.16) | 10.10 (3.18) | −0.73 |
| Vocabulary | 9.03 (3.21) | 11.10 (3.47) | −2.46* |
| Digit symbol | 4.93 (2.67) | 7.41 (3.78) | −2.88† |
| Picture completion | 9.10 (3.29) | 10.10 (3.17) | −1.68 |
| Picture arrangement | 6.18 (2.33) | 9.07 (3.36) | −3.76‡ |
| Block design | 7.52 (3.56) | 10.28 (3.99) | −2.77† |
| Object assembly | 7.28 (3.16) | 9.85 (4.01) | −2.84† |
| Memory quotient | 84.57 (12.09) | 90.48 (18.22) | −1.44 |
| Verbal memory | |||
| Auditory verbal learning 1–5 | 38.28 (7.49) | 46.34 (8.97) | −3.72‡ |
| Delayed recall | 8.75 (2.67) | 10.17 (2.74) | −2.41* |
| Recognition | 13.44 (2.81) | 14.24 (1.27) | −2.09* |
| Visual memory | |||
| Immediate recall | 13.52 (8.17) | 21.14 (9.51) | −3.27† |
| Delayed recall | 11.51 (7.29) | 19.48 (8.81) | −3.76† |
| Attention | |||
| Verbal (DSF) | 9.39 (3.16) | 10.24 (3.24) | −1.01 |
| Visual (CTT 1) | 40.17 (22.78) | 42.18 (25.37) | −0.32 |
| Executive function | |||
| Verbal | |||
| Letter fluency test | 17.68 (9.62) | 32.21 (10.21) | −5.51‡ |
| Supermarket | 10.25 (4.38) | 15.14 (5.38) | −3.76‡ |
| Animal | 12.46 (4.47) | 15.03 (4.66) | −2.13* |
| Digit span backward | 5.07 (2.88) | 6.90 (2.59) | −2.52* |
| Visual | |||
| Color trails test 2 | 76.79 (45.83) | 68.25 (41.06) | −2.08* |
| Visuospatial function | |||
| Rey complex figure copy | 23.97 (10.31) | 30.12 (8.72) | −2.41* |
| Motor function | |||
| Simple motor | |||
| Dominant hand | 18.49 (8.54) | 30.57 (12.25) | −3.29† |
| Nondominant hand | 16.42 (8.79) | 27.72 (15.94) | −2.86† |
| Fine motor | |||
| Dominant hand | 94.57 (47.99) | 115.11 (39.82) | −1.33 |
| Nondominant hand | 108.50 (32.58) | 118.10 (47.23) | −0.51 |
SD = standard deviation, FSIQ = full scale intelligence quotient, VIQ = verbal intelligence quotient, PIQ = performance intelligence quotient, DSF = digit span forward, CTT 1 = color trails test 1.
*P < 0.05; †P < 0.01; ‡P < 0.001.
Assessments for memory
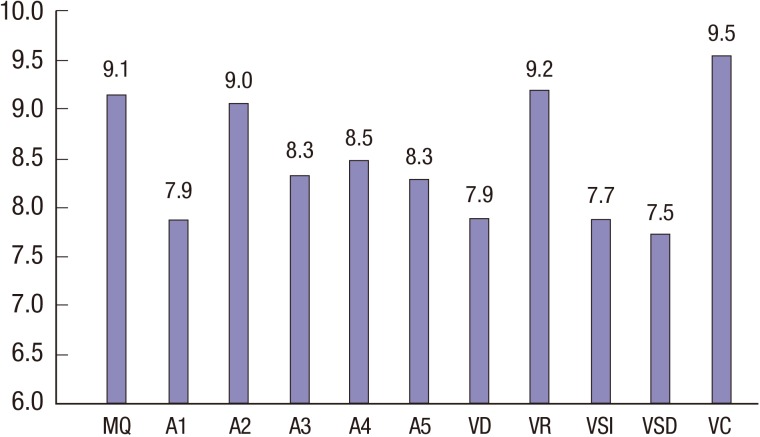
The mean estimated MQ score for the patients in this study was 86.78 (range, 45–127), which was within 1 standard deviation of the average MQ score of 100 (Table 3). Twenty-three patients (39.7%) had MQ values below 1 standard deviation of the average MQ score of 85. In addition, 5 out of 58 patients (8.6%) demonstrated scores that were 2 standard deviations below the average MQ score of 100, which indicated impairments in overall global memory function. The mean scores on all of the RKMT subtests are shown in Fig. 2. Values that were significantly lower than normal were found for the following subtests: auditory verbal 1 (T = −4.83, P < 0.001), auditory verbal 3 (T = −4.49, P < 0.001), auditory verbal 4 (T = −3.66, P < 0.001), auditory verbal 5 (T = −4.64, P = 0.001), verbal delayed recall (T = −4.94, P < 0.001), visual immediate recall (T = −3.88, P < 0.001), and visual delayed recall (T = −4.36, P < 0.001).
Fig. 2.
Mean scores on the Rey-Kim memory test of all of the patients (n = 58).
MQ=memory quotient, A1=auditory verbal 1, A2=auditory verbal 2, A3=auditory verbal 3, VD=verbal delayed recall, VR=verbal delayed recognition, VSI=visual immediate recall, VSD=visual delayed recall, VC=visual copy.
In the comparisons of the memory test scores between the patients under 8 at diagnosis (Patient Group 1) and the patients 8 or older at diagnosis (Patient Group 2), the total MQ did not differ between the groups. However, the scores on the other verbal memory and visual memory subtests were significantly lower in Patient Group 1 (Table 4). Among the memory subtests, the mean scores on the auditory verbal learning 1–5 tests were markedly lower in the patients diagnosed under 8.
Assessments for attention
Table 4 shows the mean scores for both age groups. Although the differences were not statistically significant, both the verbal and visual attention scores for Patient Group 1 were lower than those for Patient Group 2.
Assessments for executive function
For both verbal and visual executive functions, the scores of Patient Group 1 on all of the subtests were lower than those of Patient Group 2. The mean values for all of the subtests of executive function are shown in Table 4. These data show that the scores in Patient Group 1 were significantly lower than those in Patient Group 2 for the following tests: Letter Fluency Test (T = −5.51, P < 0.001), Supermarket test (T = −3.76, P < 0.001), Animal test (T = −2.13, P < 0.05), Digit Span Backward Test (T = −2.13, P < 0.05), and CTT 2 (T = −2.08, P < 0.05).
Assessments for visuospatial function
For visuospatial function, the mean score of Patient Group 1 was lower than that of Patient Group 2 in the Rey complex figure copy task (T = −2.41, P < 0.05) (Table 4).
Motor functions
For simple motor function, the mean scores of Patient Group 1 were lower than those of Patient Group 2 for both the dominant (T = −3.29, P < 0.01) and nondominant hands (T = −2.86, P < 0.01). However, no significant differences were observed for the fine motor function tests (Table 4).
Health related quality of life
In the HRQOL assessment, the PedsTotal scores were 100.59 in the patients diagnosed under the age of 8 and 90.71 in the patients diagnosed over 8 (Table 5). The results on the subtests showed slightly higher scores in Patient Group 1 than in Patient Group 2. However, the scores did not differ significantly for PWB, SFWB, EWB, or BSS. In the parent proxy-reports of the patients, the parent scores tended to be lower than those of the patients in all of the fields of the HRQOL tests. Like the patients’ scores, the mean scores of the fathers and mothers in Patient Group 1 were slightly higher than those of the father and mothers in Patient Group 2. However, there were no significant differences in the scores on the total PedsFACT-BrS or subscales.
Table 5. Means and standard deviations of the health-related quality of life (HRQOL) scores according to age at diagnosis.
| Variables | Age at diagnosis | T value | |
|---|---|---|---|
| < 8 yr (n = 29) | ≥ 8 yr (n = 29) | ||
| Mean (SD) | Mean (SD) | ||
| Patients (n = 58) | |||
| PWB | 21.43 (5.97) | 20.32 (4.82) | 0.76 |
| SFWB | 16.04 (3.31) | 13.93 (4.86) | 1.89 |
| EWB | 26.36 (6.49) | 23.75 (7.27) | 1.42 |
| BSS | 36.29 (7.24) | 32.71 (9.77) | 1.54 |
| PedsTotal | 100.59 (18.44) | 90.71 (21.48) | 1.82 |
| Fathers (n = 15) | |||
| PWB | 20.08 (6.36) | 19.09 (6.04) | 0.36 |
| SFWB | 14.14 (6.07) | 11.73 (5.62) | 1.02 |
| EWB | 27.20 (15.32) | 30.50 (8.85) | −0.49 |
| BSS | 34.29 (10.02) | 32.45 (13.20) | 0.40 |
| PedsTotal | 85.40 (37.52) | 96.38 (28.34) | 0.60 |
| Mothers (n = 53) | |||
| PWB | 19.50 (6.23) | 18.96 (5.92) | 0.31 |
| SFWB | 12.73 (6.08) | 11.88 (5.61) | 0.52 |
| EWB | 25.82 (7.76) | 30.25 (7.05) | 1.62 |
| BSS | 32.20 (9.17) | 33.11 (9.35) | −0.31 |
| PedsTotal | 89.40 (23.01) | 94.62 (20.09) | −0.64 |
SD = standard deviation, PWB = physical well-being, SFWB = social family well-being, EWB = emotional well-being, BSS = brain tumor survivor-specific.
DISCUSSION
This study is the first large-scale study to assess the neurocognitive function and QOL of Korean survivors of childhood medulloblastoma. We assessed the intelligence, motor function, verbal and visual memory, executive function, and attention of these patients. Furthermore, we assessed the HRQOL of these patients with a brain tumor-specific subscale and a pediatric Functional Assessment of Cancer Therapy.
Like other studies, we found significant changes in a wide range of neuropsychological functional domains. Maddrey et al. (5) examined the neuropsychological performance and QOL of 16 survivors of childhood medulloblastoma who survived for more than 10 years with similar test batteries and questionnaires as those used in the current study. Although the percentage of patients who survived more than 10 years in our study was only 11% (19/58), the mean age of the patients in our study (8 years) was similar to the age of the patients in the study of Maddrey et al. (7.3 years old) (5). The mean estimated IQ score in the study of Maddrey et al. (5) was 75 (range, 54–110), which was significantly lower than our mean score of 90.2. In addition, the mean FSIQ, VIQ, and PIQ scores were lower (by 10 points) than those in our study.
In order to understand the discrepancies between our results and those of the study of Maddrey et al. (5), other factors need to be considered. For example, the radiation dose could contribute to the differences in the results. The median radiation dose in the study of Maddrey et al. (5) was 3,800 cGy, whereas most of the patients in our study received doses less than 3,000 cGy, and only 7 patients received radiation doses over 3,600 cGy. The standard radiotherapy dose in earlier studies was 3,600 cGy for the craniospinal axis and 5,400 cGy for the posterior fossa (26). However, a higher radiation dose and younger age at the time of radiation are factors that have been shown to lower IQ. Thus, the total delivered radiation doses have been decreasing in recent clinical trials.
According to the recent report, larger boost volumes in addition to the higher radiation doses were also associated with poor intellectual outcomes in patients with medulloblastoma (27). Boost to the posterior fossa delivers more radiation to structures located outside the targeted area than a boost limited to the tumor bed, thus resulting in a more decline of IQ scores. In our study, only 3 patients received boost radiation so that further analysis regarding boost volumes was not conducted. Data of radiation volumes and doses are both needed to be collected for further studies.
Another explanation for the differences in the IQs between the two studies might be the difference in the percentages of long-term survivors who were included in the studies. Maddrey et al. (5) enrolled only patients who had survived for over 10 years, whereas only 11% of the patients in our study were long-term survivors. A decrease in the IQ of a patient is probable if 10 years or longer have passed after since the first diagnosis. This idea has also been suggested by other studies, including those by Palmer et al. (26) and Ris et al. (28). The study by Palmer et al. (26) evaluated 44 pediatric patients with histologically proven medulloblastoma who were diagnosed before the age of 17 and who survived more than 2 years. They obtained a mean estimated FSIQ of 83.57, which was more than 1 standard deviation below the expected population norm. They suggested that the patients lost 2.55 mean points per year on the estimated FSIQ (P = 0.0001), 2.66 mean points on the VIQ (P = 0.0001), and 1.34 mean points on the PIQ (P < 0.001). Ris et al. (28) assessed 43 patients with medulloblastoma who had a mean age of 6 years at diagnosis (range, 3–15 years). The score changes were estimated to be −4.3 FSIQ points, −4.2 VIQ points, and −4.0 nonverbal scale IQ points per year. They suggested that the intellectual losses of these patients were substantial but that some degree of intellectual preservation was possible compared with the impairments associated with radiation with conventional doses. Long-term studies of the changes in intelligence that occur according to age and radiation dose in long-term Korean survivors of medulloblastomas are necessary to better understand these IQ changes.
Our study showed that, in these patients, verbal function was higher than nonverbal function. The relative strengths of these patients in language skills may influence others to overestimate these individuals' abilities. Thus, the expectations of the performances of these patients may exceed their actual cognitive capacities (5).
For memory, the mean scores on all of the RKMT indices of both verbal and nonverbal memory function that were assessed in this study were below the standardization sample scores. The mean verbal delayed recall score of the survivors of childhood medulloblastoma was 7.9, which was at the low average level. In the visual memory tasks, the mean scores of both immediate and delayed recall were 7.7 and 7.5, which were also at the low average level. We could not compare the mean recall scores with the mean recognition task scores because we did not have a recognition task in the RKMT. In contrast to our results, Maddrey et al. (5) found both verbal and nonverbal deficits in their 10-year survivors, with more severe deficits in nonverbal memory.
The mean visual copy score on the RCFT, which assesses visuospatial function (copying as accurately as possible), in the patient group in our study was 9.5, which was at the average level. In contrast, the 10-year survivors of childhood medulloblastomas in the report of Maddrey et al. (5) showed deficits in the visuospatial test requiring the RCFT copy task.
The performances of the patient groups in our study were inconsistent in the various neurocognitive functions. The mean digit symbol score suggested a processing speed of 6.2, which was within the borderline level (29). The mean score on the digit span forward test that assessed verbal attention was 9.8, which was at the average level, while the T score on the CTT 1 that measured visual attention was 34.28, which was a mild to moderate deficit (12). In 1 executive function measure on the COWA, we were not able to compare the results with a standardization sample because no Korean norm data for the COWA exist. The mean score on the digit span backward, which is another measure of verbal executive function, was 6, which was within the borderline level, whereas the T score on the CTT 2 that measures visual executive function was 32.18, which indicated a mild to moderate deficit.
We analyzed the patients’ neurocognitive data by comparing groups that were divided according to the initial risk, radiation dose, age at diagnosis, or time after diagnosis. Of these results, statistical differences were only found in the analysis that compared the groups according to the age at diagnosis. These results were consistent with those of previous reports that neurocognitive changes were related to the patients’ ages at their first exposure to radiation. According to the study conducted by Palmer et al. (26), patients with medulloblastomas who were younger than 8.02 years when they started to receive craniospinal irradiation were at greater risk for cognitive decline than patients with medulloblastomas who were older (> 8.02 years) at the time of their craniospinal irradiation.
When we compared the neuropsychological functions of these patients by their age at diagnosis (< 8 or ≥ 8 years old), we found significant group differences in intelligence, memory, executive function, visuospatial function and simple motor function, which suggested that these neurocognitive measures were sensitive to the effects of age at diagnosis and craniospinal irradiation (18).
We examined whether there would be any differences according to age at diagnosis (before 8 years old compared to after) with general well-being and brain-tumor specific tools in both patient and parent groups. No statistical differences were found between the age groups in both the patient and parent groups. The HRQOL evaluations of our patients and parents did not show significantly lower scores. The lack of dissatisfaction with these patients' and parents' lives may be due to their adaptation and habituation to the limitations that are often observed among children with chronic medical disorders (5).
Our study had several limitations that restrict its interpretation as a cross-sectional study. First, we performed the neurocognitive assessments only once, and we did not plan repeated assessments because of the various difficult clinical situations of the Korean survivors of childhood medulloblastoma. In addition, we only included consenting patients who were able to understand study concepts. Thus, our results do not represent Korean survivors of medulloblastomas as a whole, and patients who had relatively stable intellectual functions might be included only. In addition, many other factors related to long-term complications were omitted from the analysis.
However, this study is the first study to include a large group of Korean survivors of medulloblastoma. In addition, this study included brain tumor-specific QOL assessments that were conducted by a specialized clinical neuropsychologist. In the future, a longitudinal study that lasts for more than 10 years from the time of first diagnosis and that assesses the exact neurocognitive changes in the patients’ visuospatial function, executive function, and attention is needed.
This study is the first large-scale assessment of the neurocognitive function and QOL of Korean survivors of childhood medulloblastoma. The patients exhibited significant changes in their neurocognition in a wide range of functional domains. The patients who were under 8 years old at the time of first diagnosis exhibited deficits in intelligence, memory, executive function, and visuospatial function and not in attention and simple motor function, and these cognitive deficits might be related to the earlier exposures of these patients to craniospinal irradiation and chemotherapy. A long-term follow-up study involving repeated assessments is needed in these patients after their first diagnosis in order to better understand the deficits of survivors of childhood medulloblastoma.
ACKNOWLEDGMENT
The authors thank Ms. Yoon Yi Kim, Korea Childhood Leukemia Foundation, Seoul, Republic of Korea, for her assistance with the data collection.
Footnotes
Funding: This study was supported by grants from the Korea Childhood Leukemia Foundation and the National R & D Program for Cancer Control, Ministry of Health, Welfare and Family Affairs, Republic of Korea (No. 0520300).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Park HJ, Shin HY. Data collection: Yoo HJ, Kim DS, Ra YS. Analysis and interpretation of data: Yoo HJ. Drafting of the manuscript: Yoo HJ, Kim H. Revision of the manuscript: Kim H. Approval for the final manuscript: all authors.
References
- 1.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 2.Kieffer-Renaux V, Viguier D, Raquin MA, Laurent-Vannier A, Habrand JL, Dellatolas G, Kalifa C, Hartmann O, Grill J. Therapeutic schedules influence the pattern of intellectual decline after irradiation of posterior fossa tumors. Pediatr Blood Cancer. 2005;45:814–819. doi: 10.1002/pbc.20329. [DOI] [PubMed] [Google Scholar]
- 3.Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi GC, McAlonan G, Cao G, Chan GC. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 4.Rønning C, Sundet K, Due-Tønnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg. 2005;41:15–21. doi: 10.1159/000084860. [DOI] [PubMed] [Google Scholar]
- 5.Maddrey AM, Bergeron JA, Lombardo ER, McDonald NK, Mulne AF, Barenberg PD, Bowers DC. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 6.George AP, Kuehn SM, Vassilyadi M, Richards PM, Parlow SE, Keene DL, Ventureyra EC. Cognitive sequelae in children with posterior fossa tumors. Pediatr Neurol. 2003;28:42–47. doi: 10.1016/s0887-8994(02)00471-x. [DOI] [PubMed] [Google Scholar]
- 7.Reeves CB, Palmer SL, Reddick WE, Merchant TE, Buchanan GM, Gajjar A, Mulhern RK. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 8.Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, Gibbs IC, Donaldson SS, Rosenblum RK, Varni JW, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–5500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 9.Park HJ, Nam BH, Lim HS, Shin HY, Hah JO, Kang HJ, Koo HH, Kook H, Kim DS, Kim SK, et al. Outcome of multicenter study for Korean children with medulloblastoma. Clin Pediatr Hematol Oncol. 2007;14:167–175. [Google Scholar]
- 10.Yum TH, Park YS, Oh KJ, Kim JG, Lee HY. The Manual of Korean-Wechsler Adult Intelligence Scale. Seoul: Korean Guidance Press; 1992. [Google Scholar]
- 11.Parekh PI, Blumenthal JA, Babyak MA, LaCaille R, Rowe S, Dancel L, Carney RM, Davis RD, Palmer S. INSPIRE Investigators. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005;67:425–432. doi: 10.1097/01.psy.0000160479.99765.18. [DOI] [PubMed] [Google Scholar]
- 12.Shin MS, Goo HJ. Children's Color Trails Test. Seoul: Hakjisa; 2007. [Google Scholar]
- 13.Williams J, Rickert V, Hogan J, Zolten AJ, Satz P, D’Elia LF, Asarnow RF, Zaucha K, Light R. Children’s color trails. Arch Clin Neuropsychol. 1995;10:211–223. [PubMed] [Google Scholar]
- 14.Kim HG. Rey-Kim Memory Test. Daegu: Neuropsychology Press; 1999. [Google Scholar]
- 15.Lezak MD. Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University; 1983. [Google Scholar]
- 16.Mitrushina MN, Boone KB, D'Elia LF. Handbook of Normative Data for Neuropsychological Assessment. New York, NY: Oxford University Press; 1999. [Google Scholar]
- 17.Kwak GJ, Park HW, Kim CT. Korean Wechsler Intelligence Scale for Children-III. Seoul: Special Education Publishing Co.; 2001. [Google Scholar]
- 18.Ferrans CE. Development of a quality of life index for patients with cancer. Oncol Nurs Forum. 1990;17:15–19. [PubMed] [Google Scholar]
- 19.Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Evanston, IL: Center on Outcomes, Research and Education, Evanston Northwestern Healthcare and Northwestern University; 1997. [Google Scholar]
- 20.Yoo H, Ra YS, Park HJ, Lai JS, Cella D, Shin HY, Kim DS, Kim WC, Shin YS. Validation of pediatric functional assessment of cancer therapy: patient version 2 of “brain tumor survivor” for grade school patients aged 7-12 years. Qual Life Res. 2011;20:529–535. doi: 10.1007/s11136-010-9786-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoo H, Kim DS, Shin HY, Lai JS, Cella D, Park HJ, Ra YS, Kim WC, Shin YS. Validation of the pediatric functional assessment of cancer therapy questionnaire (version 2.0) in brain tumor survivors aged 13 years and older. J Pain Symptom Manage. 2010;40:559–565. doi: 10.1016/j.jpainsymman.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Shin HY. Korean society for pediatric neuro-oncology (KSPNO) Korean J Pediatr Hematol Oncol. 2005;12:175–187. [Google Scholar]
- 23.Pendergrass TW, Milstein JM, Geyer JR, Mulne AF, Kosnik EJ, Morris JD, Heideman RL, Ruymann FB, Stuntz JT, Bleyer WA. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. J Clin Oncol. 1987;5:1221–1231. doi: 10.1200/JCO.1987.5.8.1221. [DOI] [PubMed] [Google Scholar]
- 24.Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, Burger P, Barnes P, Kun L. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031) J Clin Oncol. 2013;31:2936–2941. doi: 10.1200/JCO.2012.43.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 26.Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, Merchant TE, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 27.Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, Spiegler BJ, Malkin D, Janzen L, Mabbott D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- 28.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 29.Tulsky DS, Saklofske DH, Zhu J. Revising a Standard: an Evaluation of the Origin and Development of the WAIS-III. San Diego, CA: Academic Press; 2003. [Google Scholar]
- 30.Lai JS, Cella D, Tomita T, Bode RK, Newmark M, Goldman S. Developing a health-related quality of life instrument for childhood brain tumor survivors. Childs Nerv Syst. 2007;23:47–57. doi: 10.1007/s00381-006-0176-6. [DOI] [PubMed] [Google Scholar]