Abstract
Although pregnancy is a medical condition that contributes to bone loss, little information is available regarding bone mineral density (BMD) in puerperal women. This cross sectional study aimed to evaluate the prevalence of low BMD in puerperal women and to identify associated risk factors. We surveyed all puerperal women who had BMD measurements taken 4–6 weeks after delivery in a tertiary university hospital, and did not have any bone loss-related comorbidities. Among the 1,561 Korean puerperal women, 566 (36.3%) had low BMD at the lumbar spine, total hip, femoral neck, and/or trochanter. Multivariate analysis revealed that underweight women had a significantly higher risk of low BMD compared with obese women at pre-pregnancy (adjusted odds ratio [aOR], 3.21; 95% confidence interval [CI], 1.83–5.63). Also, women with inadequate gestational weight gain (GWG) were 1.4 times more likely to have low BMD than women with excessive GWG (aOR, 1.42; 95% CI, 1.04–1.94). One-way ANOVA showed that BMDs at the lumbar spine and total hip were significantly different between the 4 BMI groups (both P < 0.001) and also between the 3 GWG groups (both P < 0.001). In conclusion, this study identifies a high prevalence of low BMD in puerperal women and thus suggests the need for further evaluation about the change of BMD in pregnancy and postpartum period.
Keywords: Bone Density, Postpartum Period, Osteoporosis, Body Mass Index, Weight Gain, Korean
INTRODUCTION
Osteoporosis is common health problem in postmenopausal women that can have serious adverse impacts on life quality (1). Despite the importance of osteoporosis, little information is available regarding osteoporosis in premenopausal women, probably due to its low incidence in women of younger ages. However, since having a low peak bone mass early in life is an important contributing factor to developing osteoporosis later in life, bone health should be carefully monitored from an early age (2). Among several medical conditions that may decrease bone density, pregnancy is an important physiologic event experienced by most women. A pregnant woman’s body is an important source of calcium that is transferred to the fetus. The increased calcium requirement during pregnancy is usually met by increased intestinal absorption, decreased renal excretion, and the mobilization of minerals from the skeleton (3,4). Thus, during pregnancy the rate of bone resorption often exceeds the rate of bone formation, leading to bone loss (5).
Despite these important physiological changes during pregnancy, the precise effects of pregnancy on bone mineral density (BMD) have not yet been established. Small-scale prospective studies using dual energy X-ray absorptiometry (DXA) to measure BMD at pre-pregnancy and during pregnancy have reported that BMD decreases during pregnancy (6,7,8). In addition, a number of studies using ultrasound measurements of BMD have demonstrated significant decreases of BMD during pregnancy (9,10,11). However, these studies had small sample sizes and diverse research methods; thus, no established consensus has yet been reached regarding bone density in pregnant and puerperal women.
To address these issues, this study analyzed BMD in Korean puerperal women. The goals of this study were to determine the prevalence of puerperal low BMD and to identify the risk factors associated with puerperal low BMD. This is the first large-scale study of BMD in puerperal women and the first to define the risk factors in women for low BMD. The results of this study are expected to contribute to our understanding of bone density in puerperal women and to help establish guidelines for prenatal and postnatal care of women to ensure optimal bone health.
MATERIALS AND METHODS
This cross-sectional study surveyed all puerperal women who had given birth to a child at a university hospital in Korea from 2007 to 2009 and whose BMD was measured 4–6 weeks after childbirth. During the study period, 2,397 childbirths occurred. Postpartum BMD measurement was recommended to all women; 1,672 women consented to and underwent this measurement. Of these women, 110 had comorbid conditions known to be associated with secondary bone loss and were excluded. These conditions included hyperthyroidism, hypothyroidism, systemic lupus erythematous, rheumatoid arthritis, insulin-dependent diabetes mellitus, kidney disease, epilepsy, depression, schizophrenia, hematologic disease, and treatment for infertility. In addition, 55 women who delivered before 32 weeks of pregnancy were excluded. Finally, a total of 1,561 puerperal women were included in this study.
Baseline data included medical conditions, smoking status, parity, newborn birth weight, gestational age at delivery, delivery method, experience of hospitalized bed rest, treatment with dexamethasone for fetal lung maturation, calcium intake during the prenatal and postnatal periods, and breastfeeding behavior. Calcium intake was assessed by self-reported medical records. Only supplemental calcium intake was investigated; dietary calcium intake was not considered. Most calcium supplements consisted of daily multivitamins containing 125 mg calcium each. Subjects were divided into 2 groups according to the duration of calcium intake. Breastfeeding behavior during the first 6 weeks after delivery was evaluated by self-reporting and was classified as either exclusive breastfeeding or formula feeding (with or without supplemental breastfeeding).
Additional anthropometric data, including pre-pregnancy weight, pre-pregnancy height, and weight at delivery date were collected. Height and weight were used to calculate body mass index (BMI; kg/m2) at pre-pregnancy. All enrolled women were categorized according to the World Health Organization (WHO) BMI classification system for Asians as follows: underweight, BMI of 18.6 kg/m2 or less; normal weight, BMI of 18.5 to 22.9 kg/m2; overweight, BMI of 23 to 24.9 kg/m2; obese, BMI of 25 kg/m2 or higher (12). Gestational weight gain (GWG) was calculated as the difference between the maternal pre-pregnancy weight and the last weight before delivery and then classified as inadequate, adequate, or excessive according to the 2009 Institute of Medicine (IOM) recommendation (13). Weekly weight gains according to the 2009 IOM recommendations were used to adjust the recommended total GWG ranges to the gestational weeks at delivery (13).
The BMDs (g/cm2) of the lumbar spine (composite L1–L4) and the hip (total femur, femoral neck, and trochanter) were assessed 4–6 weeks postpartum by DXA using a Lunar Prodigy scanner equipped with enCORE software (GE, Madison, WI, USA). Quality assurance of the densitometric technique was checked according to the manufacturer’s protocol. The long-term percent coefficients of variance (%CV) for the different sites were 1.2% for the lumbar spine, 1.2% for the femoral neck, and 1.4% for the total hip region. The Z-scores were calculated from manufacturer-provided Korean normative data matched for sex and age. Low BMD was defined according to the National Osteoporosis Foundation guidelines as a Z-score of −2.0 or lower for the appropriate age group at any site among the lumbar spine, total hip, femoral neck, and trochanter (14).
Statistics
All maternal and newborn characteristics were summarized using descriptive statistics. The characteristics of women in the low BMD group were compared to those of women in the normal BMD group using the independent sample t-test for continuous variables and the χ2 test for categorical variables. Variables found to be significantly associated with low BMD (P < 0.10) were then entered into multivariate logistic regression analysis to identify risk factors for low BMD. The associations between the variables are expressed as odds ratios (ORs), 95% confidence intervals (95% CIs), and P values. Statistical significance was set at P < 0.05. One-way ANOVA tests were also performed to compare mean BMDs at the lumbar and hip regions for each BMI group and each gestational weight gain group. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the institutional review board of the College of Medicine of the Catholic University of Korea (KC150IS10051). The board waived informed consent from the subject patients. It was conducted in accordance with the Declaration of Helsinki.
RESULTS
The general characteristics of the study population are presented in Table 1. The pre-pregnancy BMI was classified as normal in 1,033 (66.2%) women, underweight in 265 (17.0%) women, overweight in 147 (9.4%) women, and obese in 116 (7.4%) women. Preterm delivery occurred for 134 (8.6%) women (median, 35 weeks). Information about puerperal breastfeeding was only available for 1,453 subjects, 407 of whom self-reported exclusive breastfeeding. No current smokers were in either of the groups.
Table 1. General and pregnancy-related characteristics of the study populations.
| Variables | n = 1,561 |
|---|---|
| Maternal age, yr | 32.8 ± 3.9 |
| Height, cm | 161.4 ± 5.0 |
| Pre-pregnancy weight, kg | 54.0 ± 7.4 |
| Pre-pregnancy BMI, kg/m2 | 20.8 ± 2.6 |
| Weight at delivery | 67.5 ± 8.4 |
| Weight gain during pregnancy | 13.5 ± 4.3 |
| Previous deliveries (≥ 2) | 196 (12.6%) |
| Multivitamin supplementation (> 5 mon) during pregnancy | 157 (19.0%) |
| Bed rest (> 2 wk) | 68 (4.4%) |
| Treatment of dexamethasone | 100 (6.4%) |
| Gestational age at delivery, wk | 38.7 ± 1.7 |
| Preterm delivery | 134 (8.6%) |
| Preeclampsia | 32 (2.0%) |
| Multifetal pregnancy | 51 (3.3%) |
| Newborn weight, g | 3,199.5 ± 489.8 |
| Anemia at delivery | 196 (12.6%) |
| Mode of delivery (Cesarean delivery) | 535 (34.3%) |
| Exclusive breastfeeding* | 407 (26.1%) |
All values are expressed as mean (± standard deviation) or number (%).
*Data about puerperal breastfeeding were available for 1,453 cases.
The mean lumbar spine BMD was 1.170 g/cm2 and the mean total hip BMD was 0.941 g/cm2. Out of all the women analyzed, 13.8%, 16.4%, 24.8%, and 24.1% had low BMD in the lumbar spine, total hip, femoral neck, and trochanter, respectively (Table 2). Moreover, 566 (36.3%) women had low BMD in at least one of the four measured sites, 139 (8.9%) had low BMD in two of the sites, 135 (8.6%) in three of the sites, and 87 (5.6%) in all four sites. In contrast, low-trauma fracture occurred in only 3 of the women during pregnancy (1.9 per 1,000 pregnant women). All of these women had severely low BMDs, with Z-scores less than −2.5 in each of the four sites.
Table 2. Bone mineral density (BMD) measures and proportion of low BMD.
| Skeletal sites | BMD, g/cm2 | BMD (Z-score) | Low BMD (Z-score ≤ −2) |
|---|---|---|---|
| Lumbar spine | 1.170 ± 0.136* | 215 (13.8%)† | |
| L1 | −0.59 ± 1.04 | 117 (7.5%) | |
| L2 | −0.61 ± 1.12 | 160 (10.2%) | |
| L3 | −0.04 ± 1.12 | 55 (3.5%) | |
| L4 | −0.27 ± 1.12 | 95 (6.1%) | |
| Total hip | 0.941 ± 0.115 | −0.59 ±1.04 | 256 (16.4%) |
| Femoral neck | −1.08 ± 0.93 | 387 (24.8%) | |
| Trochanter | −1.33 ± 0.88 | 376 (24.1%) |
All values are expressed as mean (± standard deviation) or number (%).
*Total BMD for the lumbar spine (L1–L4); †No. of subjects with low BMD at any lumbar spine (L1–L4).
Women with low BMD were significantly different from women with normal BMD with respect to pre-pregnancy height, pre-pregnancy weight, pre-pregnancy BMI, weight at delivery, and breastfeeding habits according to univariate analysis (all P < 0.001 except for height [P = 0.001] and breastfeeding [P = 0.042]). No significant associations were observed between age, parity, or multivitamin supplementation status during pregnancy and low BMD (P = 0.056, 0.167, and 0.145, respectively) (Table 3). Since height, weight, and BMI at pre-pregnancy and weight at delivery were significantly correlated with each other (all P < 0.001), only the BMI at pre-pregnancy was used in the following analysis.
Table 3. Comparison between puerperal women with low bone mineral density (BMD) versus women with normal BMD.
| Variables | Low BMD (n = 566) | Normal (n = 995) | P values |
|---|---|---|---|
| Maternal age, yr | 32.5 ± 3.8 | 32.9 ± 4.0 | 0.056 |
| Height, cm | 160.8 ± 4.9 | 161.7 ± 5.1 | 0.001* |
| Pre-pregnancy weight, kg | 52.2 ± 6.9 | 55.1 ± 7.5 | < 0.001† |
| Pre-pregnancy BMI, kg/m2 | 20.2 ± 2.4 | 21.1 ± 2.7 | < 0.001† |
| Weight at delivery, kg | 65.6 ± 8.0 | 68.7 ± 8.4 | < 0.001† |
| Weight gain during pregnancy, kg | 13.3 ± 4.3 | 13.5 ± 4.3 | 0.373 |
| Multipara: Yes/No | 33 (30.6%)/533 (36.7%) | 75 (69.4%)/920 (63.3%) | 0.167 |
| Multivitamin supplementation (≥ 5 mon): Yes/No | 92 (58.6%)/433 (64.8%) | 65 (41.4%)/235 (35.2%) | 0.145 |
| Bed rest: < 2 wk/≥ 2 wk | 542 (36.3%)/24 (35.3%) | 951 (63.7%)/44 (64.7%) | 0.866 |
| Dexamethasone: Yes/No | 29 (29.0%)/536 (36.7%) | 71 (71.0%)/923 (63.3%) | 0.119 |
| Preterm delivery: Yes/No | 46 (34.3%)/520 (36.4%) | 88 (65.7%)/907 (63.6%) | 0.627 |
| Preeclampsia: Yes/No | 8 (25.0%)/558 (36.5%) | 24 (75.0%)/970 (63.5%) | 0.306 |
| Multifetal pregnancy: Yes/No | 21 (41.2%)/30 (58.8%) | 30 (58.8%)/965 (63.9%) | 0.458 |
| Macrosomia (> 4 kg): Yes/No | 21 (37.5%)/545 (36.2%) | 35 (62.5%)/960 (63.8%) | 0.844 |
| Anemia at delivery: Yes/No | 75 (38.3%)/491 (36.0%) | 121 (61.7%)/874 (64.0%) | 0.532 |
| Vaginal/Cesarean delivery | 378 (36.8 %)/188 (35.1%) | 648 (63.2%)/347 (64.9%) | 0.507 |
| Exclusive breastfeeding: Yes/No | 161 (39.6%)/349 (33.9%) | 246 (60.4%)/697 (66.1%) | 0.042‡ |
*P < 0.01; †P < 0.001; ‡P < 0.05.
To attempt to adjust for any potential bias, adjusted odds ratios (aORs) for low BMD were calculated with a logistic regression model including BMI at pre-pregnancy, weight gain during pregnancy, breastfeeding status, and maternal age. The results were comparable to those obtained by univariate analysis (crude ORs) (Table 4). Women who were underweight at pre-pregnancy had an elevated risk for low BMD compared with women who were obese at pre-pregnancy. This elevated risk persisted even after adjusting for the aforementioned variables (aOR, 3.21; 95% CI, 1.83–5.63; P < 0.001). With respect to GWG, women with inadequate weight gain were 1.4 times more likely to have low BMD than women with excessive weight gain (aOR, 1.42; 95% CI, 1.04–1.94; P = 0.030). The unadjusted OR of the relationship of exclusive breastfeeding with low BMD was 1.28 (95% CI, 1.01–1.62; P = 0.042). However, after adjusting for the aforementioned variables, the risk of low BMD was no longer elevated (P = 0.116).
Table 4. Risk factors and odds ratios (ORs) for low bone mineral density (BMD).
| Variables | Low BMD | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | |
|---|---|---|---|---|
| Yes (n = 566) | No (n = 995) | |||
| BMI (kg/m2) at pre-pregnancy† | ||||
| Underweight | 132 (49.8%) | 133 (50.2%) | 3.61 (2.18–5.98)‡ | 3.21 (1.83–5.63)‡ |
| Normal | 368 (35.6%) | 665 (64.4%) | 2.01 (1.27–3.19)§ | 1.94 (1.16–3.23)∥ |
| Overweight | 41 (27.9%) | 106 (72.1%) | 1.41 (0.80–2.49) | 1.54 (0.84–2.82) |
| Obese | 25 (21.6%) | 91 (78.4%) | Ref | Ref |
| Weight gain during pregnancy | ||||
| Inadequate | 197 (44.9%) | 242 (55.1%) | 1.93 (1.46–2.57)‡ | 1.42 (1.04–1.94)∥ |
| Adequate | 246 (34.8%) | 460 (65.2%) | 1.27 (0.98–1.65) | 1.01 (0.76–1.34) |
| Excessive | 123 (29.6%) | 293 (70.4%) | Ref | Ref |
| Breastfeeding | ||||
| None or partial | 354 (33.7%) | 697 (66.3%) | Ref | Ref |
| Exclusive | 206 (40.4%) | 304 (59.6%) | 1.28 (1.01–1.62)∥ | 1.22 (0.96–1.55) |
| Maternal age, yr | ||||
| < 30 | 119 (37.7%) | 197 (62.3%) | Ref | Ref |
| 30–40 | 423 (36.5%) | 736 (63.5%) | 0.95 (0.74–1.23) | 1.06 (0.80–1.40) |
| > 40 | 24 (27.9%) | 62 (72.1%) | 0.64 (0.38–1.08) | 0.77 (0.44–1.35) |
*Adjusted ORs obtained with a logistic regression model including pre-pregnancy BMI, weight gain during pregnancy, breastfeeding status and maternal age; †Underweight, overweight, and obesity are defined as BMIs less than 18.5 kg/m2, between 23 and 24.9 kg/m2, or 25 kg/m2 or more, respectively; ‡P < 0.001; §P < 0.01; ∥P < 0.05.
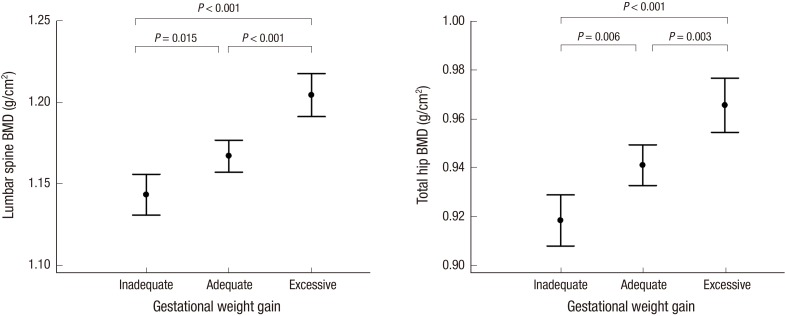
One-way ANOVA showed that the BMDs at the lumbar spine and total hip were significantly different between the 4 BMI groups (P < 0.001). Also, regarding GWG, BMDs at the lumbar spine and total hip were significantly different between the 3 GWG groups (both P < 0.001). (Figs. 1 and 2).
Fig. 1.
Mean bone mineral densities (BMDs) at the lumbar spine and total hip in the 4 body mass index (BMI) groups. Data are presented as means; vertical bars show the 95% confidence intervals. At both sites, the BMD tended to increase as the BMI increased (both P < 0.001, ANOVA test). Post hoc analysis revealed significant differences in BMD at the lumbar spine and total hip between the different BMI groups (all P < 0.05), with the exception of BMD at the lumbar spine in the overweight versus obese groups (P = 0.216).
Fig. 2.
Mean bone mineral densities (BMDs) at the lumbar spine and hip in the 3 gestational weight gain (GWG) groups. Data are presented as means; vertical bars show the 95% confidence intervals. At both sites, the BMD tended to increase as the GWG increased (both P < 0.001, ANOVA test). Post hoc analysis revealed significant differences in BMD at both sites between the different GWG groups (all P < 0.05).
DISCUSSION
This study found that the prevalence of low BMD (i.e. “below the expected range for the age”) among Korean puerperal women was quite high (36.3%). Considering the relatively young age of the subjects, the prevalence of low BMD was higher than expected. Few studies have examined the prevalence of low BMD among puerperal women, not just for Asians but also for other ethnicities. To the best of our knowledge, this is the first report to evaluate the prevalence of low BMD in puerperal women.
Low-trauma fracture occurred in three women during pregnancy. Each of these women had severely low BMD. Premenopausal women do not have a high risk of fracture, even with low BMD (15). This finding can be explained by their greater muscle mass, thicker cortices, normal trabecular connectivity, and fewer falls compared with postmenopausal women (16). However, since the high rate of bone turnover observed in pregnant women is an important risk factor for fracture fragility, pregnant women are likely to have a relatively higher risk of fracture than nonpregnant premenopausal women (3). Accordingly, pregnant and puerperal women who are at risk of severely low BMD should be strongly encouraged to strive to prevent fractures, even though the incidence of fracture is relatively low.
Low peak bone mass is known to be a critical determinant of postmenopausal osteoporosis (2). Since pregnancy is an important condition that may lower bone density in young women, it is necessary to identify pregnant or puerperal women at risk for low BMD not only for preventing fracture during the puerperal period, but also for suppressing the subsequent development of osteoporosis later in life. In this study, low pre-pregnancy BMI and inadequate GWG were found to be important risk factors for low BMD. A number of studies have reported that low BMI is significantly associated with low BMD (17,18). The present study extends these findings and suggests that BMI impacts bone density even in pregnant women. As women who have a low BMI before they become pregnant are likely to have low BMD during pregnancy, they should be more concerned about achieving optimal GWG during pregnancy. Also regular exercise and healthy eating may be helpful for bone health.
Obesity appeared to be protective against low BMD in this study. Pregnant women with normal BMI had an increased risk for low BMD compared with obese pregnant women at an odds ratio of 1.94. However, the relationship between obesity and low BMD has been quite controversial so far (19,20). Body weight is made up of lean mass and fat mass and fat mass per whole body weight is suggested to be negatively associated with BMD (21). This retrospective study did not analyze the effect of lean mass and fat mass on the BMD. Further study about the influence of body composition on the BMD during pregnancy will be necessary.
This is also the first study to indicate that achieving the optimal weight gain during pregnancy is important for maintaining a healthy BMD in pregnant women. For pregnant women, inadequate GWG indicates that the calorie intake is lower than required, which may be associated with low dietary calcium intake if calcium supplements were not taken. For the development of the fetal skeleton, 30 g of calcium is required over the course of pregnancy (3). This requirement is mainly met through an increased calcium intake, the doubling of maternal intestinal calcium absorption, increased skeletal resorption, and increased renal mineral conservation (3,4). Accordingly, low dietary calcium intake can induce excessive resorption of calcium from the skeleton, and may cause severe loss of maternal bone mineral content during pregnancy.
An increased calcium intake is expected to have a positive effect on maternal bone mineral content. Previous studies have suggested that daily intake of a calcium supplement can decrease the levels of bone resorption markers and increase postpartum lumbar BMD (22,23). However, the effect of calcium intake on BMD was difficult to analyze in the present study because daily calcium intake was not recorded. Breastfeeding is also known to be a risk factor for low BMD (3,4). The univariate analysis performed in the present study revealed that the risk of low BMD was significantly higher in the exclusively breastfeeding group. However, after adjusting the analysis according to pre-pregnancy BMI and GWG, breastfeeding did not have a significant effect on BMD. Considering the present study analyzed women within six weeks of delivery, any conclusions that can be drawn regarding the effect of breastfeeding on BMD are somewhat limited.
Although the present study did not identify the precise cause underlying the high prevalence of low BMD in puerperal women, the possibility of ethnic effects should be considered. Obesity rates of Korea are among the lowest in the OECD (24). Although relatively strict obesity criteria for Asia were applied in the present study, a relatively high percent (17.0%) of women were underweight at pre-pregnancy, whereas the rate of obesity was relatively low (7.4%). Korean women generally prefer to manage weight through diet control, even during pregnancy. In addition, Koreans get less dietary calcium from meals than Westerners do (25). These ethnic characteristics of Koreans may contribute to the high prevalence of low BMI in this study. To comprehensively understand the BMD characteristics of puerperal women, further studies of different ethnicities are required.
Also, our data do not necessarily imply that pregnancy itself is a significant risk factor for low BMD in puerperal women. Since pre-pregnancy BMD measurements were not performed for the subjects in our study group, we cannot exclude the possibility that they had already had low BMD at pre-pregnancy. Moreover, it is difficult to tell whether low BMD in the puerperal period is reversible and whether it affects peak bone mass. However, this study did identify a high prevalence of low BMD in puerperal women and thus highlights the need for interest in bone health when caring for pregnant or puerperal women, regardless of whether low BMD is caused by pregnancy.
The major finding of the present study was that approximately 36% of all puerperal women had low BMD and the risk of low BMD was higher when pre-pregnancy BMI was low and when the recommended GWG was not attained. Our study implies that women with an elevated risk for low BMD should be encouraged to achieve optimal weight gain and carefully guard against falls and fractures during pregnancy. Further studies of women of different ethnicities during the prenatal and postnatal periods are necessary to determine the extent to which our findings are broadly applicable.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Jang DG, Park IY. Literature review: Choi SK, Ko HS, Shin JC. Data management: Choi SK, Ko HS, Park IY. Statistical analysis: Kwon JY. Interpretation of the findings: Jang DG, Kwon JY, Park IY. Drafting of the manuscript: Jang DG, Kwon JY. Revision of manuscript: Kwon JY, Park IY. Final approval: all authors.
References
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 2.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010;25:1948–1957. doi: 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105–118. doi: 10.1007/s10911-005-5394-0. [DOI] [PubMed] [Google Scholar]
- 4.Ward KA, Adams JE, Mughal MZ. Bone status during adolescence, pregnancy and lactation. Curr Opin Obstet Gynecol. 2005;17:435–439. doi: 10.1097/01.gco.0000175365.65835.ad. [DOI] [PubMed] [Google Scholar]
- 5.Hellmeyer L, Ziller V, Anderer G, Ossendorf A, Schmidt S, Hadji P. Biochemical markers of bone turnover during pregnancy: a longitudinal study. Exp Clin Endocrinol Diabetes. 2006;114:506–510. doi: 10.1055/s-2006-951627. [DOI] [PubMed] [Google Scholar]
- 6.More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int. 2001;12:732–737. doi: 10.1007/s001980170048. [DOI] [PubMed] [Google Scholar]
- 7.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15:557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 8.Kaur M, Pearson D, Godber I, Lawson N, Baker P, Hosking D. Longitudinal changes in bone mineral density during normal pregnancy. Bone. 2003;32:449–454. doi: 10.1016/s8756-3282(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 9.Yamaga A, Taga M, Minaguchi H, Sato K. Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: a longitudinal study. J Clin Endocrinol Metab. 1996;81:752–756. doi: 10.1210/jcem.81.2.8636299. [DOI] [PubMed] [Google Scholar]
- 10.To WW, Wong MW, Leung TW. Relationship between bone mineral density changes in pregnancy and maternal and pregnancy characteristics: a longitudinal study. Acta Obstet Gynecol Scand. 2003;82:820–827. doi: 10.1034/j.1600-0412.2003.00227.x. [DOI] [PubMed] [Google Scholar]
- 11.Hellmeyer L, Hahn B, Fischer C, Hars O, Boekhoff J, Maier J, Hadji P. Quantitative ultrasonometry during pregnancy and lactation: a longitudinal study. Osteoporos Int. 2015;26:1147–1154. doi: 10.1007/s00198-014-2984-y. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Western Pacific Region; International Association for the Study of Obesity; International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia Pty Limited; 2000. [Google Scholar]
- 13.Institute of Medicine, National Research Council, Committee to Reexamine IOM Pregnancy Weight Guidelines (US) Weight Gain during Pregnancy: Reexamining the Guidelines. Washington, D.C.: National Academies Press; 2009. [Google Scholar]
- 14.National Osteoporosis Foundation (US) Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, D.C.: National Osteoporosis Foundation; 2008. [Google Scholar]
- 15.Martínez-Morillo M, Grados D, Holgado S. Premenopausal osteoporosis: how to treat? Reumatol Clin. 2012;8:93–97. doi: 10.1016/j.reuma.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla AK. Management of osteoporosis in a pre-menopausal woman. Best Pract Res Clin Rheumatol. 2010;24:313–327. doi: 10.1016/j.berh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Coin A, Sergi G, Benincà P, Lupoli L, Cinti G, Ferrara L, Benedetti G, Tomasi G, Pisent C, Enzi G. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos Int. 2000;11:1043–1050. doi: 10.1007/s001980070026. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, Kuroda T, Saito M, Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos Int. 2013;24:69–76. doi: 10.1007/s00198-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP. Discordance between fat mass index and body mass index is associated with reduced bone mineral density in women but not in men: the Busselton healthy ageing study. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3710-8. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger AS, Lamadrid-Figueroa H, Mercado-García A, Kordas K, Wood RJ, Peterson KE, Hu H, Hernández-Avila M, Téllez-Rojo MM. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican women. Nutr J. 2014;13:116. doi: 10.1186/1475-2891-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janakiraman V, Ettinger A, Mercado-Garcia A, Hu H, Hernandez-Avila M. Calcium supplements and bone resorption in pregnancy: a randomized crossover trial. Am J Prev Med. 2003;24:260–264. doi: 10.1016/s0749-3797(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 24.Organisation for Economic Co-operation and Development. Obesity and the economics of prevention: fit not fat (key facts ??Korea, update 2012) [Internet] [accessed on 21 February 2012]. Availabe at http://www.oecd.org/els/health-systems/49712651.pdf.
- 25.Park SY, Paik HY, Skinner JD, Spindler AA, Park HR. Nutrient intake of Korean-American, Korean, and American adolescents. J Am Diet Assoc. 2004;104:242–245. doi: 10.1016/j.jada.2003.11.015. [DOI] [PubMed] [Google Scholar]