Abstract
We evaluated and compared the effectiveness of intra-articular injection of hip joint using hyaluronic acid and steroid in patients with femoroacetabular impingement (FAI). Thirty patients with FAI clinically and radiologically were enrolled and underwent hip injection using steroid (TA) or hyaluronic acid (HA) at 0-weeks with cross-over injection at 2-weeks in patients without clinical response of decrease of pain intensity less than 2-point. Patients were followed up to 12-weeks for pain intensity (Numeric rating scale, NRS: 0-10), hip disability score (HOOS), oral medication and adverse events. In 17 patients without cross-over, HOOS at 2-weeks was improved significantly in patients with HA injection (mean increase of HOOS = 13.8 with HA vs. -2.2 with TA, P = 0.031) without difference of NRS (P = 0.943). In 13 patients with cross-over, NRS was significantly improved at 2-weeks with first TA injection (mean decrease of NRS= 1.7 with first TA vs. 0.3 with first HA, P = 0.036), without difference of HOOS (P = 0.431). At 4-weeks, NRS and HOOS were significantly different according to injection drugs (NRS: 0.9 with TA first and HA later vs. 2.7 with HA first and TA later, P = 0.001; mean increase of HOOS: 5.3 with TA first and HA later vs. 10.2 with HA first and TA later, P = 0.032). Intra-articular hip injection may be effective in FAI, with faster effect of pain improvement by TA and more delayed effect of function improvement by HA.
Keywords: Femoroacetabular Impingement, Hip, Injections, Glucocorticoids, Hyaluronic Acid
Graphical Abstract
INTRODUCTION
Femoroacetabular impingement (FAI) has been highlighted because it may cause hip pain, labral injury and early osteoarthritis in young patients (1,2,3,4). Arthroscopic surgery is the treatment of choice especially in children and adolescents for prevention of progressive degenerative change (5). However, most patients showed mixed type of FAI (6), which may need more complex surgical technique of both femoral- and acetabular-sided lesions to achieve better outcome (7,8). According to previous literatures about surgical outcome in FAI, most cases of clnical failure were related to advanced osteoarthritis to conversion to total hip replacement eventually (9,10,11,12). This may represent that patient selection is important for clinical success after surgery, and non-surgical treatment option is necessary for the patients who are concerned about poor surgical outcome or need to delay operation. However, there are only few studies of non-operative treatment in FAI (13,14,15,16,17,18). Although intra-articular injection of hip joint has been introduced traditionally and performed widely for the non-operative management of osteoarthritis of hip (19,20,21,22,23,24), to our best knowledege, there was no report about the clinical efficacy of the most widely used injection drugs, steroid and hyaluronic acid for inttra-articular injection of hip in patients with FAI, especially on comparison of therapeutic outcome of these drugs. Therefore, this prospective double-blind randomized cross-over study was aimed to assess and compare the clinical effectiveness of intra-articular injection using steroid (triamcinolone acetonide, TA) or hyaluronic acid (HA) in patients with FAI with follow-up for 12 weeks.
MATERIALS AND METHODS
Patients
Adult patients at least 20 years of age with FAI clinically by orthopedic hip surgeons and / or radiologically were eligible for inclusion. Inclusion criteria were as follows: 1) available written informed consent; 2) diagnosed of FAI clinically during the hip impingement test by orthopedic surgeon; 3) suspected of FAI, radiologically on plain radiographs including pelvic AP (standing position), Dunn, frog leg, and translateral views, showing increased alpha angle more than 55' (cam type), acetabular over-coverage (pincer type) or both (mixed type), 4) no effect after oral medication (pain killers, i.e. NSAIDs). Exclusion criteria were as follows: 1) definite radiologic findings of osteoarthritis in hip joint; 2) hypersensitivity of steroid or contrast; 3) impossible to intra-articular injection, for example skin lesion at puncture site; 4) history of intra-articular injection of hip joint; 5) history of previous operation or trauma of hip joint; 6) other cause of hip pain rather than FAI (rapid destructive hip disease, avascular necrosis of femoral head, crystalline arthropathy, inflammatory arthritis, neuropathic arthropathy, etc.); 7) uncontrolled coagulopathy; 8) pregnancy or breast feeding; 9) suspected of infection of hip joint.
Study design
All participants met inclusion/exclusion criteria by orthopedic hip surgeons were referred to radiology department for hip injection, and randomized by a nurse of radiology outpatient clinic not involved with the study team to be determined to get which drug for injection, TA or HA, keeping the orthopedic hip surgeons and patients blind to injection drug. Clinical response was defined as a reduction of pain at least 2-point of pain intensity (Numeric rating scale, NRS: 0–10) at 2-weeks, compared to baseline. Responders were follow-up to 12-weeks without additional injection, whereas non-responders were permitted to have one more hip injection at 2-weeks using alternative drug. We evaluated about pain intensity using NRS at 0-, 2-, 4-, 6-, 8-, and 12-weeks after hip injection and hip disability score using Hip dysfunction and Osteoarthritis Outcome Score (HOOS) at 0-, 2-, 4-, 8-, and 12-weeks after hip injection, as well as subjective satisfaction scale (five scale: no pain, much improved, slightly improved, no change, aggravated). Pain intensity and hip disability score were checked at 0-, 2-, 4-, 8-, and 12-weeks during patients’ visit at outpatient clinic, but only pain intensity using NRS at 6-weeks was determined by phone-call interview. Adverse events after injection and change oral medication were also asked during follow-up visit or phone-call interview.
Intervention
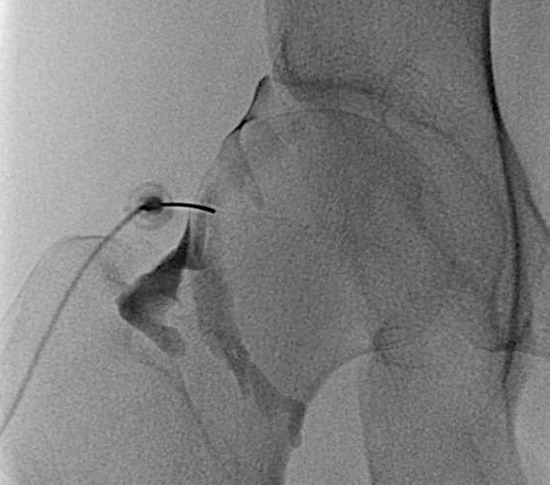
All intra-articular injection of hip was done fluoroscopically using 22-gauge spinal needle by musculoskeletal radiologists, blind to whether the patient was included in this study or not. We targeted at the lateral edge of head-neck junction of femur in supine position (Fig. 1) and confirmed the intra-articular location of the needle using contrast injection (2–3 mL). Steroid injectate was prepared to mix Triamcinolone acetate 20 mg (Triam 40 mg/1 mL, Shin Poong Pharm, Seoul, Korea) and normal saline 1.5 mL (total 2 mL). We used ready-made syringe form of Sodium hyaluronate (Hyruan plusInj. 2 mL/syringe, LG Life Sciences, Seoul, Korea).
Fig. 1.
Intra-articular hip injection is done after targeting lateral side of femur-neck junction on supine position of patients fluoroscopically. After confirmation of intra-articular location of spinal needle using small amount of contrast, steroid or hyaluronic acid is injected slowly.
Analysis of effectiveness of hip injection
The number of patients with clinical response at 2-weeks was determined. Pain intensity and HOOS were evaluated at 2- and 4-weeks according to the presence of cross-over injection. Oral medication and side effects related to injection during 12 weeks follow-up were also investigated.
Statistical considerations
The proportion of patients with clinical response in both arms was analyzed using Fisher’s exact test. Pain intensity using NRS and hip disability score using HOOS were evaluated using the Mann-Whitney U-test by statistic software (PASW, version 17.0; SPSS, Chicago, IL, USA). For evaluation of sub-group according to presence of cross-over injection, mixed-effects ML regression was used using the statistical package software Stata 12 (Stata Corp, College station, TX, USA). A P value of less than 0.05 was considered a significant difference.
Ethics statement
This prospective study was approved by the institutional review board of Seoul National University Bundang Hospital, and written informed consent was obtained from all the participants (B-1105/128-013).
RESULTS
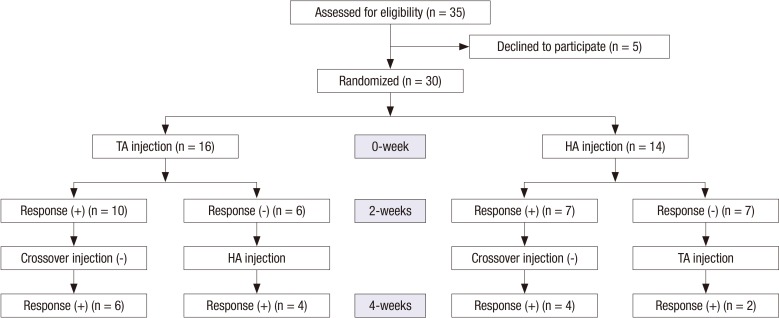
From July, 2011 to December, 2012, thirty-five patients were referred to radiology department for injection, in which five denied the participation in this study (5/35, 14.3%). Flow diagram was shown in Fig. 2.
Fig. 2.
Flow diagram shows the study flow from enrollment to follow-up during 4 weeks, as well as the number of clinical responders.
Patient demographics
Thirty patients were enrolled in the study (11 males and 19 females; mean age, 37 years; range, 24-51 years), and thirteen had cross-over injection (13/30, 43.3%). There were twelve patients with cam type, six with pincer type, and twelve of mixed type of FAI. Twelve patients had right hip injections, seven had left, and eleven had both at 0-weeks. Thirteen patients had cross-over injection at 2-weeks using alternative drug. Total forty three hip joints were evaluated for each side in this study. Only twelve patients were possible in follow-up at 12-weeks (12/30, 40%).
Proportion of clinical responders at 2-weeks
Forty-one hip joints of 27 patients were available about pain intensity at 2-weeks. In 17 patients without cross-over injection, six patients showed clinical response in ten patients after first TA injection (6/10, 60%), and four had clinical response in seven with first HA injection (4/7, 57.1%) without significant difference (Fisher’s exact test, P = 0.429). In thirteen patients with cross-over injection, four patients showed the clinical response among six patients with first TA injection group (4/6, 66.7%), whereas two demonstrated clinical response in seven with first HA injection (2/7, 28.6%) without significant difference (Fisher’s exact test, P = 0.467). Overall, ten patients had clinical response among sixteen patients with first TA injection (10/16, 62.5%), whereas six revealed clinical response in fourteen (6/14, 42.9%) without significant difference (Fisher’s exact test, P = 0.085). These results are shown in Fig. 2.
Pain intensity
Pain intensity using NRS was listed on Table 1. Overall, pain intensity decreased during 12 weeks of follow-up from baseline with mean pain intensity 6.8 becoming 2.1 at 12-weeks. Mean pain intensity was 6.8 at 0-weeks and decreased 4.8 at 2-weeks in total of 30 patients. In 17 patients without cross-over injection, mean pain intensity was 7 at 0-weeks and was improved to 3.5 at 2-weeks without significant difference according to injection drug. In 13 patients with cross-over injection, mean pain intensity changed from 6.7 at 0-weeks to 5.7 at 2-weeks, of which decrement was less than that of patients without cross-over injection. There was no significant difference between patients with first TA or HA injection in cross-over group, also.
Table 1. Pain intensity and hip disability score during 12 weeks.
| Parameters | 0-weeks | 2-weeks | 4-weeks | 6-weeks | 8-weeks | 12-weeks |
|---|---|---|---|---|---|---|
| Mean pain intensity (NRS) | ||||||
| Total (n = 30) | 6.8 (5–10) | 4.8 (0–10) | 3.8 (1–8) | 2.7 (0–7) | 2.1 (0–5) | 2.1 (0–6) |
| TA (n = 16) | 7.1 (5–10) | 4.5 (2–8) | 3.9 (1–8) | 2.6 (0–7) | 1.8 (0–5) | 2 (0–6) |
| HA (n = 14) | 6.6 (5–9) | 5.2 (6–10) | 3.8 (2–6) | 2.8 (0–5) | 2.6 (1–4) | 2.4 (1–5) |
| Cross-over injection (−) (n = 17) | 7 (5–10) | 3.5 (0–8) | 3 (1–5) | 2.5 (0–5) | 2.1 (1–4) | 2 (0–5) |
| Only TA (n = 10) | 7 (5–10) | 3.4 (2–5) | 2.4 (1–4) | 2.1 (0–3) | 1.8 (1–3) | 1.5 (0–2) |
| Only HA (n = 7) | 7 (5–8) | 3.7 (0–8) | 3.4 (2–5) | 2.8 (0–5) | 2.4 (1–4) | 3 (1–5) |
| Cross-over injection (+) (n = 13) | 6.7 (5–10) | 5.7 (2–10) | 4.6 (1–8) | 2.9 (0–7) | 2.1 (5–5) | 2.2 (0–6) |
| TA first & HA later (n = 6) | 7.1 (5–10) | 5.4 (2–8) | 4.9 (1–8) | 3.1 (0–7) | 1.7 (0–5) | 2.3 (0–6) |
| HA first & TA later (n = 7) | 6.3 (5–9) | 6 (3–10) | 4.2 (3–6) | 2.7 (1–4) | 2.8 (2–3) | 2 (2–2) |
| Mean hip disability score (HOOS) | ||||||
| Total (n = 30) | 55.79 (15–89) | 64.1 (24.38–98.75) | 64.32 (14.38–94.38) | NC | 65.75 (13.75–97.50) | 67.62 (24.38–100) |
| TA (n = 16) | 45.03 (15.5–74.23) | 59.60 (24.38–80.96) | 53.60 (14.38–84.38) | NC | 63.13 (23.13–97.50) | 61.67 (24.38–100) |
| HA (n = 14) | 63.34 (30–89) | 63.97 (32.5–98.75) | 69.15 (51.88–94.38) | NC | 78.98 (72.5–86.25) | 70 (63.75–81.25) |
| Cross-over injection (−) (n = 17) | 61.40 (16.25–89) | 64.33 (24.38–98.75) | 69.78 (16.88–94.38) | NC | 56.72 (23.13–94.38) | 59 (24.38–81.88) |
| Only TA (n = 10) | 52.8 (16.25–72.5) | 58.75 (24.38–80) | 60.96 (16.88–82.5) | NC | 56.72 (23.13–94.38) | 58.55 (24.38–81.88) |
| Only HA (n = 7) | 70 (53.13–89) | 70.84 (38.75–98.75) | 78.6 (70–94.38) | NC | NC | NC |
| Cross-over injection (+) (n = 13) | 51.65 (15–88.75) | 63.91 (32.5–80.96) | 60.96 (14.38–84.38) | NC | 69.37 (13.75–97.5) | 71.02 (38.13–100) |
| TA first & HA later (n = 6) | 45.79 (15–74.23) | 70.68 (65.63–80.96) | 60.57 (14.38–84.38) | NC | 59.75 (13.75–97.5) | 71.89 (38.13–100) |
| HA first & TA later (n = 7) | 58.16 (30–88.75) | 59.40 (32.5–80.5) | 61.59 (51.88–76.30) | NC | 79.98 (72.5–86.25) | 69.58 (63.75–81.25) |
TA, allocated to triamcinolone acetate injection firstly; HA, allocated to hyaluronic acid firstly; NRS, numeric rating scale (0–10); HOOS, hip dysfunction and osteoarthritis outcome score; NC, not checkable.
Range of NRS in parenthesis.
Function
Hip disability score using HOOS was shown in Table 1. In total 30 patients, initial mean HOOS was 55.79 and was slightly improved as 64.1 at 2-weeks and 67.62 at 12-weeks. In the group without cross-over injection, mean HOOS showed no significant improvement and there was no significant difference according to injection drugs. In patients with cross-over injection, mean HOOS increased from 51.65 at baseline to 63.91 at 2-weeks and 71.02 at 12-weeks, without significant difference regardless of first injection drugs.
Patients without cross-over injection
Among total thirty patients, 17 patients had only single hip injection without cross-over at 2-week follow-up. Mean decrease of pain intensity was 3.7 in both patients with TA or HA injections without significant difference (estimated difference of pain intensity = −0.05; 95% confidence interval (CI) = −1.53, 1.43; mixed-effects ML regression, P = 0.943). However, HOOS was improved significantly in patients with HA injection (mean increase of HOOS = 13.8) compared to patients with TA injection (mean increase of HOOS = −2.2) (estimated difference of HOOS = 20.27; 95% CI = 1.83, 38.71; mixed-effects ML regression, P = 0.031).
Patients with cross-over injection
Thirteen patients had a cross-over injection using alternative drug at 2-weeks after 1st hip injection, in which six patients had TA and seven had HA as first injection drug. Mean decrease of pain intensity at 2-weeks in the patients with first TA injection was 1.7, whereas 0.3 in patients with first HA injection, with signifcant difference (estimated difference of pain intensity = −0.73; 95% CI = −1.42, -0.05; mixed-effects ML regression, P = 0.036). However, there was no difference of HOOS at 2-weeks according to cross-over drug (mean increase of HOOS = 4.5 in the patients with first TA injection and 1.2 in the patients with first HA injection; estimated difference of HOOS = −1.16; 95% CI = −4.06, 1.73; mixed-effects ML regression, P = 0.431).
At 4-weeks after first hip injection, mean decrease of pain intensity was 0.9 in patients with first TA and second HA injections and 2.7 in patients with first HA first and second TA injections, with significant difference (estimated difference of pain intensity = 2.60; 95% CI = 1.14, 4.07; P = 0.001). HOOS was also improved in both groups, with mean increase of HOOS as 5.3 in the patients with first TA and second HA injections and 10.2 in patients with first HA and second TA injections showing significant difference (estimated difference of HOOS = −9.16; 95% CI = -17.5, 0.79; mixed-effects ML regression, P = 0.032).
Changes of oral medication
Compared with pre-injection state of 0-weeks, nineteen patients said “much improved” or “no pain” after hip injection, and “decreased” or “stopped” their oral medication (Table 2). The degree of patients’ satisfaction was different according to neither presence of cross-over injection nor injected drugs.
Table 2. Overall patients’ satisfaction and changes of oral medication during 12 weeks.
| Parameters | No. of patients |
|---|---|
| Patients′ satisfaction | |
| No change | 4 |
| Slightly improved | 7 |
| Much improved | 19 |
| Changes of oral medication | |
| No change | 11 |
| Decreased medication | 4 |
| Stopped medication | 15 |
Subjective patients’ satisfaction scale (5-scale: no pain, much improved, slightly improved, no change, aggravated).
Adverse events after hip injection
A total of eight patients complained of adverse reactions after hip injections. Among them, facial flushing was the most common symptom in six patients after TA injection, and menstrual irregularity was second frequent in three. After HA injection, swelling with pain at injection site occurred in three patients (Table 3).
Table 3. Adverse events after hip injection during 12 weeks.
| Adverse events | Type of drug | |
|---|---|---|
| TA | HA | |
| Facial flushing | 4 | |
| Menstrual irregularity | 3 | |
| Itching | 2 | 1 |
| Decreased appetite | 1 | |
| Pain at injection site | 1 | 1 |
| Swelling at injection site | 1 | |
TA, triamcinolone acetonide; HA, hyaluronic acid.
DISCUSSION
FAI is known that it may be a precursor condition of early osteoarthritis of hip joint especially in relatively young patients (1,2,3,4,5). Arthroscopic operation is a treatment option, but decision of early operation may be stressful both to patients and surgeons because of relative young age of patients and expectation of the effect by conservative treatment (8,9,10,11,12). However, the effectiveness of non-operative treatments such as physical therapy, oral medication, and intra-articular injection is not well established yet and there were only several studies about that (13,14,15,16,17,18). On the contrary, many reports revealed the efficacy of intra-articular injection as a treatment of osteoarthritis using HA or steroid drug (19,20,21,22,23,24).
In our study, we aimed to evaluate the effectiveness of each drug, HA or TA as conservative management of FAI, as well as to compare the clinical outcome between two drugs by permission of cross-over injection. Based on our results, there was no difference of decrease of pain intensity, improvement of hip function at 2-weeks as immediate clinical response, according to first injected drugs and the proportion of clinical responders was not different between two drugs.
However, after dividing the groups by the presence of cross-over injection, hip disability was more improved in patients with HA injection in the group without cross-over, likely to the result of previous literature in which the viscosupplementation showed the significant effect for hip function for hip osteoarthritis (19).
In patients with a cross-over injection, pain intensity decreased larger at 2-weeks in the patients with first TA injection than with first HA injection, and also more improved at 4-weeks in group with first HA and later TA injections than with first TA and later HA injections. These results may indicate that pain intensity might be more influenced by TA rather than HA, showing initial larger improvement of pain by TA injection.
In terms of hip function, HOOS was not different at 2-weeks in cross-over group, but more improved at 4-weeks in the patients with HA first and TA second injections than with TA first and HA second injection. This might mean that hip function may show more delayed improvement than pain intensity and HA may be more effective for hip function than TA based on both results from no cross-over and cross-over groups.
For adverse events, facial flushing and menstrual irregularity appeared after TA injection, maybe related to hormone-related effect. Whereas, local problems such as pain or swelling at injection site were noted after HA injection, probably due to high viscosity of HA.
For the efficacy of intra-articular injection of FAI, there were only a few studies (13,14,15,16,17). However, previous literature showed different and limited results for therapeutic response of steroid and hyaluronate, that could not allow to determine a intra-articular injection as treatment option and compare the outcome between steroid and hyaluronate. Our study demonstrated the effectiveness of two widely used drugs, HA and TA, for intra-articular injection about improvement of pain intensity and hip function during 12 weeks, especially short term effect at 2-weeks after hip injection, as well as the comparison of the efficacy of two drugs with permission of cross-over. To our best knowledge, there was no study to compare the efficacy of the injection drugs.
This study has some limitations. First, we could enroll just small number of patients, in which became a smaller size after grouping according to the presence of cross-over injection. Second, a very small number of patients could be followed up during 12 weeks. There might be some patients having recurred hip pain even after 12 weeks of study period, which could not be evaluated in this study. Third, we excluded the patients with definite hip osteoarthritis on plain radiographs, but the patients with minimal osteoarthritis may be included in this study, because we did not conduct hip MRI for evaluation of cartilagenous lesion.
In conclusion, intra-articular hip injection with TA or HA may be effective as a conservative treatment in patients with FAI. TA can be use to obtain faster effect in pain relief, whereas HA can be used to obtain more delayed effect in functional improvement.
ACKNOWLEDGMENT
We especially thank to So-Yeon Ahn, an expert statistician of our hospital, for effort about statistical analysis of data, An-Joo Lee, a nurse of our clinic for patient randomization of this study, and Su Yong Shin, a researcher of radiologic department for 6-week phone-call interview.
Footnotes
Funding: This research was supported by the SNUBH research fund (No. 11-2011-008).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study conception and design: Lee JW, Lee GY. Acquisition of data: Lee YK, Lee GY, Lee JW, Lee E. Analysis and interpretation of data: Lee GY, Lee YK, Lee E. Drafting of manuscript: Lee GY, Lee YK. Critical revision: Lee JW, Kang HS. Final approval of manuscript and submission: all authors.
References
- 1.Bedi A, Kelly BT. Femoroacetabular impingement. J Bone Joint Surg Am. 2013;95:82–92. doi: 10.2106/JBJS.K.01219. [DOI] [PubMed] [Google Scholar]
- 2.Imam S, Khanduja V. Current concepts in the diagnosis and management of femoroacetabular impingement. Int Orthop. 2011;35:1427–1435. doi: 10.1007/s00264-011-1278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sink EL, Kim YJ. Femoroacetabular impingement: current clinical evidence. J Pediatr Orthop. 2012;32(Suppl 2):S166–71. doi: 10.1097/BPO.0b013e318259f30d. [DOI] [PubMed] [Google Scholar]
- 4.Tijssen M, van Cingel R, Willemsen L, de Visser E. Diagnostics of femoroacetabular impingement and labral pathology of the hip: a systematic review of the accuracy and validity of physical tests. Arthroscopy. 2012;28:860–871. doi: 10.1016/j.arthro.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy AA, Rosenfeld SB. Orthopedic perspectives on femoroacetabular impingement. Pediatr Radiol. 2013;43(Suppl 1):S83–9. doi: 10.1007/s00247-012-2580-2. [DOI] [PubMed] [Google Scholar]
- 6.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 7.Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23:1295–1302. doi: 10.1016/j.arthro.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25:299–308. doi: 10.1016/j.csm.2005.12.006. [ix.] [DOI] [PubMed] [Google Scholar]
- 9.Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement in athletes. Am J Sports Med. 2011;39(Suppl):7S–13S. doi: 10.1177/0363546511404144. [DOI] [PubMed] [Google Scholar]
- 10.Ilizaliturri VM, Jr, Orozco-Rodriguez L, Acosta-Rodríguez E, Camacho-Galindo J. Arthroscopic treatment of cam-type femoroacetabular impingement: preliminary report at 2 years minimum follow-up. J Arthroplasty. 2008;23:226–234. doi: 10.1016/j.arth.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369–376. doi: 10.1016/j.arthro.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 13.Abate M, Scuccimarra T, Vanni D, Pantalone A, Salini V. Femoroacetabular impingement: is hyaluronic acid effective? Knee Surg Sports Traumatol Arthrosc. 2014;22:889–892. doi: 10.1007/s00167-013-2581-1. [DOI] [PubMed] [Google Scholar]
- 14.Ayeni OR, Farrokhyar F, Crouch S, Chan K, Sprague S, Bhandari M. Pre-operative intra-articular hip injection as a predictor of short-term outcome following arthroscopic management of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2014;22:801–805. doi: 10.1007/s00167-014-2883-y. [DOI] [PubMed] [Google Scholar]
- 15.Kivlan BR, Martin RL, Sekiya JK. Response to diagnostic injection in patients with femoroacetabular impingement, labral tears, chondral lesions, and extra-articular pathology. Arthroscopy. 2011;27:619–627. doi: 10.1016/j.arthro.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Krych AJ, Griffith TB, Hudgens JL, Kuzma SA, Sierra RJ, Levy BA. Limited therapeutic benefits of intra-articular cortisone injection for patients with femoro-acetabular impingement and labral tear. Knee Surg Sports Traumatol Arthrosc. 2014;22:750–755. doi: 10.1007/s00167-014-2862-3. [DOI] [PubMed] [Google Scholar]
- 17.Park JS, Jang YE, Nahm FS, Lee PB, Choi EJ. Efficacy of intra-articular steroid injection in patients with femoroacetabular impingement. Korean J Pain. 2013;26:154–159. doi: 10.3344/kjp.2013.26.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5:418–426. doi: 10.1016/j.pmrj.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Abate M, Pulcini D, Di Iorio A, Schiavone C. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des. 2010;16:631–640. doi: 10.2174/138161210790883859. [DOI] [PubMed] [Google Scholar]
- 20.Deshmukh AJ, Panagopoulos G, Alizadeh A, Rodriguez JA, Klein DA. Intra-articular hip injection: does pain relief correlate with radiographic severity of osteoarthritis? Skeletal Radiol. 2011;40:1449–1454. doi: 10.1007/s00256-011-1120-8. [DOI] [PubMed] [Google Scholar]
- 21.Kruse DW. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr Rev Musculoskelet Med. 2008;1:227–233. doi: 10.1007/s12178-008-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micu MC, Bogdan GD, Fodor D. Steroid injection for hip osteoarthritis: efficacy under ultrasound guidance. Rheumatology (Oxford) 2010;49:1490–1494. doi: 10.1093/rheumatology/keq030. [DOI] [PubMed] [Google Scholar]
- 23.Migliore A, Massafra U, Bizzi E, Lagana B, Germano V, Piscitelli P, Granata M, Tormenta S. Intra-articular injection of hyaluronic acid (MW 1,500-2,000 kDa; HyalOne) in symptomatic osteoarthritis of the hip: a prospective cohort study. Arch Orthop Trauma Surg. 2011;131:1677–1685. doi: 10.1007/s00402-011-1353-y. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer AI, Bockow BI, Brander VA, Yates JW, MacCarter DK, Gudger GK, Haller S, Lake SL, Magilavy DB. Hylan G-F 20 improves hip osteoarthritis: a prospective, randomized study. Phys Sportsmed. 2010;38:35–47. doi: 10.3810/psm.2010.06.1781. [DOI] [PubMed] [Google Scholar]