Abstract
Background and Purpose
Asthma presents as a heterogeneous syndrome characterized by airway obstruction, inflammation and hyper‐reactivity (AHR). Spleen tyrosine kinase (Syk) mediates allergen‐induced mast cell degranulation, a central component of allergen‐induced inflammation and AHR. However, the role of Syk in IgE‐mediated constriction of human small airways remains unknown. In this study, we addressed whether selective inhibition of Syk attenuates IgE‐mediated constriction and mast cell mediator release in human small airways.
Experimental Approach
Human precision cut lung slices (hPCLS) ex vivo derived from non‐asthmatic donors were incubated overnight with human IgE, dexamethasone, montelukast, antihistamines or a selective Syk inhibitor (SYKi). High‐affinity IgE receptor (FcεRI) activation by anti‐IgE cross‐linking was performed, and constriction and mediator release measured. Airway constriction was normalized to that induced by maximal carbachol stimulation. Syk expression (determined by qPCR and immunoblot) was also evaluated in human primary airway smooth muscle (HASM) cells to determine whether Syk directly modulates HASM function.
Key Results
While dexamethasone had little effect on FcεR‐mediated contraction, montelukast or antihistamines partially attenuated the response. SYKi abolished anti‐IgE‐mediated contraction and suppressed the release of mast cell or basophil mediators from the IgE‐treated hPCLS. In contrast, SYKi had little effect on the non‐allergic contraction induced by carbachol. Syk mRNA and protein were undetectable in HASM cells.
Conclusions and Implications
A selective Syk inhibitor, but not corticosteroids, abolished FcεR‐mediated contraction in human small airways ex vivo. The mechanism involved FcεRI receptor activation on mast cells or basophils that degranulate causing airway constriction, rather than direct actions on HASM.
Abbreviations
- CysLTs
cysteinyl LTs
- HASM
human airway smooth muscle
- hPCLS
human precision cut lung slices
- Syk
spleen tyrosine kinase
- SYKi
Syk inhibitor
Tables of Links
| TARGETS |
|---|
| Enzymes a |
| Syk, spleen tyrosine kinase |
| Nuclear Hormone Receptors b |
| Glucocorticoid receptor, NR3C1 |
| GPCRs c |
| CysLT1 receptor |
| Histamine H1 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b ,cAlexander et al., 2015a,b,c).
Introduction
Asthma, a disorder characterized by airway inflammation and hyper‐responsiveness, contributes to substantial morbidity and mortality worldwide (Vijverberg et al., 2013). Mast cells play a pivotal role in modulation of airway inflammation and bronchoconstriction in allergic asthma (Reuter et al., 2010). Current treatments directed towards the actions of mast cells includes anti‐IgE therapy, histamine and LT receptor antagonists. Whether corticosteroids directly modulate human mast cell function remains controversial (Croxtall et al., 2000; Liu et al., 2007; Zhou et al., 2008). Although these therapeutic approaches are effective in asthma, they are limited by heterogeneity of response, cost and adverse effects. Mast cells may also play an important role in promoting a severe persistent asthma phenotype because omalizumab, a monoclonal antibody that binds selectively to IgE to mitigate mast cell and basophil activation, improves clinical outcomes in these patients. Overall, targeting the mast cell and basophils through novel signalling pathways and the use of small molecule inhibitors may offer a unique therapeutic advantage in improving asthma management.
Spleen tyrosine kinase (Syk), a member of the ZAP70 family of non‐receptor protein kinases, mediates downstream signalling of immunoreceptor tyrosine‐based activation motif (ITAM)‐associated receptors (Mocsai et al., 2010). ITAM‐associated receptors, predominantly found on haematopoietic cells, modulate innate immunity, co‐activation of inflammatory cells and effector/adaptive immune responses. Evidence suggests that cross‐linking at the high‐affinity IgE receptor (FcεRI) activates Syk and that Syk is necessary and sufficient to mediate mast cell and basophil activation and de novo synthesis of eicosanoids, chemokines and cytokines (Gilfillan and Tkaczyk, 2006).
Inhibition of Syk attenuates FcεR‐mediated mast cell degranulation and allergen‐induced airway inflammation (Matsubara et al., 2006b). The Syk inhibitor (SYKi) R406 inhibits allergic airway responses in mice sensitized to ovalbumin (Matsubara et al., 2006a, b). Further, antisense oligonucleotides and, more recently, siRNA also attenuated allergen‐induced rodent responses (Stenton et al., 2002; Huang et al., 2013). Our data in rats and non‐human primates show that inhibition of Syk abrogated FcεR‐mediated tracheal extravasation, allergen‐induced airway inflammatory cell trafficking and bronchoconstriction (Moy et al., 2013). Given species difference in mast cell function and in allergen responses, the role of Syk in modulating human small airway function remains unclear. Using a low MW inhibitor for Syk (Moy et al., 2013), we investigated whether Syk inhibition modulated Fcε‐dependent airway constriction and mediator release in human precision cut lung slices (hPCLS). Here, we have demonstrated that Syk inhibition abolished FcεR‐mediated contraction while profoundly decreasing mediator release. These data suggest that Syk inhibition may modulate human small airway function in asthma.
Methods
Use of human tissue
The human tissues used here (trachea and whole lung) were obtained from the National Disease Research Interchange (Philadelphia, PA, USA) and from the International Institute for the Advancement of Medicine (Edison, NJ, USA). All tissue was de‐identified. Our Institutional IRB follows the NIH guidelines that de‐identified tissue is exempt from requiring consents and is not considered human subject research. Tissues were derived, post mortem, from non‐asthmatic and non‐smoker subjects. The demographics of the donors of the whole lungs are shown in Supplemental Table 1 and for the tracheas in Supplemental Table 2.
Isolation and culture of human airway smooth muscle
Human airway smooth muscle (HASM) cells were derived from tracheas, within 24 hr of excision from the donor as described previously (Panettieri et al., 1989). The cells were cultured in Ham's F‐12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 100 U·mL−1 penicillin, 0.1 mg·mL−1 streptomycin and 2.5 mg·mL−1 amphotericin B, and this medium was replaced every 72 h. HASM cells in subculture for up to five passages, were used, because these cells retain the expression of native contractile protein, as demonstrated by immunocytochemical staining for smooth muscle actin and myosin (Panettieri et al., 1989). The RAMOS cell line (2G6.4C10, catalog#CRL‐1923, from ATCC, Manassas, VA) were cultured in RPMI1640 with 10% vol/vol FBS. Cells were maintained between 0.3 and 1x106 cells mL‐1 in suspension culture.
Generation of precision cut lung slices and airway constriction assays
The hPCLS were prepared as previously described (Cooper et al., 2009; Cooper et al., 2011). Briefly, whole human lungs from non‐asthmatic donors were dissected and inflated using 2% (w/v) low melting point agarose (Lonza, Allendale, NJ). Once the agarose had set, the lobe was sectioned and cores of 8 mm diameter were made. The cores that contained a small airway by visual inspection were sliced at a thickness of 250 μm (Precisionary Instruments VF300 Vibratome, Greenville, NC, USA) and collected in wells containing supplemented Ham's F‐12 medium. Suitable airways (≤1 mm diameter) on slices were selected on the basis of the following criteria: presence of a full smooth muscle wall, presence of beating cilia and unshared muscle walls at airway branch points to eliminate possible counteracting contractile forces. Each slice contained ∼98% parenchyma tissue; hence, all airways situated on a slice had sufficient parenchymal tissue to impart basal tone. Adjacent slices containing contiguous segments of the same airway were paired and served as controls and were incubated at 37°C in a humidified air‐CO2 (95–5%) incubator. Sections were placed in fresh media every 2–3 h. during the remainder of day 1 and all of day 2 to remove agarose and endogenous substances released that variably confound the production of inflammatory mediators and/or alter airway tone (Cooper et al., 2009; Cooper et al., 2011). In some experiments, slices were pre‐incubated with antibody to human IgE (4 μg·mL−1; Calbiochem, Billerica, MA) for 18 h and then incubated in the presence or absence of the SYKi (R406; 10–1000 nM; (1S,4R)‐4‐hydroxy‐2,2,dimethyl‐4‐(5‐((4‐methylpryimidin‐2‐yl)amino)phenyl)thiazol‐2‐yl)cyclohexane‐1‐carboxylic acid; Supporting Information Fig. S2) for 20 min prior to assessment of contraction. A time course was performed measuring luminal area every 30 s for 10 min, or until no further constriction was observed following the administration of anti‐IgE (20 μg·mL−1). Slices were incubated for an additional 20 min, and luminal area was assessed at 30 min following FcεR cross‐linking, and culture supernatants were assessed for histamine and cysteinyl LT (CysLT) levels. To assess maximum contraction and assess specificity of the SYKi, carbachol (Cch; 100 μM) was added and the percentage of airway occlusion measured and compared with baseline.
To assess luminal area, lung slices were placed in a 12‐well plate in media and held in place using a platinum weight with nylon attachments. The airway was located using a microscope (Nikon Eclipse; model no. TE2000‐U; magnification, ×40) connected to a live video feed (Evolution QEi; model no. 32‐0074A‐130 video recorder). Airway luminal area was measured using image‐pro plus software (version 6.0; Media Cybernetics, Rockville, Maryland, USA) and presented in units of μm2 (Cooper et al., 2009; Cooper et al., 2011). After functional studies, the area of each airway at baseline and at the end of the time course or dose of agonist was calculated using image‐pro plus software. A maximum drug effect (E max) value for each airway was derived from a concentration–response curve.
Histamine and lipid analysis
Mediator release was evaluated in human lung slices according to Moy et al. (2013). The supernatant from each well was collected after the constriction recording. CysLT (Enzo Life Sciences, Farmingdale, NY, USA) and histamine (Cisbio Assays, Bedford, MA, USA) levels were evaluated by ELISA or homogeneous time resolved fluorescence respectively.
Determination of expression of SYK in HASM
We obtained RNA‐Seq results for transcripts of SYK, using the methods described in a previously published study (Himes et al., 2015). Briefly, primary ASM cells were isolated from twelve white, non‐smoking donors with no chronic illness or medication use. The ASM cell culture has been described previously (Panettieri et al., 1989; Cooper et al., 2010). Passages 4 to 7 HASM cells maintained in Ham's F12 medium supplemented with 10% FBS, CaCl2, buffered with HEPES, penicillin/streptomycin, primocin, and additional L‐glutamine were used in all experiments. The F12 medium was used for culture because it provides Ca2+ levels that are consistent with observing contractility of muscles in that media. Total RNA was extracted from cells using the miRNAeasy mini kit (Qiagen Sciences, Inc., Germantown, MD). Approximately 1 μg of RNA from each sample was used to generate RNA‐Seq cDNA libraries for sequencing using the TruSeq RNA Sample Prep Kit v2 (Illumina, Inc., San Diego, CA). Sequencing of 75 bp paired‐end reads was performed with an Illumina HiSeq 2000 instrument at Partners Personalized Medicine (Boston, MA). Taffeta scripts (https://github.com/blancahimes/taffeta) were used to analyze RNA‐Seq data, which included trimming of adapters using trimmomatic (v.0.32) (Bolger et al., 2014) and using FastQC (Andrews et al.,) (v.0.11.2) to obtain overall QC metrics. Trimmed reads for each sample were used to estimate transcript counts with Kallisto software and the hg38 human genome as reference (Bray et al., 2016). The RNA‐Seq data is available at the Gene Expression Omnibus Web site (http://www.ncbi.nlm.nih.gov/geo/) under accession GSE58434.
PCR and immunoblot analysis
Non‐asthmatic HASM (passage 3‐4) cells were stimulated overnight with TNFα (10 ng mL‐1), IFNγ (1000 U), or TNFα+IFNγ (Roche Life Sciences, Indianapolis, IN). Total RNA was purified from ASM cells and RAMOS cells using the RNAeasy kit (Qiagen, Gaithersburg, MD) and 550 ng of RNA was reverse transcribed using iScript reverse transcriptase (Biorad, Hercules, CA). Quantitative transcript analysis was carried out using Taqman assays (Invitrogen, Grand Island, NY) for Syk (Hs00895384_m1), and GAPDH (Hs99999905_m1) on an ABI7900 quantitative PCR machine, and relative gene expression determined using ΔΔCT analysis. Whole cell lysates from HASM and RAMOS cells were generated using RIPA buffer. 70 μg of total protein was loaded into Criterion gels (Biorad). Gels were transferred to nitrocellulose via the iBlot system (Invitrogen), blocked, immunoblotted for Syk (Santa Cruz, Dallas, TX), and imaged and analyzed using the Odyssey Imager and software (LI‐COR, Lincoln, NE).
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Results are presented as means ± SEM, unless otherwise indicated. graphpad prism software was used to determine statistical significance evaluated by a paired Student's t‐test for two groups or one way ANOVA (with Bonferroni's post test) for multiple groups. P‐values of <0.05 were considered significant.
Materials
The drugs used were provided as follows: carbachol (carbamoyl choline), dexamethasone, fexofenadine, and histamine from Sigma Aldrich, St. Louis, MO; SYKi (R406) was a kind gift from Merck, ; montelukast from Cayman Chemical Company (Ann Arbor, MI); C5a from Complement Technologies (Tyler, TX).
Results
SYKi inhibits FcεR‐dependent airway constriction in normal hPCLS
To address whether Syk inhibition modulated FcεR‐dependent contraction, hPCLS were prepared from nine non‐asthmatic donors. All donors manifested no chronic illnesses, as shown in Supporting Information Table S1.
We and others showed that in rodent models, Syk inhibition attenuated allergen‐induced airway hyper‐responsiveness and inflammation (Moy et al., 2013). Here, as shown in Figure 1 , Syk inhibition attenuated FcεR‐dependent contraction in hPCLS, dose‐dependently, and nearly completely abrogated the response at 1 μM concentration. To address whether SYKi inhibition ws maintained over time, experiments were performed to examine SYKi effects over 30 min. As shown in Figure 1B, SYKi (1 μM) sustained complete blockade of FcεR‐dependent contraction, whereas the 300 nM concentration was less efficacious. At 100 nM, 300 nM and 1 μM, FcεR‐induced airway constriction was also diminished as compared with 10 nM or no SYKi, as shown in Figure 1C. To characterize whether SYKi affected non‐mast cell‐dependent contraction, hPCLS were treated with SYKi and carbachol‐induced contraction was measured. As shown in Figure 1D, SYKi had no effect on carbachol‐induced contraction.
Figure 1.
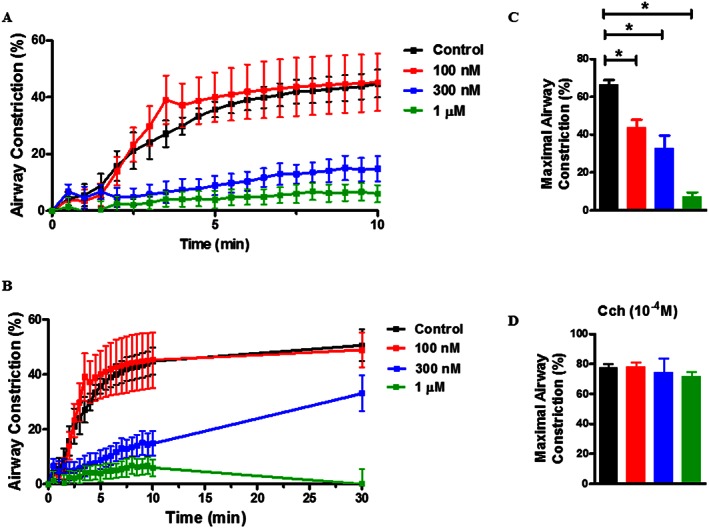
SYKi attenuates FcεR cross‐linking‐induced airway constriction but does not attenuate carbachol‐induced contraction in hPCLS. (A) Representative time course of airway constriction to FcεR cross‐linking assessed in the absence/presence of SYKi (0.1, 0.3, 1 μM) over a course of 10 min. Constriction was measured compared with baseline luminal area and expressed as % airway constriction. (B) Representative plot of inhibition measured out to 30 min following cross‐linking. (C) Maximal constriction following FcεR cross‐linking was compared. (D) Following cross‐linking, hPCLS were stimulated with carbachol (Cch; 100 μM) and % airway constriction measured. Data shown are means ± SEM and are from nine individual donors. * P ≤ 0.05, significantly different as indicated.
A SYKi, a CysLT inhibitor and an antihistamine modulate FcεR‐dependent airway constriction
To compare efficacy of SYKi, antihistamines and LT modifiers on FcεR‐dependent airway constriction, hPCLS were treated with SYKi, montelukast, fexofenadine or dexamethasone (Figure 2). The doses of the inhibitors chosen are those derived from previous studies that demonstrated maximum target inhibition with limited off‐target effects (Moy et al., 2013). SYKi (1 μM) markedly inhibited FcεR‐dependent contraction. Fexofenadine and montelukast partially inhibited FcεR‐induced contraction. The relative efficacy in the compounds inhibiting FcεR‐dependent bronchoconstriction was represented as I max (maximal inhibition) as shown in Figure 2, panel B. Montelukast, fexofenadine and SYKi significantly impaired FcεR‐induced airway constriction whereas dexamethasone had little effect. Collectively, these data suggest that inhibition of Syk has a greater effect on FcεR‐dependent contraction in human airways than treatment with montelukast, fexofenadine or dexamethasone. Because SYKi profoundly affected FcεR‐dependent constriction, and because Syk and Lyn expression has been described in cultured HASM cells (Gounni et al., 2005; Redhu et al., 2009; Redhu et al., 2011; Balhara et al., 2014), we next tested the effects of SYKi on HASM cell contraction. Accordingly, we assessed Syk expression in HASM cells and RAMOS cells. Total mRNA was prepared from cultured HASM cells and RAMOS cells (a B lymphocyte cell line), and quantitative RT‐PCR was performed. Lysates were also generated from these cells following treatment with control buffer, TNFα, IFNγ or TNFα/IFNγ for immunoblot analysis. As shown in Supporting Information Fig. S1, the human RAMOS cells robustly expressed Syk total mRNA (1A) and protein (1B) whereas HASM cells showed no expression at either the transcriptional or translational level. These data are consistent with our observations that SYKi has little effect on carbachol‐induced contraction and that the effects of SYKi, as demonstrated in hPCLS, are more likely to be mediated through mast cells or basophils found in the submucosa of the hPCLS.
Figure 2.
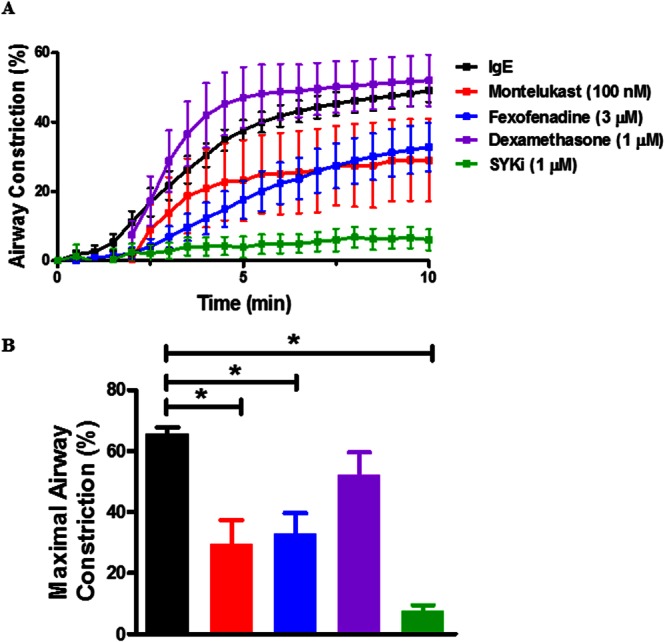
SYKi markedly attenuates FcεR cross‐linking‐induced airway constriction in comparison with fexofenadine, montelukast and dexamethasone. (A) The effects of montelukast (10 μM), fexofenadine (3 μM) and dexamethasone (1 μM) were compared with that of SYKi (1 μM) in four donors. (B) Maximal response following FcεR cross‐linking in the presence of inhibitors was compared (I max). Data shown are means SEM and are from five to eight individual donors. * P ≤ 0.05, significantly different as indicated; ANOVA, (P < 0.0001).
SYKi effects on FcεR‐dependent histamine and CysLT secretion
To characterize whether SYKi modulates FcεR‐dependent release of histamine and CysLT, slices were incubated with varying concentrations of SYKi. As shown in Figure 3A/B, pretreatment with SYKi profoundly decreased histamine levels in the supernatant after FcεR cross‐linking. We also examined CysLT release following FcεR cross‐linking. In Figure 3C, we show that SYKi pretreatment with SYKi, but not with montelukast, dose‐dependently decreased FcεR‐dependent release of CysLT from hPCLS. These data suggest that SYKi that abolished FcεR‐dependent airway constriction at doses similar to those at which it markedly diminished CysLT and histamine levels, that are likely to be due to mast cell degranulation.
Figure 3.
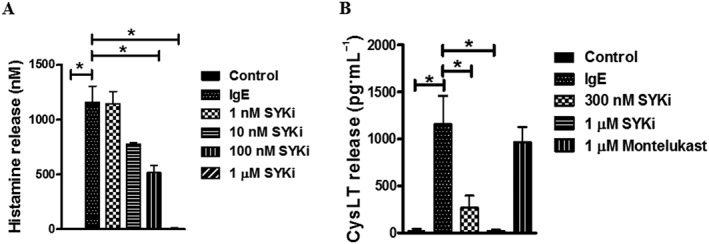
SYKi attenuates histamine and LT secretion in hPCLS. (A) SYKi (1 nM–1 μM) or montelukast (1 μM) were incubated with hPCLS 1 h prior to FcεR cross‐linking. Supernatants were collected at 30 min after cross‐linking, and histamine release measured by homogeneous time resolved fluorescence (ANOVA P = 0.007). (B) LT release in the presence/absence of SYKi (300 nM, 1 μM) or montelukast (1 μM) was measured by ELISA (ANOVA P = 0.0002). Data shown are means SEM and are from lung slices from four to seven donors for each treatment. * P ≤ 0.05, significantly different as indicated.
Discussion
Allergic asthma, a chronic respiratory disorder marked by inflammation, recurrent reversible airflow obstruction and airway hyper‐responsiveness, remains a significant cause of morbidity and mortality worldwide. Despite the use of corticosteroids, long‐acting bronchodilators and LT modifiers, there exists an unmet need to block mast cell function in the allergic subset of this disease. Syk, a kinase that mediates much of the signalling downstream of the high‐affinity FcεRI, plays a key role in activation and degranulation of mast cells and basophils. Some lowMW kinase inhibitors have been developed to inhibit this kinase and have shown efficacy in human trials of allergic rhinitis. Few studies have addressed the value of this therapeutic approach in allergic asthma. The current study demonstrates specificity of SYKi in attenuation of FcεR cross‐linking‐induced bronchoconstriction and release of inflammatory mediators from hPCLS. We also show data to suggest that the target of SYKi is most likely to be mast cells and basophils rather than the HASM, as indicated by expression patterns in the primary human cells we tested.
Current treatments targeting mast cells or products of mast cell activation and degranulation are effective in some, but not all patients, or have been contraindicated as treatment for asthma. In the past, second generation antihistamines showed improvements in symptom scores and β2 agonist use, compared with placebo, in asthma. However, the therapeutic doses that were most effective were associated with adverse effects including fatigue and drowsiness (Grant et al., 1995; Malick and Grant, 1997; Larsen, 2001). Therefore, use of antihistamines is not considered a first line treatment for asthma. We used fexofenadine, a second generation antihistamine, as a comparator to attenuate airway constriction, induced by FcεR cross‐linking, that provided findings consistent with our previous work (Cooper et al., 2009; Cooper et al., 2011). Use of montelukast, an LT receptor antagonist, is effective in a subset of patients with asthma. A recent review of paediatric clinical studies (Massingham et al., 2014) showed that montelukast, compared with inhaled corticosteroids (ICSs), showed comparable improvement in asthma symptom scores but that improvement in pulmonary function tests favoured ICS treatment over montelukast, and the risk of asthma exacerbation was greater in the montelukast treatment group. Most studies have concluded that first‐line treatment of asthma in a paediatric patient population should be ICS, but that in a subset of that population montelukast treatment may be effective, as outlined in the Expert Panel Report 3 of the Guidelines for the Diagnosis and Management of Asthma. Given the heterogeneity of the response of asthma patients to LT modifiers, a therapy with broader effects may be more useful in the treatment of allergic asthma. Again we used montelukast as an industry standard in a model of FcεR cross‐linking‐induced contraction. We found that montelukast partly attenuated airway constriction to FcεR cross‐linking that was not as effective as SYKi‐induced ablation of contraction. Use of steroids in asthma therapy has been a mainstay for many years. Inhaled steroid therapy improved bronchodilator responses to β2 agonists, decreased inflammatory cell influx when used as maintenance therapy and modulated inflammatory mediator elaboration from airway cells (Chung et al., 2009). Experimental evidence in human basophils suggests that glucocorticoid treatment of the cells prior to FcεR cross‐linking attenuated not only IL‐4 release but histamine release (Schleimer et al., 1981; Schroeder et al., 1997). In a study using purified lung mast cells, however, dexamethasone had little effect on release of PGD2, PGF2α, PGE2, LTs and histamine with anti‐IgE treatment. Surprisingly, TxB2 was the only mediator modulated by dexamethasone treatment given prior to the FcεR cross‐linking (Schleimer et al., 1983). We used dexamethasone to assess steroid effects on FcεR cross‐linking‐induced contraction and found that dexamethasone had little effect on this response, in our model. Accordingly, our data suggest that bronchoconstriction predominantly occurs with activation of mast cells and basophils that then release of a range of contractile agonists. Our data show that the SYKi inhibited not only FcεR cross‐linking‐induced contraction, but also the release of LTs and histamine. Syk inhibition provides a target to inhibit several allergen‐triggered events, bronchconstriction following cross‐linking of FcεRI, elaboration of inflammatory mediators involved in modulation of contractile responses and perpetuation of inflammation associated with allergic asthma. Targeting Syk, a kinase proximal to the receptor, conveys therapeutic advantage over other approaches that are currently used in to alleviate symptoms of allergic asthma.
Given the inhibition that we observed in the hPCLS system, we sought to identify the potential cellular target of SYKi. Others have shown that primary HASM express FcεRI, and upon incubation with IgE and subsequent stimulation with anti‐IgE release IL‐4, IL‐5, IL‐13 and eotaxin and that cross‐linking of FcεRI evokes in intracellular calcium flux consistent with contraction of the muscle (Gounni et al., 2005). In bronchial smooth muscle derived post mortem from normal patients, attenuation of Syk expression with a lentiviral vector decreased promoter activity at the IL‐8, IP‐10, RANTES and eotaxin promoters in response to FcεR cross‐linking (Redhu et al., 2009). To assess whether HASM was a potential target for SYKi, we examined Syk expression at both the mRNA and protein levels in primary HASM. In contrast to the findings from Redhu et al., we failed to show any basal levels or TNFα‐stimulated expression of mRNA or protein for Syk. RAMOS cells served as a positive control for both mRNA and protein expression. A potential reason for this discrepancy could be the sources of HASM that we used compared with the previous studies. We, however, have shown in primary human mast cells, the expression of Syk (Moy et al., 2013), suggesting that the inhibition of airway constriction that is observed following treatment of the tissue with SYKi is likely to be due to activation of mast cells and not a direct effect on HASM. Addititionally, Wang et al. (2015) demonstrated that genetic ablation of Syk attenuated airway hyper‐responsiveness following ovalbumin challenge and exposure to PM2.5 and ozone, but had little to no effect on methacholine‐induced contractility of the lungs in vivo and in the PCLS. Here, we demonstrated that SYKi had little effect on methacholine‐, histamine‐ and KCl‐induced bronchoconstriction in hPCLS (Supporting Information Fig. S3 A & B, KCl – data not shown). Additionally, we showed that SYKi has little effect on C5a peptide‐induced airway constriction (Supporting Information Fig. S3 C). Together, these data suggest a selective effect of SYKi on the contraction induced by FcεRI cross‐linking.
The hPCLS system that we utilized for the functional studies examining FcεR cross‐linking‐induced airway constriction has made use of a passive sensitization paradigm of FcεR sensitization (Cooper et al., 2009; Cooper et al., 2011). Despite the limitations of this model, the hPCLS provide a novel model of integrated structural cell function, devoid of trafficking leukocytes that are likely to contribute to the airway inflammatory response. Given this limitation, the activation of contraction in response to this stimulation is dependent upon resident immune cells, namely, mast cells. Despite the dose‐ and time‐dependent inhibition of Syk in this system, the specific effects of SYKi‐mediated Syk inhibition on mast cell function are difficult to assess in this model.
Our data support Syk inhibition as a novel therapeutic approach to prevent IgE‐FcεRI‐mediated airway constriction and mediator release from some of the cells in the airways. Further studies are needed to determine whether Syk inhibition offers therapeutic advantages over current state‐of‐the‐art therapy in asthma.
Author contributions
C.J.K.‐W. wrote the manuscript. C.J.K.‐W., Y.J., G.B., P.C., S.A. and R.A.P. Jr. contributed to experimental design. C.J.K.‐W., Y.J., G.B., P.C., D.Z., M.C., E.S. and S.S. performed experiments and analysed data, together with B.E.H. Y.J., G.B.,S.A. and R.A.P. Jr. edited the manuscript. A.N. synthesized compound.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Demographics of hPCLS donors.
Table S2 Demographics of trachea donors; source of HASM.
Figure S1 Syk is expressed in RAMOS cells but not in HASM. HASM were incubated in the presence or absence of TNFα (50 ng·mL−1), IFNγ (1000 U), or the combination of TNFα and IFNγ. Both mRNA (exposure time – A) and protein (overnight ‐ B) expression were assessed and compared with RAMOS cells, which are known to express high levels of Syk. (C) Expression of syk in HASM as assessed by RNAseq. Data are representative of 6 individual donors, with bars representing mean + SEM.
Figure S2 Chemical structure and IUPAC name for SYKi.
Figure S3 Effect of SYKi on (A) methacholine‐, (B) histamine‐, and (C) C5a‐induced constriction of human small airways. Data in (A) and (B) are represented as % of KCl‐induced airway constriction, where SYKi no effect on KCl‐induced constriction.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This study was funded by Merck Research Laboratories.
Koziol‐White, C. J. , Jia, Y. , Baltus, G. A. , Cooper, P. R. , Zaller, D. M. , Crackower, M. A. , Sirkowski, E. E. , Smock, S. , Northrup, A. B. , Himes, B. E. , Alves, S. E. , and Panettieri, R. A. Jr (2016) Inhibition of spleen tyrosine kinase attenuates IgE‐mediated airway contraction and mediator release in human precision cut lung slices. British Journal of Pharmacology, 173: 3080–3087. doi: 10.1111/bph.13550.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. FastQC A Quality Control tool for High Throughput Sequence Data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Balhara J, Redhu NS, Shan L, Gounni AS (2014). IgE regulates the expression of smMLCK in human airway smooth muscle cells. PLoS One 9: e93946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics Epub 2014/04/04. doi:10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L (2016). Near‐optimal probabilistic RNA‐seq quantification. Nat Biotechnol. 34: 525–527. [DOI] [PubMed] [Google Scholar]
- Chung KF, Caramori G, Adcock IM (2009). Inhaled corticosteroids as combination therapy with beta‐adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol 65: 853–871. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Mesaros AC, Zhang J, Christmas P, Stark CM, Douaidy K et al (2010). 20‐HETE mediates ozone‐induced, neutrophil‐independent airway hyper‐responsiveness in mice. PLoS One 5: e10235. Epub 2010/04/28. doi: 10.1371/journal.pone.0010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L et al. (2009). TLR3 activation stimulates cytokine secretion without altering agonist‐induced human small airway contraction or relaxation. Am J Physiol 297: L530–L537. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Zhang J, Damera G, Hoshi T, Zopf DA, Panettieri RA Jr (2011). C‐027 inhibits IgE‐mediated passive sensitization bronchoconstriction and acts as a histamine and serotonin antagonist in human airways. Allergy Asthma Proc 32: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ (2000). Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor‐dependent, transcription‐independent mechanism. Br J Pharmacol 130: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C (2006). Integrated signalling pathways for mast‐cell activation. Nat Rev Immunol 6: 218–230. [DOI] [PubMed] [Google Scholar]
- Gounni AS, Wellemans V, Yang J, Bellesort F, Kassiri K, Gangloff S et al. (2005). Human airway smooth muscle cells express the high affinity receptor for IgE (Fc epsilon RI): a critical role of Fc epsilon RI in human airway smooth muscle cell function. J Immunol 175: 2613–2621. [DOI] [PubMed] [Google Scholar]
- Grant JA, Nicodemus CF, Findlay SR, Glovsky MM, Grossman J, Kaiser H et al. (1995). Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo‐controlled trial. J Allergy Clin Immunol 95: 923–932. [DOI] [PubMed] [Google Scholar]
- Himes BE, Koziol‐White C, Johnson M, Nikolos C, Jester W, Klanderman B, et al (2015) Vitamin D Modulates Expression of the Airway Smooth Muscle Transcriptome in Fatal Asthma. PLoS One. 10:e0134057. doi: 10.1371/journal.pone.0134057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZY, Kim MK, Kim‐Han TH, Indik ZK, Schreiber AD (2013). Effect of locally administered Syk siRNA on allergen‐induced arthritis and asthma. Mol Immunol 53: 52–59. [DOI] [PubMed] [Google Scholar]
- Larsen JS (2001). Do antihistamines have a role in asthma therapy? Pharmacotherapy 21: 28S–33S. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhou J, Zhang LD, Wang YX, Kang ZM, Chen YZ et al. (2007). Rapid inhibitory effect of corticosterone on histamine release from rat peritoneal mast cells. Horm Metab Res 39: 273–277. [DOI] [PubMed] [Google Scholar]
- Malick A, Grant JA (1997). Antihistamines in the treatment of asthma. Allergy 52: 55–66. [DOI] [PubMed] [Google Scholar]
- Massingham K, Fox S, Smaldone A (2014). Asthma therapy in pediatric patients: a systematic review of treatment with montelukast versus inhaled corticosteroids. J Pediatr Health Care 28: 51–62. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Koya T, Takeda K, Joetham A, Miyahara N, Pine P et al. (2006a). Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol 34: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Li G, Takeda K, Loader JE, Pine P, Masuda ES et al. (2006b). Inhibition of spleen tyrosine kinase prevents mast cell activation and airway hyperresponsiveness. Am J Respir Crit Care Med 173: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy LY, Jia Y, Caniga M, Lieber G, Gil M, Fernandez X et al. (2013). Inhibition of spleen tyrosine kinase attenuates allergen‐mediated airway constriction. Am J Respir Cell Mol Biol 49: 1085–1092. [DOI] [PubMed] [Google Scholar]
- Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI (1989). A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 256: C329–C335. [DOI] [PubMed] [Google Scholar]
- Redhu NS, Saleh A, Lee HC, Halayko AJ, Ziegler SF, Gounni AS (2011). IgE induces transcriptional regulation of thymic stromal lymphopoietin in human airway smooth muscle cells. J Allergy Clin Immunol 128: 892–896.e892. [DOI] [PubMed] [Google Scholar]
- Redhu NS, Saleh A, Shan L, Gerthoffer WT, Kung SK, Halayko AJ et al. (2009). Proinflammatory and Th2 cytokines regulate the high affinity IgE receptor (FcepsilonRI) and IgE‐dependant activation of human airway smooth muscle cells. PLoS One 4: e6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Stassen M, Taube C (2010). Mast cells in allergic asthma and beyond. Yonsei Med J 51: 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer RP, Lichtenstein LM, Gillespie E (1981). Inhibition of basophil histamine release by anti‐inflammatory steroids. Nature 292: 454–455. [DOI] [PubMed] [Google Scholar]
- Schleimer RP, Schulman ES, MacGlashan DW Jr, Peters SP, Hayes EC, Adams GK 3rd et al. (1983). Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest 71: 1830–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JT, MacGlashan DW Jr, MacDonald SM, Kagey‐Sobotka A, Lichtenstein LM (1997). Regulation of IgE‐dependent IL‐4 generation by human basophils treated with glucocorticoids. J Immunol 158: 5448–5454. [PubMed] [Google Scholar]
- Stenton GR, Ulanova M, Dery RE, Merani S, Kim MK, Gilchrist M et al. (2002). Inhibition of allergic inflammation in the airways using aerosolized antisense to Syk kinase. J Immunol 169: 1028–1036. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland‐van der Zee AH, Koenderman L (2013). Clinical utility of asthma biomarkers: from bench to bedside. Biologics 7: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Khanna N, Wu J, Godri Pollitt K, Evans GJ, Chow CW et al. (2015). Syk mediates airway contractility independent of leukocyte function. Allergy 70: 429–435. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu DF, Liu C, Kang ZM, Shen XH, Chen YZ et al. (2008). Glucocorticoids inhibit degranulation of mast cells in allergic asthma via nongenomic mechanism. Allergy 63: 1177–1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographics of hPCLS donors.
Table S2 Demographics of trachea donors; source of HASM.
Figure S1 Syk is expressed in RAMOS cells but not in HASM. HASM were incubated in the presence or absence of TNFα (50 ng·mL−1), IFNγ (1000 U), or the combination of TNFα and IFNγ. Both mRNA (exposure time – A) and protein (overnight ‐ B) expression were assessed and compared with RAMOS cells, which are known to express high levels of Syk. (C) Expression of syk in HASM as assessed by RNAseq. Data are representative of 6 individual donors, with bars representing mean + SEM.
Figure S2 Chemical structure and IUPAC name for SYKi.
Figure S3 Effect of SYKi on (A) methacholine‐, (B) histamine‐, and (C) C5a‐induced constriction of human small airways. Data in (A) and (B) are represented as % of KCl‐induced airway constriction, where SYKi no effect on KCl‐induced constriction.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


