Abstract
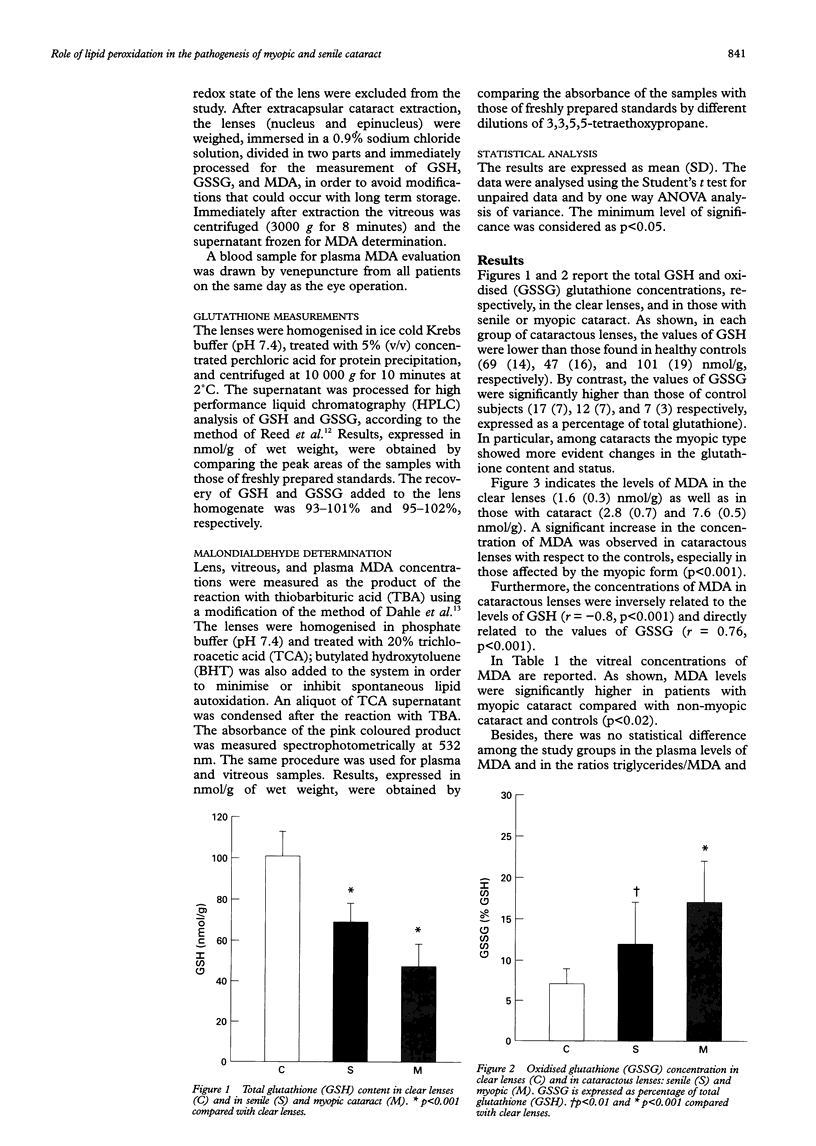
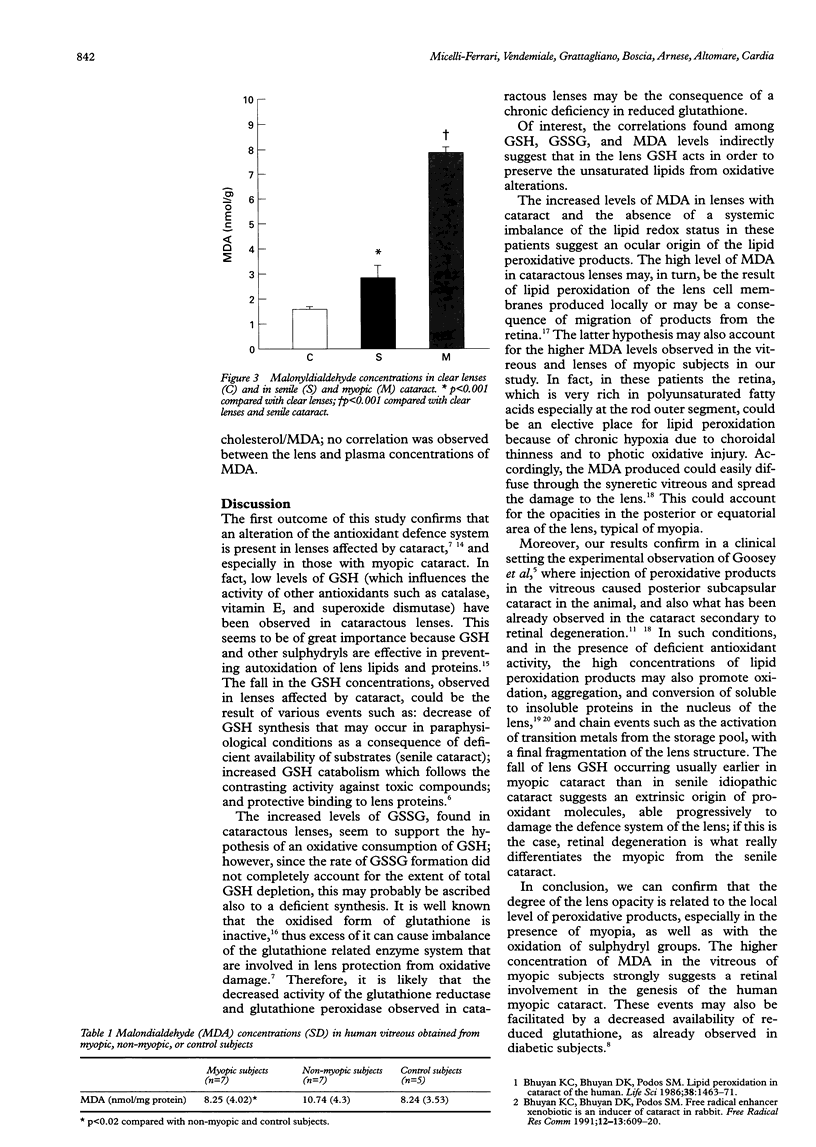
AIMS/BACKGROUND: Increased production of free radicals, consumption of antioxidant, and oxidation of unsaturated lipids have been observed recently in cataractous lenses and active participation of the retina in human cataractogenesis has been proposed. To verify this hypothesis, the total (GSH) and oxidised (GSSG) glutathione concentrations were assayed in the lens and the malondialdehyde (MDA) levels assayed in the vitreous and in the lens of normal controls and patients with senile or myopic cataract. METHODS: The study was conducted on 34 lenses (nucleus and epinucleus) (nine clear lenses, 14 lenses with idiopathic senile cataract, and 11 lenses affected by severe myopic cataract) and vitreous of 19 (seven non-myopic, seven myopic, and five control) subjects. Glutathione determination was performed following the method of Reed, while malondialdehyde was assayed using a modification of the method of Dahle. RESULTS: Cataractous lenses showed a decreased content of GSH and increased concentration of GSSG compared with clear lenses. A higher oxidative consumption of GSH was found in myopic cataracts compared with senile ones. Also, increased levels of MDA were observed both in cataractous lenses and in the vitreous of myopic patients compared with the control and the senile ones. CONCLUSION: The observed alterations strongly suggest that retinal lipid peroxidation might play a key role in human cataractogenesis, especially in the myopic type.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajiboye R., Harding J. J. The non-enzymic glycosylation of bovine lens proteins by glucosamine and its inhibition by aspirin, ibuprofen and glutathione. Exp Eye Res. 1989 Jul;49(1):31–41. doi: 10.1016/0014-4835(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Altomare E., Vendemiale G., Grattagliano I., Angelini P., Micelli-Ferrari T., Cardia L. Human diabetic cataract: role of lipid peroxidation. Diabete Metab. 1995 Jun;21(3):173–179. [PubMed] [Google Scholar]
- Babizhaev M. a., Arkhipenko Iu V., Kagan V. E. Aktivnost' antioksidantnykh fermentov i metabolizm perekisnykh soedinenii v khrustalike pri kataraktogeneze. Biull Eksp Biol Med. 1987 Feb;103(2):143–146. [PubMed] [Google Scholar]
- Bhuyan K. C., Bhuyan D. K., Podos S. M. Free radical enhancer xenobiotic is an inducer of cataract in rabbit. Free Radic Res Commun. 1991;12-13 Pt 2:609–620. doi: 10.3109/10715769109145837. [DOI] [PubMed] [Google Scholar]
- Bhuyan K. C., Bhuyan D. K., Podos S. M. Lipid peroxidation in cataract of the human. Life Sci. 1986 Apr 21;38(16):1463–1471. doi: 10.1016/0024-3205(86)90559-x. [DOI] [PubMed] [Google Scholar]
- Calvin H. I., Medvedovsky C., Worgul B. V. Near-total glutathione depletion and age-specific cataracts induced by buthionine sulfoximine in mice. Science. 1986 Aug 1;233(4763):553–555. doi: 10.1126/science.3726547. [DOI] [PubMed] [Google Scholar]
- DAHLE L. K., HILL E. G., HOLMAN R. T. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962 Aug;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- Fecondo J. V., Augusteyn R. C. Superoxide dismutase, catalase and glutathione peroxidase in the human cataractous lens. Exp Eye Res. 1983 Jan;36(1):15–23. doi: 10.1016/0014-4835(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Garland D., Zigler J. S., Jr, Kinoshita J. Structural changes in bovine lens crystallins induced by ascorbate, metal, and oxygen. Arch Biochem Biophys. 1986 Dec;251(2):771–776. doi: 10.1016/0003-9861(86)90389-9. [DOI] [PubMed] [Google Scholar]
- Goosey J. D., Tuan W. M., Garcia C. A. A lipid peroxidative mechanism for posterior subcapsular cataract formation in the rabbit: a possible model for cataract formation in tapetoretinal diseases. Invest Ophthalmol Vis Sci. 1984 May;25(5):608–612. [PubMed] [Google Scholar]
- Huang L. L., Zhang C. Y., Hess J. L., Bunce G. E. Biochemical changes and cataract formation in lenses from rats receiving multiple, low doses of sodium selenite. Exp Eye Res. 1992 Nov;55(5):671–678. doi: 10.1016/0014-4835(92)90172-o. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Rogers K. M., Augusteyn R. C. Glutathione reductase in normal and cataractous human lenses. Exp Eye Res. 1978 Dec;27(6):719–721. doi: 10.1016/0014-4835(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Simonelli F., Nesti A., Pensa M., Romano L., Savastano S., Rinaldi E., Auricchio G. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp Eye Res. 1989 Aug;49(2):181–187. doi: 10.1016/0014-4835(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Bodaness R. S., Gery I., Kinoshita J. H. Effects of lipid peroxidation products on the rat lens in organ culture: a possible mechanism of cataract initiation in retinal degenerative disease. Arch Biochem Biophys. 1983 Aug;225(1):149–156. doi: 10.1016/0003-9861(83)90018-8. [DOI] [PubMed] [Google Scholar]


