Abstract
The Akt signal transduction pathway controls most hallmarks of cancer. Activation of the Akt cascade promotes a malignant phenotype and is also widely implicated in drug resistance. Therefore, the modulation of Akt activity is regarded as an attractive strategy to enhance the efficacy of cancer therapy and irradiation. This pathway consists of phosphatidylinositol 3 kinase (PI3K), mammalian target of rapamycin, and the transforming serine-threonine kinase Akt protein isoforms, also known as protein kinase B. DNA-targeted agents, such as platinum agents, taxanes, and antimetabolites, as well as radiation have had a significant impact on cancer treatment by affecting DNA replication, which is aberrantly activated in malignancies. However, the caveat is that they may also trigger the activation of repairing mechanisms, such as upstream and downstream cascade of Akt survival pathway. Thus, each target can theoretically be inhibited in view of improving the potency of conventional treatment. Akt inhibitors, e.g., MK-2206 and perifosine, or PI3K modulators, e.g., LY294002 and Wortmannin, have shown some promising results in favor of sensitizing the cancer cells to the therapy in vitro and in vivo, which have provided the rationale for incorporation of these novel agents into multimodality treatment of different malignancies. Nevertheless, despite the acceptable safety profile of some of these agents in the clinical studies, with regard to the efficacy, the results are still too preliminary. Hence, we need to wait for the upcoming data from the ongoing trials before utilizing them into the standard care of cancer patients.
Keywords: Phosphatidylinositol 3 kinase/Akt, Platinum, Taxane, Antimetabolite, Radiation
Core tip: The Akt pathway plays an important role in resistance to several cytotoxic agents, targeted drugs and radiation. Exposure to these drugs will stimulate the Akt survival pathway leading to a decreased response to these drugs. In model systems inhibition of the Akt pathway enhanced the cytotoxicity of drugs like taxanes, antimetabolites, platinum analogs, several targeted drugs and radiation. Akt inhibitors offer a new opportunity to increase the efficacy of currently used drugs and of radiotherapy.
AKT PATHWAY SIGNALING OVERVIEW
The Akt signal transduction pathway controls most hallmarks of cancer, including metabolism, cell survival, cell cycle progression, regulation of apoptosis, protein synthesis, motility, and genomic instability by phosphorylation of the substrates[1]. Aberrant loss or gain of Akt activation has been associated with the development of various diseases, e.g., diabetes, autoimmune diseases, and cancer[2-5].
The Akt pathway consists of phosphatidylinositol 3 kinase (PI3K), mammalian target of rapamycin (mTOR), and the transforming serine-threonine kinase Akt protein isoforms (further referred to as Akt), also known as protein kinase B (PKB) and phosphate and tensin homologue (PTEN) as a critical tumor suppressor. PI3K enzymes phosphorylate phosphatidylinositol-4,5-biphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3) at the cell membrane that are required for the recruitment and activation of Akt[6,7] (Figure 1). These phospholipids are constitutively elevated in most cancer cells. Docking of Akt to the cell membrane causes a conformational change, which in turn leads to phosphorylation of the two critical amino acid residues, threonine 308 and serine 473, and finally leads to the activation of Akt[8]. After the activation, Akt is translocated to intracellular compartments where it phosphorylates several substrate proteins. The downstream targets of Akt are numerous due to the multiple interactions with its consensus sequence[1]. In summary, the most important effects of Akt activation are: (1) cell survival through inhibition of BAD, caspase-9, and FOX[9-11]; (2) cell proliferation and gluconeogenesis through inhibition of GSK3, P21, P27, etc.[12]; and (3) protein synthesis and cell growth through activation of mTOR[13] (Figure 1).
Figure 1.
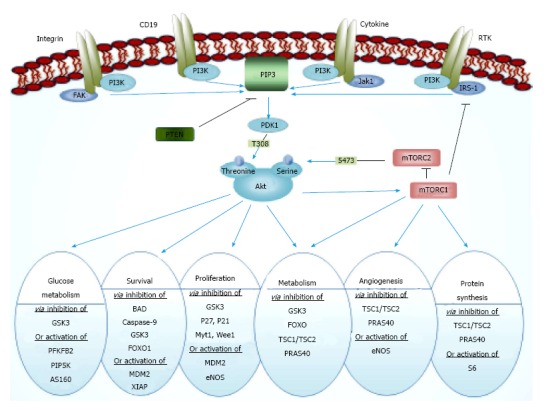
Phosphatidylinositol 3 kinase/Akt pathway. Activated RTKs activate PI3K through direct binding or through tyrosine phosphorylation of scaffolding adaptors, such as IRS1, which then bind and activate PI3K. PI3K phosphorylates PIP2 to generate PIP3, in a reaction that can be reversed by the PIP3 phosphatase PTEN. Activation of PI3K results in membrane recruitment and thus activation of Akt protein. Akt regulates cell growth and many other cellular processes through its effects on mTOR pathways and thus regulates glucose metabolism, protein synthesis, mitochondrial metabolism, lipid metabolism, adipogenesis, lipogenesis, angiogenesis, autophagy, proliferation and cell growth. Other targets of Akt include insulin receptor substrate-1 (IRS-1), glycogen synthase kinase 3 (GSK3), phosphodiesterase-3B (PDE-3), B cell lymphoma-2-associated death promoter (BAD), human caspase-9, Forkhead box (FOX) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcription factors, endothelial nitric oxide synthase (eNOS), Rapidly Accelerated Fibrosarcoma (Raf) kinases, P21CIP1/WAF1 (P21; a potent cyclin-dependent kinase inhibitor), P27Kip1, Tuberous Sclerosis Complex 2 (TSC2; also known as Tuberin), X-linked inhibitor of apoptosis protein (XIAP; also known as inhibitor of apoptosis protein 3 and baculoviral IAP repeat-containing protein 4), and Mouse Double Minute 2. Following the activation, Akt phosphorylates and blocks the molecules involved in the apoptotic pathway, including FOX, Caspase-9 and BAD. In addition to the inhibition of proapoptotic factors, Akt can activate the transcription of antiapoptotic genes through the activation of the transcription factor Rel/NFκB. Akt also phosphorylates and activates IκB, which results in IκB degradation by the proteasome. This allows NFκB to translocate from the cytoplasm to the nucleus and activate transcription of a variety of substrates including anti-apoptotic IAP genes, such as the c-IAP1 and c-IAP2. IRS1: Insulin receptor substrate 1; PI3K: Phosphatidylinositol 3-kinase; PIP3: Phosphatidylinositol-3,4,5-trisphosphate; PTEN: Phosphate and tensin homologue; RTK: Receptor tyrosine kinase.
To date, 3 Akt family members have been identified in mammals, i.e., Akt1 (also known as PKBα), Akt2 (PKBβ) and Akt3 (PKBγ). Having shown highly conserved properties, these homologues may be activated by the same mechanism[14]. However, being encoded by three different regions at 14q32, 19q13, and 1q44, respectively, these three isoforms are distinct substrates with distinct physiological outcomes, and also opposing to each other. Accumulating evidence casts Akt1 and Akt2 function almost in contrary to each other in modulating phenotypes associated with migration and invasion. Akt isoforms contain an N-terminal pleckstrin homology (PH) domain, a central catalytic domain, and a C-terminal regulatory region. The PH domain can bind phosphatidylinositol lipids (e.g., PIP3) with high affinity and targets Akt to the cell membrane[15].
Regulation of Akt, on the other hand, is mainly achieved through PTEN, which antagonizes PI3K. PTEN is a tumor suppressor gene that is frequently mutated in different types of cancer, and loss of PTEN leads to elevation of PI3K lipid products and thus activating the Akt pathway[16]. Thus, PTEN negatively regulates the Akt pathway, while loss of PTEN results in overactive Akt, which induces proliferation and promotes survival by inhibiting apoptosis[10,17]. Among the three Akt isoforms, Akt2, is exclusively having carcinogenic properties in PTEN-deficient solid tumors[18].
Despite many breakthroughs in elucidating the cancer behavior and possible mechanisms leading to developing different treatments, resistance is still a problem. The main goal of cytotoxic cancer therapy is to eliminate irregularly dividing cancer cells by targeting DNA synthesis or the mitotic apparatus. Different molecules, genes, proteins and signal transduction pathways are involved in this complicated process[1,19,20]. Resistance is often related to uptake, metabolism or alterations in the target. Besides, many studies demonstrated the modulation of key signaling pathways by the DNA-targeted therapies (reviewed in the following sections). The PI3K/Akt signaling pathway being mutated in a high percentage of malignancies[20] is widely implicated in tumor growth, which may also render tumor cells resistant to chemotherapeutic drugs[5]. Thus, inhibition of this pathway should foil local tumor growth. Many trials are underway to investigate whether adding inhibitors targeting PI3K/Akt pathway may improve the efficacy of the conventional regimen by reducing the apoptotic threshold[21]. Here, we review the literature on the potential value of modulating Akt pathway in view of improving the cytotoxicity of DNA-targeted anticancer drugs and radiotherapy.
METHODS: A SYSTEMATIC BEST EVIDENCE REVIEW
We looked for publications studying the effects of the approved or tested DNA-targeted cytotoxic agents on the Akt signaling using the Medline via PubMed database. The inclusion criteria consisted of studies on modulation of Akt signaling by DNA-targeted cytotoxic agents, i.e., platinum agents (cisplatin, carboplatin, oxaliplatin), taxanes (paclitaxel, docetaxel), antimetabolites (gemcitabine, fluorouracil, pemetrexed), and radiation in glioblastoma, mesothelioma and lung, ovary, and pancreas cancers, including their synonyms, with no language restriction as of September 2014. Search terms related to the Akt modulation were “p-Akt” OR “pAkt” OR “phospho-Akt” OR “phosphorylat* Akt” OR “Akt phosphorylation” OR “Akt inhibition” OR “Akt modulation” OR “inhibit* Akt” OR “inactivation of Akt” OR “Akt inactivation” OR “activation of Akt” OR “inactivating Akt”. Because of a large body of data on this subject, here we narrowed the scope of the current review down to the preclinical and clinical data on the simultaneous administration of cisplatin, paclitaxel, gemcitabine, or pemetrexed with some of the most clinically relevant PI3K/Akt modulators, i.e., PI3K inhibitors, (e.g., LY294002, Wortmannin), PI3K/mTOR inhibitors (e.g., BEZ235), or Akt inhibitors (e.g., perifosine, MK2206), which were tested in combinations with DNA-targeted agents in any of the five types of cancers. We excluded papers not meeting the inclusion criteria as well as retracted publications, duplicates, or non-original papers, i.e., review articles, letters and editorials, comments, and case reports.
For clinical data, we searched for all the registered trials, being planned or performed to study the effect of PI3K/Akt inhibitors, i.e., MK2206, perifosine, LY294002, Wortmannin, BEZ235, in combination with any of the nine DNA-targeted modalities discussed above, i.e., carboplatin, cisplatin, oxaliplatin, paclitaxel, docetaxel, fluorouracil, gemcitabine, pemetrexed, and radiation. All published full text papers or abstracts as well as those with preliminary results in any languages fulfilling selection criteria were included with no time period restriction. Studies with prior administration of anticancer medications, or combination of drugs targeting other signaling pathways other than Akt, PI3K, and mTOR were excluded. The databases that were used in this phase included the US clinical trials registry (clinicaltrials.gov), NIH clinical research studies (clinicalstudies.info.nih.gov), worldwide clinical trials listings (www.clinicaltrialssearch.org/5-cancer-clinical-trials.html), and the WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch). The latter included 13 registries (Nationalities: Australian, New Zealand, Chinese, American, Canadian, European, Dutch, Brazilian, Indian, Korean, Cuban, German, Iranian, Japanese, Pan African, Sri Lankean, and Thai clinical trials).
AN OVERVIEW ON PI3K/AKT INHIBITORS
Many compounds have been developed to inhibit PI3K, Akt, and mTOR signaling, among which only few were tested in clinical settings (Figure 2). However, they did not yet have significant clinical benefit, except for idelalisib (GS-1101, a p110δ-selective inhibitor), which is the first Food and Drug Administration approved PI3K inhibitor[22], and some mTOR inhibitors, e.g., rapamycin and its analogs. There are six general classes of these agents targeting the Akt network: Pan-class I PI3K inhibitors, isoform-selective PI3K inhibitors, rapamycin analogues (rapalogues), active-site mTOR inhibitors, pan-PI3K-mTOR inhibitors and Akt inhibitors[23] (Table 1). Isoformspecific PI3K inhibitors targeting PI3Kβ, inhibitors of ribosomal protein S6 kinase β1 (S6K), PDK1 inhibitors and isoform-selective Akt kinase inhibitors (Akt1 and 2) are also under investigations soon to be tested in the clinic[23].
Figure 2.
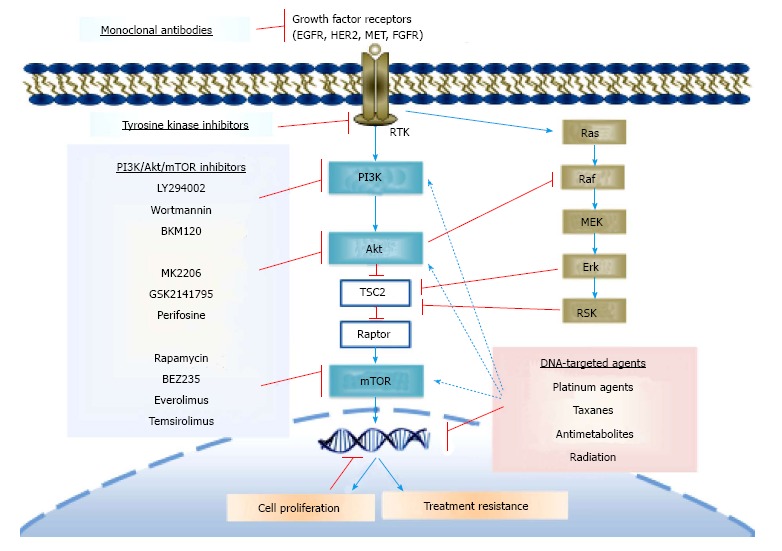
A schematic figure showing the complementary effects of phosphatidylinositol 3-kinase/Akt inhibitors with platinum agents, taxanes, antimetabolites, tumor antibiotics, and radiation resulting in a better cytotoxic profile.
Table 1.
Drugs targeting phosphatidylinositol 3 kinase/Akt/mammalian target of rapamycin pathway
| PI3K/Akt/mTOR subgroups | Agents | Clinical Stage |
| Pan-PI3K inhibitors | XL147 | Phase II |
| BKM120 | Phase III | |
| GDC0941 | Phase II | |
| Rapalogues (mTORC1 inhibitors) | Sirolimus | Phase III |
| Everolimus | Approved | |
| Temsirolimus | Approved | |
| Ridaforolimus | Phase III | |
| mTORC1/2 inhibitors | INK128 | Phase II |
| AZD8055 | Phase I | |
| OSI027 | Phase I | |
| PI3K–mTOR inhibitors | BEZ235 | Phase II |
| XL765 | Phase II | |
| GSK1059615 | Phase I | |
| Isoform-specific PI3K inhibitors | CAL-101 (p110δ) | Phase III |
| INK1117 (p110α) | Phase I | |
| BYL719 (p110α) | Phase II | |
| Akt inhibitors | Perifosine | Phase III |
| MK-2206 | Phase II | |
| GDC0068 | Phase II | |
| GSK690693 | Phase I |
PI3K: Phosphatidylinositol 3 kinase; mTOR: Mammalian target of rapamycin.
PI3K is upstream of Akt pathway, and with its inhibition all the subsequent signaling will potentially be down-regulated. Emerging clinical data show limited single-agent activity of inhibitors targeting PI3K. However, the drugs targeting PI3K pathway usually modulate the myriad substrates, including Akt and/or mTOR. LY294002, Wortmannin, and perifosine as PI3K/Akt inhibitors, BEZ235 as dual PI3K/mTOR inhibitor, and MK2206 as a specific Akt inhibitor are some of the commonly used drugs in this category that modulate Akt signaling. LY294002 is a morpholine-containing chemical compound that is a potent reversible inhibitor of PI3K signaling. Wortmannin is a steroid metabolite of the fungi Penicillium funiculosum with an irreversible inhibitory effect on PI3K. Perifosine is an orally active alkylphospholipid analog, which targets cell membrane and modulates different signaling pathways, and Akt in particular[24,25].
INDIRECT ALTERATION OF AKT SIGNALING AND ITS MODULATION
We finally selected 65 papers being suited to this review (Figure 3; Table 2) by which combination of a PI3K/Akt inhibitor with any of cisplatin, paclitaxel, gemcitabine, or pemetrexed was studied. The results are precisely discussed in the following sections.
Figure 3.
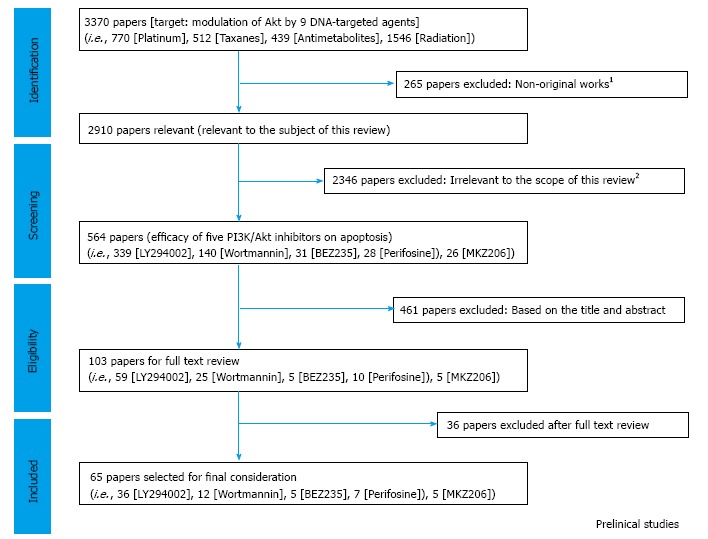
Review flow diagram of the publication selection in preclinical category. 1Exclusion criteria include the retracted publication, duplicate publication or non-original papers, including review articles, letters and editorials, comments, case reports, etc.; 2According to the scope of the current review: Efficacy of Akt modulation by 9 DNA-targeted agents in 5 types of cancer, including lung cancer, malignant mesothelioma, pancreatic cancer, ovarian cancer, and malignant glioma. PI3K: Phosphatidylinositol 3 kinase; EGFR: Epidermal growth factor receptor; mTOR: Mammalian target of rapamycin.
Table 2.
Systematic chart of searching methodology and the results based on PubMed
| DNA-targeted therapies | Agents |
Akt modulation |
|||||||
| All papers1 | Non-original2 | Relevant papers3 | PI3K/Akt inhibitors4 | ||||||
| LY294002 | Wortmannin | BEZ235 | Perifosine | MK2206 | |||||
| Platinum | Carboplatin | 68 | 6 | 51 | 3 | 1 | 0 | 0 | 4 |
| Cisplatin | 631 | 15 | 589 | 85 | 22 | 8 | 9 | 5 | |
| Oxaliplatin | 71 | 5 | 59 | 5 | 2 | 0 | 0 | 0 | |
| Taxane | Docetaxel | 149 | 13 | 127 | 9 | 2 | 3 | 1 | 3 |
| Paclitaxel | 363 | 18 | 331 | 47 | 15 | 4 | 2 | 5 | |
| Antimetabolite | Fluorouracil | 191 | 5 | 174 | 18 | 6 | 0 | 2 | 3 |
| Gemcitabine | 213 | 14 | 191 | 16 | 8 | 3 | 1 | 1 | |
| Pemetrexed | 35 | 3 | 32 | 5 | 2 | 1 | 0 | 0 | |
| Radiation | Irradiation/radiation | 1649 | 186 | 1356 | 151 | 82 | 12 | 13 | 5 |
| Total articles | (9 agents) | 3370 | 265 | 2910 | 339 | 140 | 31 | 28 | 26 |
Terms used in the search: Query agent/radiation, and Akt, within whole the article with no language limitation, as of September, 2014;
Including the retracted publication, duplicate publication or non-original papers, including Review articles, Letters and Editorials, Comments, Case Reports, etc.;
Relevant with regard to the modulation of Akt by the query agent. [p-Akt OR pAkt OR "phospho-Akt" OR (phosphorylat* Akt) OR “Akt phosphorylation” OR “Akt inhibition” OR “Akt modulation” OR (inhibit* Akt) OR “inactivation of Akt” OR “Akt inactivation” OR “activation of Akt” OR “inactivating Akt”];
Suppressing Akt cascade by a PI3K/Akt inhibitor to sensitize the query agent. PI3K: Phosphatidylinositol 3 kinase.
Effect of platinum analogs on Akt signaling
Cisplatin, carboplatin and oxaliplatin are the 3 most commonly used anticancer platinum analogs. The main antitumor properties of cisplatin are attributed to the formation of platinum-DNA adducts causing DNA bending[26], which interferes with DNA replication, transcription and other nuclear functions leading to the inhibition of cellular proliferation and tumor growth. Intrinsic and acquired resistance limits the efficacy of platinum drugs in cancer treatment. Decreased drug uptake along with increased influx or inactivation by sulfhydryl molecules, such as glutathione, or increased DNA adduct repair can result in platinum resistance[27,28]. Wang and Lippard[29] suggested that induction of signaling pathways might be an alternative mechanism of resistance to platinum analogs.
Some data suggest that cell death induced by cisplatin may occur through regulation of cell cycle[30-32]. Mitsuuchi et al[32] demonstrated that PI3K, Akt1, and Akt2 are required for p53 protein expression and the full induction of p21 in ovarian cancer cells treated with cisplatin. Liu et al[33] suggested that Akt1 expression regulates cisplatin resistance in lung cancer cells through mTOR pathway, and its inhibition may sensitize cells to cisplatin. Moreover, amplification of a catalytic subunit of PI3K (PIK3CA) was also found to be associated with the risk of resistance to platinum-based chemotherapy in a group of patients with ovarian cancer, but not with the overall survival[34]. Thus available data support the probable role of Akt amplification/overexpression in platinum-resistance in vitro and in vivo[33,35-43].
The PTEN (encoding PIP3 phosphatase) and PIK3CA (encoding the PI3K catalytic isoform p110α) are the two most frequently altered mutated tumor suppressor and oncogenes, respectively[23]. Moreover, a low level of the PTEN expression is associated with amplified PIK3CA expression and finally PI3K/Akt activity[42]. Other data suggest that loss of the FHIT (fragile histidine triad; an inhibitor of Akt signaling) and overexpression of Redd1 (an inhibitor of mTOR signaling) are associated with cisplatin resistance in lung cancer cell lines[44-46]. Furthermore, overexpression of ADAM17 (a disintegrin and metalloproteinase-17) has been found to be associated with hypoxia-induced cisplatin resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway in vitro[47]. ADAM17 is a member of the metalloproteinase superfamily involved in the cleavage of ectodomain of many transmembrane proteins. Besides, prostate apoptosis response-4 (a proapoptotic tumor suppressor protein) downregulation was associated with cisplatin resistance in pancreatic cancer cells through upregulation of PI3K/Akt signaling in vivo[31]. Given that cisplatin activates PI3K/Akt signaling, downregulation of this pathway may bypass cisplatin-resistance. Akt pathway overactivation may decrease cisplatin sensitivity and cause treatment resistance even in platinum sensitive cells, whereas downregulation of Akt can boost the drug sensitivity and resistance to platinum compounds like cisplatin[36,39].
Effect of Akt-inhibition on platinum sensitivity
Some studies did not find treatment sensitization by adding LY294002 to cisplatin[48,49]. However, the PI3K/Akt inhibitors, LY294002, Wortmannin, or BEZ235 in combination with cisplatin showed synergistic or additive effects against malignant mesothelioma and lung cancer[44,50-55], pancreatic cancer[30], ovarian cancer[41-43,56-61], as well as glioblastoma[62,63] in vitro and in vivo (Table 3).
Table 3.
Studies evaluating the efficacy of phosphatidylinositol 3 kinase phosphatidylinositol 3 kinase/Akt modulators on the apoptotic profile of cisplatin, paclitaxel, gemcitabine and pemetrexed
| PI3K/Akt inhibitor and DNA-targeted agent combination |
Akt modulation (phosphorylation) |
|||||||
|
Lung cancer and mesothelioma |
Pancreatic cancer |
Ovarian cancer |
Malignant glioma |
|||||
| Synergistic | Antagonistic | Synergistic | Antagonistic | Synergistic | Antagonistic | Synergistic | Antagonistic | |
| LY294002/Cisplatin | 1[51]a | 1[50] | 2[30,31] | - | 6[42,56-60] | 1[49] | 2[62,63]b | 1[48] |
| LY294002/Paclitaxel | 2[64,65] | 2[50,66] | - | - | 7[32,56,67-71]b | - | 1[63] | - |
| LY294002/Gemcitabine | 1[72] | 1[73] | 4[74-77]b | 1[78] | 1[56] | - | - | - |
| LY294002/Pemetrexed | 1[79] | 1[50] | - | - | - | - | - | - |
| Wortmannin/Cisplatin | 1[52] | - | - | 3[41-43] | - | - | - | |
| Wortmannin/Paclitaxel | 1[80] | - | - | - | 1[70] | - | 1[80] | - |
| Wortmannin/Gemcitabine | - | - | 5[74,81-84] | - | - | - | - | - |
| Wortmannin/Pemetrexed | 1[79] | - | - | - | - | - | - | - |
| BEZ235/Cisplatin | 1[53] | - | - | - | 1[61] | - | - | - |
| BEZ235/Paclitaxel | - | - | - | - | 1[61] | - | - | - |
| BEZ235/Gemcitabine | - | - | 1[85] | - | - | - | - | - |
| BEZ235/Pemetrexed | 1[86] | - | - | - | - | - | - | - |
| Perifosine/Cisplatin | 2[44,54] | - | - | - | 2[25,87] | - | - | - |
| Perifosine/Paclitaxel | - | - | - | - | 1[88] | - | - | - |
| Perifosine/Gemcitabine | 1[54]b | - | - | - | 1[89] | - | - | - |
| Perifosine/Pemetrexed | - | - | - | - | - | - | - | - |
| MK2206/Cisplatin | 2[51,55] | - | - | - | - | - | - | - |
| MK2206/Paclitaxel | - | - | - | - | - | - | - | - |
| MK2206/Gemcitabine | 2[54,90]b | - | - | - | - | - | - | - |
| MK2206/Pemetrexed | - | 1[54]c | - | - | - | - | - | - |
| Total | 17 | 6 | 12 | 1 | 24 | 1 | 4 | 1 |
Chowdhry et al[51]. Reporting an increased sensitivity, but synergism not evaluated;
Shingu et al[62,63], Kawaguchi et al[69], Pinton et al[54] additive enhancement of proliferative inhibition;
Holcomb et al[76] LY294002 combination with gemcitabine showed additive effects on proliferative inhibition in PANC-1 and synergistic in PaCa2+ pancreatic cancer cell lines. However, pAkt levels rebounded at later time points. PI3K: Phosphatidylinositol 3 kinase.
Perifosine increased the antineoplastic activity of cisplatin in ovarian[25,87], endometrial[91], and lung[44,54,55,92] cancer cells by activating apoptotic pathways and thus enhancing the cytotoxicity of cisplatin. Likewise, the specific Akt inhibitor MK-2206 showed synergism when combined with cisplatin in lung cancer[51,55], gastric cancer[93], and nasopharyngeal carcinoma cells[94] in vitro and in vivo. It can be concluded that activation of the Akt survival pathway plays a pivotal role in platinum resistance, and inhibition of Akt may enhance the effect of this type of anticancer drug.
Effect of taxanes on Akt signaling
Taxanes, e.g., paclitaxel and docetaxel, are frontline therapy for several cancers. They stabilize microtubules, leading to cell cycle arrest through centrosomal impairment, induction of abnormal spindles and suppression of spindle microtubule dynamics, finally triggering apoptosis[95]. Microtubules are critical for the integrity of the segregated DNA during mitosis. However, inherent or acquired resistance to taxanes may compromise their therapeutic efficacy[96,97].
Resistance to taxanes includes increased efflux, and modification in tubulins. Akt pathway activation contributed to an increased resistance to paclitaxel or docetaxel in epithelial ovarian cancer, prostate cancer, and breast cancer cells[98-101]. Activation of Akt1 by HER2/PI3K may also lead to taxane resistance in breast adenocarcinoma cells[102]. Moreover, PIK3CA gene, encoding a catalytic subunit of the PI3K, is mutated and/or amplified in various neoplasms, such as ovarian cancer. Its amplification strongly decreased the sensitivity and thus response to platinum with/without taxanes in patients with ovarian carcinoma[34].
There are also crosstalks between PI3K/Akt pathway with BAD and ERK[41,68], and inhibition of these cascades sensitized ovarian cancer cells to taxanes. Therefore, in order to sensitize taxane treatment, PI3K/Akt cascade can be considered as a suitable target.
Effect of Akt-inhibition on taxane sensitivity
LY294002, Wortmannin, BEZ235, or perifosine-mediated inhibition of the PI3K/Akt-dependent survival pathway enhanced paclitaxel cytotoxicity in various cancers, e.g., malignant glioma[63,80], lung[50,64-66,80], esophageal[64,80], and ovarian cancer cells[32,56,61,67-70,88] (Table 3). However, there are some data not in favor of the combination. LY294002 did not potentiate cisplatin, pemetrexed, or paclitaxel in A549 lung adenocarcinoma cells harboring K-ras mutation and wild-type EGFR[50]. Likewise, inactivation of PI3K/Akt signaling by LY294002 did not result in significant alteration of sensitivity of human ovarian carcinoma A2780 cells to paclitaxel[48]. Similarly, the combination of paclitaxel with LY294002 was antagonistic in vitro when dexamethasone was also administered; although dexamethasone did not alter the Akt activity[66].
Activation of NFκB is linked to Akt-dependent transcription of pro-survival genes[103]. Thus, LY294002-mediated suppression of the PI3K/Akt survival pathway with secondary inhibition of NFκB transcriptional activity is associated with enhancement of paclitaxel cytotoxicity in lung, esophageal and ovarian cancer cells[64,104,105], which indicates that NFκB may be the crucial intermediary step connecting Akt to the intrinsic susceptibility of cancer cells to paclitaxel.
Additionally, the Akt inhibitor MK-2206 augmented the efficacy of paclitaxel and carboplatin combination in gastric cancer[106], breast cancer[107], and melanoma cells[108]. However, addition of MK-2206 to paclitaxel alone had no additive inhibitory effect on growth of nasopharyngeal carcinoma cells in vitro[90]. Furthermore, Hirai et al[90] found that synergy of MK-2206 with docetaxel was dependent on the treatment sequence, in which a schedule of MK-2206 before docetaxel was not effective in terms of growth inhibition. Dual inhibition of PI3K and mTORC1/2 by BEZ235 may overcome docetaxel resistance in human castration resistant prostate cancer in vitro and in vivo[109]. Thus, modulation of the PI3K/Akt signaling may increase the efficacy and potency of taxanes according to in vitro and in vivo data. However, the effect may have been masked by inclusion of platinum in several studies, indicating that in some studies, the effect might be via platinum.
Effect of antimetabolites on Akt signaling
Antimetabolites are a large group of anticancer drugs widely used in combination therapy of various leukemias and solid tumors. They interfere with DNA and RNA synthesis and therefore the growth of tumor[110]. Anti-metabolites are categorized as pyrimidine analogs [e.g., 5-fluorouracil (5-FU), gemcitabine], purine analogs (e.g., azathioprine, mercaptopurine), and antifolates (e.g., methotrexate, pemetrexed). In the present review, we mainly focused on two commonly used antimetabolites gemcitabine and 5-FU, as well as the novel anti-folate pemetrexed.
Effect of gemcitabine on Akt signaling and effect of Akt inhibition: Gemcitabine is used in the treatment of various carcinomas, such as lung cancer, bladder cancer, breast cancer, and lymphomas[111]. A substantial number of potential biomarkers for sensitivity or resistance to gemcitabine have been characterized, including ribonucleotide reductase, deoxycytidine kinase, cytidine deaminase and human equilibrative transporter-1[112,113]. Additional mechanisms of resistance may exist, possibly not involving metabolism and direct targets[74,75,112,114].
Gemcitabine resistance in breast cancer cells may also be mediated by activation of the PI3K/Akt signaling pathway through phosphorylated Akt[115], so that inhibitors of PI3K/Akt might reverse the resistance to gemcitabine. Moreover, involvement and overexpression of PI3K and phosphorylated Akt in pancreatic carcinoma tissues has been reported in gemcitabine-resistant cells in vitro[73,78,84]. Rad51 overexpression may also mediate gemcitabine resistance through Akt or ERK1/2 activation in non-small cell lung cancer (NSCLC) cells, which could be overcome by downregulation of Rad51 or inhibition of Akt and ERK1/2 proteins[72]. Although Akt phosphorylation status is tailored as a predictive biomarker for gemcitabine resistance in NSCLC patients[116], gemcitabine may also reduce Akt phosphorylation without affecting the Akt overall expression[117]. Wilson et al[73] reported a weak correlation between phosphorylated S6K and phosphorylated Akt, suggesting the existence of Akt-independent regulation of mTOR-mediated resistance to apoptosis. Overall, inhibition of PI3K/Akt signaling may enhance the gemcitabine cytotoxic profile.
Wortmannin enhanced the efficacy of gemcitabine by a 5-fold increase of apoptosis in murine pancreatic xenografts[81]. A synergistic effect of Wortmannin, LY29 4002, and BEZ235 with gemcitabine was also reported in ovarian cancer[56] and pancreatic carcinoma[74-77,81-85] in vitro and in vivo (Table 3). Although gemcitabine induces cell cycle arrest at the G1 and early S phases, PI3K/Akt activation does not seem to influence gemcitabine-induced cell cycle arrest[84]. Likewise, perifosine has shown additivity in combination with gemcitabine by inhibiting Akt1 and Akt3 axis, and interfering Akt upstream, EGFR, and MET phosphorylation[54]. Perifosine also enhanced the gemcitabine-mediated antitumor effect on pancreatic cancer cells through blocking p70S6K1 (S6K1) activation, and thus disrupting S6K1-Gli1 association and subsequent Gli1 activation[89]. Besides, Akt[118], mTOR[119], and MAPK[120] may also activate Gli1. Likewise, the Akt inhibitor MK2206 enhanced the effect of gemcitabine on growth inhibition in vitro and in vivo[90]. In the contrary, Arlt et al[78] found that NFκB, rather than PI3K/Akt, activity conferred resistance to gemcitabine in a panel of five pancreatic carcinoma cell lines, which was strongly diminished by NFκB inhibitors, and not by LY294002. Overall, the PI3K/Akt inhibitors have been efficacious in improving gemcitabine cytotoxicity.
Effect of FU on Akt signaling and effect of Akt inhibition: 5-FU is an antimetabolite that acts by inhibition of thymidylate synthase (TS) and can be incorporated into RNA and DNA altering the cancer cell replication and proliferation[121]. 5-FU-based regimens are often used in adjuvant chemotherapy regimens and treatment of various advanced malignancies, such as colon cancer, head and neck cancer, breast cancer, but depending on the disease and stage of the tumor, intrinsic resistance to 5-FU can be as high as 50%[122]. Resistance to 5-FU has often been associated with an increased TS expression, both transient and permanent[123]. Other factors, such as enzymes involved in pyrimidine metabolism, i.e., increased dihydropyrimidine dehydrogenase, decreased orotate phosphoribosyltransferase, or altered folate metabolism have been associated with 5-FU resistance[121,124,125]. Moreover, 5-FU has major effects on glycosylation pathways as well[126], which may indirectly have effects on signaling pathways. Hence, evidence is accumulating that 5-FU resistance is associated with altered signaling.
Smad4 deficiency may also contribute to 5-FU resistance through upregulation of vascular endothelial growth factor expression, which is associated with increased vascular density[127,128]. Zhang et al[129] found that loss of Smad4 in colorectal cancer patients may induce resistance to 5-FU through activation of Akt pathway. Akt can interact with Smad molecules to regulate transforming growth factor beta (TGF-β) signaling that is involved in transmitting chemical signals from the cell surface to the nucleus[130-132]. In summary, suppression of PI3K/Akt signaling may potentiate 5-FU.
The combination of LY294002 with 5-FU was synergistic via downregulation of PI3K/Akt signaling in Smad4-deficient colorectal cancer cells[129]. Likewise, sequential combination of 5-FU and LY294002 induced synergistic cytotoxicity and overcame intrinsic and acquired resistance of 5-FU via downregulation of Akt and mitochondria-dependent apoptosis in an Epstein-Barr virus positive gastric cancer cell line[133]. Wortmannin also promoted 5-FU antitumor activity in oral squamous cell carcinoma[134] and breast cancer cells[135]. In colorectal cancer cell lines, preclinical studies indicate that perifosine and BEZ235 may enhance the cytotoxic effects of 5-FU, likely through the NFκB and thus PI3K/Akt pathway[136]. As a result, PI3K/Akt pathway is a rational target for sensitizing the tumor cells to 5-FU.
Effect of pemetrexed on Akt signaling and effect of Akt inhibition: Pemetrexed (Alimta; formerly known as LY231514), a multitargeted antifolate, inhibits thymidylate synthase (TS), dihydrofolate reductase, and the de novo purine nucleotide synthesis[137]. Pemetrexed is currently used as a single agent, but more often in combination with cisplatin for first line treatment of non-squamous NSCLC and malignant pleural mesothelioma[138-140]. Resistance to pemetrexed has been associated with TS upregulation in a colon cancer cell line[123,141], a transport deficiency, decreased activation by folylpolyglutamate synthetase and increased efflux[139]. Members of the ATP-binding cassette (ABC) transporters including P-glycoprotein (Pgp/ABCB1), multidrug resistance proteins (MRPs/ABCC) as well as breast cancer resistance protein (BCRP/ABCG2) as ATP-dependent drug efflux transporters may play roles in pemetrexed resistance[142]. The PI3K/Akt pathway regulates ABCG2-mediated drug efflux, which induces drug resistance[86,143-145].
Pemetrexed can also activate Akt signaling[79,143,146,147], although its molecular mechanisms is not completely understood. Chen et al[79] have observed a pemetrexed-induced cell apoptosis and a parallel increase in sustained Akt phosphorylation and nuclear accumulation in the NSCLC A549 cell line, and postulated that the activated Akt may play a proapoptotic role, while Giovannetti et al[147] observed that pemetrexed increased EGFR phosphorylation and slightly reduced Akt phosphorylation and enhanced apoptosis in six NSCLC cell lines[143,146] and malignant pleural mesothelioma (MPM) cells, particularly when combined with EGFR inhibitor erlotinib or carboplatin.
Adding a PI3K/Akt inhibitor may further increase pemetrexed antineoplastic effect. LY294002 and Wortmannin decreased the pemetrexed-stimulated Akt and GSK3β phosphorylated activation in the NSCLC A549 cell line[79] (Table 3). Perifosine antagonized the effect of pemetrexed in MPM cells by interfering upstream of Akt, affecting EGFR and MET phosphorylation[54]. Likewise, BEZ235 enhanced the antineoplastic effect of pemetrexed in malignant pleural mesothelioma by decreasing ABCG2-mediated drug efflux at the cell surface[86], which may be of therapeutic value in combination regimens. These data suggest that combining pemetrexed with a PI3K/Akt inhibitor may result in a better antineoplastic effect in various tumors.
Effects of irradiation and chemoradiation on Akt signaling: The combination of radiation with cytotoxic chemotherapy has become a standard treatment option for the majority of inoperable, locally advanced cancers, including brain, head and neck, lung, and gastrointestinal malignancies[148]. However, resistance to irradiation compromises therapeutic efficacy leading to tumor recurrence or metastasis. Tumors that recur after a successful radiation are often associated with radioresistance[149]. Resistance to radiotherapy is predominantly related to efficient repair of the DNA damage induced by X-ray. Both normal and neoplastic cells have several types of repair pathways, usually starting with the recognition and excision of the lesion, and then insertion of a new nucleotide. Regulation of several of these repair enzymes is mediated through methylation of the gene or activation of various protein kinases[150]. Given the complex biology underlying the interactions between the targeted agent and chemoradiation, comprehensive preclinical investigations are critical to design the rational combination[148].
Different combinations of drugs and radiation have been studied to improve efficacy and lessen toxicity. Chemotherapeutic drugs that perturb nucleotide metabolism have the potential to produce substantial sensitization of tumor cells to radiation treatment. Redistribution of cells into S-phase of the cell cycle and depletion of deoxynucleotide pools are probable mechanisms for gemcitabine and 5-FU, which made them potent radiosensitizers[151,152].
Radiation can activate multiple signaling pathways in cells[153], such as EGFR and several downstream proteins, i.e., PI3K/Akt, MAPK JNK, p38, NFκB, etc., stimulating DNA repair and thus causing radioresistance and survival of tumor cells. Loss of PTEN[154], as well as KRAS mutations[155,156] and NF-κB activation[157,158] also are associated with radioresistance, making the DNA less susceptible to ionizing radiation. Additionally, the ability of radiation to activate signaling pathways may depend on the expression of growth factor receptors, RAS mutation, and autocrine or paracrine ligands such as TGF-α, TGF-β, HB-EGF, neuregulins, and interleukin 6[153].
Effect of Akt-inhibition on radiation sensitivity: Alkylphosphocholines may potentiate the effect of radiation if given before or together with radiotherapy[159]. Targeting the PI3K pathway by LY294002 led to radiosensitization in glioblastoma[154] and human bladder cancer cell xenografts[160] in vivo. BEZ235 has also shown a modest antitumor response in vivo, while the combination of BEZ235 and ionizing radiation provided a longer survival and led to a smaller tumor volume when compared to radiation alone[161]. Likewise, the PI3K inhibitor BKM120 inhibited the radiation-induced activation of Akt[162]. This induced suppression of DNA-double-strand breaks repair and increased apoptosis, which resulted in increased sensitivity of liver cancer cells to irradiation[162]. Perifosine showed some radiosensitization in squamous cell carcinoma[163-165], malignant glioma[166], lymphoma[167], and prostate cancer[168]. In contrast, one study failed to show any favorable results with perifosine in terms of increasing its anticancer potency, despite a significant reduction in the level of phosphorylated Akt as well as Akt activity in vitro and in vivo[169]. Overall, given the activation of PI3K/Akt pathway by radiation, addition of a PI3K/Akt inhibitor may potentiate the therapeutic index of the conventional chemoradiation therapy.
PI3K/AKT INHIBITORS IN THE CLINIC
In the trial databases, we found 147 studies (Figure 4), which were designed to test the clinical profile of the six PI3K/Akt inhibitors. Only 11 trials - three published - were planned to assess the efficacy and safety of any of the PI3K/Akt inhibitors in combination with DNA-targeted agents (Table 4). We could not find any clinical trials being reported or registered to study the safety and efficacy of LY294002 or Wortmannin.
Figure 4.
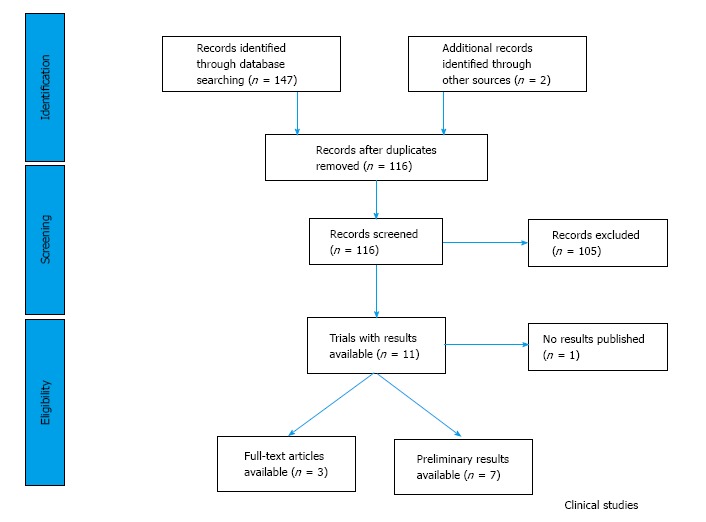
Review flow diagram of the publication selection in clinical category.
Table 4.
Clinical trials on phosphatidylinositol 3 kinase/Akt inhibitors in combination with DNA-targeted agents
| PI3K/Akt inhibitor | Target(s) | Combination arm(s) | Condition | Trial phase/status | Trial/registration |
| MK-2206 | Akt | Carboplatin + paclitaxel, docetaxel, erlotinib | Locally advanced, metastatic solid tumors | I/completed (published) | NCT00848718/February 2009[170] |
| Paclitaxel, trastuzumab | HER2-overexpressing advanced solid tumors | I/completed (abstract is published) | NCT01235897/November 2010[171] | ||
| Paclitaxel | Adult solid neoplasm, | I/ongoing (unpublished) | NCT01263145/December 2010 | ||
| recurrent or metastatic breast cancer | |||||
| Perifosine | PI3K/Akt | Docetaxel, prednisone | Neoplasms | I/completed (abstract is published) | NCT00399087/November 2006[172] |
| Docetaxel | Recurrent ovarian cancer | I/completed (abstract is published) | NCT00431054/February 2007[173] | ||
| Paclitaxel | Neoplasms | I/completed (abstract is published) | NCT00399126/November 2006[174] | ||
| Gemcitabine | Neoplasms | I/completed (abstract is published) | NCT00398697/November 2006[175] | ||
| Radiation | Solid tumors | I/published | Vink et al[176] | ||
| Radiation | Biochemically recurrent, hormone-sensitive prostate cancer with previous prostatectomy and/or radiation therapy | II/published | Chee et al[177] | ||
| BEZ235 | PI3K/mTOR | BEZ235 + paclitaxel, BKM120 + paclitaxel, BEZ235 + paclitaxel + trastuzumab, BKM120 + paclitaxel + trastuzumab | Metastatic or locally advanced solid tumors | I/completed (abstract is published) | NCT01285466/January 2011[178] |
| Paclitaxel | Inoperable locally advanced breast cancer, metastatic breast cancer | I and II/completed (abstract is published) | NCT01495247/September 2011[179] |
HER2: Human epidermal growth factor receptor 2; PI3K: Phosphatidylinositol 3 kinase; mTOR: Mammalian target of rapamycin.
MK-2206
From 36 trials on MK-2206, registered between April 2008 (the first trial) and May 2013 (the last trial), to evaluate the safety and efficacy of MK-2206; only three suited our selection criteria (Table 4).
In a phase I study with 72 patients with advanced solid tumor, Molife et al[170] (clinicaltrials.gov; NCT008 48718) demonstrated that MK-2206 (45 or 60 mg every other day) plus carboplatin [area under the curve 6.0 mg/mL (AUC 6)] and paclitaxel (200 mg/m2), docetaxel (75 mg/m2), or erlotinib (100 or 150 mg daily) was well-tolerated, with early evidence of antitumor activity. The main dose-limiting toxicities included skin rash, febrile neutropenia, tinnitus, and stomatitis. Common drug-related toxicities included fatigue, nausea, and rash.
In a phase I trial (NCT01235897) on 17 patients[171], MK2206 in combination with weekly paclitaxel (80 mg/m2 weekly) with or without trastuzumab (2 mg/kg weekly after a 1-time loading dose of 4 mg/kg) has been tested in patients with human epidermal growth factor receptor 2 (HER2)-overexpressing solid tumor malignancies (11 breast, 3 gastric, 1 esophageal). The highest safe dose of MK-2206 was found to be 135 mg weekly [The Best Disease Response by Response Evaluation Criteria in Solid Tumor (RECIST) scoring was used for evaluation of the treatment toxicity in 15 patients]. Two patients experienced dose-limiting toxicities, while 64% showed tumor response and 29% had no disease progression[171]. Although all patients experienced different adverse events due to the treatment, serious or life threatening adverse events were reported in 5/17 (29.41%) participants. Based on these results, the authors concluded that MK2206 weekly at a dose of 135 mg in combination with weekly paclitaxel and trastuzumab was safe and well tolerated.
A phase I trial (NCT01263145) is ongoing to determine the maximum tolerated dose, safety and antitumor activity of MK2206 and paclitaxel combination in patients with locally advanced or metastatic solid tumors or metastatic breast cancer. Thus, based on this scant evidence, we cannot conclude for or against administration of MK-2206 in combination with the available DNA-targeted agents.
Perifosine
From 42 trials on perifosine, registered between June 2000 (first trial) and August 2014 (last trial), only four studies as well as two published papers met our selection criteria for the current review.
Three trials evaluated the safety and efficacy of docetaxel or paclitaxel with perifosine (50, 100, and 150 mg/d). However, the results, despite the completion of the studies, have not yet been published (Table 4). In the first open-label study (NCT00399087) on 39 patients[172], daily doses up to 150 mg perifosine in combination with 75 mg/m2 per 3 wk docetaxel with/without prednisolone was tolerated. Furthermore, the maximum tolerated dose for the weekly perifosine was 1200 mg. In the second trial (NCT00431054)[173] the safety and efficacy of the combination of docetaxel and perifosine were studied on 22 patients with epithelial cancer of the ovary, fallopian tube cancer or gynecologic primary peritoneal cancer, which were platinum resistant or refractory. The third phase I trial (NCT00399126)[174] studied the gastrointestinal toxicity and cancer progression on the combination of daily perifosine with weekly or every 3-wk paclitaxel in 12 cancer patients. The preliminary results showed that the combination was well tolerated without increasing the toxicities being expected from using each drug alone.
Perifosine in combination with gemcitabine has also been studied in a non-randomized open-label phase I trial (NCT00398697) on 22 patients[175]. The preliminary results showed that 150 mg daily perifosine might have some manageable toxicities without affecting the pharmacokinetic of perifosine.
The feasibility and tolerability of daily perifosine and radiation combination have also been studied in two independent successive trials. Vink et al[176] tested this combination in 21 radio-naive patients with solid tumors, of which 17 had NSCLC. Dysphasia and pneumonitis were the main complications of radiotherapy, and nausea, fatigue, vomiting, diarrhea, and anorexia as major drug-related toxicities in the population. One hundred and fifty milligram daily perifosine combined with fractionated radiotherapy was considered a safe modality. Chee et al[177] conducted a phase II trial in 25 patients with biochemically recurrent, hormone-sensitive prostate cancer with previous prostatectomy and/or radiation therapy. However, only 20% of patients met the primary endpoint of prostate-specific antigen reduction, defined as a decrease by ≥ 50% from the pretreatment value. Accordingly, we should wait for the results of ongoing and future studies before coming to the conclusion if perifosine may add to the potency of DNA-targeted therapies.
BEZ235
From 23 trials on BEZ235, registered between February 2008 (first trial) and December 2013 (last trial), only two met our selection criteria for the final review, evaluating the safety and efficacy of the combination with any of the named DNA-targeted agents.
A phase I multi-center, open-label, 4-arm dose-escalation study (NCT01285466)[178] is ongoing to evaluate the safety and efficacy of oral BEZ235 and BKM120 in combination with weekly paclitaxel in patients with advanced solid tumors and weekly paclitaxel/trastuzumab in patients with HER2+ metastatic breast cancer. The preliminary results[178] showed that of 35 patients who received BEZ235 at 400-800 mg/d and paclitaxel at 70-80 mg/m2 per week dose-limiting toxicities occurred in 5 patients, 29% discontinued due to adverse effects and 63% due to progressive disease. Thus, they concluded that the safety over efficacy of this regimen would be questionable.
Additionally, a dose-finding phase I followed by an open-label, randomized phase II trial (NCT01495247)[179] of oral BEZ235 given twice daily (bid) with paclitaxel in patients with HER2 negative, locally advanced or metastatic breast cancer have recently been completed. The preliminary results with 18 patients showed that the determined maximum tolerated dose of BEZ235 (200 mg bid) in combination with paclitaxel (80 mg/m2 per week) was not reached, and the trial has been terminated[179]. Thus, based on these two preliminary results, BEZ235 seems not safe in combination with paclitaxel for patients with solid tumors.
CONCLUSION
The Akt pathway is clearly important for the regulation of cell proliferation and survival. Its activation is an additional resistance mechanism for current chemoradiotherapy. Therefore, modulation of Akt activity is an attractive strategy to enhance the efficacy of treatment. However, insight in the mechanism of protection is incomplete and warrants further research. This lack of knowledge hampers to properly evaluate combinations in clinic, while current clinical trials are too preliminary to draw conclusions, despite having several drugs that are relatively safe and efficacious. Thus, the Akt modulation is an attractive target to improve the toxicity and safety profile of classical antitumor compounds and irradiation. Nevertheless, studies were inadequate and therefore inconclusive regarding the additive effect of PI3K/Akt inhibitors to the standard regimens. Accordingly, more in-depth preclinical and clinical studies as well as a critical appraisal are warranted to find congruent rational avenues to designing solid studies on any of the combinations.
Footnotes
Conflict-of-interest statement: There are no conflicts of interests.
Manuscript source: Invited manuscript
Specialty Type: Oncology
Country of Origin: The Netherlands
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 24, 2016
First decision: May 16, 2016
Article in press: August 1, 2016
P- Reviewer: Kabir A, Lyakhovich A S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/- mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 4.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 6.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 7.Zhao JJ, Roberts TM. PI3 kinases in cancer: from oncogene artifact to leading cancer target. Sci STKE. 2006;2006:pe52. doi: 10.1126/stke.3652006pe52. [DOI] [PubMed] [Google Scholar]
- 8.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 9.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 10.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344 Pt 2:427–431. [PMC free article] [PubMed] [Google Scholar]
- 14.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem. 2000;275:30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Tsukada S, Satterthwaite A, Havlik MH, Park H, Takatsu K, Witte ON. Activation of Bruton’s tyrosine kinase (BTK) by a point mutation in its pleckstrin homology (PH) domain. Immunity. 1995;2:451–460. doi: 10.1016/1074-7613(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 16.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Chin YR, Yuan X, Balk SP, Toker A. PTEN-deficient tumors depend on AKT2 for maintenance and survival. Cancer Discov. 2014;4:942–955. doi: 10.1158/2159-8290.CD-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 21.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 22.FDA approves first PI3K inhibitor. Nature reviews Drug discovery. 2014;13:644–645. [Google Scholar]
- 23.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Sawah E, Chen X, Marchion DC, Xiong Y, Ramirez IJ, Abbasi F, Bou Zgheib N, Chon HS, Wenham RM, Apte SM, et al. Perifosine, an AKT inhibitor, modulates ovarian cancer cell line sensitivity to cisplatin-induced growth arrest. Gynecol Oncol. 2013;131:207–212. doi: 10.1016/j.ygyno.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 26.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34:155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 27.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 28.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara M, Izuishi K, Sano T, Hossain MA, Kimura S, Masaki T, Suzuki Y. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J Exp Clin Cancer Res. 2008;27:76. doi: 10.1186/1756-9966-27-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan J, You Y, Xu T, Yu P, Wu D, Deng H, Zhang Y, Bie P. Par-4 downregulation confers cisplatin resistance in pancreatic cancer cells via PI3K/Akt pathway-dependent EMT. Toxicol Lett. 2014;224:7–15. doi: 10.1016/j.toxlet.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Mitsuuchi Y, Johnson SW, Selvakumaran M, Williams SJ, Hamilton TC, Testa JR. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 2000;60:5390–5394. [PubMed] [Google Scholar]
- 33.Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 34.Kolasa IK, Rembiszewska A, Felisiak A, Ziolkowska-Seta I, Murawska M, Moes J, Timorek A, Dansonka-Mieszkowska A, Kupryjanczyk J. PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther. 2009;8:21–26. doi: 10.4161/cbt.8.1.7209. [DOI] [PubMed] [Google Scholar]
- 35.Hövelmann S, Beckers TL, Schmidt M. Molecular alterations in apoptotic pathways after PKB/Akt-mediated chemoresistance in NCI H460 cells. Br J Cancer. 2004;90:2370–2377. doi: 10.1038/sj.bjc.6601876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecol Oncol. 2004;94:785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Westfall SD, Skinner MK. Inhibition of phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to carboplatin and allows adjunct chemotherapy treatment. Mol Cancer Ther. 2005;4:1764–1771. doi: 10.1158/1535-7163.MCT-05-0192. [DOI] [PubMed] [Google Scholar]
- 38.Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, Eng C, Wu H, Song M, Dorigo O. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011;17:2373–2384. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahne JC, Honig A, Meyer SR, Gambaryan S, Walter U, Wischhusen J, Häussler SF, Segerer SE, Fujita N, Dietl J, et al. Downregulation of AKT reverses platinum resistance of human ovarian cancers in vitro. Oncol Rep. 2012;28:2023–2028. doi: 10.3892/or.2012.2041. [DOI] [PubMed] [Google Scholar]
- 40.Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int J Cancer. 2008;122:534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- 42.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 43.Ohta T, Ohmichi M, Hayasaka T, Mabuchi S, Saitoh M, Kawagoe J, Takahashi K, Igarashi H, Du B, Doshida M, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147:1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- 44.Wu DW, Lee MC, Hsu NY, Wu TC, Wu JY, Wang YC, Cheng YW, Chen CY, Lee H. FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA reduction. Oncogene. 2015;34:2505–2515. doi: 10.1038/onc.2014.184. [DOI] [PubMed] [Google Scholar]
- 45.Jin HO, Hong SE, Kim JH, Choi HN, Kim K, An S, Choe TB, Hwang CS, Lee JH, Kim JI, et al. Sustained overexpression of Redd1 leads to Akt activation involved in cell survival. Cancer Lett. 2013;336:319–324. doi: 10.1016/j.canlet.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Semba S, Trapasso F, Fabbri M, McCorkell KA, Volinia S, Druck T, Iliopoulos D, Pekarsky Y, Ishii H, Garrison PN, et al. Fhit modulation of the Akt-survivin pathway in lung cancer cells: Fhit-tyrosine 114 (Y114) is essential. Oncogene. 2006;25:2860–2872. doi: 10.1038/sj.onc.1209323. [DOI] [PubMed] [Google Scholar]
- 47.Wang XJ, Feng CW, Li M. ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem. 2013;380:57–66. doi: 10.1007/s11010-013-1657-z. [DOI] [PubMed] [Google Scholar]
- 48.Lou X, Zhou Q, Yin Y, Zhou C, Shen Y. Inhibition of the met receptor tyrosine kinase signaling enhances the chemosensitivity of glioma cell lines to CDDP through activation of p38 MAPK pathway. Mol Cancer Ther. 2009;8:1126–1136. doi: 10.1158/1535-7163.MCT-08-0904. [DOI] [PubMed] [Google Scholar]
- 49.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 50.Ito S, Igishi T, Takata M, Ueda Y, Matsumoto S, Kodani M, Takeda K, Izumi H, Sakamoto T, Yamaguchi K, et al. Synergistic cell growth inhibition by the combination of amrubicin and Akt-suppressing agents in K-ras mutation-harboring lung adenocarcinoma cells: implication of EGFR tyrosine kinase inhibitors. Int J Oncol. 2014;44:685–692. doi: 10.3892/ijo.2014.2249. [DOI] [PubMed] [Google Scholar]
- 51.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang B, Zhang K, Liu Z, Hao F, Wang M, Li X, Yin Z, Liang H. Secreted clusterin gene silencing enhances chemosensitivity of a549 cells to cisplatin through AKT and ERK1/2 pathways in vitro. Cell Physiol Biochem. 2014;33:1162–1175. doi: 10.1159/000358685. [DOI] [PubMed] [Google Scholar]
- 53.Herrera VA, Zeindl-Eberhart E, Jung A, Huber RM, Bergner A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011;31:849–854. [PubMed] [Google Scholar]
- 54.Pinton G, Manente AG, Angeli G, Mutti L, Moro L. Perifosine as a potential novel anti-cancer agent inhibits EGFR/MET-AKT axis in malignant pleural mesothelioma. PLoS One. 2012;7:e36856. doi: 10.1371/journal.pone.0036856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gálvez-Peralta M, Flatten KS, Loegering DA, Peterson KL, Schneider PA, Erlichman C, Kaufmann SH. Context-dependent antagonism between Akt inhibitors and topoisomerase poisons. Mol Pharmacol. 2014;85:723–734. doi: 10.1124/mol.113.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fekete M, Santiskulvong C, Eng C, Dorigo O. Effect of PI3K/Akt pathway inhibition-mediated G1 arrest on chemosensitization in ovarian cancer cells. Anticancer Res. 2012;32:445–452. [PubMed] [Google Scholar]
- 57.Liu Y, Chen X, Luo Z. [Combined inhibition of PI3K and MEK has synergistic inhibitory effect on the proliferation of cisplatin-resistant ovarian cancer cells] Xibao Yu Fenzi Mianyixue Zazhi. 2014;30:592–596. [PubMed] [Google Scholar]
- 58.Peng DJ, Wang J, Zhou JY, Wu GS. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun. 2010;394:600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karam AK, Santiskulvong C, Fekete M, Zabih S, Eng C, Dorigo O. Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskeleton (Hoboken) 2010;67:535–544. doi: 10.1002/cm.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nonaka M, Itamochi H, Kawaguchi W, Kudoh A, Sato S, Uegaki K, Naniwa J, Sato S, Shimada M, Oishi T, et al. Activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway overcomes cisplatin resistance in ovarian carcinoma cells. Int J Gynecol Cancer. 2012;22:922–929. doi: 10.1097/IGC.0b013e31824f0b13. [DOI] [PubMed] [Google Scholar]
- 61.Kudoh A, Oishi T, Itamochi H, Sato S, Naniwa J, Sato S, Shimada M, Kigawa J, Harada T. Dual inhibition of phosphatidylinositol 3’-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach for mucinous adenocarcinoma of the ovary. Int J Gynecol Cancer. 2014;24:444–453. doi: 10.1097/IGC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 62.Shingu T, Yamada K, Hara N, Moritake K, Osago H, Terashima M, Uemura T, Yamasaki T, Tsuchiya M. Growth inhibition of human malignant glioma cells induced by the PI3-K-specific inhibitor. J Neurosurg. 2003;98:154–161. doi: 10.3171/jns.2003.98.1.0154. [DOI] [PubMed] [Google Scholar]
- 63.Shingu T, Yamada K, Hara N, Moritake K, Osago H, Terashima M, Uemura T, Yamasaki T, Tsuchiya M. Synergistic augmentation of antimicrotubule agent-induced cytotoxicity by a phosphoinositide 3-kinase inhibitor in human malignant glioma cells. Cancer Res. 2003;63:4044–4047. [PubMed] [Google Scholar]
- 64.Nguyen DM, Chen GA, Reddy R, Tsai W, Schrump WD, Cole G, Schrump DS. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg. 2004;127:365–375. doi: 10.1016/j.jtcvs.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 65.Lan L, Sun T, Yang C, Xiong D, Liu D, Pang J, Yang L, Zhang L, Ren Y. [Effects of combined therapy of Phosphatidylinositol 3p-Kinase and Paclitaxel in human lung cancer nude mice model.] Zhongguo Feiai Zazhi. 2008;11:165–171. doi: 10.3779/j.issn.1009-3419.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 66.Morita M, Suyama H, Igishi T, Shigeoka Y, Kodani M, Hashimoto K, Takeda K, Sumikawa T, Shimizu E. Dexamethasone inhibits paclitaxel-induced cytotoxic activity through retinoblastoma protein dephosphorylation in non-small cell lung cancer cells. Int J Oncol. 2007;30:187–192. [PubMed] [Google Scholar]
- 67.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3’-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 68.Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E, Nakatsuji Y, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- 69.Kawaguchi W, Itamochi H, Kigawa J, Kanamori Y, Oishi T, Shimada M, Sato S, Shimogai R, Sato S, Terakawa N. Simultaneous inhibition of the mitogen-activated protein kinase kinase and phosphatidylinositol 3’-kinase pathways enhances sensitivity to paclitaxel in ovarian carcinoma. Cancer Sci. 2007;98:2002–2008. doi: 10.1111/j.1349-7006.2007.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim SH, Juhnn YS, Song YS. Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann N Y Acad Sci. 2007;1095:82–89. doi: 10.1196/annals.1397.012. [DOI] [PubMed] [Google Scholar]
- 71.Shi XY, Cai XJ, Lei JX, Cao FJ, Pan DF, Chen P. [Reversal effect of PI-3K/Akt pathway inhibitor LY294002 on multidrug resistance of ovarian cancer cell line A2780/Taxol] Aizheng. 2008;27:343–347. [PubMed] [Google Scholar]
- 72.Tsai MS, Kuo YH, Chiu YF, Su YC, Lin YW. Down-regulation of Rad51 expression overcomes drug resistance to gemcitabine in human non-small-cell lung cancer cells. J Pharmacol Exp Ther. 2010;335:830–840. doi: 10.1124/jpet.110.173146. [DOI] [PubMed] [Google Scholar]
- 73.Wilson SM, Barbone D, Yang TM, Jablons DM, Bueno R, Sugarbaker DJ, Nishimura SL, Gordon GJ, Broaddus VC. mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids. Am J Respir Cell Mol Biol. 2008;39:576–583. doi: 10.1165/rcmb.2007-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng SSW MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- 75.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299–2306. doi: 10.1158/1078-0432.ccr-03-0488. [DOI] [PubMed] [Google Scholar]
- 76.Holcomb B, Yip-Schneider MT, Matos JM, Dixon J, Kennard J, Mahomed J, Shanmugam R, Sebolt-Leopold J, Schmidt CM. Pancreatic cancer cell genetics and signaling response to treatment correlate with efficacy of gemcitabine-based molecular targeting strategies. J Gastrointest Surg. 2008;12:288–296. doi: 10.1007/s11605-007-0406-6. [DOI] [PubMed] [Google Scholar]
- 77.Ke XY, Wang Y, Xie ZQ, Liu ZQ, Zhang CF, Zhao Q, Yang DL. LY294002 enhances inhibitory effect of gemcitabine on proliferation of human pancreatic carcinoma PANC-1 cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33:57–62. doi: 10.1007/s11596-013-1071-5. [DOI] [PubMed] [Google Scholar]
- 78.Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, Schäfer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 79.Chen KC, Yang TY, Wu CC, Cheng CC, Hsu SL, Hung HW, Chen JW, Chang GC. Pemetrexed induces S-phase arrest and apoptosis via a deregulated activation of Akt signaling pathway. PLoS One. 2014;9:e97888. doi: 10.1371/journal.pone.0097888. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Yu K, Lucas J, Zhu T, Zask A, Gaydos C, Toral-Barza L, Gu J, Li F, Chaudhary I, Cai P, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005;4:538–545. doi: 10.4161/cbt.4.5.1660. [DOI] [PubMed] [Google Scholar]
- 81.Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- 82.Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- 83.Pham NA, Tsao MS, Cao P, Hedley DW. Dissociation of gemcitabine sensitivity and protein kinase B signaling in pancreatic ductal adenocarcinoma models. Pancreas. 2007;35:e16–e26. doi: 10.1097/mpa.0b013e318095a747. [DOI] [PubMed] [Google Scholar]
- 84.Motoshige H, Oyama K, Takahashi K, Sakurai K. Involvement of phosphatidylinositol 3-kinase/Akt pathway in gemcitabine-induced apoptosis-like cell death in insulinoma cell line INS-1. Biol Pharm Bull. 2012;35:1932–1940. doi: 10.1248/bpb.b12-00298. [DOI] [PubMed] [Google Scholar]
- 85.Awasthi N, Yen PL, Schwarz MA, Schwarz RE. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J Cell Biochem. 2012;113:784–791. doi: 10.1002/jcb.23405. [DOI] [PubMed] [Google Scholar]
- 86.Fischer B, Frei C, Moura U, Stahel R, Felley-Bosco E. Inhibition of phosphoinositide-3 kinase pathway down regulates ABCG2 function and sensitizes malignant pleural mesothelioma to chemotherapy. Lung Cancer. 2012;78:23–29. doi: 10.1016/j.lungcan.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Engel JB, Schönhals T, Häusler S, Krockenberger M, Schmidt M, Horn E, Köster F, Dietl J, Wischhusen J, Honig A. Induction of programmed cell death by inhibition of AKT with the alkylphosphocholine perifosine in in vitro models of platinum sensitive and resistant ovarian cancers. Arch Gynecol Obstet. 2011;283:603–610. doi: 10.1007/s00404-010-1457-6. [DOI] [PubMed] [Google Scholar]
- 88.Sun H, Yu T, Li J. Co-administration of perifosine with paclitaxel synergistically induces apoptosis in ovarian cancer cells: more than just AKT inhibition. Cancer Lett. 2011;310:118–128. doi: 10.1016/j.canlet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Xin Y, Shen XD, Cheng L, Hong DF, Chen B. Perifosine inhibits S6K1-Gli1 signaling and enhances gemcitabine-induced anti-pancreatic cancer efficiency. Cancer Chemother Pharmacol. 2014;73:711–719. doi: 10.1007/s00280-014-2397-9. [DOI] [PubMed] [Google Scholar]
- 90.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 91.Engel JB, Honig A, Schönhals T, Weidler C, Häusler S, Krockenberger M, Grunewald TG, Dombrowski Y, Rieger L, Dietl J, et al. Perifosine inhibits growth of human experimental endometrial cancers by blockade of AKT phosphorylation. Eur J Obstet Gynecol Reprod Biol. 2008;141:64–69. doi: 10.1016/j.ejogrb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Elrod HA, Lin YD, Yue P, Wang X, Lonial S, Khuri FR, Sun SY. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- 93.Li B, Wang L, Chi B. Upregulation of periostin prevents P53-mediated apoptosis in SGC-7901 gastric cancer cells. Mol Biol Rep. 2013;40:1677–1683. doi: 10.1007/s11033-012-2218-3. [DOI] [PubMed] [Google Scholar]
- 94.Ma BB, Lui VW, Hui CW, Lau CP, Wong CH, Hui EP, Ng MH, Tsao SW, Li Y, Chan AT. Preclinical evaluation of the AKT inhibitor MK-2206 in nasopharyngeal carcinoma cell lines. Invest New Drugs. 2013;31:567–575. doi: 10.1007/s10637-012-9896-5. [DOI] [PubMed] [Google Scholar]
- 95.Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3:193–203. doi: 10.2174/1568009033481967. [DOI] [PubMed] [Google Scholar]
- 96.Markman M. Taxanes in the management of gynecologic malignancies. Expert Rev Anticancer Ther. 2008;8:219–226. doi: 10.1586/14737140.8.2.219. [DOI] [PubMed] [Google Scholar]
- 97.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 98.Silasi DA, Alvero AB, Illuzzi J, Kelly M, Chen R, Fu HH, Schwartz P, Rutherford T, Azodi M, Mor G. MyD88 predicts chemoresistance to paclitaxel in epithelial ovarian cancer. Yale J Biol Med. 2006;79:153–163. [PMC free article] [PubMed] [Google Scholar]
- 99.Ji D, Deeds SL, Weinstein EJ. A screen of shRNAs targeting tumor suppressor genes to identify factors involved in A549 paclitaxel sensitivity. Oncol Rep. 2007;18:1499–1505. [PubMed] [Google Scholar]
- 100.Kosaka T, Miyajima A, Shirotake S, Suzuki E, Kikuchi E, Oya M. Long-term androgen ablation and docetaxel up-regulate phosphorylated Akt in castration resistant prostate cancer. J Urol. 2011;185:2376–2381. doi: 10.1016/j.juro.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12:1676–1687. doi: 10.1158/1535-7163.MCT-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J, Fan Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 103.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 104.Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, Kawagoe J, Takahashi K, Yada-Hashimoto N, Seino-Noda H, et al. Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin Cancer Res. 2004;10:7645–7654. doi: 10.1158/1078-0432.CCR-04-0958. [DOI] [PubMed] [Google Scholar]
- 105.Yang YI, Lee KT, Park HJ, Kim TJ, Choi YS, Shih IeM, Choi JH. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis. 2012;33:2488–2498. doi: 10.1093/carcin/bgs302. [DOI] [PubMed] [Google Scholar]
- 106.Almhanna K, Cubitt CL, Zhang S, Kazim S, Husain K, Sullivan D, Sebti S, Malafa M. MK-2206, an Akt inhibitor, enhances carboplatinum/paclitaxel efficacy in gastric cancer cell lines. Cancer Biol Ther. 2013;14:932–936. doi: 10.4161/cbt.25939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, Miller TW, Arteaga CL, Mills GB, Gonzalez-Angulo AM, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18:5816–5828. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rebecca VW, Massaro RR, Fedorenko IV, Sondak VK, Anderson AR, Kim E, Amaravadi RK, Maria-Engler SS, Messina JL, Gibney GT, et al. Inhibition of autophagy enhances the effects of the AKT inhibitor MK-2206 when combined with paclitaxel and carboplatin in BRAF wild-type melanoma. Pigment Cell Melanoma Res. 2014;27:465–478. doi: 10.1111/pcmr.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yasumizu Y, Miyajima A, Kosaka T, Miyazaki Y, Kikuchi E, Oya M. Dual PI3K/mTOR inhibitor NVP-BEZ235 sensitizes docetaxel in castration resistant prostate cancer. J Urol. 2014;191:227–234. doi: 10.1016/j.juro.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 110.Peters GJ, van der Wilt CL, van Moorsel CJ, Kroep JR, Bergman AM, Ackland SP. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther. 2000;87:227–253. doi: 10.1016/s0163-7258(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 111.Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1:7–17. doi: 10.1517/14796694.1.1.7. [DOI] [PubMed] [Google Scholar]
- 112.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2’,2’-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5:19–33. doi: 10.1016/s1368-7646(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 113.Elnaggar M, Giovannetti E, Peters GJ. Molecular targets of gemcitabine action: rationale for development of novel drugs and drug combinations. Curr Pharm Des. 2012;18:2811–2829. doi: 10.2174/138161212800626175. [DOI] [PubMed] [Google Scholar]
- 114.Galmarini CM, Clarke ML, Falette N, Puisieux A, Mackey JR, Dumontet C. Expression of a non-functional p53 affects the sensitivity of cancer cells to gemcitabine. Int J Cancer. 2002;97:439–445. doi: 10.1002/ijc.1628. [DOI] [PubMed] [Google Scholar]
- 115.Yang XL, Lin FJ, Guo YJ, Shao ZM, Ou ZL. Gemcitabine resistance in breast cancer cells regulated by PI3K/AKT-mediated cellular proliferation exerts negative feedback via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 2014;7:1033–1042. doi: 10.2147/OTT.S63145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danesi R, Altavilla G, Giovannetti E, Rosell R. Pharmacogenomics of gemcitabine in non-small-cell lung cancer and other solid tumors. Pharmacogenomics. 2009;10:69–80. doi: 10.2217/14622416.10.1.69. [DOI] [PubMed] [Google Scholar]
- 117.Giovannetti E, Mey V, Danesi R, Basolo F, Barachini S, Deri M, Del Tacca M. Interaction between gemcitabine and topotecan in human non-small-cell lung cancer cells: effects on cell survival, cell cycle and pharmacogenetic profile. Br J Cancer. 2005;92:681–689. doi: 10.1038/sj.bjc.6602382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz I Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, et al. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009;48:703–712. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- 121.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 122.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 123.Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 124.Soong R, Diasio RB. Advances and challenges in fluoropyrimidine pharmacogenomics and pharmacogenetics. Pharmacogenomics. 2005;6:835–847. doi: 10.2217/14622416.6.8.835. [DOI] [PubMed] [Google Scholar]
- 125.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11:282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 126.Peters GJ, Pinedo HM, Ferwerda W, de Graaf TW, van Dijk W. Do antimetabolites interfere with the glycosylation of cellular glycoconjugates? Eur J Cancer. 1990;26:516–523. doi: 10.1016/0277-5379(90)90029-s. [DOI] [PubMed] [Google Scholar]
- 127.Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, Zhou JR, Thiagalingam S. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011;71:998–1008. doi: 10.1158/0008-5472.CAN-09-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Samuel S, Fan F, Dang LH, Xia L, Gaur P, Ellis LM. Intracrine vascular endothelial growth factor signaling in survival and chemoresistance of human colorectal cancer cells. Oncogene. 2011;30:1205–1212. doi: 10.1038/onc.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang B, Zhang B, Chen X, Bae S, Singh K, Washington MK, Datta PK. Loss of Smad4 in colorectal cancer induces resistance to 5-fluorouracil through activating Akt pathway. Br J Cancer. 2014;110:946–957. doi: 10.1038/bjc.2013.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 131.Drabsch Y, ten Dijke P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31:553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 132.Samanta D, Datta PK. Alterations in the Smad pathway in human cancers. Front Biosci (Landmark Ed) 2012;17:1281–1293. doi: 10.2741/3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. doi: 10.1186/1471-2407-10-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iwase M, Yoshiba S, Uchid M, Takaoka S, Kurihara Y, Ito D, Hatori M, Shintani S. Enhanced susceptibility to apoptosis of oral squamous cell carcinoma cells subjected to combined treatment with anticancer drugs and phosphatidylinositol 3-kinase inhibitors. Int J Oncol. 2007;31:1141–1147. [PubMed] [Google Scholar]
- 135.Vandermoere F, El Yazidi-Belkoura I, Adriaenssens E, Lemoine J, Hondermarck H. The antiapoptotic effect of fibroblast growth factor-2 is mediated through nuclear factor-kappaB activation induced via interaction between Akt and IkappaB kinase-beta in breast cancer cells. Oncogene. 2005;24:5482–5491. doi: 10.1038/sj.onc.1208713. [DOI] [PubMed] [Google Scholar]
- 136.Richardson PG, Eng C, Kolesar J, Hideshima T, Anderson KC. Perifosine , an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin Drug Metab Toxicol. 2012;8:623–633. doi: 10.1517/17425255.2012.681376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ, Patel VF, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–1123. [PubMed] [Google Scholar]
- 138.Adjei AA. Pemetrexed: a multitargeted antifolate agent with promising activity in solid tumors. Ann Oncol. 2000;11:1335–1341. doi: 10.1023/a:1008379101017. [DOI] [PubMed] [Google Scholar]
- 139.Galvani E, Peters GJ, Giovannetti E. Thymidylate synthase inhibitors for non-small cell lung cancer. Expert Opin Investig Drugs. 2011;20:1343–1356. doi: 10.1517/13543784.2011.617742. [DOI] [PubMed] [Google Scholar]
- 140.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 141.Sigmond J, Backus HH, Wouters D, Temmink OH, Jansen G, Peters GJ. Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol. 2003;66:431–438. doi: 10.1016/s0006-2952(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 142.Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat. 2006;9:227–246. doi: 10.1016/j.drup.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G, Peters GJ. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol. 2008;73:1290–1300. doi: 10.1124/mol.107.042382. [DOI] [PubMed] [Google Scholar]
- 144.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Porcelli L, Assaraf YG, Azzariti A, Paradiso A, Jansen G, Peters GJ. The impact of folate status on the efficacy of colorectal cancer treatment. Curr Drug Metab. 2011;12:975–984. doi: 10.2174/138920011798062274. [DOI] [PubMed] [Google Scholar]
- 146.Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del Tacca M, Danesi R. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 147.Giovannetti E, Zucali PA, Assaraf YG, Leon LG, Smid K, Alecci C, Giancola F, Destro A, Gianoncelli L, Lorenzi E, et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer. 2011;105:1542–1553. doi: 10.1038/bjc.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 2014;4:280–291. doi: 10.1158/2159-8290.CD-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Debeb BG, Xu W, Woodward WA. Radiation resistance of breast cancer stem cells: understanding the clinical framework. J Mammary Gland Biol Neoplasia. 2009;14:11–17. doi: 10.1007/s10911-009-9114-z. [DOI] [PubMed] [Google Scholar]
- 150.Julsing JR, Peters GJ. Methylation of DNA repair genes and the efficacy of DNA targeted anticancer treatment. Oncol Discov. 2014;2:3. [Google Scholar]
- 151.McGinn CJ, Shewach DS, Lawrence TS. Radiosensitizing nucleosides. J Natl Cancer Inst. 1996;88:1193–1203. doi: 10.1093/jnci/88.17.1193. [DOI] [PubMed] [Google Scholar]
- 152.McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleosides. Semin Radiat Oncol. 2001;11:270–280. doi: 10.1053/srao.2001.26002. [DOI] [PubMed] [Google Scholar]
- 153.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 154.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009;4:43. doi: 10.1186/1748-717X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bernhard EJ, Stanbridge EJ, Gupta S, Gupta AK, Soto D, Bakanauskas VJ, Cerniglia GJ, Muschel RJ, McKenna WG. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–6600. [PubMed] [Google Scholar]
- 156.Kim IA, Bae SS, Fernandes A, Wu J, Muschel RJ, McKenna WG, Birnbaum MJ, Bernhard EJ. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 2005;65:7902–7910. doi: 10.1158/0008-5472.CAN-05-0513. [DOI] [PubMed] [Google Scholar]
- 157.Kim JY, Lee S, Hwangbo B, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. NF-kappaB activation is related to the resistance of lung cancer cells to TNF-alpha-induced apoptosis. Biochem Biophys Res Commun. 2000;273:140–146. doi: 10.1006/bbrc.2000.2909. [DOI] [PubMed] [Google Scholar]
- 158.Loftus JC, Dhruv H, Tuncali S, Kloss J, Yang Z, Schumacher CA, Cao B, Williams BO, Eschbacher JM, Ross JT, et al. TROY (TNFRSF19) promotes glioblastoma survival signaling and therapeutic resistance. Mol Cancer Res. 2013;11:865–874. doi: 10.1158/1541-7786.MCR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Principe P, Coulomb H, Broquet C, Braquet P. Evaluation of combinations of antineoplastic ether phospholipids and chemotherapeutic drugs. Anticancer Drugs. 1992;3:577–587. doi: 10.1097/00001813-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 160.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, McKenna WG. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 161.Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, Amici A, Boothman DA, Scaglioni PP. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu WL, Gao M, Tzen KY, Tsai CL, Hsu FM, Cheng AL, Cheng JC. Targeting Phosphatidylinositide3-Kinase/Akt pathway by BKM120 for radiosensitization in hepatocellular carcinoma. Oncotarget. 2014;5:3662–3672. doi: 10.18632/oncotarget.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berkovic D, Gründel O, Berkovic K, Wildfang I, Hess CF, Schmoll HJ. Synergistic cytotoxic effects of ether phospholipid analogues and ionizing radiation in human carcinoma cells. Radiother Oncol. 1997;43:293–301. doi: 10.1016/s0167-8140(97)01909-9. [DOI] [PubMed] [Google Scholar]
- 164.Belka C, Jendrossek V, Pruschy M, Vink S, Verheij M, Budach W. Apoptosis-modulating agents in combination with radiotherapy-current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–554. doi: 10.1016/j.ijrobp.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 165.Vink SR, Lagerwerf S, Mesman E, Schellens JH, Begg AC, van Blitterswijk WJ, Verheij M. Radiosensitization of squamous cell carcinoma by the alkylphospholipid perifosine in cell culture and xenografts. Clin Cancer Res. 2006;12:1615–1622. doi: 10.1158/1078-0432.CCR-05-2033. [DOI] [PubMed] [Google Scholar]
- 166.Rübel A, Handrick R, Lindner LH, Steiger M, Eibl H, Budach W, Belka C, Jendrossek V. The membrane targeted apoptosis modulators erucylphosphocholine and erucylphosphohomocholine increase the radiation response of human glioblastoma cell lines in vitro. Radiat Oncol. 2006;1:6. doi: 10.1186/1748-717X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- 168.Gao Y, Ishiyama H, Sun M, Brinkman KL, Wang X, Zhu J, Mai W, Huang Y, Floryk D, Ittmann M, et al. The alkylphospholipid, perifosine, radiosensitizes prostate cancer cells both in vitro and in vivo. Radiat Oncol. 2011;6:39. doi: 10.1186/1748-717X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de la Peña L, Burgan WE, Carter DJ, Hollingshead MG, Satyamitra M, Camphausen K, Tofilon PJ. Inhibition of Akt by the alkylphospholipid perifosine does not enhance the radiosensitivity of human glioma cells. Mol Cancer Ther. 2006;5:1504–1510. doi: 10.1158/1535-7163.MCT-06-0091. [DOI] [PubMed] [Google Scholar]
- 170.Molife LR, Yan L, Vitfell-Rasmussen J, Zernhelt AM, Sullivan DM, Cassier PA, Chen E, Biondo A, Tetteh E, Siu LL, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol. 2014;7:1. doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chien AJ, Truong TG, Melisko ME, Moasser MM, Kelley RK, Korn M, Ko AH, Pantoja N, Reinert A, Grabowsky JA, et al. Phase Ib dose-escalation trial of the AKT inhibitor MK2206 in combination with paclitaxel and trastuzumab in patients with HER2-overexpressing cancer. ASCO Meeting Abstracts. 2013;31:2605. [Google Scholar]
- 172.Cervera A, Bernhardt B, Nemunaitis JJ, Ebrahimi B, Birch R, Richards DA, Smith GB, Allerton JP, Henderson IC. Perifosine can be combined with docetaxel without dose reduction of either drug. ASCO Meeting Abstracts. 2006;24:13066. [Google Scholar]
- 173.Ebrahimi B, Nemunaitis JJ, Shiffman R, Birch R, Diaz-Lacayo M, Berdeaux DH, Rettenmaier MA, Goggins TF, Henderson IC. A phase 1 study of daily oral perifosine with weekly paclitaxel. ASCO Meeting Abstracts. 2006;24:13117. [Google Scholar]
- 174.Goggins TF, Nemunaitis JJ, Shiffman R, Birch R, Berdeaux DH, Rettenmaier M, Diaz-Lacayo M, Henderson IC. A phase 1 study of daily oral perifosine with every 3-week paclitaxel. ASCO Meeting Abstracts. 2006;24:13134. [Google Scholar]
- 175.Weiss S, Nemunaitis JJ, Diaz-Lacayo M, Birch R, Ebrahimi B, Berdeaux DH, Allerton JP, Gardner LR, Henderson IC. A phase 1 study of daily oral perifosine and weekly gemcitabine. ASCO Meeting Abstracts. 2006;24:13084. [Google Scholar]
- 176.Vink SR, Schellens JH, Beijnen JH, Sindermann H, Engel J, Dubbelman R, Moppi G, Hillebrand MJ, Bartelink H, Verheij M. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006;80:207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 177.Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 178.Rodon Ahnert J, Schuler MH, Machiels JPH, Hess D, Paz-Ares L, Awada A, von Moos R, Steeghs N, Cruz Zambrano C, De Mesmaeker P, et al. Phase lb study of BEZ235 plus either paclitaxel in advanced solid tumors or PTX plus trastuzumab (TZ) in HER2 breast cancer (BC) ASCO Meeting Abstracts. 2014;32:621. [Google Scholar]
- 179.Gil-Martin M, Fumoleau P, Isambert N, Urruticoechea A, Beck J, Csonka D, di Tomaso E, Gaur A, Roiffe K, Urban P, et al. Abstract P2-16-22: A dose-finding phase lb study of BEZ235 in combination with paclitaxel in patients with HER2-negative, locally advanced or metastatic breast cancer. Cancer research. 2013;73:P2–16-22. [Google Scholar]


