Abstract
Dies1/VISTA induces embryonic stem-cell differentiation, via BMP-pathway, but also acts as inflammation regulator and immune-response modulator. Dies1 inhibition in a melanoma-mouse model led to increased tumour-infiltrating T-cells and decreased tumour growth, emphasizing Dies1 relevance in tumour-microenvironment. Dies1 is involved in cell de/differentiation, inflammation and cancer processes, which mimic those associated with Epithelial-to-Mesenchymal-Transition (EMT). Despite this axis linking Dies1 with EMT and cancer, its expression, modulation and relevance in these contexts is unknown. To address this, we analysed Dies1 expression, its regulation by promoter-methylation and miR-125a-5p overexpression, and its association with BMP-pathway downstream-effectors, in a TGFβ1-induced EMT-model, cancer cell-lines and primary samples. We detected promoter-methylation as a mechanism controlling Dies1 expression in our EMT-model and in several cancer cell-lines. We showed that the relationship between Dies1 expression and BMP-pathway effectors observed in the EMT-model, was not present in all cell-lines, suggesting that Dies1 has other cell-specific effectors, beyond the BMP-pathway. We further demonstrated that: Dies1 expression loss is a recurrent event in GC, caused by promoter methylation and/or miR-125a-5p overexpression and; GC-microenvironment myofibroblasts overexpress Dies1. Our findings highlight Dies1 as a novel player in GC, with distinct roles within tumour cells and in the tumour-microenvironment.
Dies1/VISTA (ENSG00000107738, ENSMUSG000000201011) is a type-I membrane protein, which induces terminal differentiation of embryonic stem cells (ESCs) into neurons or cardiomyocytes, via the Bone Morphogenetic Protein (BMP)-signalling pathway2. This occurs under the control of a regulatory loop involving BMP4 activation of miR-125a-5p, which in turn is capable of directly repressing Dies1 transcription, halting ESC differentiation3. Dies1 also plays a crucial regulatory role during adipocyte differentiation, however independently of BMP-signalling. In particular, upregulation of Dies1 was demonstrated exclusively in differentiated fat cells and its knockdown inhibited adipocyte differentiation4. Dies1 has also been implicated in inflammation, due to the demonstration of chronic multi-organ inflammation and increased inflammatory chemokines’ serum levels in Dies1-knockout mice5. Although this mouse model lacked systemic or organ-specific autoimmune disease, an increased population of activated peripheral T-cells was observed, in agreement with previous data showing that antigen presenting cells (APCs) expressing Dies1/VISTA are able to directly suppress T-cell proliferation6. Similar observations were reported by Yoon et al., which have also shown that Dies1 (DD1α) works as a homophilic receptor in T-cells, as well as a key molecule interacting simultaneously with apoptotic cells and macrophages, facilitating phagocytic engulfment. Interestingly, this process was shown to be tightly controlled by p53, as Dies1 was shown to be a downstream target of this tumour suppressor gene7. Supporting the role of Dies1 as an immune-response regulator, Le Mercier et al. have also shown that Dies1 blockade, using a monoclonal antibody, caused an increase in tumour-infiltrating T-cells and decreased tumour growth, in an inducible melanoma transgenic mouse model6. Altogether, the described roles credited to Dies1 span the fields of differentiation, inflammation and cancer.
Epithelial to mesenchymal transition (EMT) occurs naturally during embryogenesis, but may also occur associated with pathological contexts of chronic inflammation, fibrosis and cancer8,9,10. This process increases cellular plasticity, through a large scale cellular reprogramming, that promotes a shift from differentiated, polarized epithelial cells to dedifferentiated mesenchymal-like cells8,9,10,11. EMT is thought to bestow cancer cells with de novo migratory and invasion properties, enabling cancer progression and distant organ colonization. In addition, EMT has been shown to generate cells with increased stem-like properties, either by enrichment in stem cell subpopulations and/or increased expression of stem cell markers, such as CD44 and CD2411,12,13. EMT can be triggered in epithelial cells, by inflammatory mediators such as TGFβ1, TNFα and interleukins thought to be produced by immune cells from the microenvironment. As a consequence, EMT inducers like Snail and Twist become overexpressed and downregulate the EMT hallmark protein E-cadherin14. These molecules are all important players in cancer with impact in cancer progression and patient´s prognosis9,15,16,17. EMT is therefore a process also involved in differentiation, inflammation and cancer.
Increased exposure of epithelial cells to inflammatory factors during chronic inflammation can favour tumourigenesis18,19. This association between inflammation and tumourigenesis may provide a mechanistic explanation for the augmented cancer risk in some inflammatory disorders. This is particularly valid for the gastrointestinal tract, which is highly exposed to pro-inflammatory factors produced by the gut microbiome, presenting a persistent chronic low-level inflammation state20. Upon oncogenic transformation, epithelial cells exposed to inflammatory signals may be more resistant to elimination by the immune system, which in turn promotes tumour progression20. Several studies have shown that areas of gastric adenocarcinoma frequently overlap with regions of chronic inflammation. In fact, upon chronic inflammation, parietal cells and chief cells in the gastric mucosa may be lost, leading to a reduction in signals for growth and differentiation of gastric progenitors21. Transgenic mice with specific ablation of parietal cells, revealed an accumulation of undifferentiated progenitors with increased levels of proliferation and occurrence of intestinal metaplasia, a crucial step in Correa’s pathway for gastric adenocarcinoma development22. During the process of gastric tumourigenesis, signalling pathways such as the PI3K/AKT, Wnt/β-catenin, Notch and TGF-β become activated and may trigger cancer-related EMT, either transiently or persistently23,24,25,26. Loss of CDH1/E-cadherin expression and increased cancer stem cell self-renewal are processes that often take place when gastric cancer (GC) is developing26. GC is not the single example of inflammation-associated cancer, as colorectal cancer risk also increases with the severity of inflammation in the setting of ulcerative colitis27. In this nonspecific inflammatory condition, characterized by alternating periods of active disease and remission, it has been shown consistent up-regulation of an EMT-related signature, specifically in the intestinal mucosa of active ulcerative colitis patients’27. Altogether these examples support the existence of an axis involving cellular differentiation, chronic inflammation, EMT and tumour progression. Interestingly, this axis appears to overlap the diverse roles of Dies1 as differentiation inducer, inflammation regulator and cancer immunity modulator. In the present study, we used a TGFβ1-induced EMT model, epithelial cancer cell lines and cancer samples to dissect the mechanisms underlying Dies1 expression and disclose its role in epithelial carcinogenesis.
Results
In the present study, we analysed the expression and regulation of Dies1 in a TGFβ1-induced EMT model, epithelial cancer cell line models and cancer samples, aiming at disclosing a role for this molecule in epithelial carcinogenesis, and the mechanisms that may control its expression and signalling.
Dies1 expression and its promoter methylation status vary along EMT and MET
We started by assessing Dies1 mRNA expression variation in an EMT/MET in vitro model, that we established using the spontaneously immortalized normal mammary epithelial cell line, EpH428. By subjecting the parental EpH4 cells (E-cells) to a 7-day treatment with the inflammatory cytokine TGFβ1, we obtained a culture of mesenchymal cells (M-cells), with a classical fibroblastoid morphology, downregulation or functional inactivation of several epithelial markers (for example, Ocln and Mgat3 downregulation, and E-cadherin deregulation28), and upregulation of mesenchymal markers (i.e. Vim, Zeb2, Twist1). Upon TGFβ1 removal from the culture medium, M-cells re-acquired a cobblestone morphology, creating a Reversed-Epithelial cell population (RE-cells). Overall, RE-cells displayed an expression pattern for epithelial and mesenchymal markers similar to E-cells, with a recovery of an epithelial-like signature28. Our EMT/MET in vitro model simulates the dynamics observed during reversible inflammation-induced cellular dedifferentiation, with M-cells constituting a less differentiated population in opposition to the more differentiated E and RE-cells.
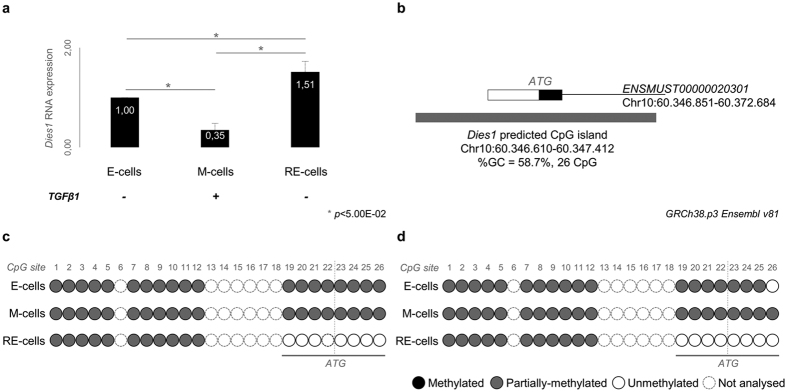
By qRT-PCR we observed that Dies1 expression became significantly downregulated in M-cells when compared to E-cells (p = 1.00E-03, Fig. 1a). Interestingly, in RE-cells Dies1 expression was recovered to levels even higher than in the original E-cells (p = 1.30E-03 for M vs. RE-cells, p = 1.40E-02 for E vs. RE-cells, Fig. 1a). We next investigated whether this alteration in Dies1 RNA expression could be caused by alterations in the methylation status of its gene promoter. Using Ensembl database1 and the web tool “CpG Island Searcher” 29, we detected a possible CpG island encompassing 26 CpG sites at the 5′-end of Dies1 locus (GRCm38.p4, Chr10:60.346.610-60.347.412, %GC = 58.7%, Fig. 1b). Sequencing of E, M and RE-cells bisulfite-treated DNA revealed that at least 7/8 CpG sites were recurrently methylated in E and M cells, and demethylated in RE-cells (CpG sites 19-26, examples of 2 biological replicates in Fig. 1c and Supplementary Fig. S1). Importantly, these CpG sites overlap with the start codon of Dies1 (ATG at chr10: 60.347.022 for ENSMUST00000020301)1. The fluctuation in Dies1 expression in RE-cells could therefore be associated with the methylation pattern at these specific CpG sites: E and M-cells presenting lower expression and denser methylation, while RE-cells presenting increased Dies1 expression and some degree of demethylation.
Figure 1. Dies1 expression and regulation across an in vitro model of EMT and MET.
(a) Dies1 expression in E-, M- and RE-cells. Asterisks stand for significantly distinct comparisons (p < 5.00E-02). (b) Schematic representation of Dies1 predicted CpG island in mouse. White rectangle for Dies1 5′UTR in exon 1. Black rectangle for coding region of exon 1. Grey rectangle for the predicted CpG island. (c,d) Two examples of the results obtained for the direct-sequencing of Dies1 predicted CpG island. Representation of each CpG site using white circles for unmethylated CpG sites, grey circles for partially-methylated CpG sites and black circles for fully methylated CpG sites.
Dies1 modulates the expression of its downstream effectors ID2 and ID3 in an EMT/MET model
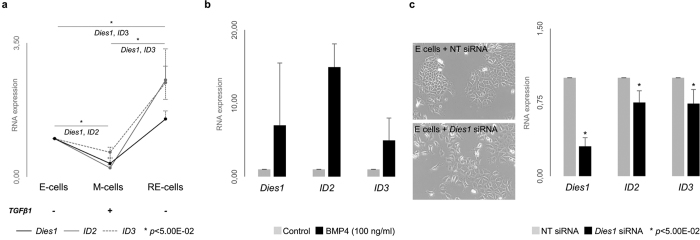
Previous studies have shown that Dies1 is a crucial modulator of stem cell differentiation, exerting its regulatory action in either a BMP-pathway dependent or independent manner2,4. To understand whether Dies1 effects were BMP-signalling dependent in our EMT/MET model, we assessed the expression of ID2 and ID3, which have been previously described as downstream effectors of Dies1 and BMP2. By qRT-PCR, we verified that ID2 and ID3 closely followed the expression pattern of Dies1 across EMT/MET (Fig. 2a). ID2 and ID3 were both downregulated in M-cells (ID2 statistically significant: p = 5.91E-03 for E vs. M-cells) and upregulated in RE-cells (ID3 statistically significant: p = 2.98E-02 for M vs. RE-cells and 4.15E-02 for E vs. RE-cells). To prove that Dies1 was under the control of the BMP-pathway, we submitted E-cells to a 48h-treatment with exogenous BMP4. In fact, we observed an overall increase in Dies1 expression (Fig. 2b) as well as of ID2 and ID3, demonstrating that Dies1 is modulated by BMP signalling in E-cells. Next, to understand whether the fluctuation of ID2 and ID3 expression was induced by Dies1 expression changes, we specifically inhibited Dies1 expression in E-cells by RNAi. Upon 70% inhibition of Dies1 expression, both ID2 and ID3 were significantly downregulated (NT siRNA vs. Dies1 siRNA: p = 5.66E-03 for ID2 and p = 3.51E-03 for ID3, Fig. 2c). These results demonstrate that ID2 and ID3 expression is modulated by Dies1 in E-cells.
Figure 2. Dies1 and BMP-pathway effectors expression in E-, M-, RE- cells and in E-cells with specific inhibition of Dies1.
(a) Expression of Dies1, ID2 and ID3 in E-, M- and RE-cells. Asterisks stand for significantly distinct comparisons (p < 5.00E-02). (b) Expression of Dies1, ID2 and ID3 in E-cells after exogenous stimulation with BMP4 (Dies1 expression fold change from 2.0x to 16.8x, ID2 expression fold change from 11.3x to 17.2x, and ID3 expression fold change from 1.5x to 7,1x). (c) Brightfield images of E-cells transfected with Non-Targeting siRNA (NT siRNA) and Dies1 siRNA and expression of Dies1, ID2 and ID3.
The expression of Dies1 and its downstream targets is generally downregulated in epithelial cancer cell lines and may be explained by epigenetic mechanisms
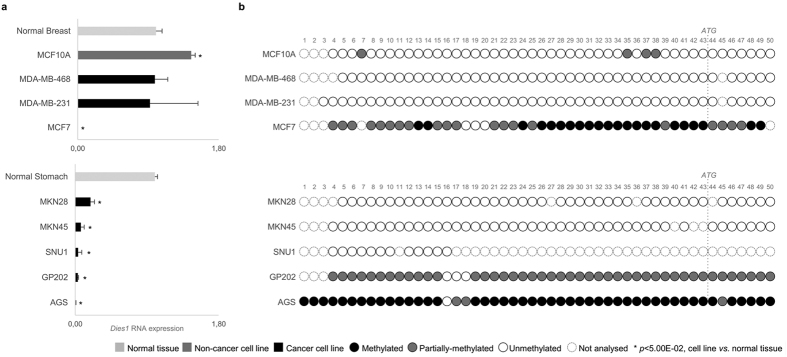
Given the observed variation of Dies1 expression during EMT/MET and the increasing relevance of these processes for cancer progression13,23,30, we evaluated the expression of Dies1 in 12 cell lines derived from epithelial cancer: 3 from breast, 5 from gastric and 4 from colon. We also analysed normal breast, gastric and colon tissues and an immortalized normal breast epithelial cell line (MCF10A) (Fig. 3a, Supplementary Fig. S2). Dies1 was found significantly downregulated in 9/13 cancer cell lines in comparison with corresponding normal tissues and/or normal cancer cell line: 1/4 breast; 3/4 colon; and 5/5 gastric cancer cell lines (Fig. 3a, Supplementary Fig. S2). Importantly, all GC cell lines displayed consistently very low or no expression of Dies1 in comparison with normal stomach (p < 1.16E-03, Fig. 3a).
Figure 3. Dies1 expression and promoter methylation status in a panel of breast cancer, gastric cancer and colorectal cancer cell lines.
(a) Dies1 expression in a panel of breast cancer cell lines (MDA-MB-468, MDA-MB-231 and MCF7) normalized to normal breast tissue and of gastric cancer cell lines (MKN28, MKN45, SNU1, GP202 and AGS) normalized to normal gastric tissue. Also included is the near-normal breast cell line MCF10A (non-cancer, dark grey bar). Light grey bars to normal tissues and black bars correspond to cancer cell lines. Asterisks stand for significantly distinct comparisons (p < 5.00E-02). (b) Dies1 promoter methylation status in all described cancer cell lines (and MCF10A). Representation of each CpG site using white circles for unmethylated CpG sites, grey circles for partially-methylated CpG sites, black circles for fully methylated CpG sites and dashed white circles for unassessed CpG sites.
Given that Dies1 expression could be modulated by promoter methylation, we next investigated the methylation status of Dies1 promoter in all cancer cell lines. Using the same approach described for the murine Dies1 locus, we were able to identify a CpG island upstream of the human Dies1 locus, encompassing 50 CpG sites (CpG island located at chr10:71.773.335-71.773.735, GRCh38.p3 Ensembl v81, Fig. 3b, Supplementary Fig. S2). The breast cancer cell line MCF7 displayed low Dies1 expression levels and concomitant widespread methylation across Dies1 promoter. In contrast, MCF10A, MDA-MB-468 and MDA-MB-231 displayed Dies1 expression at the same levels of normal breast tissue, and an almost completely demethylated Dies1 promoter. These data demonstrated a direct relationship between Dies1 expression and the methylation status of its promoter in all breast cell lines analysed (Fig. 3b). The promoter methylation analysis recurrently failed for colon cancer cell lines, with a single cell line (RKO) displaying 9 methylated CpG sites out of the 12 that we succeeded to analyse (Supplementary Fig. S2). In GC cell lines, the overall downregulation of Dies1 expression was followed by extensive promoter methylation in two cell lines (AGS and GP202), while in the other two (MKN45 and MKN28) the gene promoter remained demethylated, despite the very low Dies1 expression levels (Fig. 3b). Overall, these data suggest that Dies1 expression is downregulated in epithelial cancer derived cell lines, and that as in the EMT/MET model, Dies1 expression can be controlled by methylation at the gene promoter in a fraction of the cell lines analysed.
Given the massive Dies1 downregulation in GC cell lines, and in order to identify a potential mechanism that could downregulate Dies1 in the two GC cell lines (MKN45 and MKN28) lacking promoter methylation, we explored the expression of miR-125a-5p, known to target Dies1 3′-UTR in mouse ESCs31, in these two GC cell lines. We first confirmed that the murine Dies1 3′-UTR target region for miR-125a-5p was perfectly conserved with a region within the human Dies1 3′UTR (starting at chr10: 60.371.477, Fig. 4a). Next, we used the web tool MiRWalk 2.032 to predict all possible miRNAs targeting the human Dies1 3′UTR. We observed that all five selected databases, included within MiRWalk, consistently predicted a binding site for miR-125a-5p in the same region of the human Dies1 3′-UTR, which was seen to be conserved with the murine Dies1 3′-UTR target region for miR-125a-5p. We therefore analysed the expression of miR-125a-5p, by qRT-PCR, in four GC cell lines: GP202 and AGS with widespread Dies1 promoter methylation and; MKN45 and MKN28 without Dies1 promoter methylation. We observed that in GP202 and MKN45, miR-125a-5p was significantly downregulated in comparison with normal stomach (p < 2.32E-04, Fig. 4b), hence excluding it as responsible for Dies1 downregulation. In AGS and MKN28, miR-125a-5p was found to be overexpressed (p = 4.47E-02 for AGS and p > 5.00E-02 for MKN28) in comparison with normal stomach (Fig. 4b). These observations suggest that in AGS, promoter methylation and miR-125a-5p overexpression could be acting in concomitance, while in MKN28, Dies1 downregulation could be solely due to miR-125a-5p overexpression. To understand whether miR-125a-5p was directly targeting Dies1, we next specifically inhibited miR-125a-5p expression in MKN28 cells, using antimiRs technology.Three biological replicates were performed, all successfully inhibiting miR-125a-5p expression with concomitant increase in Dies1 expression (Fig. 4c). Of notice, Dies1 expression was found to be strongly correlated with the efficiency of miR-125a-5p inhibition (Fig. 4d, R2 = 0,94). Concerning ID2 and ID3, an overall trend for increased expression was observed upon miR-125a-5p downregulation (Fig. 4c). These results reinforce that in MKN28, Dies1 expression is under the control of miR-125a-5p. Since, it has been recently described that Dies1 is a downstream target of p53, we tried to understand whether mutations in TP53 could also constitute a mechanism underlying Dies1 downregulation in GC cells lines. Indeed, COSMIC data showed that MKN45 carries a TP53 mutation (heterozygous R110C33) that may explain Dies1 downregulation. Additionally, MKN28 also carried a TP53 mutation (homozygous I251L33) besides miR-125a-5p upregulation.
Figure 4. Dies1 expression and miR-125a-5p in gastric cancer cell lines.
(a) Conservation of the murine and human Dies1 seed region for miR-125a-5p. Asterisks stand for conserved nucleotides, dashes for complementary regions. (b) Dies1 and miR-125a-5p expression in gastric cancer cell lines in comparison with normal stomach mucosa (in log scale). Also included is a summary of the Dies1 promoter methylation status (same legend as in Fig. 1 applies), for comparison and correlation purposes. Asterisks stand for significantly distinct comparisons (p < 5.00E-02). (c) RNA expression analysis confirming miR-125a-5p inhibition (from 0,016x to 0,001x vs. negative control) and concomitance increased expression of Dies1 (from 1,58x to 1,94x vs. negative control), ID2 (from 0,87x to 2,19x vs. negative control) and ID3 (from 0,92x to 1,87x vs. negative control. (d) Representation of the RNA expression analysis results correlating miR-125a-5p expression with Dies1 expression for each biological replicate performed.
We also assessed ID2 and ID3 mRNA expression across the same panel of human cell lines (n = 13, Supplementary Fig. S2). Unlike what was observed for Dies1 in breast cancer cell lines, ID2 and ID3 were significantly downregulated in all cell lines (p < 2.72E-03), demonstrating no relationship between Dies1 and its potential downstream targets ID2 and ID3 in this cancer type. In contrast, 3/4 colon cancer cell lines exhibited ID2 or ID3 expression loss concomitantly with Dies1 downregulation (Supplementary Fig. S2). In 5/5 GC cell lines, ID2 or ID3 expression was low or undetectable in comparison with normal stomach (p < 7.67E-03), and in agreement with Dies1 downregulation. In summary, Dies1 expression downregulation seems particularly relevant in GC, as this is a consistent feature of all GC cell lines analysed. This downregulation could be explained via Dies1 promoter methylation, miR-125a-5p overexpression, or a combination of both, and even through the presence of mutant p53. Moreover, as ID2 and ID3 follow the Dies1 expression trend in GC, it is likely that they act asDies1 downstream targets also in the GC model.
Expression of Dies1 and ID3 is recurrently downregulated in primary GC samples
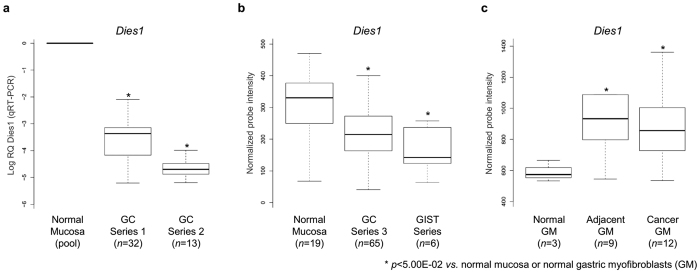
The consistent downregulation of Dies1 in GC cell lines led us to investigate the relevance of its expression in primary GC samples. We assessed, by qRT-PCR, the expression of Dies1 in 45 samples from two independent GC series available to us in light of ongoing collaborations with São João Hospital (GC series 1: 32 samples; GC series 2: 13 samples). Dies1 was significantly downregulated in both GC series in comparison to a pool of normal stomach mucosas (GC series 1: p = 5.00E-03; GC series 2: p = 1.00E-02 for GC, Fig. 5a). To re-enforce these results, we also mined the gene expression profiles of GC samples assessed by microarray technology for which data was deposited and available for analysis at the ArrayExpress database (E-GEOD-2694234). This experiment encompassed 65 GC human samples (GC series 3), 6 gastrointestinal stromal tumour (GIST series) samples, and 19 normal stomach mucosa samples, used for comparison purposes. Mimicking the results obtained for GC series 1 and 2, Dies1 expression was significantly downregulated in samples from GC series 3 and GIST samples, in comparison with normal mucosas (GC series 3: p = 1.60E-03; GIST: p = 3.40E-03, Fig. 5b). Next, we tried to understand whether Dies1 downregulation was also detected at the protein level, using three anti-Dies1 antibodies. These antibodies were tested for immunohistochemistry, immunocytochemistry and Western Blot, using normal stomach mucosa samples, as well as human cell lines. However, inconsistent and non-reproducible results were obtained, e.g. different staining patterns within the same histological structure (Supplementary Fig. S3). Therefore, Dies1 protein expression assessment was not further pursued.
Figure 5. Dies1 expression in three independent GC Series, six GIST samples and in a series of normal, adjacent and cancer-associated gastric myofibroblasts (GM).
(a) Dies1 expression in GC Series 1 and 2 normalized to a pool of normal gastric mucosa. Asterisks stand for significantly distinct comparisons (p < 5.00E-02). (b) Dies1 expression in a pool of normal mucosas, in GC Series 3 and in six GIST samples. (c) Dies1 expression in a series of normal, adjacent and cancer-associated gastric myofibroblasts.
Following the same rational used for cell lines, we were able to assess the TP53 status (WT or mutated) in the 32 GC samples encompassing Series 1. This information was available as these 32 GC samples have been subjected to Whole Genome Sequencing in a parallel ongoing project of the group. We found that 9/32 (28.1%) of GC samples displayed somatic TP53 mutations: frameshift mutation TP53_g.14066G > A; missense mutations TP53_g.12521A > C, TP53_g.13794G > A, TP53_g.13370G > A, TP53_g.13379C > T, TP53_g.13824C > T, TP53_g.13797C > T or TP53_g.13779C > T and; nonsense mutation TP53_g.12706C > T. We further assessed ID2 and ID3 expression in GC series 3 and in the GIST series. We detected a significant downregulation of ID3 in GC series 3 in comparison with the normal stomach mucosa samples (p = 1.42E-05), but ID2 expression remained unchanged in this experiment (Supplementary Fig. S4).
In summary, we observed a consistent downregulation of Dies1 in three independent GC series in comparison with normal gastric mucosa that was mimicked by ID3 expression downregulation.
Expression of Dies1 is increased in myofibroblasts from the GC microenvironment
Recent findings have shown that Dies1 is overexpressed in APCs within the tumour microenvironment, directly suppressing T-cell proliferation6. To understand whether Dies1 expression could be detected in other tumour microenvironment components, we analysed data derived from a set of gene expression microarrays experiments deposited in the ArrayExpress database (E-GEOD-44740)34, from Balabanova et al.35. We assessed Dies1 expression in primary cultures of myofibroblasts isolated from: gastric tumours (n = 12); normal tissue adjacent to gastric tumours (n = 9), and; normal tissue from healthy donors (n = 3). We observed that in both adjacent and cancer-associated gastric myofibroblasts, Dies1 expression was significantly increased in comparison with gastric tissue myofibroblasts from healthy donors (p = 3.60E-02 and p = 3.10E-02, respectively, Fig. 5c). Concerning ID2 and ID3 expression, no significant alterations were detected among the three types of gastric myofibroblasts (Supplementary Fig. S4). Overall, in cancer-associated or cancer-adjacent gastric myofibroblasts Dies1 is overexpressed, however the expression of its potential downstream targets, ID2 and ID3, does not follow the same trend. These data support distinct roles for Dies1 in tumour cells and in the surrounding microenvironment, and suggest that Dies1 may signal through distinct pathways, depending on the cell type.
Discussion
The aim of this study was to explore the relevance of Dies1 gene expression, and its potential downstream targets, in an in vitro model mimicking TGFβ1-associated inflammation and cellular transdifferentiation, and in epithelial cancer. We started by assessing Dies1 expression and its promoter methylation status in a TGFβ1-induced EMT in vitro model. This model was designed to recreate a TGFβ1-induced inflammation process known to be relevant for both cellular differentiation and cancer. We verified that epithelial cells treated with TGFβ1 to trigger EMT, significantly decrease Dies1 expression without any promoter methylation changes. However, when cells recovered from EMT regaining an epithelial-like phenotype, Dies1 became overexpressed and specific promoter CpG sites became demethylated. These results are particularly interesting when considering that EMT is a process that can create cells with increased self-renewal ability and multi-lineage differentiation potential36. Following on this idea, it is valid to assume that cells that underwent EMT became dedifferentiated in comparison with the original epithelial population (Dies1 expression decreased), and that upon TGFβ1 removal, cells differentiated towards an epithelial phenotype (Dies1 expression increased). It seems therefore that in the present model, and as previously described in ESCs2, Dies1 expression is necessary for the reacquisition of a more differentiated phenotype, and may act as a regulator of cellular differentiation. We also show for the first time that Dies1 expression is controlled by promoter methylation.
Following the data described for ESCs2, in our EMT/MET model the expression variation of BMP-signalling effectors ID2 and ID3 closely mimicked that of Dies1, supporting Dies1 role as a differentiation regulator acting in a BMP-dependent manner. Aloia et al. had shown that the Dies1 role in ESCs differentiation was associated and dependent to BMP-signalling: on one hand, upon Dies1 knockdown, ID1/2/3 expression was lost and ESCs were not allowed to differentiate; on the other hand, when combining Dies1 knockdown with chemical suppression of the BMP-signalling receptor Alk3, ESCs overcame Dies1 absence and became more differentiated. To understand whether this crosstalk between Dies1 and BMP-signalling was also valid in our model, we first stimulated this pathway in our E-cells with exogenous BMP4 treatment and observed an increase in Dies1 expression, as well as ID2/ID3. This observation proved that Dies1 was under the control of the BMP-pathway in our model. Next, we specifically inhibited Dies1 expression in E-cells, as Dies1 expression was too low in M-cells to allow further downregulation. Dies1 depletion led to a decrease in ID2/ID3 expression, in agreement with Aloia et al. data, supporting a bidirectional relationship between Dies1 and BMP-signalling in our model. Also of notice was the fact that BMP4 expression was found to be specifically downregulated in M-cells, which could explain the expression downregulation of Dies1 in these cells (Supplementary Fig. S1). In fact, M-cells are generated by exogenous treatment with TGF-β1, which might be diverting the shared cofactor Smad4 signaling from the BMP-pathway (likely active in E-cells) towards the TGFβ-pathway. Nevertheless, further experiments ought to be performed to confirm this hypothesis. Taken together, our results suggest a role for Dies1 in TGFβ1-induced EMT/MET, with increased Dies1 expression associated with more differentiated cellular states (E- and RE-cells).
In light of the above results and literature data associating Dies1 with cancer, we assessed Dies1 expression and its promoter methylation status across a panel of epithelial cancer cell lines. We observed a complete correlation between Dies1 expression and promoter methylation status in breast cancer cell lines, as those that were demethylated expressed Dies1 and those that were methylated did not. To the best of our knowledge, this is the first epigenetic mechanism leading to Dies1 downregulation described in breast cancer cell lines. We excluded an exclusive relationship between Dies1 and BMP-signalling in the breast cancer model by assessing the expression of ID2/ID3, as these genes were systematically downregulated independently of Dies1 expression. In addition, one of the breast cancer cell lines used, MDA-MB-468, has an expression-impairing deletion in the Smad4 gene, hence decoupling Dies1 expression from BMP-signalling37. The observations in the breast cancer cell lines are in line with findings by Ren et al.4 who showed that Dies1 silencing inhibited adipogenesis without altering the levels of BMP-pathway regulators such as phospho-Smad1, even in the presence of the BMP-receptor ligand, BMP44. Altogether, these data mainly demonstrate the limited role for Dies1 in breast cancer and highlight the fact that the BMP-pathway and the expression of its targets is independent from Dies1.
In the colon model, although ID2/ID3 expression paralleled Dies1 expression in all cell lines analysed, 2/3 cell lines are Smad4-deficient38,39 and therefore irresponsive to the BMP-signalling. This suggests that, as seen for the breast cancer cell lines, Dies1 expression is independent from BMP-signalling in colon cancer.
In the present study, the most consistent cancer-associated data was obtained for the GC model. We have shown that Dies1 is downregulated in all GC cell lines assessed, regardless of their differentiation status (poorly differentiated: AGS, SNU1, MKN45, GP202; moderately differentiated: MKN28) or their EMT signature (metastable: MKN45, MKN28, AGS; mesenchymal-like: SNU1)40,41,42,43,44,45. We were able to demonstrate that Dies1 promoter methylation could control Dies1 expression in some cell lines, while miR-125a-5p expression, a known transcription repressor of Dies1 in ESCs31, can control Dies1 expression in others. In fact, we proved that in MKN28 cells, which displayed no methylation at the promoter level, Dies1 expression is under the control of miR-125a-5p, as its targeted inhibition led to an overexpression of Dies1. Synergistic effects may also be underlying Dies1 expression downregulation in GC cell lines: for example, AGS cells displayed the lowest levels of Dies1, likely due to the co-occurrence of promoter methylation and miR-125a-5p overexpression. Other mechanisms may also help explaining Dies1 downregulation, such as p53 mutation status, in light of Yoon et al. study. For example, MKN45 cells, for which neither miR-125a-5p overexpression nor Dies1 promoter methylation was detected, encode a p53 heterozygous mutation (R110C33), which could explain Dies1 downregulation. In fact, p53 mutations were also detected in MKN28 (homozygous I251L33), strengthening the existence of combinatory mechanisms underlying Dies1 massive downregulation. Nevertheless, p53 mutation status per se may not predict Dies1 expression, given that breast cancer cells MDA-MB-468 and MDA-MB-231, display high levels of Dies1 and mutations in p53 (homozygous R273H and heterozygous R280K33, respectively). Beyond the described genetic/epigenetic mechanisms, we may not exclude a contribution of BMP signalling to the regulation of Dies1 expression, as we have observed that Dies1, ID2 and ID3 were concomitantly decreased in all GC cells lines.
The GC cell line data was reinforced by data obtained from three different series of primary GC samples. Strikingly, Dies1 was significantly downregulated in all GC samples analysed (n = 110 samples), in comparison to normal stomach mucosa. We tried to understand whether Dies1 mRNA downregulation was reflected in Dies1 protein expression loss, using several antibodies and techniques. However, inconsistent results were obtained, showing that further studies should be performed, using other available antibodies, to address whether Dies1 protein expression is also lost in GC.
Interestingly, 9 out of the 32 GC samples from Series 1 displayed somatic TP53 mutations, which may help explaining Dies1 downregulation. No information was available concerning TP53 status in Series 2. Dies1 was also downregulated in a small series of gastrointestinal stromal tumours (GIST), a fact not surprising given the mesenchymal origin of these tumours46, which recalled Dies1 downregulation in our epithelial cells that undergo EMT. Of notice is the fact that Dies1 downregulation was followed by ID3 downregulation, strengthening the association between Dies1 and BMP-signalling in GC.
Following on the observations that Dies1 is an immune-response modulator when expressed in cells from the tumour microenvironment6,7,47,48, we found that Dies1 expression was significantly upregulated in cancer-adjacent and cancer-associated gastric myofibroblasts, in comparison with normal gastric myofibroblasts. In fact, the secretome of cancer-associated gastric myofibroblasts has been shown to impact tumour growth, reinforcing the relevance of stromal cells in the tumour microenvironment49. Moreover, when overexpressed in APCs from the tumour microenvironment, Dies1 actively suppressed T-cell proliferation, both in vitro and in vivo, leading to a decreased anti-tumoral immune response and promoting tumour growth in a T-cell dependent manner47. Our observations suggest that Dies1 may also have a role in other tumour microenvironment components, such as cancer-associated myofibroblasts. In summary, we disclosed promoter methylation as an epigenetic mechanism controlling Dies1 expression in an inflammation-induced EMT model and in several epithelial cancer cell lines. We have also shown that the relationship between Dies1 expression and BMP-signalling, found for the EMT model, may be mimicked or not in different cancer types. This suggests that Dies1 may have other cell-specific effectors, beyond the BMP-pathway. Our results demonstrated that Dies1 loss of expression is a recurrent event in GC, in concomitance with promoter methylation and in association, or not, with miR-125a-5p overexpression, and even TP53 mutations. Finally, we have also shown that in GC, ID3 is likely a downstream effector of Dies1, and that GC myofibroblasts overexpress Dies1, which may be acting together with immune cells to refrain the anti-tumoral immune response, enabling tumour growth.
Methods
Cell culture
EpH4 cell line (E-cells) was cultured in D-MEM/F12-Glutamax™ (Invitrogen) supplemented with fetal bovine serum (5%, Lonza), penicillin-streptomycin (1%, Invitrogen) and insulin (5 μg/ml, Sigma-Aldrich). EpH4 cell line was kindly provided by Dr. Angela Burleigh and Dr. Calvin Roskelley from British Columbia Cancer Agency (Vancouver, Canada). Mesenchymal cell cultures (M-cells) were obtained by adding transforming-growth-factor-β1 (TGFβ1, Sigma-Aldrich) during 7 days. Reverted-epithelial cell cultures (RE-cells) were obtained by replacing TGFβ1-enriched medium by normal culture medium for another 4 days. Cell lines MCF10A, MDA-MB-468, MDA-MB-231, MCF7, MKN28, SNU1, MKN45, AGS, SW480, HCT116, HT29 and RKO were acquired from ATCC and cultured using recommended mediums (RPMI/DMEM, Invitrogen, 10% fetal bovine serum Lonza and 1% penicillin-streptomycin, Invitrogen). GP202 was obtained from Ipatimup’s Cell Line Bank40 and cultured using recommended mediums (RPMI, Invitrogen, 10% fetal bovine serum Lonza and 1% penicillin-streptomycin, Invitrogen). All cell lines authentication was performed at the Ipatimup’s Cell Lines Bank, using STR amplification (Promega-Powerplex16, Identifiler).
RNA extraction and expression quantification
RNA was extracted using mirVana miRNA Isolation Kit (Invitrogen) following manufacturer’s instructions from: (1) 3 biological replicas of EpH4 cell line stages (E, M, RE-cells); (2) all described cell lines and; (3) randomly selected tissue specimens from three GC series (GC Series 1, n = 32 GC samples, GC Series 2, n = 13 GC samples and n = 10 normal gastric mucosa from an available IPATIMUP/Hospital São João dataset, informed consent obtained from all patients, study approved by Hospital S. João Ethics Committee). Of notice, for Series 1 and Series 2, GC cases were reviewed by an expert pathologist and RNA was extracted from microdissected gastric tumours in regions displaying more than 70% of gastric cancer cells. RNA from pools of normal stomach, colon and breast tissue was acquired from Stratagene (Agilent Technologies). RNA was reversed-transcribed using Superscript-II-Reverse-Transcriptase and random-hexamers (Invitrogen). Quantitative-Real-Time-PCR (qRT-PCR) was performed using RNA from 3 biological replicas (E, M, RE-cells and cancer cell lines described), in a ABI Prism 7000 Sequence Detection System and using TaqMan Gene Expression Assays (Applied Biosystems) or PrimeTime qPCR Assays (Integrated DNA Technologies), for the following genes: mouse CDH1 (Mm00486909_g1), Vim (Mm01333430_m1), ID2 (Mm00711781_m1), ID3 (Mm00492575_m1), Dies1 (Mm00472312_m1) and; human Dies1 (Hs00735289_m1), ID2 (Hs00171409_m1), ID3 (Hs00747379_m1) and miR-125a-5p (hsa-miR-125a-5p). The endogenous controls used were mouse and human GAPDH (Mm99999915_g1, Hs02758991_g1) for cell lines and human 18S for GC series samples (Hs99999901_s1). All data was analysed by the comparative 2(−ΔΔCT) method50 and two-tailed Student’s T-test or Wilcoxon signed-rank test applied51.
DNA extraction and methylation analysis
DNA from 3 independent biological replicas of E, M, RE-cells and described human cancer cell lines was extracted using Invisorb Spin Tissue Mini Kit, following manufacturer’s instructions (STRATEC Molecular). DNA was subjected to bisulfite-conversion, using Epitect Bisulfite Kit, following manufacturer’s instructions (Qiagen). Murine/human Dies1 promoter methylation analysis was carried out within the CpG islands bioinformatically predicted using the following criteria: (1) genomic area with ≥500 bp; (2) a percentage of GC ≥55 and; (3) the observed/expected CpG dinucleotides ≥0.65 [32]. Using the Ensembl database version 81 (GRCh38.p3)1, the web tool “CpG Island Searcher”29 and the described criteria, a single CpG island was predicted within/in the vicinity of murine Dies1 locus at chr10:60.346.610-60.347.412. Concerning human Dies1, the same strategy was used and the predicted CpG island was located at chr10:71.773.335-71.773.735 (antisense strand). Custom designed primers (Sigma-Aldrich) used for bisulfite-treated-DNA amplification of murine/human Dies1 promoter CpG island regions were: murine forward primer: 5′-GGGAAGTGGTTGGTTGGATA-3′; murine reverse primer: 5′-CCCATCCTACCCCCTACACT-3′; human forward primer 1: 5′-GAGGTAGATTTATTTTTTAGGT-3′; human reverse primer 1: 5′-CTATCTTCTCCCAAC-3′; human forward primer 2: 5′-AGGTAGTTTTTTTATA-3′ and human reverse primer 2: 5′-CTATCTTCTCCCAACTT-3′ (Sigma-Aldrich). Bisulfite-PCR products were sequenced for methylation-status determination using the same custom-designed primers in a 3130 Genetic Analyzer (Applied Biosystems).
Short-interference-RNA experiments
Short-interference-RNA experiments were performed using mouse Dies1 siGENOME-SMARTpool (100 nM for 72 hours, Thermo Fisher Scientific), ON-TARGET plus Non-targeting siRNA #4 (100 nM for 72 hours, Thermo Fisher Scientific) as non-targeting control and, Lipofectamine 2000 (Thermo Fisher Scientific) as transfection agent.
BMP4 treatment
BMP-pathway stimulation was performed using rhBMP4 (100 ng/mL for 48 hours, Immunotools).
AntiMir experiments
AntiMiR experiments were performed using anti-miR miRNA inhibitor for hsa-miR-125a-5p or anti-miR Negative Control #1 (50 nM for 24 hours, Ambion) and Lipofectamine 3000 (Thermo Fisher Scientific) as transfection agent.
Data mining of GC series
Microarray data from GC and gastrointestinal stromal tumours (GIST) human samples (GC series 3) collected from ArrayExpress34, ID: E-GEOD-26942, for probes ILMN_2205963 (Dies1), ILMN_1793990 (ID2) and ILMN_1732296 (ID3). Microarray data from normal myofibroblasts, adjacent myofibroblasts and cancer-associated gastric myofibroblasts collected from ArrayExpress, ID: E-GEOD-44740 for probes 225372_at (Dies1), 213931_at (ID2) and 207826_s_at (ID3)35. Analysed data from both experiments was used for boxplot representation and statistical significance assessed using Wilcoxon signed-rank test in R51.
Additional Information
How to cite this article: Oliveira, P. et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci. Rep. 6, 34860; doi: 10.1038/srep34860 (2016).
Supplementary Material
Acknowledgments
IPATIMUP integrates the i3S Research Unit, which is partially supported by FCT, the Portuguese Foundation for Science and Technology. This work was funded by: 1) FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT/ Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274); 2) FCT Fellowships (SFRH/BPD/89764/2012 to PO; SFRH/BPD/86543/2012 to JC; SFRH/BD/63300/2009 to VC; SFRH/BPD/104208/2014 to BS and; 3) Salary support to JP from iFCT Program 2012, POPH - QREN Type 4.2, European Social Fund and Portuguese Ministry of Science and Technology (MCTES).
Footnotes
Author Contributions P.O., J.C. and C.O. were involved in the study’s conception, design, and funding; P.O., J.C., S.R., M.A., I.R., V.C., S.V. and B.S. conducted the study; P.O., J.C., S.R., M.A. and C.O. analysed the data; P.O., J.C. and C.O. wrote the manuscript; P.O., J.C., J.P., R.A., D.H. and C.O. reviewed the manuscript; and all authors gave final approval of the manuscript.
References
- Cunningham F. et al. Ensembl 2015. Nucleic acids research 43, D662–D669, 10.1093/nar/gku1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia L., Parisi S., Fusco L., Pastore L. & Russo T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. The Journal of biological chemistry 285, 7776–7783, 10.1074/jbc.M109.077156 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista M. et al. miR-125b regulates the early steps of ESC differentiation through dies1 in a TGF-independent manner. International journal of molecular sciences 14, 13482–13496, 10.3390/ijms140713482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Beech C. & Smas C. M. The immunoglobulin superfamily protein differentiation of embryonic stem cells 1 (dies1) has a regulatory role in preadipocyte to adipocyte conversion. Plos one 8, e65531, 10.1371/journal.pone.0065531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proceedings of the National Academy of Sciences of the United States of America 111, 14846–14851, 10.1073/pnas.1407447111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mercier I. et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer research 74, 1933–1944, 10.1158/0008-5472.CAN-13-1506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. W. et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 349, 1261669, 10.1126/science.1261669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Dedhar S., Kalluri R. & Thompson E. W. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of cell biology 172, 973–981, 10.1083/jcb.200601018 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A., Brabletz T. & Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nature cell biology 16, 488–494, 10.1038/ncb2976 (2014). [DOI] [PubMed] [Google Scholar]
- Kalluri R. & Weinberg R. A. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation 119, 1420–1428, 10.1172/JCI39104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X. & Weinberg R. A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends in cell biology 25, 675–686, 10.1016/j.tcb.2015.07.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. J. & Yang M. H. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Bioscience reports 31, 449–455, 10.1042/BSR20100114 (2011). [DOI] [PubMed] [Google Scholar]
- Mani S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715, 10.1016/j.cell.2008.03.027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Portillo F. & Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. The International journal of developmental biology 48, 365–375, 10.1387/ijdb.041794hp (2004). [DOI] [PubMed] [Google Scholar]
- Khan M. A., Chen H. C., Zhang D. & Fu J. Twist: a molecular target in cancer therapeutics. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 34, 2497–2506, 10.1007/s13277-013-1002-x (2013). [DOI] [PubMed] [Google Scholar]
- Sanchez-Tillo E. et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cellular and molecular life sciences: CMLS 69, 3429–3456, 10.1007/s00018-012-1122-2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nes J. G. et al. Co-expression of SNAIL and TWIST determines prognosis in estrogen receptor-positive early breast cancer patients. Breast cancer research and treatment 133, 49–59, 10.1007/s10549-011-1684-y (2012). [DOI] [PubMed] [Google Scholar]
- Coussens L. M. & Werb Z. Inflammation and cancer. Nature 420, 860–867, 10.1038/nature01322 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R. & Karin M. Immunity, inflammation, and cancer. Cell 140, 883–899, 10.1016/j.cell.2010.01.025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner-Feigl S., Kesselring R. & Strober W. Chronic inflammation and the development of malignancy in the GI tract. Trends in immunology 36, 451–459, 10.1016/j.it.2015.06.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G. & Wang T. C. Inflammation, atrophy, and gastric cancer. The Journal of clinical investigation 117, 60–69, 10.1172/JCI30111 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Karam S. M. & Gordon J. I. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. The Journal of biological chemistry 271, 3671–3676 (1996). [PubMed] [Google Scholar]
- Lu W. D., Zuo Y., Xu Z. & Zhang M. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. World journal of gastroenterology 21, 4564–4573, 10.3748/wjg.v21.i15.4564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. Journal of experimental & clinical cancer research: CR 33, 38, 10.1186/1756-9966-33-38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer cells. Oncogene 33, 2737–2747, 10.1038/onc.2013.238 (2014). [DOI] [PubMed] [Google Scholar]
- Peng Z., Wang C. X., Fang E. H., Wang G. B. & Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World journal of gastroenterology 20, 5403–5410, 10.3748/wjg.v20.i18.5403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. et al. Mobilization of epithelial mesenchymal transition genes distinguishes active from inactive lesional tissue in patients with ulcerative colitis. Human molecular genetics 24, 4615–4624, 10.1093/hmg/ddv192 (2015). [DOI] [PubMed] [Google Scholar]
- Pinho S. S. et al. Loss and recovery of Mgat3 and GnT-III Mediated E-cadherin N-glycosylation is a mechanism involved in epithelial-mesenchymal-epithelial transitions. PloS one 7, e33191, 10.1371/journal.pone.0033191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai D. & Jones P. A. The CpG island searcher: a new WWW resource. In silico biology 3, 235–240 (2003). [PubMed] [Google Scholar]
- Pang M. F. et al. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene, 10.1038/onc.2015.133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi S. et al. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26, 3957–3968, 10.1096/fj.12-211607 (2012). [DOI] [PubMed] [Google Scholar]
- Dweep H. & Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nature methods 12, 697, 10.1038/nmeth.3485 (2015). [DOI] [PubMed] [Google Scholar]
- Forbes S. A. et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic acids research 43, D805–D811, 10.1093/nar/gku1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson H. et al. ArrayExpress update–an archive of microarray and high-throughput sequencing-based functional genomics experiments. Nucleic acids research 39, D1002–D1004, 10.1093/nar/gkq1040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanova S. et al. The neuroendocrine phenotype of gastric myofibroblasts and its loss with cancer progression. Carcinogenesis 35, 1798–1806, 10.1093/carcin/bgu086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula V. L. et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem cells 28, 1435–1445, 10.1002/stem.467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winter J. P., Roelen B. A., ten Dijke P., van der Burg B. & van den Eijnden-van Raaij A. J. DPC4 (SMAD4) mediates transforming growth factor-beta1 (TGF-beta1) induced growth inhibition and transcriptional response in breast tumour cells. Oncogene 14, 1891–1899, 10.1038/sj.onc.1201017 (1997). [DOI] [PubMed] [Google Scholar]
- Pohl M. et al. SMAD4 mediates mesenchymal-epithelial reversion in SW480 colon carcinoma cells. Anticancer Res. 30, 2603–2613, 30/7/2603 (2010). [PubMed] [Google Scholar]
- Kaiser C. et al. Chemogenomic analysis identifies Macbecin II as a compound specific for SMAD4-negative colon cancer cells. Chem Biol Drug Des. 75, 360–368, JPP949 10.1111/j.1747-0285.2010.00949.x (2010). [DOI] [PubMed] [Google Scholar]
- Gartner F., David L., Seruca R., Machado J. C. & Sobrinho-Simoes M. Establishment and characterization of two cell lines derived from human diffuse gastric carcinomas xenografted in nude mice. Virchows Arch 428, 91–98 (1996). [DOI] [PubMed] [Google Scholar]
- Motoyama T., Hojo H. & Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn. 36, 65–83 (1986). [DOI] [PubMed] [Google Scholar]
- Huang N. et al. MiR-338-3p inhibits epithelial-mesenchymal transition in gastric cancer cells by targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget 6, 15222–15234, 10.18632/oncotarget.3835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. et al. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochemical and biophysical research communications 358, 925–930, 10.1016/j.bbrc.2007.05.023 (2007). [DOI] [PubMed] [Google Scholar]
- Yano K., Imaeda T. & Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. American journal of physiology. Gastrointestinal and liver physiology 294, G336–G343, 10.1152/ajpgi.00328.2007 (2008). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-kB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget 6, 8323–8338, 10.18632/oncotarget.3200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. C. et al. Beyond the GIST: mesenchymal tumors of the stomach. Radiographics: a review publication of the Radiological Society of North America, Inc. 33, 1673–1690, 10.1148/rg.336135507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. The Journal of experimental medicine 208, 577–592, 10.1084/jem.20100619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J. L., Sempere L. F., Broughton T., Wang L. & Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer immunology research 2, 510–517, 10.1158/2326-6066.CIR-14-0072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg C. et al. Release of TGFbetaig-h3 by gastric myofibroblasts slows tumor growth and is decreased with cancer progression. Carcinogenesis 33, 1553–1562, 10.1093/carcin/bgs180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.