Abstract
Background
Metastatic castration-resistant prostate cancer (mCRPC) often involves bone, and bone-targeted therapy (BTT) has become part of the overall treatment strategy.
Objective
Investigation of outcomes for concomitant BTT in a post hoc analysis of the COU-AA-302 trial, which demonstrated an overall clinical benefit of abiraterone acetate (AA) plus prednisone over placebo plus prednisone in asymptomatic or mildly symptomatic chemotherapy-naïve mCRPC patients.
Design, setting, and participants
This report describes the third interim analysis (prespecified at 55% overall survival [OS] events) for the COU-AA-302 trial.
Intervention
Patients were grouped by concomitant BTT use or no BTT use.
Outcome measurements and statistical analysis
Radiographic progression-free survival and OS were coprimary end points. This report describes the third interim analysis (prespecified at 55% OS events) and involves patients treated with or without concomitant BTT during the COU-AA-302 study. Median follow-up for OS was 27.1 mo. Median time-to-event variables with 95% confidence intervals (CIs) were estimated using the Kaplan-Meier method. Adjusted hazard ratios (HRs), 95% CIs, and p values for concomitant BTT versus no BTT were obtained via Cox models.
Results and limitations
While the post hoc nature of the analysis is a limitation, superiority of AA and prednisone versus prednisone alone was demonstrated for clinical outcomes with or without BTT use. Compared with no BTT use, concomitant BTT significantly improved OS (HR 0.75; p = 0.01) and increased the time to ECOG deterioration (HR 0.75; p < 0.001) and time to opiate use for cancer-related pain (HR 0.80; p = 0.036). The safety profile of concomitant BTT with AA was similar to that reported for AA in the overall intent-to-treat population. Osteonecrosis of the jaw (all grade 1/2) with concomitant BTT use was reported in <3% of patients.
Conclusions
AA with concomitant BTT was safe and well tolerated in men with chemotherapy-naïve mCRPC. The benefits of AA on clinical outcomes were increased with concomitant BTT.
Patient summary
Treatment of advanced prostate cancer often includes bone-targeted therapy. This post hoc analysis showed that in patients with advanced prostate cancer who were treated with abiraterone acetate and prednisone in combination with bone-targeted therapy, there was a continued trend in prolongation of life when compared with patients treated with prednisone alone.
Trial registration
Keywords: Abiraterone acetate, Bone-targeted therapy, Chemotherapy-naïve, Metastatic castration-resistant prostate cancer
1. Introduction
Prostate cancer is the second most commonly diagnosed malignancy in men, with a 5-yr prevalence estimate of 3 200 000 men worldwide [1]. In the USA, prostate cancer is the second leading cause of cancer-related death in men [2]. Most patients who present with metastatic disease at diagnosis or with disease recurrence after potentially curative therapy with prostatectomy or radiation therapy respond to castration with androgen deprivation therapy [3]; however, progression to castration-resistant disease usually occurs within 2–3 yr [3,4]. Ultimately, almost all patients who progress after androgen deprivation therapy develop metastatic castration-resistant prostate cancer (mCRPC) [3,4], and more than 90% of patients with mCRPC develop bone metastases, which produces significant morbidity in many men and is associated with increased mortality [5,6].
Bone-targeted therapy (BTT) delays the development of symptoms and reduces the risk of bone-specific morbidities [7–10]. The BTT agents bisphosphonates and denosumab inhibit osteoclast-mediated bone resorption and can prevent bone loss and increase bone mineral density in patients with prostate cancer receiving androgen deprivation therapy [10–12]. Zoledronic acid and denosumab are effective for the prevention of skeletal-related events in patients with metastatic prostate cancer [13]. Historically, bone-seeking radiopharmaceuticals have been used as a palliative treatment for pain in patients with metastatic prostate cancer [14]. Radium-223 was the first radiopharmaceutical agent shown to improve survival for symptomatic patients with mCRPC and no visceral disease, and was recently approved for this indication [15].
Abiraterone acetate is converted in vivo to abiraterone, which inhibits the enzyme 17α-hydroxylase/C17,20-lyase and blocks extragonadal and testicular androgen biosynthesis, a key target for blocking tumor growth in mCRPC [16,17].
Study COU-AA-302 compared the efficacy and safety of abiraterone acetate plus low-dose prednisone (hereafter referred to as abiraterone) compared to prednisone alone in asymptomatic or mildly symptomatic men with chemotherapy-naïve mCRPC [20]. Abiraterone doubled time to radiographic progression-free survival (rPFS) compared to prednisone alone (median 16.5 vs 8.3 mo). All secondary endpoints significantly favored abiraterone over prednisone alone [20].
The third interim analysis (IA3) of study COU-AA-302 (56% of expected death events) confirmed that patients treated with abiraterone had a statistically significant improvement in rPFS (HR 0.52; p < 0.0001), The overall survival (OS) analysis favored abiraterone over prednisone alone (median 35.3 vs 30.1 mo, HR 0.79; p = 0.0151; prespecified statistical boundary α = 0.0035), and the OS benefit of abiraterone was supported in an exploratory multivariate analysis (HR 0.74; p = 0.0017) that adjusted for baseline prognostic factors [22]. In addition, analyses of prespecified measures of patient-reported outcomes confirmed that abiraterone treatment delayed pain progression and deterioration in functional status compared with prednisone alone [21,22]. The objective of the present post hoc analysis of study COU-AA-302 was to evaluate the clinical benefits and safety of abiraterone and concomitant BTT in chemotherapy-naïve mCRPC patients.
2. Patients and methods
Study COU-AA-302 (NCT00887198) is a phase 3, multinational, randomized, double-blind, placebo-controlled study being conducted at 151 sites in 12 countries. Patients were enrolled from April 2009 to June 2010, and the study is ongoing. The ethics review boards at all participating institutions approved the study, and all patients gave written informed consent.
2.1 Study design
The study design and the primary and secondary efficacy endpoints have been described previously [20]. The study included male patients with mCRPC aged ≥18 yr who were medically or surgically castrated, had tumor progression, and were asymptomatic or mildly symptomatic. Patients with visceral metastases were excluded, as were patients who had received previous therapy with ketoconazole for >7 d.
Patients were randomized at a 1:1 ratio to receive abiraterone acetate 1 g daily and prednisone 5 mg twice daily (abiraterone group) or placebo and prednisone 5 mg twice daily (prednisone alone group) via a centralized interactive web/voice response system in this double-blind, placebo-controlled multinational study. A permuted block randomization was used. The treatment was in continuous 28-d cycles [20]. Patients were stratified by Eastern Cooperative Oncology Group performance status (ECOG PS) score (0 vs 1). Concomitant BTT use was defined as the use of at least one of the following classes of bone drugs during the study: bisphosphonate, RANKL inhibitor, radiopharmaceutical, or other bone-targeted agent. Patients had the first recorded dose of BTT in the study after randomization, and although new BTT was not allowed per protocol, 7% of patients treated with BTT were actually treated after study entry. The primary end point of rPFS was defined as time from randomization to radiographic progression, as previously described [20]. Secondary endpoints were time to prostate-specific antigen (PSA) progression according to Prostate Cancer Working Group 2 criteria, time to initiation of cytotoxic chemotherapy, time to opiate use for cancer-related pain, and time to deterioration in ECOG PS score by ≥1 point [23]. Adverse events (AEs) of special interest included events related to mineralocorticoid excess (hypertension, hypokalemia, fluid retention).
2.2. Statistical analyses
The present analyses represent IA3 data at 56% of expected death events. The medians for time-to-event variables with 95% confidence intervals (CIs) were estimated using the Kaplan-Meier method. Patients were grouped by concomitant BTT use or no BTT use. Since most patients who used concomitant BTT received it from study start (336/353, 95%), concomitant BTT use was treated as a baseline covariate. However, sensitivity analyses treating concomitant BTT use as time-dependent were also conducted and showed similar results. The adjusted HRs, 95% CIs, and p values for concomitant BTT use versus no BTT use were obtained through Cox models that also included treatment and key baseline factors (PSA, lactate dehydrogenase, alkaline phosphatase, hemoglobin, and whether a patient had bone metastases only at entry) as covariates. The treatment effect on all endpoints within each subgroup was assessed using a stratified Cox regression model, and p values were determined by stratified log-rank test. No adjustment for multiple testing was made for this post hoc analysis; significance was declared for p ≤ 0.05.
3. Results
A total of 353 patients (34% [184/546] of the abiraterone group, 31% [169/542] of the prednisone group) were receiving concomitant BTT. Of these 353 patients, 93% were treated with zoledronic acid, 6% with denosumab, and the remaining 1% with other BTT. Among the 353 patients who received concomitant BTT, 336 (95%) were receiving BTT at baseline. Another 17 (5%) patients started BTT during the study. Baseline patient characteristics were generally similar among the treatment arms (Table 1). As expected, there was a higher prevalence of bone disease and a higher percentage of patients with a baseline Gleason score ≥8 among patients with BTT use. In the BTT group, 93% (329/353) of patients had bone disease at study entry, with a median time of 4 yr from the time of initial diagnosis to first dose of the study drug. In the group without BTT use, 76% (555/735) of patients had bone disease at study start, with a median time of 6 yr from the time of initial diagnosis to first dose of study drug. BTT use in patients without bone disease at study start was compatible with the dosing used for bone loss prevention, not the level used for bone metastases. The primary and secondary endpoint results obtained at the time of the IA3 have been described in detail previously [22].
Table 1.
Baseline patient characteristics and covariates (intent-to-treat population)
| Characteristic | Abiraterone (n = 546) | Prednisone (n = 542) | ||
|---|---|---|---|---|
| BTT (n = 184) | No BTT (n = 362) | BTT (n = 169) | No BTT (n = 373) | |
| Median age, yr (IQR) | 70.0 (64.0–76.0) | 71.0 (65.0–78.0) | 70.0 (65.0–76.0) | 70.0 (63.0–76.0) |
| Gleason score ≥8 at initial diagnosis, n/N (%) | 95/167 (57) | 168/321 (52) | 89/157 (57) | 165/351 (47) |
| Median PSA at study entry, ng/ml (IQR) | 42.5 (16.4–128.2) | 41.5 (16.1–110.4) | 34.2 (11.3–91.9)a | 39.1 (18.2–98.1)b |
| ECOG PS at study entry, n (%) | ||||
| 0 or 1 | 184 (100) | 361 (100) | 169 (100) | 373 (100) |
| 2 | 0 | 1 (0.3) | 0 | 0 |
| Extent of disease, n (%) | ||||
| Bone | 172 (94) | 280 (78) | 157 (93) | 275 (74) |
| Soft tissue or nodec | 76 (41) | 191 (53) | 58 (34) | 213 (57) |
| Other | 2 (1) | 2 (0.6) | 1 (0.6) | 6 (2) |
| Bone metastasis only at study entry, n (%) | 100 (54) | 138 (38) | 95 (56) | 147 (39) |
| Median alkaline phosphatase, IU/l (IQR) | 91.5 (66.0–140.5) | 94.0 (72.0–133.0) | 91.0 (65.5–137.0)d | 89.0 (69.0–139.0)e |
| Median lactate dehydrogenase, U/l (IQR) | 192.0 (164.0–223.0)f | 184.5 (160.0–207.5)g | 187.0 (161.0–222.0)h | 183.0 (165.0–210.0)i |
| BTT use, n (%) | ||||
| At baseline | 170 (31) | NA | 157(29) | NA |
| New starts during study | 14 (3) | NA | 12 (2) | NA |
| Type of BTT, n (%)j | ||||
| Zoledronic acid | 172 (32) | NA | 158 (29) | NA |
| Other bisphosphonates | 7 (1) | NA | 8 (2) | NA |
| Denosumab | 16 (3) | NA | 6 (1) | NA |
| Otherk | 2 (0.4) | NA | 3 (0.6) | NA |
BTT = bone-targeted therapy; ECOG PS = Eastern Cooperative Oncology Group performance status; IQR = interquartile range; NA = not applicable; PSA = prostate-specific antigen.
n = 167;
n = 372.
Metastatic lesions other than liver or visceral metastases on computed tomography or magnetic resonance imaging. If lymph node metastasis was the only evidence of metastasis, it must be ?2 cm in diameter. Patients with visceral metastases were excluded.
n = 168;
n = 371;
n = 183;
n = 360;
n = 166;
n = 370.
Patients could receive more than one BTT at the same time.
Methylsulfonylmethane, strontium ranelate.
3.1. Effect of concomitant BTT use versus no BTT use
The effect of concomitant BTT use on clinical endpoints was evaluated using data from both treatment arms. The interaction effects of concomitant BTT use and treatment group were not statistically significant for any of the endpoints (Table 2; p = 0.13–1.0). Point estimates of HRs for all efficacy endpoints were <1.0. BTT use was associated with significantly longer OS (p = 0.012; risk reduction 25%), longer time to deterioration in ECOG PS (p < 0.001, risk reduction 25%), and longer time to opiate use for cancer-related pain (p = 0.036, risk reduction 20%; Table 2). BTT use was not associated with significantly longer time to chemotherapy initiation or to PSA progression.
Table 2.
Clinical outcomes for treatment with and without concomitant bone-targeted therapy (BTT)
| BTT | No BTT | HR (95% CI)a | p value | |
|---|---|---|---|---|
| Median rPFS (mo) | 13.6 | 11.0 | 0.86 (0.72–1.02) | 0.08 |
| Median overall survival (mo) | NE | 30.3 | 0.75 (0.60–0.94) | 0.012 |
| Median time to opiate use for CaRP (mo) | NE | 27.9 | 0.80 (0.65–0.99) | 0.036 |
| Median time to chemotherapy initiation (mo) | 22.4 | 21.1 | 0.92 (0.76–1.10) | 0.4 |
| Median time to ECOG PS deterioration (mo) | 14.3 | 11.1 | 0.75 (0.64–0.87) | <0.001 |
| Median time to PSA progression (mo) | 8.3 | 8.3 | 0.88 (0.75–1.03) | 0.11 |
CaRP = cancer-related pain; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; NE = not estimable; PSA = prostate-specific antigen; rPFS = radiographic progression-free survival.
Stratified Cox model with concomitant BTT use, treatment, and key baseline parameters (PSA, lactate dehydrogenase, alkaline phosphatase, hemoglobin, and whether a patient had bone metastases only at entry) as factors. When the interaction effect of treatment and BTT use was included in the models, the p values were not significant (rPFS, p = 0.18; OS, p = 0.13; time to opiate use for Ca-RP, p = 1.0; time to chemotherapy initiation, p = 0.9; time to ECOG PS deterioration, p = 0.18; time to PSA progression, p = 0.8).
3.2. Subgroup analysis
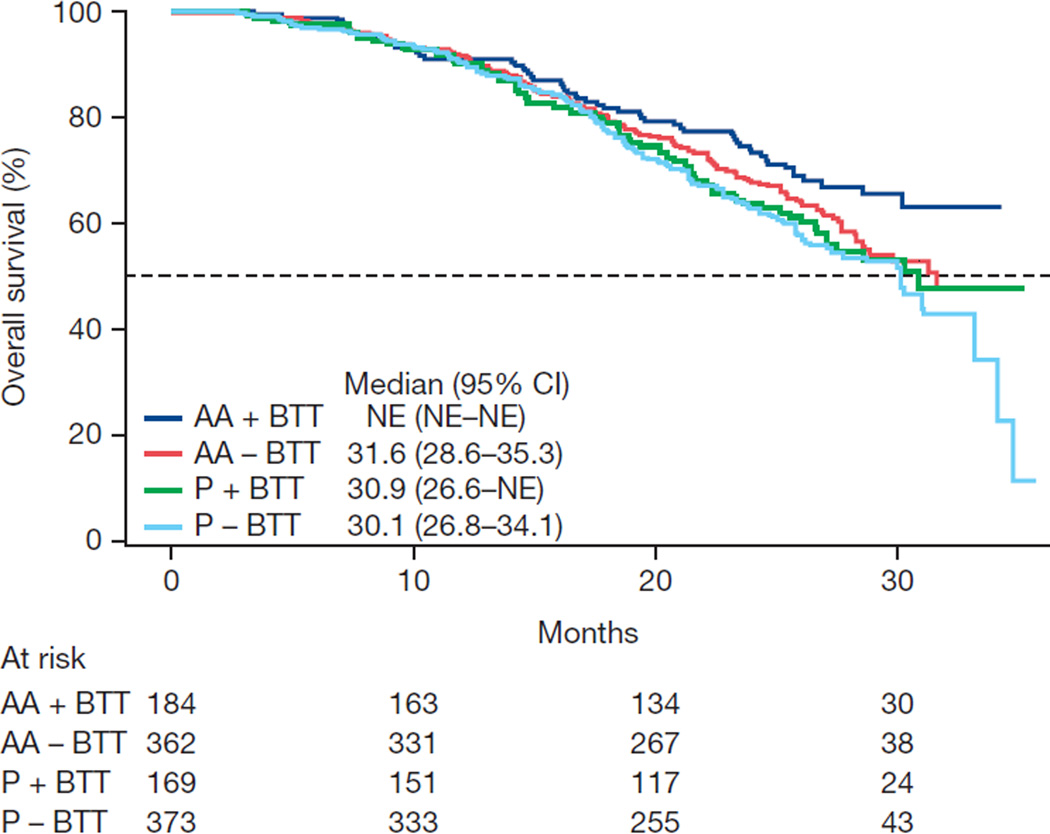
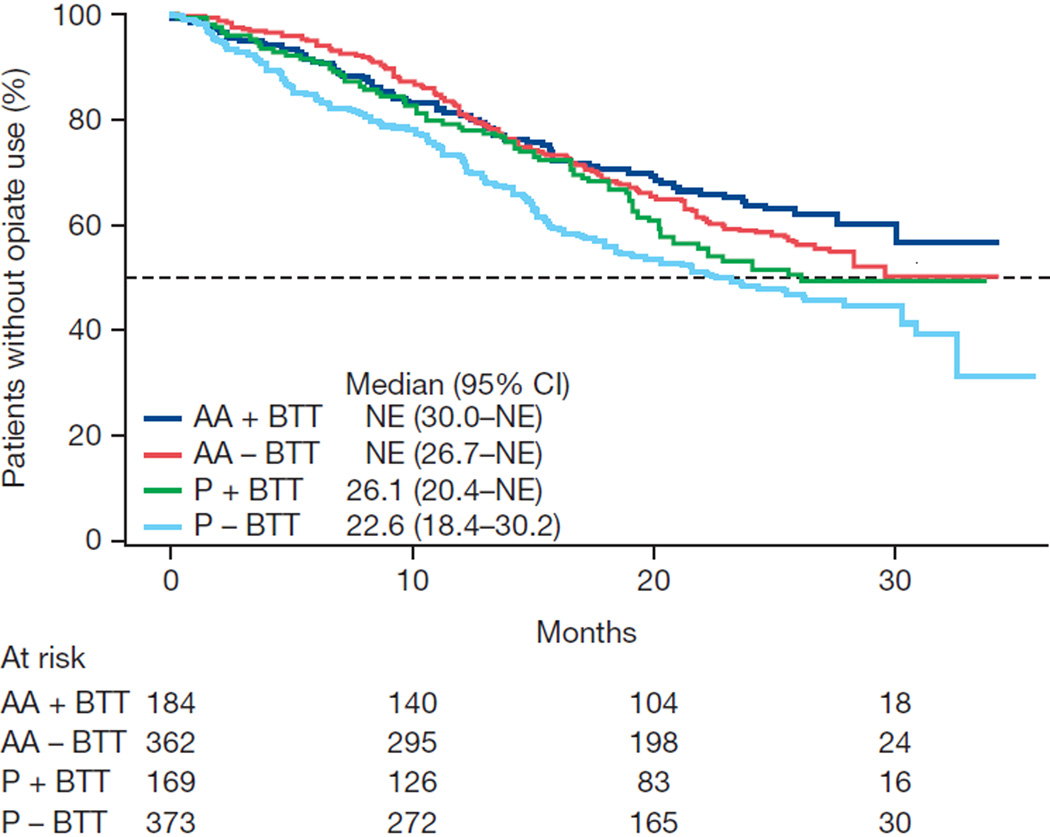
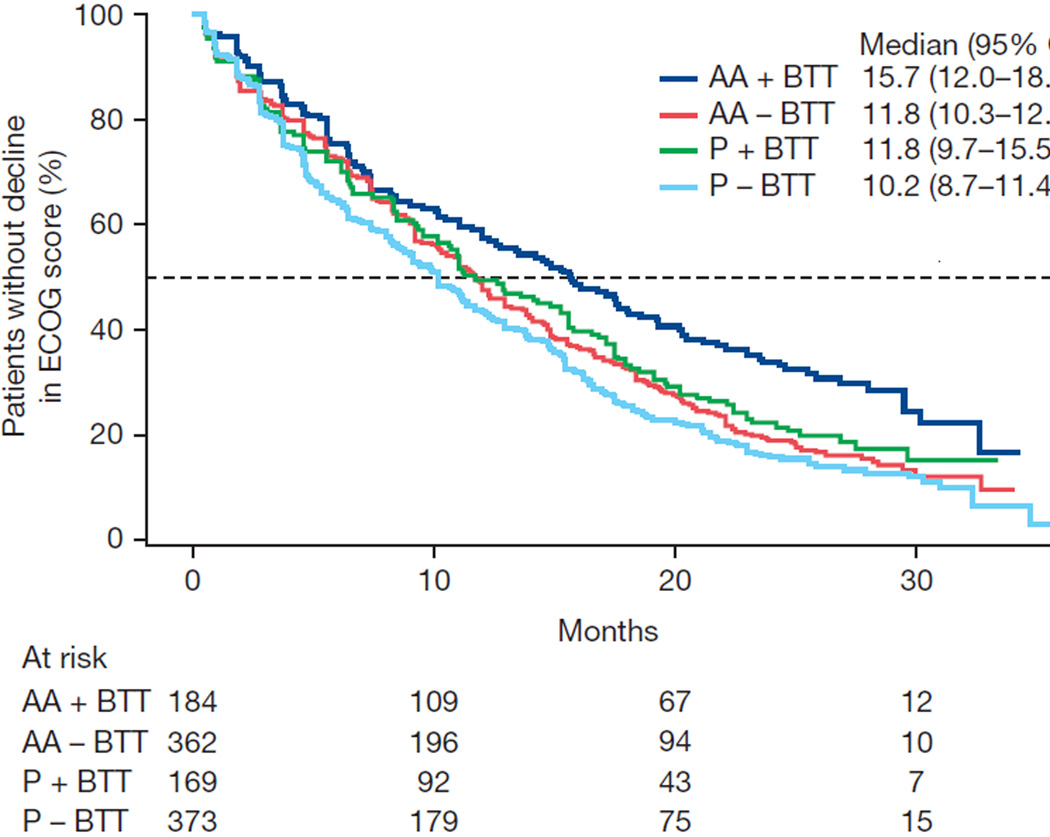
Exploratory associations with clinical outcomes were evaluated within the subgroups of patients with and without BTT use. Favorable results for abiraterone were observed for all endpoints with or without BTT use, although statistical significance was not consistently achieved because of the reduced sample size in each subgroup (Table 3). The Kaplan-Meier data for time-to-event outcomes stratified by BTT use in each of the treatment arms illustrate the effect of BTT use and beneficial effects on OS, time to opiate use, and ECOG PS deterioration (Fig. 1).
Table 3.
Comparison of clinical outcomes for different treatments within bone-targeted therapy subgroups using a stratified Cox model
| Bone-target therapy | No bone-targeted therapy | |||
|---|---|---|---|---|
| Abiraterone | Prednisone | Abiraterone | Prednisone | |
| Median rPFS (mo) | 16.6 | 10.4 | 16.3 | 8.2 |
| Adjusted HR (95% CI) | 0.63 (0.48–0.84) | 0.48 (0.40–0.58) | ||
| p valuea | 0.001 | <0.0001 | ||
| Median overall survival (mo) | NE | 30.9 | 31.6 | 30.1 |
| Adjusted HR (95% CI) | 0.71 (0.50–1.00) | 0.84 (0.67–1.05) | ||
| p value | 0.050 | 0.13 | ||
| Median time to opiate use for CaRP (mo) | NE | 26.1 | NE | 22.6 |
| Adjusted HR (95% CI) | 0.74 (0.53–1.04) | 0.70 (0.56–0.87) | ||
| p valuea | 0.078 | 0.001 | ||
| Median time to chemotherapy initiation (mo) | 27.1 | 17.5 | 26.1 | 16.7 |
| Adjusted HR (95% CI) | 0.66 (0.49–0.89) | 0.59 (0.48–0.72) | ||
| p valuea | 0.006 | <0.0001 | ||
| Median time to ECOG PS deterioration (mo) | 15.7 | 11.8 | 11.8 | 10.2 |
| Adjusted HR (95% CI) | 0.76 (0.59–0.97) | 0.87 (0.74–1.02) | ||
| p valuea | 0.025 | 0.086 | ||
| Median time to PSA progression (mo) | 11.1 | 5.6 | 11.0 | 5.6 |
| Adjusted HR (95% CI) | 0.54 (0.42–0.70) | 0.50 (0.42–0.60) | ||
| p valuea | <0.0001 | <0.0001 | ||
CaRP = cancer-related pain; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; NE = not estimable; PSA = prostate-specific antigen; rPFS = radiographic progression-free survival.
Stratified log-rank test.
Fig. 1.
Kaplan-Meier estimates of (A) overall survival, (B) time to opiate use for cancer-related pain, and (C) time to deterioration in Eastern Cooperative Oncology Group (ECOG) performance status score by ≥1 point. AA = abiraterone acetate; BTT = bone-targeted therapy; CI = confidence interval; NE = not estimable; P = prednisone.
3.3. Safety
As expected given the mechanisms of action of the drugs, the incidence of AEs observed for concomitant BTT was similar to that observed in the overall population [20] and in patients without concomitant BTT (Table 4). Grade 3/4 AEs with or without BTT in the abiraterone and prednisone treatment subgroups were similar to those reported previously [20,22]. AEs of special interest were similar among treatment subgroups. In addition, grade 3 or 4 mineralocorticoid-related AEs were similar across treatment subgroups. Increases in alanine aminotransferase were more common with abiraterone. Osteonecrosis of the jaw with concomitant BTT use was reported in <3% of patients across the treatment groups; all cases were grade 1/2. Hypocalcemia was observed in 12 patients across the treatment groups, including one report each of grade 3 or 4 hypocalcemia in the prednisone with BTT group and abiraterone without BTT.
Table 4.
Treatment-emergent adverse events by treatment subgroup
| Abiraterone | Prednisone | |||
|---|---|---|---|---|
| BTT (n = 183) | No BTT (n = 359) | BTT (n = 168) | No BTT (n = 372) | |
| TE AEs, n (%) | 181 (99) | 357 (99) | 163 (97) | 361 (97) |
| Grade 3/4 TE AEs, n (%) | 85 (46) | 182 (51) | 68 (41) | 167 (45) |
| TE serious AEs, n (%) | 62 (34) | 126 (35) | 45 (27) | 101 (27) |
| Grade 3/4 AEs, n (%) | 47 (26) | 109 (30) | 37 (22) | 86 (23) |
| Abiraterone | Prednisone | |||||||
|---|---|---|---|---|---|---|---|---|
| BTT (n = 183) | No BTT (n = 359) | BTT (n = 168) | No BTT (n = 372) | |||||
| TE AEs leading to treatment discontinuation, n (%) |
15 (8) | 43 (12) | 16 (10) | 37 (10) | ||||
| TEAE leading to death, n (%) | 6 (3) | 15 (4) | 6 (4) | 10 (3) | ||||
| Osteonecrosis of the jaw, n (%)a | 5 (3) | 1 (<1) | 4 (2) | 0 (0) | ||||
| AEs of special interest, n (%) | Total | Grade 3/4 | Total | Grade 3/4 | Total | Grade 3/4 | Total | Grade 3/4 |
| Fluid retention/edemab | 47 (26) | 2 (1) | 111 (31) | 3 (<1) | 37 (22) | 5 (3) | 93 (25) | 4 (1) |
| Hypertensionb | 44 (24) | 10 (5) | 74 (21) | 13 (4) | 26 (15) | 6 (4) | 47 (13) | 11 (3) |
| Cardiac disordersb | 32 (17) | 10 (5) | 81 (23) | 26 (7) | 38 (23) | 9 (5) | 57 (15) | 10 (3) |
| Hypokalemiab | 32 (17) | 3 (2) | 61 (17) | 11 (3) | 21 (13) | 3 (2) | 48 (13) | 7 (2) |
| ALT increasedb | 18 (10) | 9 (5) | 1 (<1) | 1 (<1) | 8 (5) | 0 | 19 (5) | 4 (1) |
BTT = bone-targeted therapy; TE = treatment-emergent; AE = adverse event; ALT = alanine aminotransferase.
All events were grade 1/2.
Reflects collection of terms based on specific Standardized Medical Dictionary for Regulatory Activities queries.
4. Discussion
This post hoc analysis is the first to evaluate the association between BTT and clinical outcomes in patients receiving abiraterone versus prednisone alone for mCRPC. We demonstrate that concomitant administration of abiraterone and BTT appears to be safe and well tolerated, and that the efficacy of abiraterone is maintained with concomitant BTT, with a possible added benefit of delaying the need for opiates to control pain. Delaying symptoms from bone metastases as mCRPC progresses is central to therapeutic management [24]. Prostate cancer patients with bone metastases have a greater risk of skeletal morbidity, which can impair quality of life (QoL) [25]. The findings that concomitant BTT use was associated with improvements in OS, time to opiate use for cancer-related pain, and time to ECOG PS deterioration are clinically relevant in the context of the mCRPC patient population. In the present analysis, all treatment group comparisons favored abiraterone over prednisone alone, and concomitant BTT was associated with increased effectiveness of abiraterone regarding clinical outcomes. Abiraterone with or without BTT was associated with a beneficial effect on survival, time to opiate use for cancer-related pain, and time to ECOG PS deterioration. These findings support and corroborate the patient-reported QoL outcomes from study COU-AA-302, in which abiraterone delayed pain progression, pain interference, and health-related QoL degradation compared with prednisone alone [22,26], and the outcomes from study COU-AA-301, in which abiraterone delayed pain progression and time to the first skeletal-related event in men with mCRPC previously treated with docetaxel [27].
Among patients treated with abiraterone, BTT was associated with a longer time to ECOG PS deterioration. Compared with prednisone with BTT, abiraterone in combination with BTT delayed the median time to deterioration in ECOG PS by 3.9 mo. Patients receiving abiraterone in combination with BTT also had a longer time to treatment with cytotoxic chemotherapy, an observation that supports the findings of previous reports [20,22]. These associations are consistent with an interaction between abiraterone and BTT use that may benefit clinical outcomes. In fact there is accumulating preclinical and clinical evidence of a potential anticancer effect of bisphosphonates [13]. In a retrospective analysis of a phase 3 clinical trial on zoledronic acid versus placebo, zoledronic acid–mediated normalization of bone markers was associated with improved survival in men with bone metastases from CRPC [28].
A limitation of our post hoc analysis is that as a retrospective analysis it may not account for unknown variables that may have had an impact on outcomes. Among the 353 patients who received BTT, 93% were treated with zoledronic acid. Furthermore, BTT use was not randomized, and the treatment effect was evaluated in subgroups, resulting in smaller sample sizes. Thus, these results are hypothesis-generating and may not represent the effects or interactions of abiraterone and other types of BTT, such as RANKL inhibitors. However, this post hoc analysis was conducted from a prespecified interim analysis, and the association between BTT use and outcomes was evaluated in patient subgroups that were well balanced among treatment arms. Moreover, the sample size in the current analysis is similar to that reported in a retrospective analysis showing a significant delay in the median time to a second skeletal-related event with zoledronic acid versus placebo in 422 men with advanced prostate cancer [29].
Safety data indicate that, as expected, there was no additional toxicity associated with abiraterone in combination with BTT compared to abiraterone without BTT. AEs that were mechanism-based and secondary to mineralocorticoid excess resulting from 17α-hydroxylase/C17,20-lyase blockade occurred at similar rates in patients with or without concomitant BTT. Moreover, treatment-emergent AEs, including those leading to treatment discontinuation or death, were similar across treatment subgroups.
5. Conclusions
The results of this post hoc analysis of study COU-AA-302 show that the safety profile of treatment with abiraterone plus prednisone or prednisone alone with concomitant BTT is similar to that without concomitant BTT in chemotherapy-naïve patients with mCRPC. Concomitant BTT use was associated with longer OS, time to opiate use for cancer-related pain, and time to ECOG PS deterioration. The abiraterone treatment effect was maintained with or without concomitant BTT use for all endpoints. Moreover, this post hoc analysis suggests an added clinical benefit of abiraterone in combination with BTT in reducing symptomatic progression.
In patients with metastatic castration-resistant prostate cancer, treatment with abiraterone acetate and prednisone with concomitant bone-targeted therapy showed improved clinical benefit compared to prednisone alone, as measured by overall survival, time to deterioration of Eastern Cooperative Oncology Group performance status, and time to opiate use for cancer-related pain.
Acknowledgments
Financial disclosures: Fred Saad certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Fred Saad is a consultant or advisor to and has received honoraria and research funding from Astellas and Janssen. Neal Shore is a consultant or advisor to Algeta, Amgen, Bayer, BNI, Dendreon, Ferring, Janssen, Millennium, and Sanofi. Dana E. Rathkopf provides uncompensated research funding for Celgene, Janssen/J&J, Medication/Astellas, Millenium, and Novartis. She is also a consultant and advisor to Johnson & Johnson. Matthew R. Smith is a consultant and advisor to and receives research funding from Janssen. Johann S. de Bono is a consultant and advisor to and has received honoraria from Johnson & Johnson. Christopher J. Logothetis is a consultant or advisor to and has received honoraria and research funding from Astellas, BMS, J&J, Novartis, Pfizer, and Exelixis. Paul de Souza is a consultant and advisor to and has received honoraria from Janssen Australia Pty Ltd. Karim Fizazi is a consultant and advisor to and has received honoraria from Janssen. Paul Mainwaring is a consultant and advisor to and has received honoraria from Janssen, Roche, and Sanofi. Tomasz M. Beer receives research funding from Astellas Pharma Global, Janssen Research & Development (formerly Cougar Biotechnology), and Medivation. He receives consulting fees from Janssen Japan, and other fees from Research to Practice supported in part by Astellas and Medivation. Scott North is a consultant or advisor to Astellas and Janssen, and has received honoraria from Astellas, GSK, Janssen, Novartis, and Pfizer. Thomas A. Griffin, Peter De Porre, Anil Londhe, Thian Kheoh, and Arturo Molina are employees of Janssen Research and Development and hold stock in Johnson & Johnson. Howard I. Scher has received research funding from the Prostate Cancer Foundation, Aragon, Bristol-Myers Squibb, Exelixis, Janssen Research & Development, Janssen Global Services, and Medivation; has acted as a consultant or advisor to Dendreon, Endo/Orion Pharmaceuticals, Genentech, Novartis, and Ortho Biotech Oncology Research & Development (now Janssen Research & Development; proceeds donated); and has acted as an uncompensated consultant or advisor to Aragon, Celgene, Exelixis, Foundation Medicine, Janssen, Johnson & Johnson Pharmaceutical Research & Development, Medivation, Millennium Pharmaceuticals, and Takeda Pharmaceutical Company. Charles J. Ryan is a consultant and advisor to and has received honoraria of Millennium, and has received honoraria and research funding from Janssen. Hendrik Van Poppel, Peter F.A. Mulders, Yves Fradet, and Eric J. Small have nothing to disclose.
John D. Hainsworth
Funding/Support and role of the sponsor: This work was supported by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, Cougar Biotechnology unit). Writing assistance was provided by Ann P. Tighe of PAREXEL and was funded by Janssen Global Services, LLC. The sponsor played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Fred Saad had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Saad, de Bono, Griffin, Londhe, Kheoh, Molina, Ryan.
Acquisition of data: Saad, Shore, Rathkopf, Smith, de Bono, Logothetis, de Souza, Fizazi, Mulders, Mainwaring, Hainsworth, Beer, North, Fradet, Small, Scher, Ryan.
Analysis and interpretation of data: Saad, de Bono, Griffin, De Porre, Londhe, Kheoh, Scher, Molina, Ryan.
Drafting of the manuscript: Saad, de Bono, De Porre, Londhe, Molina, Ryan.
Critical revision of the manuscript for important intellectual content: Saad, Shore, Rathkopf, Smith, de Bono, Logothetis, de Souza, Fizazi, Mulders, Mainwaring, Hainsworth, Beer, North, Fradet, Griffin, De Porre, Londhe, Kheoh, Small, Scher, Molina, Ryan.
Statistical analysis: Londhe, Kheoh.
Obtaining funding: Saad.
Administrative, technical, or material support: Saad.
Supervision: Saad, Shore, Rathkopf, Smith, de Bono, Logothetis, de Souza, Fizazi, Mulders, Mainwaring, Hainsworth, Beer, North, Fradet, Griffin, De Porre, Londhe, Kheoh, Small, Scher, Molina, Ryan.
Other: None.
References
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat Rev. 2013;39:275–289. doi: 10.1016/j.ctrv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 6.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 7.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nat Rev Urol. 2013;10:522–528. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]
- 8.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–899. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 13.El-Amm J, Freeman A, Patel N, Aragon-Ching JB. Bone-targeted therapies in metastatic castration-resistant prostate cancer: evolving paradigms. Prostate Cancer. 2013;2013:210686. doi: 10.1155/2013/210686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal J, Antonarakis ES. Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett. 2012;323:135–146. doi: 10.1016/j.canlet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson S, Franzen L, Parker C, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20–26. doi: 10.1016/j.clgc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17:3876–3883. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 17.Molina A, Belldegrun A. Novel therapeutic strategies for castration resistant prostate cancer: inhibition of persistent androgen production and androgen receptor mediated signaling. J Urol. 2011;185:787–794. doi: 10.1016/j.juro.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 18.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14:1193–1199. doi: 10.1016/S1470-2045(13)70424-8. [DOI] [PubMed] [Google Scholar]
- 22.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–825. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan PW, Mulani PM, Fishman M, Sleep D. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Qual Life Res. 2007;16:571–575. doi: 10.1007/s11136-006-9156-2. [DOI] [PubMed] [Google Scholar]
- 25.Bishr M, Saad F. Preventing bone complications in prostate cancer. Curr Opin Support Palliat Care. 2012;6:299–303. doi: 10.1097/SPC.0b013e328356da87. [DOI] [PubMed] [Google Scholar]
- 26.Basch EM, de Bono JS, Scher HI, et al. Pain control and delay in time to skeletal-related events (SREs) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone acetate (AA): long-term follow-up. J Clin Oncol. 2012;30(Suppl 5):183. [Google Scholar]
- 27.Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 28.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 29.Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]