Abstract
A tight link exists between dietary factors and irritable bowel syndrome (IBS), one of the most common functional syndromes, characterized by abdominal pain/discomfort, bloating and alternating bowel habits. Amongst the variety of foods potentially evoking “food sensitivity”, gluten and other wheat proteins including amylase trypsin inhibitors represent the culprits that recently have drawn the attention of the scientific community. Therefore, a newly emerging condition termed non-celiac gluten sensitivity (NCGS) or non-celiac wheat sensitivity (NCWS) is now well established in the clinical practice. Notably, patients with NCGS/NCWS have symptoms that mimic those present in IBS. The mechanisms by which gluten or other wheat proteins trigger symptoms are poorly understood and the lack of specific biomarkers hampers diagnosis of this condition. The present review aimed at providing an update to physicians and scientists regarding the following main topics: the experimental and clinical evidence on the role of gluten/wheat in IBS; how to diagnose patients with functional symptoms attributable to gluten/wheat sensitivity; the importance of double-blind placebo controlled cross-over trials as confirmatory assays of gluten/wheat sensitivity; and finally, dietary measures for gluten/wheat sensitive patients. The analysis of current evidence proposes that gluten/wheat sensitivity can indeed represent a subset of the broad spectrum of patients with a clinical presentation of IBS.
Keywords: Biomarkers, Dietary factors, Functional bowel disorder, Gluten, Wheat
Introduction
Patients with functional bowel disorders (FBDs) manifest variable combinations of intestinal symptoms without structural and/or biochemical abnormalities. The latter concept has been challenged by growing evidence showing low-grade inflammatory changes in the gut and altered gut-brain axis signaling.1,2 According to the Rome III classification, FBDs include the irritable bowel syndrome (IBS), functional bloating (FB), functional constipation, functional diarrhea, and unspecified FBD, and they are attributed to abnormalities likely originating from the small bowel, colon, and rectum.3,4 Since FBDs lack objective biomarkers, their diagnosis is based on the clinical symptoms reported by patients, physical examination, and the exclusion of alarm symptoms/signs (eg, blood in stools, anemia, weight loss, and others). Although FBDs are not regarded as life threatening, these conditions can significantly worsen the patient’s quality of life. Indeed, FBDs are responsible for prolonged absenteeism from work as well as for suboptimal performance in the workplace with relevant social costs.5,6
Amongst FBDs, IBS is certainly the most common clinical entity affecting up to 20% of the general population.7 Classically, an IBS diagnosis revolves around abdominal pain/discomfort in conjunction with altered bowel habits. The clinical phenotypes include IBS with constipation, with diarrhea (IBS-D), alternating bowel or “mixed” (the most frequent pattern in Western industrialized countries), and unsubtyped according to stool frequency and consistency.3,8,9 The pathogenesis underlying IBS is only partly understood and notoriously referred to as multifactorial being attributable to dysfunction of the gut-brain axis. In this context, recognized mechanisms in IBS span a wide spectrum including gut dysmotility, low-grade inflammation, visceral hypersensitivity, changes of gut microbiome, infections, altered gut barrier function, and genetic and psychosocial factors.10–14
The role of dietary factors in IBS pathogenesis is a topic of great interest.15–17 Indeed, more than 60% of patients with IBS relate the occurrence of bloating and abdominal pain to the ingestion of certain foods. The majority of these patients report worsening of symptoms between 15 minutes to a few hours after meal intake.18 However, only recent animal and human studies have focused on the key role of specific foods in altering gut physiology.
The aim of the present review is to provide an overview highlighting the major aspects of the complex interplay existing between foods and gut function with relevance to IBS. Specifically, the reader will have an update on the role of gluten/wheat sensitivity as potential dietary triggers evoking gut dysfunction and symptoms in IBS.
Pathogenesis of Irritable Bowel Syndrome
IBS is a heterogeneous disorder, with multiple clinical presentations and likely different causes. The pathophysiology of IBS is still not well understood, limiting the capacity to effectively treat the disorder.19 Enteric infections are the strongest environmental triggers for IBS, constituting the well-characterized subgroup of post-infective IBS,20 which is associated with dysbiosis, low-grade inflammation and altered intestinal permeability.21 These mechanisms have also been proposed in the general IBS population, but results are not as consistent as in post-infective IBS.22 In addition to enteric infection, other environmental and psychosocial triggers have been linked with IBS. Interestingly, many of these triggers induce visceral hypersensitivity, changes in gut microbiota, and altered levels of enteric hormones and neurotransmitters which may explain symptom generation.19,22,23 Alterations in gastrointestinal transit, which may be caused by stress,24 have also been reported in IBS patients. Although IBS is considered to affect mainly the colon, several studies have reported motility alterations also in the esophagus, stomach and small intestine, which often correlate with patients’ symptoms.25
Several studies and a recent meta-analysis26–28 have demonstrated bile acid (BA) malabsorption, at least in a sub-population of IBS-D. In a study of 119 patients with IBS,28 32% had abnormal colonic transit measured by scintigraphy at 24 or 48 hours, with accelerated transit in 48% of IBS-D patients; the causes of abnormal transit are unclear. BA sequestrants have been proposed as a possible therapeutic option, however, the mechanism of action of these BA sequestrants to ameliorate bowel function is not yet clearly demonstrated.29
Of all the possible non-infectious environmental triggers, food is a likely candidate that can affect a variety of physiologic parameters important in IBS such as motility, visceral perception, brain-gut interactions, microbiota composition, permeability, immune activation, and neuro-endocrine function.30 Among the foods reported to associate with IBS symptoms, those rich in carbohydrates,19 gluten and wheat31,32 are common. However, food sensitivity or allergy cannot be always confirmed in patients that recognize a specific food, as a trigger of their symptoms.31 Therefore, a better understanding of the dietary factors involved in IBS and their underlying mechanisms is key to determine the real benefit of exclusion diets in IBS.
Dietary Factors in Irritable Bowel Syndrome
Ingestion of a meal activates an array of complex mechanisms enabling the digestive system to perform the complex task of digestion and absorption of nutrients as well as expulsion of waste. Under normal circumstances, our gut has adapted to respond with a tightly regulated system of neuro-immune interactions necessary to maintain proper gut function and homeostasis. Dietary factors, however, can be harmful in certain circumstances and can cause intolerance, allergy, or hypersensitivity via a number of different mechanisms. Food intolerance, such as lactose intolerance, classically relates to a reaction to food components based on some metabolic deficiency.33–35 Food allergy and hypersensitivity instead refer to undesirable immune-based reactions that cause symptoms. The most common food sensitivities have been related to proteins in nuts, wheat, and milk.36 Some of these reactions are typically IgE mediated (allergy), while others recognize non-IgE immune pathways.
Patients with IBS often report worsening of symptoms by a wheat containing diet.37 Gluten is a group of immunogenic proteins in wheat, which causes celiac disease, an inflammatory and autoimmune disease, in genetically susceptible people.38 This led to the hypothesis that a subgroup of IBS patients could develop mild immune or functional alterations in the absence of celiac disease.39 Gluten proteins are indeed insufficiently degraded by gut proteases, leaving undigested peptides that could trigger innate immune mechanisms that may be of importance in IBS. A mouse study determined that gluten sensitization causes altered smooth muscle contractility and altered barrier function, which are mechanisms commonly associated with IBS. It is important to stress that other proteins in wheat, such as α-amylase/trypsin inhibitors (ATIs)40 and wheat lectin agglutinin,41 have recently shown to induce innate immune pathways. ATIs have been shown to trigger a toll-like-receptor (TLR)-4 mediated activation40 and it remains to be determined whether this is associated with gut functional changes. In addition to protein fractions, wheat contains a group of carbohydrates, called fructans. These are members of fermentable oligosaccharide, disaccharide, mono-saccharides, and polyols (FODMAPs) that are poorly absorbed in the small intestine.37,40,42 These substrates are very important for gut health, supporting microbiota diversity and short chain fatty acid production43 but under certain circumstances could cause, through excessive bacterial colonic fermentation, symptoms such as bloating and altered bowel habits.19,44 Regardless of the offending component, the withdrawal of wheat from the diet in a subgroup of patients with IBS has indeed been shown to improve symptoms.45 Although unselected IBS patients display clinical improvement after wheat withdrawal, only a proportion may truly reflect underlying gluten sensitivity (Table 1).46–56 The absence of specific biomarkers and the great proportion of patients with placebo effect observed in IBS trials, are 2 barriers for identifying the specific role of wheat components in generating IBS symptoms. Therefore, double-blind-placebo-controlled-crossover (DBPCC) trials are a reliable way to establish whether wheat and its fractions are involved in IBS symptom induction.50,51,53,56
Table 1.
Evidence for the Effect of Gluten-free Diet in Irritable Bowel Syndrome
| Author | Study design | Population | Intervention | Control diet | Outcome | Conclusion |
|---|---|---|---|---|---|---|
| Wahnschaffe et al, 200146 | Observational | 102 IBS-D; 41 CD; 30 controls | GFD for 6 months in 26 IBS-D | - | Stool frequency; immune markers (AGA, tTGA) in duodenal aspirate; IELs; HLA-DQ2 status (in all patients) | Improved stool frequency and decreased immune markers in duodenal aspirates of HLA-DQ2+ IBS-D patients after GFD |
| Wahnschaffe et al, 200747 | Observational | 145 IBS-D; 74 CD; 57 IBD | GFD for 6 months in 41 IBS-D | - | Stool frequency; serum immune markers (AGA, tTGA); HLA-DQ2 status | Improved stool frequency and decreased immune markers in HLA-DQ2+ IBS-D patients after GFD |
| Biesiekierski et al, 201148 | DB-randomized controlled trial | 34 IBS that improved on GFD for 6 wk; Marsh 0; 56% HLADQ2/8+ | Gluten challenge (bread/muffin) 16 g/ day for 6 wk | Gluten-free muffin/bread | GI symptoms; intestinal permeability; fecal lactoferrin | Gluten triggered gut symptoms and tiredness in 68% of IBS patients |
| Sapone et al, 201149 | Observational | 26 NCGS; 42 CD; 39 disease controls (dyspepsia) | GFD | - | GI and extra-intestinal symptoms; intestinal permeability | Gluten evoked innate immune activation in the absence of changes in mucosal barrier function |
| Carroccio et al, 201350 | DB-randomized controlled trial | 276 IBS with Marsh 0–1 and negative skin Prick IgE that improved with wheat withdrawal for 4 wk | Wheat challenge (capsules containing wheat) for 2 wk | Placebo (capsules containing xylose) for 2 wk | GI symptom changes according to VAS increase (> 20 mm) | 1/3 of IBS reacted to wheat challenge; wheat sensitive patients had eosinophil infiltration in duodenal and colonic mucosa |
| Biesiekierski et al, 201351 | DB-cross-over challenge | 22 IBS that improved on GFD for 6 wks; Marsh 0; 56% HLA-DQ2/8+ | 2-wk low-FODMAPs period, followed by high-gluten (16 g/day); 2-wk washout. Meals provided | Low-gluten (2 g/day) or (16 g whey protein/day) for 1 wk | GI symptom changes according to VAS increase (> 20 mm) | No evidence of specific or dose-dependent effects of gluten in IBS patients on previous low-FODMAPs diet |
| Vazquez-Roque et al, 201352 | Randomized controlled trial | 57 IBS-D screened; 45 IBS-D randomized (50% HLA-DQ2/8+) | 23 patients on GFD for 4 wk Meals provided | 22 patients on GCD for 4 wk | GI symptoms and transit; intestinal permeability; cytokine profile; TJ proteins | Gluten alters intestinal barrier in patients with in HLA-DQ2/8+ IBS-D patients |
| Rodrigo et al, 201453 | Observational | 97 IBS and fibromyalgia; 58 Marsh I and 39 Marsh 0 | GFD for 1 yr | - | Fibromyalgia questionnaire; health assessment questionnaire (SF36) | Symptom improvement in IBS/fibromyalgia patients after GFD |
| Di Sabatino et al, 201554 | DB-cross over challenge | 92 with suspected NCGS/NCWS responding to GFD; 61 randomized | Gluten challenge (capsules = 4.375 g/day) for 1 wk; 1-wk washout | Placebo (capsules containing rice starch) | GI and extraintestinal symptom changes according to VAS increase | Gluten evoked overall GI and extraintestinal symptom worsening in some NCGS patients |
| Zanini et al, 201555 | DB-cross-over challenge | 112 with suspected NCGS/NCWS; 53 enrolled; 35 actually challenged | Gluten containing flour for 10 days; 2-wk washout | Gluten-free flour | GSRS | Gluten challenge evoked symptom recurrence in 1/3 of NCGS/NCWS patients |
| Elli et al, 201656 | DB-cross-over challenge | 140 FGIDs; 101 responding to GFD; 98 actually enrolled and randomized | Gluten challenge (capsules = 5.6 g/day) for 1 wk | Placebo (capsules containing rice starch) | GI symptom changes according to VAS increase (> 3 cm); SF36 | Gluten challenge triggered symptom recurrence in 14% of patients with FGIDs |
IBS-D, diarrhea predominant irritable bowel syndrome; CD, celiac disease; GFD, gluten-free diet; AGA, anti-gliadin antibodies; tTGA, tissue transglutaminase antibodies; IELs, intra-epithelial lymphocytes; HLA, human leukocyte antigen; IBD, inflammatory bowel disease; DB, double blind; GI, gastrointestinal; NCGS, non-celiac gluten sensitivity; IBS, irritable bowel syndrome; IgE, immunoglobulin E; VAS, visual analogue scale; FODMAPs, fermentable oligo-, di-, and monosaccharides and polyols; GCD, gluten containing diet; TJ, tight junction; SF36, Short Form (36-item) questions health survey; NCWS, non-celiac wheat sensitivity; GSRS, gastrointestinal symptom rating scale; FGIDs, functional gastrointestinal disorders.
Experimental Evidence for a Role of Wheat Components in Irritable Bowel Syndrome
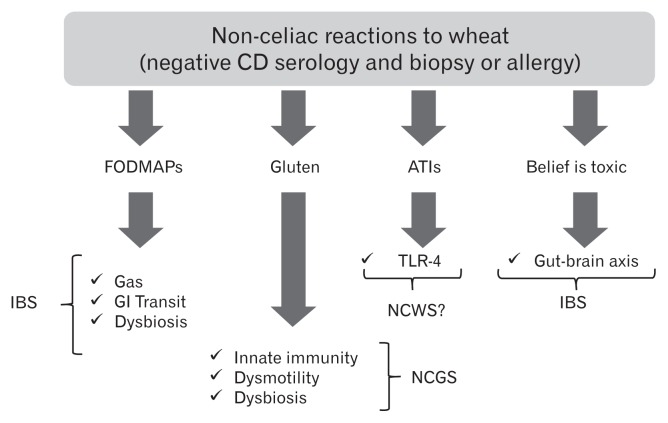
Different mechanisms have been proposed to explain how gluten may trigger gastrointestinal symptoms in the absence of celiac disease (Figure). In vitro studies have demonstrated that digests of gliadin increase the expression of co-stimulatory molecules and the production of proinflammatory cytokines in monocytes and dendritic cells.40,57,58 Certain “toxic” (that only stimulates the innate immune response) gliadin-derived peptides such as the 31–43mer, may evoke epithelial cell dysfunction, increased IL-15 production and enterocyte apoptosis.59 Recent studies have demonstrated increased expression of TLR-2 in the intestinal mucosa of non-celiac compared to celiac patients, suggesting a role of the innate immune system in the pathogenesis of non-celiac reactions to gluten or other wheat components.49 Other studies have shown that monocytes from HLA-DQ2+ non-celiac individuals spontaneously release 2–3 fold more IL-8 than monocytes from HLA-DQ2 negative patients. This suggests that patients without celiac disease (no enteropathy and negative specific serology), but with positive HLA-DQ2 status, may represent a subpopulation reacting mildly to gluten.60 In terms of gut dysfunction, gluten sensitization in mice has been shown to induce acetylcholine release, one of the main excitatory neurotransmitters in the gut, from the myenteric plexus.57 This correlates with increased smooth muscle contractility and a hypersecretory status with increased ion transport and water movements.57 These functional effects induced by gluten were not accompanied by mucosal atrophy, and were not observed after sensitization with non-gluten proteins. Interestingly gluten-induced gut dysfunction was particularly notable in mice transgenic for the human celiac gene HLA-DQ8.57
Figure.
Putative mechanisms for symptom generation by wheat components. CD, celiac disease; FODMAPs, fermentable oligo-, di-, and mono-saccharides and polyols; ATIs, α-amylase/trypsin inhibitors; IBS, irritable bowel syndrome; GI, gastrointestinal; TLR-4, toll-like-receptor-4; NCWS, non-celiac wheat sensitivity; NCGS, non-celiac gluten sensitivity.
ATIs, a group of wheat proteins that confer resistance of the grain to pests, are strong inducers of innate immune responses via TLR4 and via the myeloid differentiation factor 88-dependent and -independent pathway.40 This activation occurs both in vitro and in vivo after oral ingestion of purified ATIs or gluten, while gluten-free cereals display no or minimal activities.61 The role of ATIs in IBS is not yet known, however there is clear description of a mechanism that could be involved in the generation of gut dysfunction and symptoms. These mechanisms are different from those proposed for gluten and thus it is conceivable that they could co-exist in given patients or have a synergistic effect.
Clinical Evidence for a Role of Wheat Components in Irritable Bowel Syndrome
IBS and celiac disease are common conditions, and they may overlap by chance. However, celiac disease is 3–4 times more common in IBS patients compared to controls suggesting that both disorders could be mechanistically associated.62 Active screening by celiac serology and biopsy reveals that 4% of patients labeled as IBS have underlying celiac disease.62–65 This overlap between the 2 conditions is different from the issue of wheat components, including gluten, causing functional symptoms in patients without celiac disease.39 Clinical studies have revealed that a subgroup of IBS patients in whom celiac disease and wheat allergy were ruled out, displayed intestinal and extra-intestinal symptoms after wheat ingestion. Three consensus conferences defined this new syndrome as non-celiac gluten sensitivity (NCGS) or, alternatively, non-celiac wheat sensitivity (NCWS) given the possibility for immune reactions or intolerances to other wheat components as explained above.66–68 It is important to bear in mind the complexity of food hypersensitivities and intolerances, many of which could coexist in the same patient. This constitutes a confounder that we must be aware of for the design and interpretation of clinical trials.69
The prevalence of NCGS/NCWS is still not clearly established mainly due to the hitch in standardizing international diagnostic criteria. In United States this condition seems to be identified more frequently in tertiary referral centers than in primary care. Data obtained in primary care practice, from the National Health and Nutrition Examination Survey (NHANES), indicate that the estimated prevalence of NCGS/NCWS was 0.6% over 7762 subjects.70 On the other hand, data published from the Celiac Disease Center in Baltimore (University of Maryland), showed a prevalence of suspected NCGS/NCWS of 6% over 5896 subjects.66 Moreover, an Italian multicenter study prospectively evaluated the prevalence of NCGS/NCWS and celiac disease in pediatric and adult centers for gluten related disorders.71 Among 12 255 subjects consecutively investigated during a one-year survey, 391 (3.2%) cases of suspected NCGS/NCWS and 340 (2.8%) celiac patients were identified. Based on these results, it is possible to estimate a ratio between NCGS/NCWS and celiac disease of 1.15:1, data in line with the NHANES survey. Although NCGS/NCWS can occur at any age, this condition seems to occur more frequently in adulthood with a mean age at diagnosis of 40 years. The female gender, similarly to IBS, is more affected by this sensitivity with a ratio up to 5:1.71
Some clinical trials have attempted to investigate underlying functional and immune abnormalities in patients with a clinical picture of NCGS/NCWS. Initial results showed that there is increased expression of TLR2 in the small intestine of patients with NCGS/ NCWS.49 This was in contrast with the normal expression of IL-17A, IL-6, IFN-γ, IL-17, and IL-21 in the duodenal mucosa of these patients, which seemed to rule out the contribution of adaptive immunity.72 Recent work has suggested increased IFN-γ levels in small intestinal biopsies of NCGS/NCWS patients following a short-term gluten challenge.73 Although this cytokine is a key mediator of T-helper 1 adaptive immune responses, it could also be produced by innate cells such as intraepithelial lymphocytes (IELs).73 Another relevant pathogenic aspect that has been investigated in NCGS/NCWS pertains to possible functional changes, such as the increase of intestinal permeability. Using the lactulose/mannitol test, IBS patients with self-reported sensitivity to gluten did not exhibit significant alterations in sugar permeability.49 This may be related to the population involved or technical difficulties in the interpretation of clinical permeability tests. However, in contrast to this study, Vazquez-Roque et al,52 using the same method, found increased intestinal permeability in a subgroup of HLA-DQ2/DQ8+ NCGS/ NCWS patients with IBS-D, once again raising the possibility of a particularly vulnerable subpopulation. This underscores the importance of careful patient phenotyping in studies involving functional symptoms and adverse reactions to food components. It also raises the concept of a subgroup of genetically predisposed individuals carrying celiac markers, but without active celiac disease, that may be more sensitive to low-grade inflammation or functional changes induced by gluten. This will need to be confirmed in large clinical trials.
An initial study enrolling IBS patients fulfilling criteria for NCGS/NCWS showed symptom recurrence following gluten reintroduction.48 In a second trial from the same group,51 IBS patients that responded to a gluten-free diet were challenged with low dose of gluten (2 g/day), high dose of gluten (16 g/day) or whey protein (16 g/day) after 2 weeks of low dose FODMAPs diet. After the challenge, gluten evoked neurological, but not intestinal, symptoms. It is worthwhile to note that patients increased their symptoms with all challenges, including placebo, and that gluten challenge did not show a dose response. Carroccio et al50 demonstrated that IBS patients randomized to receive whole wheat vs placebo, had a significant worsening of their intestinal and extra-intestinal symptoms after wheat ingestion. A recent DBPCC trial used pure gluten vs rice starch (control)-containing capsules to clarify the exact role played by gluten in symptom generation in patients with highly suspected NCGS/NCWS.54 The results showed that pure gluten ingestion induced recurrence of a variety of symptoms including, bloating, abdominal pain, foggy mind, aphthous stomatitis, headache and depression, but not in all of NCGS patients. Two recent DBPCC trials55,56 performed in IBS patients self-diagnosed as “gluten/wheat sensitive” strongly suggested that gluten/wheat was responsible for symptom generation in up to one-third of IBS patients. Overall the results indicated that gluten and other wheat proteins cause symptoms in the absence of celiac disease in a subset of the IBS population (Table 2).
Table 2.
Clinical Trials on Gluten Challenge or Gluten-free Diet in Patients with Irritable Bowel Syndrome
| Supportive | Not supportive |
|---|---|
| Gluten challenge causes symptoms in IBS | No dose response to low/high gluten in IBS |
| Biesiekierski et al, 201148 | Biesikierski et al, 201351 |
| Di Sabatino et al, 201554a | |
| Zanini et al, 201555a | |
| Elli et al, 201656 | |
| IBS + celiac genes respond to GFD | |
| Wahnschaffe et al, 200747 | |
| Vazquez-Roque et al, 201352 | |
| Wheat challenge triggers symptoms in IBS | |
| Carroccio et al, 201250 |
Studies performed in non-celiac gluten sensitivity/non-celiac wheat sensitivity patients.
IBS, irritable bowel syndrome; GFD, gluten-free diet.
The dilemma is that a gluten-free diet may also be low in FODMAPs. Some studies have assessed the efficacy of low-FODMAPs diet in IBS with variable results (Table 3). However, none of these trials included a placebo group, thus introducing a possible bias hampering the actual value of a low-FODMAPs diet both in terms of diagnosis and as a therapeutic intervention. A recent DBPC trial (without crossover) comparing a low-FODMAP diet with traditional dietary advice in IBS, showed no difference among the 2 strategies, thus renewing controversy of the specificity of therapeutic effect of FODMAPs exclusion in the general IBS population.74 Moreover, this study indicated that patients are more likely to react positively to FODMAPs restriction, when already reducing FODMAPs from their diet before initiation of the trial. Finally, the data on the effect of FODMAPs in IBS were pooled in three systematic reviews, and one of them included a meta-analysis. In the systematic reviews from Rao et al75 and Moayyedi et al76 the authors showed that the data could not be combined for meta-analysis and concluded that low-FODMAPs is of uncertain benefit in IBS. In a third review,77 with less rigorous inclusion criteria, the authors were able to pool the data and found that low FODMAP diet was effective in IBS. However, the results from this meta-analysis should be taken cautiously due to a great heterogeneity in population included and the comparator for the intervention (Table 3).
Table 3.
Systematic Reviews and Meta-analysis on the Effect of FODMAPs in Patients with Irritable Bowel Syndrome
| Author | Clear eligibility criteria | RCTs eligible | Meta-analysis performed |
|---|---|---|---|
| Moayyedi et al, 201576 | Yes | 1 | Not possible |
| Rao et al, 201575 | Yes | 4 | Not possible |
| Marsh et al, 201577 | No | 6 | Conducted |
FODMAPs, fermentable oligo-, di-, and mono-saccharides and polyols; RCTs, randomized controlled trials.
Can We Diagnose Non-celiac Reactions to Wheat/Gluten?
NCGS/NCWS is often considered a self-diagnosis, usually reported by the patient. For the physician, it is based on the thorough evaluation of the clinical features according to the indications proposed by the Consensus Conferences on this syndrome.66–68 Identification of biomarkers that allow us to diagnose NCGS/NCWS with more specificity and differentiate it from the unselected IBS population will be key to define and manage this condition.78,79
The clinical picture of patients with NCGS/NCWS is characterized by symptoms occurring shortly after consumption of wheat/ gluten containing meals and disappearing or recurring in a few hours/days after specific withdrawal or challenge.66–68 Symptoms that characterize IBS, such as bloating, abdominal discomfort/pain, altered bowel habits and tiredness, are present in NCGS/NCWS. It has been suggested that a clinical distinction can be made between general IBS and NCGS/NCWS because of more extra-intestinal manifestations involving the central and/or peripheral nervous system, joint/muscle (“fibromyalgia-like”) and skin manifestations in the latter.71 Due to the lack of biomarkers, the diagnosis of NCGS/ NCWS remains highly presumptive being based only on clinical and exclusion criteria.80,81 The improvement of symptoms after a gluten-free diet, which is regarded as the major diagnostic criteria for NCGS/NCWS, might be due to a placebo effect which often follows the elimination of some foods from the diet.82,83 In this context it is important to stress the negative potential influence (“nocebo” effect) generated by media on the “deleterious effect of wheat consumption”. There is no scientific evidence to support that gluten/ wheat consumption is deleterious to the overall population and such sensitivity seems to be limited to a subpopulation of patients that present with IBS symptoms. Despite some disproportionate press on one hand, and some healthy skepticism on the other, there is no doubt that awareness and interest in NCGS/NCWS continues to grow. A specific diagnosis requires the development of a biomarker. Antibodies to native gliadin (AGA) have been suggested as a potential diagnostic marker for NCGS/NCWS diagnosis, in the absence of specific celiac serology such as tissue transglutaminase, endomysial and deamidated gliadin antibodies.84 AGA have been detected in the sera of about half of NCGS/NCWS patients being predominantly of the IgG class. Although AGA are not specific for NCGS/NCWS being detectable in various conditions, ie, autoimmune disorders, connective tissue diseases and even in healthy controls, their positivity, especially at high titers, in patients with a clinical picture suggestive of gluten/wheat sensitivity may be regarded as a diagnostic adjunct. In parallel to symptom resolution, AGA normalized in almost all patients with NCGS/NCWS within 6 months of gluten-free diet.85
In cases with suspected NCGS/NCWS, the most important clinical issue is to rule out celiac disease while the patient is on a gluten-containing diet. Patients with NCGS/NCWS have normal duodenal mucosal histology, although an increased number of IELs ranging from 25 to 40/100 epithelial cells are found in at least 40% of cases, suggesting an accompanying low-grade inflammation.71 There is no increase of T-cell receptor γ/δ IELs in biopsies of patients with NCGS/NCWS. Data on HLA complex and NCGS/ NCWS are unclear. Some studies found that HLA-DQ2 and/or HLA-DQ8 genes were present in ~50% of NCGS/NCWS patients, which is slightly higher than in the general population.84 On the other hand, some studies have suggested that IBS patients who carry celiac susceptibility genes are more likely to respond to the gluten-free diet.52 This apparent controversy may be explained by the fact that overall the NCGS/NCWS population may be heterogeneous and responsive to multiple stimuli. It could be speculated that those with celiac susceptibility genes may be more prone to develop mild immune responses and gut dysfunction to gluten. Up to 20% of NCGS/NCWS show mild laboratory abnormalities, such as low levels of ferritin, folic acid, vitamin D and B12, most likely related to a minimal inflammatory state in the intestinal mucosa.86 Evidence of osteopenia detected by bone densitometry has been found in about 50% of NCGS/NCWS.87 Finally, recent data reported a high prevalence of serum autoantibodies (antinuclear antibodies, ANA) and a frequent association with autoimmune disorders (ie, Hashimoto’s thyroiditis) in patients with NCGS/NCWS.88,89
Dietary Options in Irritable Bowel Syndrome
The gluten-free diet as a treatment for celiac disease is well established. No specific guidelines are yet available for the treatment of IBS patients with NCGS/NCWS. Gluten is a common ingredient of many food items and its complete removal is almost impossible to achieve. Exposure to as little as 10–50 mg of gluten (a breadcrumb) can cause intestinal lesions and symptoms in celiac patients, although the threshold for gluten tolerance is unknown in the NCGS/NCWS population. Moreover, there seems to be an individual level of tolerance in NCGS/NCWS.66–68 Experts in the field recommend that investigations to rule out celiac disease and wheat allergy should be performed in people who consider themselves as gluten/wheat sensitive before starting a gluten-free diet.66
Patients should be warned that an inappropriate dietary restriction can cause nutritional deficiencies. Because gluten-rich grains are important sources of nutrients in the general diet, their exclusion could potentially have major effects on nutritional status. Lower caloric90–93 and fiber intake,90 but a higher intake of total and saturated fat91,92 was observed in the diet of celiac patients compared to healthy control subjects on a gluten containing diet. Lower levels of folate, niacin, vitamin B12, vitamin E, vitamin A, phosphorus, calcium, zinc, and selenium were described in the diet of celiac individuals compared to control subjects.90–93 Currently, it is still difficult to draw a conclusion on the nutritional adequacy of a gluten-free diet because of discordant results noticed from the studies regarding macro- and micro-nutrient intake. However, the evidence suggests that following a gluten-free diet may be detrimental if not properly evaluated and medically indicated.
Dietary restriction has been shown to affect the richness and composition of small intestinal and fecal microbiota, reducing beneficial bacterial groups such as Firmicutes.94,95 It is necessary to consider that after any restriction diet, adaptations in gut physiology and microbial metabolism could increase sensitivity to the subsequent re-introduction of gluten/wheat or FODMAPs.
The persistence of symptoms after a period of gluten-free diet (at least 6 weeks) in patients with suspected NCGS/NCWS suggests that other food sensitivities/intolerances may be responsible for symptom generation.37 In this respect, the patient may benefit from a low-FODMAPs trial excluding rapidly absorbable carbohydrates such as those naturally contained in legumes, onions, honey, pears, water melon, dry fruits, fennel, and dairy products.
Conclusions
Foods can be triggers of gastrointestinal and extra-intestinal symptoms in a proportion of IBS patients. Inside the spectrum of the so-called food hypersensitivity, NCGS/NCWS has been recognized as a newly identified gluten-related disorder which presents clinically with overlapping symptoms of IBS.66–68 Along with gluten, other wheat and food components, have emerged as functional digestive symptom triggers. Within wheat, a potential culprit of symptom generation is ATI, a still poorly investigated soluble protein fraction of wheat.40 A role for FODMAPs, detectable not only in gluten-containing cereals (wheat, rye, and barley), but also in milk, honey, and legumes, has been proposed, although a recent DBPC trial did not find this restriction more efficient than traditional dietary advice for IBS patients.74 We have discussed the evidence for some specific dietary components, such as gluten and other wheat components, to cause functional digestive symptoms and extra-intestinal manifestations matching the current criteria for IBS. As with overall IBS, the mechanisms underlying symptom generation in the subgroup of patients with NCGS/NCWS are still poorly understood. Our review focused on clinical features with the intent to expand current knowledge in this area of gastroenterology. A better understanding of food hypersensitivity and intolerances as well as the development of biomarkers will enable physicians to design tailored dietary approaches to treat patients with food-related functional bowel disorders.
Acknowledgements
We thank Dr Paul Moayedi for scientific discussions and construction of Table 2. M. Ines Pinto-Sanchez received a CIHR-CAG Health Professional Fellowship Award.
Footnotes
Financial support: Elena F Verdu is funded by CIRH MO#142773 and holds a Canada Research Chair. Roberto De Giorgio is supported by the Ricerca Finalizzata RER2009 (Ita-MNGIE) by the Italian Ministry of Public Health; Telethon (Grant GGP15171) and by RFO (Ricerca Fondamentale Orien-tata) from University of Bologna, Italy.
Conflicts of interest: None.
Author contributions: Roberto De Giorgio, Umberto Volta, and Elena F Verdu: concept and design of review and critical revision of the manuscript for important intellectual content; and Maria Ines Pinto-Sanchez, Elisa Boschetti, Giacomo Caio, Umberto Volta, Roberto De Giorgio, and Elena F Verdu: writing of manuscript.
References
- 1.Malagelada C, Drozdzal M, Seguí S, et al. Classification of functional bowel disorders by objective physiological criteria based on endoluminal image analysis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G413–G419. doi: 10.1152/ajpgi.00193.2015. [DOI] [PubMed] [Google Scholar]
- 2.Ke J, Qi R, Liu C, et al. Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol Motil. 2015;27:1796–1803. doi: 10.1111/nmo.12692. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Simrén M, Svedlund J, Posserud I, Björnsson ES, Abrahamsson H. Health-related quality of life in patients attending a gastroenterology out-patient clinic: functional disorders versus organic diseases. Clin Gastroenterol Hepatol. 2006;4:187–195. doi: 10.1016/S1542-3565(05)00981-X. [DOI] [PubMed] [Google Scholar]
- 6.Cogliandro RF, Antonucci A, De Giorgio R, et al. Patient-reported outcomes and gut dysmotility in functional gastrointestinal disorders. Neurogastroenterol Motil. 2011;23:1084–1091. doi: 10.1111/j.1365-2982.2011.01783.x. [DOI] [PubMed] [Google Scholar]
- 7.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 9.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39:201–215. doi: 10.1016/j.dld.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 13.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto-Sanchez MI, Bercik P, Verdu EF. Motility alterations in celiac disease and non-celiac gluten sensitivity. Dig Dis. 2015;33:200–207. doi: 10.1159/000371400. [DOI] [PubMed] [Google Scholar]
- 15.Burden S. Dietary treatment of irritable bowel syndrome: current evidence and guidelines for future practice. J Hum Nutr Diet. 2001;14:231–241. doi: 10.1046/j.1365-277X.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 16.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–1214. doi: 10.1016/j.jada.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y) 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 18.Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 19.Bolino CM, Bercik P. Pathogenic factors involved in the development of irritable bowel syndrome: focus on a microbial role. Infect Dis Clin North Am. 2010;24:961–975. ix. doi: 10.1016/j.idc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez LA, Ruigómez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565–566. doi: 10.1136/bmj.318.7183.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235–245. vi–vii. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Houghton LA, Lea R, Morris J, Reilly B, Whorwell PJ. Bloating and distention in irritable bowel syndrome: the role of visceral sensation. Gastroenterology. 2008;134:1882–1889. doi: 10.1053/j.gastro.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri M, Neri M. Motility disorders and stress. Dig Dis Sci. 1989;34:1777–1786. doi: 10.1007/BF01540058. [DOI] [PubMed] [Google Scholar]
- 25.McKee DP, Quigley EM. Intestinal motility in irritable bowel syndrome: is IBS a motility disorder? Part 2. Motility of the small bowel, esophagus, stomach, and gall-bladder. Dig Dis Sci. 1993;38:1773–1782. doi: 10.1007/BF01296098. [DOI] [PubMed] [Google Scholar]
- 26.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:438–448. doi: 10.1111/apt.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chey WD. Food: the main course to wellness and illness in patients with irritable bowel syndrome. Am J Gastroenterol. 2016;111:366–371. doi: 10.1038/ajg.2016.12. [DOI] [PubMed] [Google Scholar]
- 31.Ford AC, Vandvik PO. Irritable bowel syndrome: dietary interventions. BMJ Clin Evid. 2015;2015 pii:0410. [PMC free article] [PubMed] [Google Scholar]
- 32.Bhat K, Harper A, Gorard DA. Perceived food and drug allergies in functional and organic gastrointestinal disorders. Aliment Pharmacol Ther. 2002;16:969–973. doi: 10.1046/j.1365-2036.2002.01256.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26(suppl 3):128–131. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff SC, Mayer J, Wedemeyer J, et al. Colonoscopic allergen provocation (COLAP): a new diagnostic approach for gastrointestinal food allergy. Gut. 1997;40:745–753. doi: 10.1136/gut.40.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuomo R, Andreozzi P, Zito FP, Passananti V, De Carlo G, Sarnelli G. Irritable bowel syndrome and food interaction. World J Gastroenterol. 2014;20:8837–8845. doi: 10.3748/wjg.v20.i27.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philpott H, Yu S, Rao S. It’s all in the mix: diagnosis and management of food intolerance. Clin Gastroenterol Hepatol. 2016;14:1221–1222. doi: 10.1016/j.cgh.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 37.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169–178. doi: 10.1136/gutjnl-2015-309757. [DOI] [PubMed] [Google Scholar]
- 38.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–2408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. 2013;5:771–787. doi: 10.3390/nu5030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson PR, Muir JG, Newnham ED. Other dietary confounders: FODMAPS et al. Dig Dis. 2015;33:269–276. doi: 10.1159/000371401. [DOI] [PubMed] [Google Scholar]
- 43.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basilisco G, Marino B, Passerini L, Ogliari C. Abdominal distension after colonic lactulose fermentation recorded by a new extensometer. Neurogastroenterol Motil. 2003;15:427–433. doi: 10.1046/j.1365-2982.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- 45.Ferch CC, Chey WD. Irritable bowel syndrome and gluten sensitivity without celiac disease: separating the wheat from the chaff. Gastroenterology. 2012;142:664–666. doi: 10.1053/j.gastro.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Wahnschaffe U, Ullrich R, Riecken EO, Schulzke JD. Celiac disease-like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology. 2001;121:1329–1338. doi: 10.1053/gast.2001.29572. [DOI] [PubMed] [Google Scholar]
- 47.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–850. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 49.Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroccio A, Mansueto P, D’Alcamo A, Iacono G. Non-celiac wheat sensitivity as an allergic condition: personal experience and narrative review. Am J Gastroenterol. 2013;108:1845–1852. doi: 10.1038/ajg.2013.353. [DOI] [PubMed] [Google Scholar]
- 51.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328. e1–e3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911. e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigo L, Blanco I, Bobes J, de Serres FJ. Effect of one year of a gluten-free diet on the clinical evolution of irritable bowel syndrome plus fibromyalgia in patients with associated lymphocytic enteritis: a case-control study. Arthritis Res Ther. 2014;16:421. doi: 10.1186/s13075-014-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Sabatino A, Volta U, Salvatore C, et al. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: a randomized, double-blind, placebo-controlled, cross-over trial. Clin Gastroenterol Hepatol. 2015;13:1604–1612. e3. doi: 10.1016/j.cgh.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Zanini B, Baschè R, Ferraresi A, et al. Randomised clinical study: gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment Pharmacol Ther. 2015;42:968–976. doi: 10.1111/apt.13372. [DOI] [PubMed] [Google Scholar]
- 56.Elli L, Tomba C, Branchi F, et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients. 2016;8:84. doi: 10.3390/nu8020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdu EF, Huang X, Natividad J, et al. Gliadin-dependent neuro-muscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 58.Nikulina M, Habich C, Flohé SB, Scott FW, Kolb H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–1933. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- 59.Chirdo FG, Arranz E. Cereal proteins: immunostimulatory and toxic peptides. Valladolid: Omnia Science Monographs; 2016. pp. 141–156. [Google Scholar]
- 60.Cinova J, Palová-Jelíinková L, Smythies LE, et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201–209. doi: 10.1007/s10875-006-9061-z. [DOI] [PubMed] [Google Scholar]
- 61.Schuppan D, Zevallos V. Wheat amylase trypsin inhibitors as nutritional activators of innate immunity. Dig Dis. 2015;33:260–263. doi: 10.1159/000371476. [DOI] [PubMed] [Google Scholar]
- 62.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–658. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 63.Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 64.Zuo XL, Li YQ, Li WJ, et al. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin Exp Allergy. 2007;37:823–830. doi: 10.1111/j.1365-2222.2007.02727.x. [DOI] [PubMed] [Google Scholar]
- 65.Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:359–365. e1. doi: 10.1016/j.cgh.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 66.Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Catassi C, Bai JC, Bonaz B, et al. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–3853. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the salerno experts’ criteria. Nutrients. 2015;7:4966–4977. doi: 10.3390/nu7064966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mansueto P, D’Alcamo A, Seidita A, Carroccio A. Food allergy in irritable bowel syndrome: The case of non-celiac wheat sensitivity. World J Gastroenterol. 2015;21:7089–7109. doi: 10.3748/wjg.v21.i23.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol. 2013;48:921–925. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 71.Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. doi: 10.1186/1741-7015-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80. doi: 10.1159/000260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brottveit M, Beitnes AC, Tollefsen S, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842–850. doi: 10.1038/ajg.2013.91. [DOI] [PubMed] [Google Scholar]
- 74.Bohn L, Storsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407. e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 75.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FOD-MAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 76.Moayyedi P, Quigley EM, Lacy BE, et al. The effect of dietary intervention on irritable bowel syndrome: a systematic review. Clin Transl Gastroenterol. 2015;6:e107. doi: 10.1038/ctg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55:897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 78.Rostami K, Hogg-Kollars S. A Patient’s Journey. Non-coeliac gluten sensitivity. BMJ. 2012;345:e7982. doi: 10.1136/bmj.e7982. [DOI] [PubMed] [Google Scholar]
- 79.Volta U, De Giorgio R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol. 2012;9:295–299. doi: 10.1038/nrgastro.2012.15. [DOI] [PubMed] [Google Scholar]
- 80.Biesiekierski JR, Newnham ED, Shepherd SJ, Muir JG, Gibson PR. Characterization of adults with a self-diagnosis of nonceliac gluten sensitivity. Nutr Clin Pract. 2014;29:504–509. doi: 10.1177/0884533614529163. [DOI] [PubMed] [Google Scholar]
- 81.Aziz I, Lewis NR, Hadjivassiliou M, et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol. 2014;26:33–39. doi: 10.1097/01.meg.0000435546.87251.f7. [DOI] [PubMed] [Google Scholar]
- 82.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1–4. doi: 10.1056/NEJM199507063330101. [DOI] [PubMed] [Google Scholar]
- 83.Di Sabatino A, Corazza GR. Nonceliac gluten sensitivity: sense or sensibility? Ann Intern Med. 2012;156:309–311. doi: 10.7326/0003-4819-156-4-201202210-00010. [DOI] [PubMed] [Google Scholar]
- 84.Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–685. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 85.Caio G, Volta U, Tovoli F, De Giorgio R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014;14:26. doi: 10.1186/1471-230X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molina-Infante J, Santolaria S, Sanders DS, Fernández-Bañares F. Systematic review: noncoeliac gluten sensitivity. Aliment Pharmacol Ther. 2015;41:807–820. doi: 10.1111/apt.13155. [DOI] [PubMed] [Google Scholar]
- 87.Carroccio A, Soresi M, D’Alcamo A, et al. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: a prospective observation study. BMC Med. 2014;12:230. doi: 10.1186/s12916-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carroccio A, D’Alcamo A, Cavataio F, et al. High proportions of people with nonceliac wheat sensitivity have autoimmune disease or antinuclear antibodies. Gastroenterology. 2015;149:596–603. e1. doi: 10.1053/j.gastro.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 89.Volta U, Caio G, De Giorgio R. Is autoimmunity more predominant in nonceliac wheat sensitivity than celiac disease? Gastroenterology. 2016;150:282. doi: 10.1053/j.gastro.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 90.Wild D, Robins GG, Burley VJ, Howdle PD. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment Pharmacol Ther. 2010;32:573–581. doi: 10.1111/j.1365-2036.2010.04386.x. [DOI] [PubMed] [Google Scholar]
- 91.Kinsey L, Burden ST, Bannerman E. A dietary survey to determine if patients with coeliac disease are meeting current healthy eating guidelines and how their diet compares to that of the British general population. Eur J Clin Nutr. 2008;62:1333–1342. doi: 10.1038/sj.ejcn.1602856. [DOI] [PubMed] [Google Scholar]
- 92.Dall’Asta C, Scarlato AP, Galaverna G, Brighenti F, Pellegrini N. Dietary exposure to fumonisins and evaluation of nutrient intake in a group of adult celiac patients on a gluten-free diet. Mol Nutr Food Res. 2012;56:632–640. doi: 10.1002/mnfr.201100515. [DOI] [PubMed] [Google Scholar]
- 93.Bardella MT, Fredella C, Prampolini L, Molteni N, Giunta AM, Bianchi PA. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am J Clin Nutr. 2000;72:937–939. doi: 10.1093/ajcn/72.4.937. [DOI] [PubMed] [Google Scholar]
- 94.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102:1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 95.Nistal E, Caminero A, Vivas S, et al. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie. 2012;94:1724–1729. doi: 10.1016/j.biochi.2012.03.025. [DOI] [PubMed] [Google Scholar]