Abstract
To systematically review the effects of probiotics on central nervous system function in animals and humans, to summarize effective interventions (species of probiotic, dose, duration), and to analyze the possibility of translating preclinical studies. Literature searches were conducted in Pubmed, Medline, Embase, and the Cochrane Library. Only randomized controlled trials were included. In total, 38 studies were included: 25 in animals and 15 in humans (2 studies were conducted in both). Most studies used Bifidobacterium (eg, B. longum, B. breve, and B. infantis) and Lactobacillus (eg, L. helveticus, and L. rhamnosus), with doses between 109 and 1010 colony-forming units for 2 weeks in animals and 4 weeks in humans. These probiotics showed efficacy in improving psychiatric disorder-related behaviors including anxiety, depression, autism spectrum disorder (ASD), obsessive-compulsive disorder, and memory abilities, including spatial and non-spatial memory. Because many of the basic science studies showed some efficacy of probiotics on central nervous system function, this background may guide and promote further preclinical and clinical studies. Translating animal studies to human studies has obvious limitations but also suggests possibilities. Here, we provide several suggestions for the translation of animal studies. More experimental designs with both behavioral and neuroimaging measures in healthy volunteers and patients are needed in the future.
Keywords: Animals, Anxiety, Depression, Humans, Probiotics
Introduction
The microbiota, the ecological community of commensal, symbiotic, and pathogenic microorganisms literally sharing our body space, includes more than 10 times the number of host cells to human cells.1 The majority of the microbiome lives in the gastrointestinal (GI) tract and is composed of 10 100 trillion microorganisms, containing 100 times as many genes as our genome.2 Symbiosis of the gut microbiota (GM) can maintain a normal physiology in the host, while dysbiosis of the GM can shift the balance and may induce diseases.
Recent studies have found a role for the GM in the gut-brain axis, which can alter minds and behaviors through the central nervous system (CNS).3–8 Maintaining GM symbiosis is important for retaining healthy CNS functions. Sudo’s study was the first linking the GM and CNS, showing an increased hypothalamic-pituitary-adrenal (HPA) stress response and decreased brain-derived neurotrophic factor (BDNF) levels in the hippocampus of germ-free (GF) mice.9 Recently, researchers have found a relationship between the GM and CNS-related disorders in humans, such as Parkinson’s disease and autism: overall diversity and individual genus abundances were associated with their symptoms.10–12
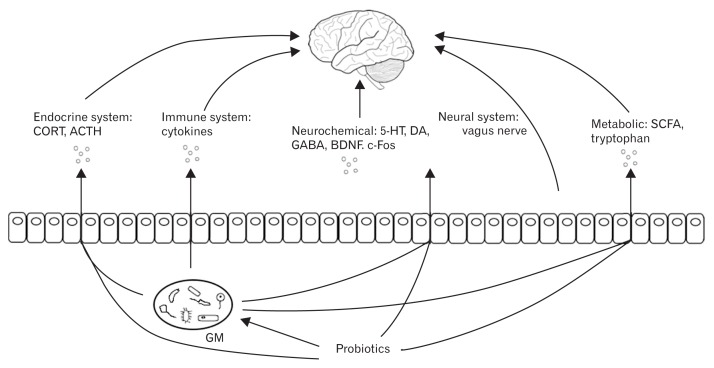
Probiotics are defined as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host”.13,14 Some types of probiotics have been used to treat gastrointestinal disorders, such as irritable bowel syndrome (IBS).15–18 Also, probiotics have been studied in relation to altering visceral pain responses.19 Recently they have been reported to have an influence in the CNS by altering the GM composition.3,8 Studies using probiotics to change CNS functions have increased over the last 10 years. The CNS functions mostly reported being altered by probiotics are psychiatric disorders and memory abilities. Studies in animals and humans have found, and reviews have summarized, potential mechanisms underlining these probiotic effects (Fig. 1). First, probiotics may directly alter CNS biochemistry, such as by affecting levels of BDNF, γ-aminobutyric acid (GABA), serotonin (5 hydroxytryptamine; 5 HT), and dopamine (DA), thus influencing mind and behavior.20–24 Both the vagus and the enteric nerves are involved in this gut-brain interaction and can be affected by certain probiotics.22,23 The HPA stress response, which regulates mood and emotion, has frequently been shown to be attenuated by probiotics, decreasing corticosteroid (CORT) levels.25 The immune system can be influenced by probiotics, limiting pro-inflammatory cytokine production and inflammation, which, in turn, can affect the endocrine and nervous systems.26,27 Probiotics manipulate GM by increasing microbiota diversity and beneficial bacteria compositions.28–30 Improved GM changes metabolites, such as short-chain fatty acids and tryptophan, which can indirectly improve CNS function.26,31
Figure 1.
Mechanisms of probiotic effects on the central nervous system. Probiotics influence central nervous system (CNS) function through direct and indirect mechanisms. Probiotics affect the hypothalamic-pituitary-adrenal (HPA) axis, by altering corticosteroid (CORT) and/or adrenocorticotropic hormone (ACTH) levels. The immune system is influenced by limited pro-inflammatory cytokine production and inflammation, and this, in turn, has effects on the CNS. Probiotics can also directly alter CNS biochemistry, such as by affecting brain-derived neurotrophic factor (BDNF), c-Fos, γ-aminobutyric acid (GABA), 5 hydroxytryptamine (5-HT), and dopamine (DA) levels, thus influencing mind and behavior. The vagus and enteric nerves are also involved in this gut-brain interaction and are affected by certain probiotics. Probiotics manipulate the gut microbiota (GM) by increasing microbiota diversity and beneficial bacteria composition. An “improved” GM changes metabolites, such as short-chain fatty acids (SCFAs) and tryptophan, and so improves CNS function indirectly. The GM also interacts with the endocrine, immune, and neural systems.
While there are reviews describing effects of probiotics on CNS function, there has been no previous systematic review that analyzes all the current animal and human studies in the field and describes the most effective probiotic interventions. Furthermore, animal studies in this area outnumber human studies, because behavioral experiments in animals are better established and standardized, while clinical studies in humans on this topic started to increase a few years ago and need translation from preclinical studies. However, from preclinical animal models to clinical trials in humans, there is no direct translation. To bridge the gap between preclinical and clinical studies, a systematic review is needed to summarize effective probiotic interventions on CNS function. We first sought to describe CNS functions that can be influenced by probiotics. Second we provide information about probiotic interventions including strain, dosage, and duration. Furthermore, we analyze and discuss the potential translation of animal models to human studies.
Methods
Search Strategy
This systematic review was conducted according to guidelines of the “Cochrane Handbook for Systematic Reviews of Interventions”, following the Preferred Items for Systematic Reviews and Meta-analysis guidelines.32,33 Relevant studies were found by searches in the Pubmed, Medline, Embase, and Cochrane Library databases. Articles from 1950 to April 2016 were initially searched using the search terms “(probiotic OR gut microbiota) AND (behavior OR central nervous system)”. Additional citations were sought using references in articles retrieved from searches. We only included articles written in English. The first search was undertaken by analyzing text words contained in the title and abstract, and of the key words describing the articles. The second search was conducted according to the citations from all identified reports and relevant review articles.
Study Selection
We included all animal and human studies using different strains of probiotics. In human studies reports of both healthy volunteers and patients were considered.
The abstracts of the retrieved papers were screened for matching the following criteria: (1) the study included a probiotic intervention and (2) the study tested CNS function. After exclusion of non-relevant studies, the remaining articles were screened for the following criteria: (1) the study was described as a randomized controlled study (RCT), (2) the study was described as double-blind if studied in human participants, (3) the study involved use of probiotics in single- and/or multi-strains and those that only used pre-biotics or antibiotics were not included, and (4) the study included measures of behavioral experiments, neuropsychological measures (eg, electroencephalography, magnetoencephalography, and functional magnetic resonance imaging). Those that only involved neurophysiological measures were not included in the qualitative analysis but only in the Discussion (eg, measuring neurochemical level, HPA axis activity) because different studies tested quite divergent aspects of lower-level CNS activity.
Data Collection and Analysis
A data extraction and assessment form from the Cochrane Collaboration was used to further exclude inappropriate studies and to extract data we needed to analyze.33 Double extraction of data was conducted: important data included the source of participants (animal species/strains, patient types), intervention groups (probiotic, placebo, or any other intervention), sample size, duration of interventions, and outcomes (behavioral changes as the primary outcome and lower-level changes [eg, biochemical changes] as secondary outcomes).
We assessed the quality of each study included using the Quality Assessment Tool for Quantitative Studies and the Quality Assessment Tool for Quantitative Studies Dictionary developed by The Effective Public Health Practice Project (EPHPP) (National Collaborating Centre for Methods and Tools [2008]).34
The inclusion of both animals and human studies resulted in great heterogeneity of participants, interventions, and CNS functions measured. The outcomes of each study were described. A qualitative synthesis of selected studies was made with the aim of coming to conclusions about which probiotic interventions, at which dose, and for how long, were more/most effective with regard to certain CNS functions. Because of the heterogeneity of designs a meta-analysis was deemed to be inappropriate.
Results
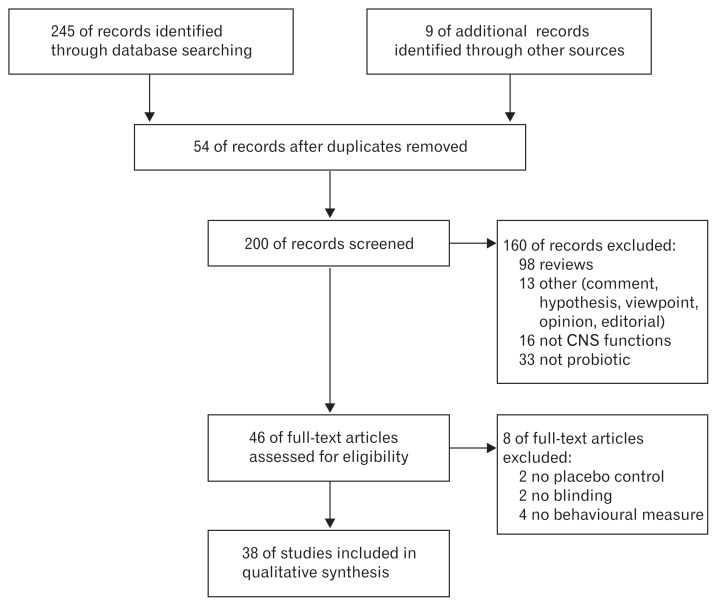
In total, 46 potentially relevant citations were obtained through the primary search strategy, which included animal and human studies, after excluding reviews (n = 98) (systematic reviews, narrative reviews, respective reviews, and systematic reviews and meta-analyses), other articles including comments, hypotheses, viewpoints, opinions, and editorials (n = 13), studies without the use of a probiotic (n = 33), and studies not focusing on CNS function (n = 16) (Fig. 2). Many articles concerned the potential, and demonstrated mechanisms of the effects, of GM and/or probiotics on CNS function. However, we only included studies that clearly described probiotics as interventions, and excluded studies only measuring GM composition when investigating CNS function. After the full screen, 8 more studies were excluded: four studies lacked a control arm or blinding, and 4 studies did not measure CNS functions at the behavioral level. In total, 38 studies remained for the qualitative analysis. Of them, 15 were in humans and 25 were in animals (2 studies were conducted in humans and animals).
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) scheme of retrieved literature. CNS, central nervous system.
Animal Studies
Of the 25 studies, 19 provided explicit information in the Materials and Methods section on the numbers of animals allocated to each treatment group, and they accounted for all the animals in their results, while 6 studies provided specifics regarding treated animals only in the pertinent areas of the Results section. Results from the quality assessment tool for quantitative studies showed all studies were strong in global ratings for selection bias, study design, confounders, blinding, data collection method, and withdrawals and dropouts (no weak rating): 15 studies described assigning animals randomly to treatment groups or control, although these animal studies were supposed to be RCTs. Only four studies might have confounders because they used both male and female animals while the other studies only used single gender animals, excluding the effect of gender on the results. Only 13 studies reported that experimenters were blinded toward the interventions or exposure status of the participants (we included the other 12 studies that did not describe blinding, because animals were not aware of the research question or intervention, and we ignored potential effects of the experimenter). Data collection tools shown in all studies were considered valid and reliable; no study reported withdrawals or drop outs.
Although all of studies examined were on rodents (mice or rats), the selection of animals was heterogeneous in some respects: strains of animals and health conditions of the animals. Studies were also heterogeneous in the CNS functions they were looking at, the experimental models they used, the probiotics they used, and the dose and duration of the probiotic interventions. Due to the heterogeneity of the included studies, we only described the results based on the interventions and measurements of the CNS functions (Table 1).
Table 1.
Studies of Effects of Probiotics on Central Nervous System Functions in Animals
| Study | Animal | CNS function | Probiotic (species, dosage [CFU/day], duration) | Outcome (behavioral level) | Secondary outcome |
|---|---|---|---|---|---|
| Liu et al,20 2016 | ELS mice Naïve mice |
Locomotor activity (open-field test) Anxiety (open-field test, elevated plus maze) Depression (sucrose-preference test, forced-swimming test) |
L. plantarum PS128 109 28 days |
Locomotor activity ↑ Anxiety (only in naïve mice) ↓ Depression (only in ELS mice) ↓ |
Corticosteroids (CORT) ↓ Cytokine: inflammatory cytokine TNF-α, IL-6 ↓, anti-inflammatory cytokine IL-10 ↑ Brain monoamines and metabolites: 5-HT ↑, 5-HIAA ↓, DA ↑, DOPAC ↓, HVA ↓ |
| Liu et al,21 2016 | GF mice | Locomotor activity (open-field test) Anxiety (elevated-plus maze) Depression (forced-swimming test) |
L. plantarum PS128 109 16 days |
Locomotor activity ↑ Anxiety ↓ Depression (−) |
CORT: NA Brain monoamines and metabolites: DA ↑, HVA ↑, 5-HT ↑ and 5-HIAA ↑ |
| Liang et al,38 2015 | SPF CRS rats | Stress (chronic-restraint stress) Depression (sucrose-preference test) Anxiety (elevated-plus maze, open-field test) Non-spatial memory (object-recognition test, object-placement test) |
L. helveticus NS8 109 Initial 21 days |
Anxiety ↓ Depression ↓ Non-spatial memory ↑ |
CORT and ACTH ↓ Cytokines: IL-10 ↑ Brain monoamines: 5-HT ↑ and NE ↑ BDNF expression ↑ |
| Wang et al,30 2015 | Ampicillin-treated rats | Anxiety (elevate-plus maze) Spatial memory (Morris water maze) |
L. fermentum N93 109 Initial 30 days |
Anxiety ↓ Spatial memory ↑ |
CORT and ACTH ↓ Brain monoamines: MR ↑, NMDA ↑, GR: NA Brain BDNF: NA Colon inflammation: myeloperoxidase activity ↓ Fecal microbiota: Bacteroides ↑, C. coccoides ↓, Firmicutes ↓, Lactobacillus ↑ |
| Smith et al,29 2014 | RagI−/− mice | Stress response (water-avoidance stress) Anxiety (light/dark box test) Non-spatial memory (novel-object test) |
L. rhamnosus R0011 + L. helveticus R0052 6 × 109 28 days |
Anxiety ↓ Non-spatial memory ↑ |
CORT: NA Brain c-Fos expression ↑ Intestinal permeability ↓ Fecal microbiota: Bacteroides ↑, Enterobacteriaceae ↑, Firmicutes ↑ |
| Luo et al,42 2014 | Hyperammonemia rats | Anxiety (elevate-plus maze) Spatial memory (Morris water maze) |
L. helveticus NS8 109 14 days |
Anxiety ↓ Spatial memory ↑ |
Neuroinflammation: PGE2 ↓, IL-1β ↓ Brain monoamines: 5-HT ↓ Plasma kynurenine pathway: KYN/TRP ↑, KA/KYN ↓ |
| Savignac et al,36 2014 | Mice | Anxiety (defensive marble burying, elevated-plus maze, open field) depression (tail-suspension test, forced-swim test) |
B. longum 1714/B. breve 1205 109 Initial 21 days |
B. longum: Anxiety ↓ Depression ↓ B. breve: Anxiety ↓ |
CORT: NA |
| Ohland et al,37 2013 | Il-10 deficient mice | Anxiety (elevated Barnes Maze) Spatial memory (elevated Barnes maze) |
L. helveticus R0052 109 21 days |
Anxiety ↓ Spatial memory ↑ |
Colon inflammation ↓ Cytokines: NA CORT ↓ SCFA metabolites: NA |
| Messaoudi et al,39 2011 | Rats | Anxiety (conditioned defensive burying) |
B. longum R0175 + L. helveticus R0052 109 14 days |
Anxiety ↓ | NA |
| Bravo et al,23 2011 | Mice | Anxiety (open arms, elevated-plus maze, fear conditioning) Depression (forced-swim test) |
L. rhamnosus JB-1 109 28 days |
Anxiety ↓ Depression ↓ |
CORT ↓ GABA receptor expression influence depending on brain areas Probiotic effect via vagus nerve |
| Bercik et al,22 2011 | Chronic colitis mice | Anxiety (step-down test) |
B. longum NCC3001 1010 14 days |
Anxiety ↓ | Colon inflammation: NA Brain BDNF expression: NA Enteric neurons excitability ↓ Probiotic effect via vagus nerve |
| Bercik et al,35 2010 | T-muris infected mice | Anxiety (light/dark behavior, step-down test) |
B. longum NCC3001/L. rhamnosus NCC4007 1010 10 days |
Anxiety ↓ (B. longum only) | Colon inflammation ↓ Plasma cytokines: NA BDNF expression ↑ (only by B. longum) Tryptophan and kynurenine: NA No effect of vagotomy |
| Singh et al,40 2012 | Rats | CFS and depression induced by forced-swim test (immobility period, post-swim fatigue time) |
L. acidophilus as LAB or LAB FB 107 7 days |
Depression ↓ (larger effect of LAB FB than LAB); | Brain oxido-nitrosative stress biomarker ↓ Cytokines: TNF-α ↓ |
| Arseneautl-Bread et al,41 2012 | MI rats | Post-MI depression (forced-swim test); social behavior (social interaction test); emotional memory (passive avoidance step-down test) |
B. longum R0175 + L. helveticus R0052 109 14 days |
Depression ↓ Social interaction ↑ Non-spatial memory ↑ |
Cytokines: pro-inflammatory cytokine IL-1β ↓ Intestinal barrier permeability ↓ |
| Desbonnet et al,27 2010 | MS rats | Depression (forced-swim test) |
B. infantis 35624 1010 Initial 40 days |
Depression ↓ | CORT: NA Cytokines: IL-10 ↓ Tryptophan: NA Brain monoamines (−) Noradrenaline ↑ |
| Desbonnet et al,26 2008 | Rats | Depression (forced-swim test) |
B. infantis 35624 1010 14 days |
No behavioral change | Cytokines: pro-inflammatory cytokines IL-6, IFN-γ ↓; anti-inflammatory cytokines IL-10 ↓ Tryptophan ↑ Brain monoamines and metabolites – 5-HIAA ↓, DOPAC ↓ Neuroendocrine: NA, CORT: NA, AVP: NA, CRF: NA |
| Liu etal,44 2015 | VaD (vascular dementia) mice | Locomotor activity (open-field test) Spatial memory (Morris water maze) |
C. butyricum WZMC1016 (CGMCC 9831) 106/107/108 42 days |
Locomotor activity ↑ Spatial memory ↑ |
Morphological change of hippocampus ↓ BDNF expression ↑ Butyrate in feces and brain ↑ Fecal bacteria diversity ↑ |
| Jeong et al,47 2015 | Aged rats | Spatial memory (Y-maze, Morris water maze) |
L. plantarum KY1032 + L. curvatus HY7601 1010 48 days |
Spatial memory ↑ | Cytokines: pro-inflammatory cytokines NF-κB, ↓ BDNF ↑ Lipidemia ↓ |
| Savignac et al,43 2015 | Mice | Non-spatial memory (object recognition, fear conditioning) Spatial memory (Barnes Maze) |
B. longum 1714/B. breve 1205 109 initial 21 days |
B. longum: Non-spatial memory ↑ Spatial memory ↑ B. breve: Non-spatial memory ↑ |
Visceral sensitivity -colon distension: NA CORT: NA |
| Gareau et al,45 2011 | C. rodentium infected mice | Memory dysfunction induced by water avoidance (novel-object test, T-maze); |
L. rhamnosus R0011 + L. helveticus R0052 6 × 109 17 days |
Non-spatial memory ↑ | CORT ↓ Colon epithelial cell hyperplasia ↓ Cytokine: pro-inflammatory cytokines IFN γ ↓ Brain BDNF and c-Fos expression ↑ Microbiota: Firmicutes ↓, Enterobacteriaceae ↓, Eubacteria rectale ↓, Lactobacillus ↑ |
| Davari et al,46 2013 | Diabetic rats | Spatial memory (Morris water maze) |
L. acidophilus 4356 + B. lactis 10140 + L. fermentum ATCC9338 2 × 1010 56 days |
Spatial memory ↑ | Hippocampal long-term potentiation ↑ Serum glucose ↓ and insulin ↑ Oxidative stress biomarkers: SOD ↑, 8-OHdG ↓ |
| Hsiao et al,31 2013 | MIA mice | Autism spectrum disorder: Anxiety (open field, marble burying); Sensory gating (prepulse inhibition); Communicative behavior (ultrasonic vocalizations); Social interaction (3-chamber social test) |
B. fragilis NCTC9343 1010 6 days |
Anxiety ↓ Sensory gating ↑ Communicative behavior ↑ Social interaction (−) |
Intestinal permeability ↓ Tryptophan metabolites: indolepyruvate ↓ Microbiota: Lachnospiraceae ↓, Bacteroidales ↓ |
| Kantak et al,48 2014 | Male mice | obsessive-compulsive-disorder-like behavior (open field, marble burying, ultrasonic vocalizations, intermale aggression) |
L. rhamnosus GG (ATCC 53103) 109 14, 28 days |
Locomotor behavior ↓ Marble burying ↓ Ultrasonic vocalizations (−) Intermale aggression (−) |
NA |
| D’Mello et al,49 2015 | Male mice | Inflammation associated sickness behavior (social exploratory) | VSL#3 1.7 × 109 10 days |
Social exploratory behavior in bile duct ligation treated mice ↑ | Intestinal permeability: NA Cytokine: pro-inflammatory cytokine TNF-α ↓ Monocyte infiltration ↓ Microglial activation ↓ Fecal Microbiota (−) |
| Takada et al,50 2016 | Male rats | Stress response to water avoidance stress |
L. casei Shirota YIT 9029 3 × 109 14 days |
NA | CORT ↓ c-Fos expression in the paraventricular nucleus ↓ Gastric vagal afferent activity ↑ Neuronal excitability of NTS ↑ |
ELS, early life stress; CORT, corticosterone; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-Hydroxyindoleacetic acid; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; GF, germ free; NA, not applicable; SPF, specific pathogen free; CRS, chronic restraint stress; ACTH, adrenocorticotropic hormone; NE, norepinephrine; BDNF, brain-derived neurotrophic factor; MR, mineralocorticoid; NMDA, N-methyl-D-aspertate; GR, glucocorticoid; RagI−/−, RagI knockout; PGE2, prostaglandin E2; KYN, L-kynurenine; TRP, tryptophan; KA, kynurenic acid; SCFA, short-chain fatty acid; GABA, gamma-Aminobutyric acid; CFS, chronic fatigue syndrome; LAB, Lactobacillus acidophilus; FB, floating bead; MI, myocardial infarction; MS, maternal separation; AVP, arginine vasopressin; CRF, corticotrophin-releasing factor; VaD, vascular dementia; NF-κB, nuclear factor-kappa B; SOD, superoxide dismutase; 8-OHdG, 8-hydroxy-2’deoxyguanosine; MIA, maternal immune activation; VSL#3, a high-concentration probiotic preparation of 8 live freeze-dried bacterial (Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus thermophiles); NTS, nucleus tractus solitary; B. breve, Bifidobacterium breve; B. fragilis, Bifidobacterium fragilis; B. infantis, Bifidobacterium infantis; B. lactis, Bifidobacterium lactis; B. longum, Bifidobacterium longum; C. butyricum, Clostridium butyricum; C. coccoides, Clostridium coccoides; C. rodentium, Citrobacter rodentium; L. acidophilus, Lactobacillus acidophilus; L. casei, Lactobacillus casei; L. curvatus, Lactobacillus curvatus; L. fermentum, Lactobacillus fermentum; L. helveticus, Lactobacillus helveticus; L. plantarum, Lactobacillus plantarum; L. rhamnosus, Lactobacillus rhamnosus.
Most of the studies (18/25) investigated the effects of a single strain of probiotics. In 18 studies using single-strain probiotics, seven used Bifidobacterium, eleven used Lactobacillus, and one used Clostridium (one used both Bifidobacterium and Lactobacillus). Almost all the studies found significant effects on measured CNS functions, except for one testing effect of Bifidobacterium infantis on depression-like behavior. The concentration of the effective probiotic interventions ranged from 107 to 1011 colony-forming units (CFU), with the most using 109 (14/25) or 1010 (6/25) CFU per animal per day. The duration of the probiotic treatments ranged from 6 to 77 days, with the most frequent period being 14 days (7/25). Effects of different probiotics on different specific CNS functions in animals were analyzed and described in the following text.
Anxiety
Twelve studies tested anxiety-like behavior in animals (mice or rats). The anxiety-like behaviors were evaluated with the elevate plus/Barnes maze, light/dark box, defensive burying, open field/arms, fear conditioning, and step-down tests. Three of them used a single strain of Bifidobacterium longum, all with positive results, ie, the animals showed less anxious behavior.22,35,36 Two studies using Lactobacillus helveticus also found reduced anxiety-like behaviors in immune-deficient mice and chronically restrained rats.29,37,38 Two studies used Lactobacillus rhamnosus but only one showed reduced anxiety behaviors.23,35 Two studies using Lactobacillus plantarum also found alleviated anxiety levels in mice after the intervention.20,21 Bifidobacterium breve and Lactobacillus fermentum were used once each and showed anxiolytic effects.30,36 Two studies using multi-strain probiotic combinations of L. rhamnosus + L. helveticus and B. longum + L. helveticus found reduced anxious behavior.29,39
Depression
Nine studies focused on depression and all reported positive results except one. Depression-like behaviors were measured with the tail-suspension, forced-swim, and sucrose-preference tests. B. infantis was used twice but only one study found reduced depression-like behavior. Each of the single strains of B. longum, B. breve, L. rhamnosus, and L. helveticus was also used once and all showed antidepressant effects.23,26,27,36,40 Two studies tested L. plantarum, but it only had an effect in mice with the early life stress of maternal separation.20,21 One study used a multi-strain combination of B. longum + L. helveticus and also showed positive effects.41
Cognitive function
Eleven studies tested cognitive function, and all showed the probiotics to be beneficial for memory performance. Spatial memory was tested with the Morris water maze and the Barnes maze tests; other non-spatial memory abilities were measured with the novel object, fear conditioning, passive avoidance step-down, and T-maze tests.
Single strains of B. longum, B. breve, and L. helveticus were effective on both spatial and non-spatial memories.37,38,42,43 Single strains of L. fermentum and Clostridium butyricum improved spatial memory ability.30,37,42,44 Multi-strain probiotics that were assessed to be effective with regard to non-spatial memory included combinations of L. rhamnosus + L. helveticus29,45 and B. longum + L. helveticus,41 and combinations of Lactobacillus acidophilus + B. lactis + L. fermentum and L. plantarum + Lactobacillus curvatus in spatial memory.46,47
Autism spectrum disorder and obsessive-compulsive disorder
Autism spectrum disorder-related behaviors were tested with the open field and marble-burying tests for anxiety, the pre-pulse inhibition test for sensorimotor, ultrasonic vocalization for communicative, and the three-chamber social test for social interaction behaviors. Bifidobacterium fragilis improved behaviors related to the ASD in maternal immune activation mice, including anxiety-like behavior, sensory gating and communicative behavior, but not social interaction behavior.31
Obsessive-compulsive disorder-related behaviors were also measured with the open field, marble burying, pre-pulse inhibition, ultrasonic vocalization and 3-chamber social tests. L. rhamnosus was found to be able to decrease obsessive-compulsive disorder-like behaviors in mice, but only locomotor ability and anxiety level. No effect was found in communicative or social interaction behaviors.48 However, a recent study investigated sickness behavior using a social investigative behavior paradigm, and found VSL#3 improved sickness behavior with increased social exploratory behaviors.49
Stress response
Four studies involved stress induction to test behavioral response to stress. Stress was induced, with water avoidance stress as an acute stressor29,45,50 and maternal separation as a chronic stressor.27 Acute stress was used to induce anxiety, memory dysfunction and HPA response; chronic stress was used to induce depression.
Anxiety behavior was not successfully induced by water avoidance stress, while memory dysfunction was induced only in Gareau’s study.45 A probiotic combination of L. rhamnosus + L. helveticus prevented non-spatial memory dysfunction induced by acute stress.45 One study only measured plasma corticosterone levels in response to acute stress and found a significant decrease due to Lactobacillus casei Shirota intervention.50 For behavioral changes caused by chronic stress exposure, B. infantis normalized depression-like behavior induced by maternal separation.27
Mechanisms of action
In addition to outcomes on behavioral levels, we also collected data at the physiological level, exploring endocrine, immune, neural chemical, and metabolic changes due to probiotics. Most of the studies investigated serum corticosteroid levels and found they were decreased by various probiotics: L. plantarum, L. helveticus, L. fermentum, L rhamnosus, and L. casei Shirota.20,23,30,37,44,45,50 Adrenocorticotropic hormone (ACTH) could also be decreased by L. helveticus and L. fermentum.30,38 Colon inflammation was alleviated and cytokine levels were influenced: inflammatory cytokines such as IL-6 and TNF-α were decreased and anti-inflammatory cytokines such as IL 10 were increased.20,30,35,37,38,40,42,45,47,49 These immune-effective probiotics were L. plantarum, L. helveticus, L. fermentum, L. acidophilus, B. longum, and L. rhamnosus. Brain monoamines, such as 5-HT and DA, could be increased by the probiotics L. plantarum, L. helveticus, and B. infantis, while their metabolites were reduced.20,26,38 GABA receptor expression could be influenced by L. rhamnosus, depending on the brain area.23 Brain BDNF and c-Fos mRNA expression increased after probiotic intervention with L. helveticus, L. plantarum, L. rhamnosus, B. longum, and C. butyricum, while c-Fos in the hypothalamus paraventricular nucleus was decreased by L. casei Shirota.35,38,44,45,47 Two studies found effects of L. rhamnosus and B. longum that were mediated via the vagus nerve (ie, no effect in vagotomized mice),22,23 and one study found L. casei Shirota to enhance gastric vagal afferent activity.50 Enteric neuron excitability was inhibited by B. longum,22 while visceral sensitivity by colon distension was unaffected.43 One study found that a probiotic formulation of B. longum + L. helveticus reduced intestinal barrier permeability.31,41 Probiotics L. helveticus, B. infantis, and B. fragilis influenced metabolites by enhancing serum tryptophan levels and inhibiting its metabolites.26,31,42 Several studies conducted microbiota analyses on the fecal samples and found fecal microbiota were altered by probiotics: for example, Bacteroides and Lactobacillus were increased and Firmicutes decreased by L. fermentum.29–31,44,45 More details are shown in Table 1.
Human Studies
In total, 15 human studies were included. All of the selected studies had strong ratings in the quality assessment tool for quantitative studies, although one of the studies did not describe the age and gender of the participants in each group.51 Among the 15 studies, 8 used a single-strain probiotic (L. casei, L. casei subsp. rhamnosus, L. casei Shirota, L. plantarum, and B. infantis), of which 2 used probiotic containing milk, and the other 7 studies used multi-strain probiotics. Eight of the 15 studies found significant effects of the probiotic interventions. Doses of the effective interventions ranged from 107 to 3.63 × 1010, and the duration of the treatments ranged from 20 days to 8 weeks. Doses around 109 (5/8) and 1010 (3/8) were used most often. Durations were most commonly 4 (6/15) and 8 (4/15) weeks. Due to the heterogeneity of the studies (eg, probiotic interventions and measurements of CNS functions), we only describe the results based on the different interventions (Table 2).
Table 2.
Studies of Probiotic Effects on Central Nervous System Functions in Humans
| Study | Participants | Probiotic | Dosage (CFU/day) and duration | CNS function | Outcome | Secondary outcome |
|---|---|---|---|---|---|---|
| Takada et al,50 2016 | 140 healthy students | L. casei Shirota YIT 9029 | 1 × 109 8 weeks |
STAI | No difference of STAI score | Change in salivary cortisol level before exam ↓ Decrease in physical symptoms ↓ |
| Mohammadi et al,52 2015 | 70 petrochemical workers | probiotic yogurt (L. acidophilus LA5 + B. lactis Bb12) + placebo capsule; Conventional yogurt (S. thermophilus and L. bulgaricus.) + probiotic capsule (L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus) |
probiotic yogurt: 107 in total Probiotic capsule: 3 × 103, 3 × 107, 7 × 109, 5 × 108, 2 × 1010; 109, 3 × 108, respectively (2.88 × 1010 in total)/6 weeks |
GHQ DASS HPA axis activity |
Improvement of GHQ and DASS in probiotics yogurt and probiotics capsule groups; no difference in HPA axis activity | NA |
| Steenbergen et al,53 2015 | 40 healthy volunteers | Ecologic Barrier: B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis (W19 and W58). | 5 × 109 4 weeks |
LEIDS-r BDI BAI |
Improvement of total score and item ‘rumination’ of LEIDS-r. No difference of scores of Beck Depression and Beck Anxiety Inventory |
NA |
| Dickerson et al,54 2014 | 65 schizophrenia patients | L. rhamnosus GG (ATCC 53103+ B. animalis subsp. lactis Bb12 |
2 × 109 14 weeks |
PANSS | No difference of toll score or positive, negative or general scores. | Severe difficulty in bowel movement ↓ |
| Vaghef-Mehrabany et al,55 2014 | 46 patients with rheumatoid arthritis | L. casei 01 | 108 8 weeks |
STAI | No difference of STAI score | Dietary: NA Cytokines: pro inflammatory cytokine TNF-α, IL-6, and IL-12 ↓, anti-inflammatory cytokine IL-10 ↑ |
| Dapoigny et al,56 2012 | 50 IBS patients | L. casei subsp. rhamnosus LCR35 | 6 × 108 4 weeks |
HADS | No difference in HADS score | IBS severity score: only clinically relevant decreased in subtype IBS-D ↓ Presence of Lactobacillus in feces: 85% of patients |
| Simrén et al,57 2010 | 74 IBS patients | Milk fermented with yoghurt bacteria L. bulgaricus + S. thermophilus and containing L. paracasei F19 + L. acidophilus La5 + B. lactis Bb12 | 2 × 1010 8 weeks |
HADS | No difference of HADS score | Diet: same among groups |
| Whorwell et al,58 2006 | 362 female IBS patients | B. infantis 35624 | 106, 108, 1010 4 weeks |
HADS | No difference in any of the dosages | IBS symptom: ↓ in 108 group |
| Reale et al,59 2012 | 72 male smokers | L. casei Shirota | 4 × 1010 3 weeks |
STAI | No difference in STAI score | Natural killer cell activity ↑ CD16+ cell ↑ BMI: NA Bowel function ↑ |
| Parracho et al,60 2010 | 15 children (4–16Y) with ASD | L. plantarum WCFS1 | 4.5 × 1010 3 weeks |
DBC | No significant difference in DBC score | Bowel function: only different in stool consistency Fecal microbiota: Lactobacillus Lab 158 ↑, Clostridium Erec482 ↓ |
| Messaoudi et al,39 2011 | 55 healthy volunteers | L. helveticus R0052 + B. longum R0175 | 3 × 109 4 weeks |
HSCL-90 HADS PSS CCL |
Improvement of anxiety, depression and problem solving, and reduced UFC level in probiotics group | Median urinary free cortisol ↓ |
| Messaoudi et al,61 2011 | 25 healthy volunteers (with lower UFC levels than median value at baseline | L. helveticus R0052 + B. longum R0175 | 3 × 109 4 weeks |
HSCL-90 HADS PSS CCL |
Improvement of anxiety and depression in probiotics group | NA |
| Rao et al,51 2009 | 35 CFS patients | L. casei Shirota | 2.4 × 1010 8 weeks |
BDI BAI |
Decreased anxiety symptoms in probiotic group | Fecal microbiota: aerobes ↑, anaerobes ↑ Bifidobacteria ↑, Lactobacillus ↑ |
| Benton et al,62 2007 | 124 healthy volunteers | L. casei Shirota (containing milk) | 6.5 × 109 10/20 days |
Questionnaire-based POMS Episodic memory (Wechsler Memory Scale) Retrieval from long-term memory Verbal fluency Eating-associated behavior NART |
Improved mood in the bottom third of the POMS depressed/elated distribution at baseline in probiotics group after 20 days Improved memory in probiotics group after 20 days |
NA |
| Tillisch et al,63 2013 | 36 healthy females | FMPP (B. lactis I-2494 [DN-173 010], containing yogurt starters include S. thermophilus I–1630, L. bulgaricus I–1632 and I–1519) and Lactococcus lactis subsp lactis I–1631 |
B. lactis : 1.25 × 1010 S. thermophilus and L. bulgaricus : 1.2 × 109 4 weeks |
Standard emotional faces-attention task for fMRI | Decreased activity to emotional faces in a large distributed network Changes in midbrain connectivity during resting state |
NA |
STAI, State-Trait Anxiety Inventory; NA, not applicable; GHQ, General Health Questionnaire; DASS, Depression Anxiety and Stress Scale; HPA, hypothalamic-pituitary-adrenal; BMI, body mass index; LEIDS-r, Leiden Index of Depression Sensitivity-Revised; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; PANSS, Positive and Negative Symptom Scale; IBS, irritable bowel syndrome; HADS, Hospital Anxiety and Depression Scale; ASD, autism spectrum disorder; IBS-D, diarrhea-predominant IBS; DBC, Development Behavior Checklist; HSCL-90, Hopkins Symptom Checklist; PSS, Perceived Stress Scale; CCL, Coping Checklist; UFC, urinary free cortisol; CFS, chronic fatigue syndrome; POMS, questionnaire-based Profile of Mood State; NART, National Adult Reading Rest; FMPP, fermented milk product with probiotic; fMRI, functional magnetic resonance imaging; B. animalis, Bifidobacterium animalis; B. breve, Bifidobacterium breve; B. bifidum, Bifidobacterium bifidum; B. infantis, Bifidobacterium infantis; B. lactis, Bifidobacterium lactis; B. longum, Bifidobacterium longum; L. acidophilus, Lactobacillus acidophilus; L. brevis, Lactobacillus brevis; L. bulgaricus, Lactobacillus bulgaricus; L. casei, Lactobacillus casei; L. helveticus, Lactobacillus helveticus; L. paracasei, Lactobacillus paracasei; L. plantarum, Lactobacillus plantarum; L. rhamnosus, Lactobacillus rhamnosus; L. salivarius, Lactobacillus salivarius; S. thermophiles, Streptococcus thermophiles.
Psychiatric conditions
Here, we summarize the studies on depression, anxiety, and/or mood together, because in most of the studies, questionnaires that tested multiple psychiatric conditions were used. Fifteen studies tested healthy participants with respect to anxiety, depression, distress levels, mood state, and behavior problems.39,51–62 The measurement tools included the General Health Questionnaire (GHQ), the Depression Anxiety and Stress Scale (DASS), the Leiden Index of Depression Sensitivity-Revised (LEIDS-r), the Positive and Negative Symptom Scale (PANSS), the State-Trait Anxiety Inventory (STAI), the Development Behavior Checklist (DBC), the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), the Hopkins Symptom Checklist (HSCL-90), the Hospital Anxiety and Depression Scale (HADS), the Perceived Stress Scale (PSS), the Coping Checklist (CCL) (also used to counter the stress of daily life), and the questionnaire-based Profile of Mood State (POMS). Due to the different questionnaires, scales, and their combinations used in these studies, we only report here whether the probiotics treatment improved mental health/mood.
One study compared a probiotic capsule (containing Lactobacillus casei, L. acidophilus, L. rhamnosus, Lactobacillus bulgaricus, B. breve, B. longum, and Streptococcus thermophilus) and a probiotic yogurt (containing B. lactis and L. acidophilus) with the combination of conventional yogurt and a placebo capsule, and found the former two were more effective in alleviating distress, anxiety, and depression in petrochemical workers.52 A recent study using multi-strain probiotics found improvement in the LEIDS-r score, which is predictive of depression.53 Two studies by Messaoudi et al39,61 found probiotic formulations of B. longum and L. helveticus could improve anxiety and depression in all participants, and also in those who had lower urinary free cortisol levels at baseline. One study using L. casei Shirota-containing milk improved mood only in the bottom third of the depressed distribution at baseline.62 A study in chronic fatigue syndrome patients also used L. casei Shirota and found decreased anxiety levels following treatment.51 One recent study, also using L. casei Shirota, found decreased salivary cortisol levels in university students in response to stress, although no significant difference in STAI score was observed.50
However, other studies found no significant effect of their probiotic interventions. Patients with schizophrenia showed no change in PANSS score after treatment with L. rhamnosus for 14 weeks.54 Patients with rheumatoid arthritis showed no change in anxiety levels, as assessed with the STAI after 8 weeks of L. casei.55 Three studies conducted in IBS patients all looked into HADS scores before and after interventions, but found no effect of L. casei or fermented milk with L. paracasei and L. acidophilus.56–58 In healthy male smokers, a 3-week intervention with L. casei showed no effect on STAI score.59 In children with ASD, a 3-week intervention with L. plantarum did not change the DBC score.60
Memory and other cognitive abilities
The study of Benton et al62 measured different memory and cognitive abilities in healthy participants, including episodic memory, tested with the Wechsler Memory Scale, retrieval from long-term memory, verbal fluency, eating-associated behavior, and premorbid intelligence, tested with the National Adult Reading Test. However, L. casei Shirota decreased memory abilities in all participants compared with the placebo, and had no effect on verbal fluency or eating-associated behavior.
Neuroimaging study
There was only one neuroimaging study, using functional magnetic resonance imaging (fMRI), investigating the change in brain activity to emotional stimuli and basal brain activation after intake of a fermented milk product with probiotic (FMPP) containing B. lactis with yogurt starters, S. thermophilus, L. bulgaricus, and Lactococcus lactis subsp. lactis.63 The FMPP decreased activity of a large distributed network including affective, viscerosensory, and somatosensory cortices to emotional faces, and changed midbrain connectivity during the resting state.
Mechanisms of action
Two studies found reduced cortisol levels in saliva and urine after probiotic interventions with L. casei Shirota and multi-strain L. helveticus + B. longum, respectively.39,50 The immune system could be improved by the probiotic L. casei, with evidence of reduced pro-inflammatory cytokines (TNF-α, IL-6, and IL-12), increased regulatory cytokines (eg, IL-10), and increased natural killer cell activity in smokers.55,59 Only one study in humans investigated metabolites of the tryptophan pathway but did not find any significant change by probiotics.52 Many of the human studies looked at bowel function, and they did find reduced difficulties in bowel movement, IBS severity, and symptoms in patients.54,56,58,60 Microbiota analysis helped to confirm that fecal microbiota were altered by probiotic intervention: Lactobacillus was increased and Clostridium decreased by L. plantarum, whereas Bifidobacteria and Lactobacillus were increased by L. casei Shirota.51,60 More details are shown in Table 2.
Summarizing all the studies in animals and humans that focused on CNS functions, including psychiatry conditions (anxiety and depression) and memory abilities, Bifidobacterium and Lactobacillus were the probiotics used most frequently. Doses ranged from 107 to 4 × 1010 CFU per day and most studies used 109 and 1010 CFU in animals and 3 × 109 CFU in humans. The duration of intake ranged from 1 week to 6 months with the most frequent durations of 2 weeks in animals and 4 weeks in humans.
Discussion
The number of studies using probiotics to improve central nervous system function has increased over the past 10 years, though with a focus on effects in animals. Researchers have used various strains of probiotics and studied various CNS functions. Summarizing the divergent findings motivated us to perform a systematic review of this research area. Previously, there was no systematic review or meta-analysis of the effects of probiotics on CNS function in animals and humans. To date, there are a few reviews on probiotic effects on infantile colic, which may reflect peripheral nervous system action, and one recent study reviewing only human studies.64–66 Similarly, among the 56 RCTs so far which tested probiotics in adults with IBS that have or have not shown effects on peripheral (bowel) functions,15 none have investigated whether any CNS effect was affected or improved. We identified studies applying probiotics as single- or multiple-strain preparations in animals and humans. Because of the diversity of the interventions and the CNS functions tested in these studies, we did not perform a meta-analysis.
Effects of Probiotics
Combining all the studies in animals and humans, probiotics appear to have a positive effect in improving central nervous system function. However, a publication bias toward positive results cannot be excluded. Based on currently available studies, we can see that most of the studies used Bifidobacterium and Lactobacillus preparations, and most of them were effective in improving specific CNS functions. Again, however, negative results in relation to other functions may have been unreported, even in otherwise positive studies. Doses of 109 and 1010 CFU have been used in most studies showing an effect on behaviors. An intake of the probiotic for 2 weeks in animals and 4 weeks in humans is apparently sufficient to elicit measurable effects.
B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were the most commonly used preparations in these studies, as single- or multi-strain preparations, all of which were able to improve anxiety, depression, and memory related behaviors, based on animal models. All of these probiotics are regarded as “good” bacteria, presumably inhibiting the growth of harmful bacteria or pathogens and/or improving the immune system.67–69 These probiotics were also found to reduce the symptoms of gastrointestinal disease, such as irritable bowel syndrome.15,16,58,70 Probiotics may play an important role in gut-brain axis communication, thereby benefiting both the brain and the gut.
While some studies found no significant effect of probiotic intervention, the evidence is inadequate to conclude that the interventions were ineffective because some difficulties and/or weak points exist. For example, schizophrenia as a severe mental illness, and being closely related to a genetic disposition, may be a case in which probiotics can hardly be expected to have a significant effect on changing symptoms.54 Probiotic doses in some studies were below the supposed effective dosages (at least 109 CFU), such as 108 CFU in the study in rheumatoid arthritis patients and 106, 5 × 107, and 108 CFU in IBS patients.55–58 Also, in two studies, one in male smokers and one in children with ASD, the intervention periods were 3 weeks, shorter than the effective period, which seems to be 4 weeks, that can make a measurable effect.59,60
Also, caution is warranted when drawing conclusions from the human studies that used psychological questionnaires and/or scales rather than behavioral or neuropsychological experiments, resulting in subjective biases. The clinical efficacy of probiotic interventions and guidelines for their administration in diseases such as diarrhea, allergies, IBS, and inflammatory bowel disease has been addressed in previous reviews.15,71,72 More studies investigating probiotic effects in mental disorders are needed.
Mechanisms of Action of Probiotic Effects
The current state-of-the-art suggests several mechanisms: the endocrine system, immune system, action of enteric neurons, and commensal bacteria (or their metabolic activity). This evidence has come primarily from preclinical studies, while a few clinical studies have analyzed cortisol and cytokine levels in saliva and plasma. The HPA axis activity has been linked closely to mood disorders and memory abilities.73,74 Many probiotics reduced HPA axis activity by decreasing CORT and/or ACTH levels, including most of the Lactobacillus strains tested: L. plantarum, L. helveticus, L. fermentum, L. rhamnosus, and L. casei.19,22,29,36,39,43,44,49 However, single strains of Bifidobacterium such as B. infantis, B. longum, and B. breve had no effect on CORT levels.27,29,36 BDNF is the key for neurogenesis and synaptic plasticity, which structurally support CNS function.75,76 Lactobacillus (L. plantarum, L. helveticus, L. fermentum), Bifidobacterium (B. longum), and C. butyricum increased brain BDNF.34,37,43,46 Neuronal activation can be indicated by the nuclear localization of c-Fos; the effect of c Fos in the CNS depends on its location. The combination of L. rhamnosus + L. helveticus improved c Fos expression in the hippocampus and improved memory ability, while L. casei decreased it in the paraventricular nucleus of hypothalamus region and alleviated stress responses.45,50 Neurotransmitters 5-HT, DA, and GABA are closely related to psychiatric conditions, and were influenced directly by many strains of probiotic (L. plantarum, L. helveticus, L. fermentum, L. rhamnosus, and B. infantis). The vagus nerve has been proposed as a pathway of probiotic effects because neurochemical and behavioral changes due to L. rhamnosus and B. longum were not seen in vagotomized animals. Direct evidence for the role of the vagus nerve also comes from studies showing that gastric vagal afferent activity was enhanced by L. casei. The excitability of the enteric nervous system, which is connected to the vagus nerve, has been shown to be modulated by B. longum.22
Probiotics also alter CNS function indirectly through several other pathways. L. helveticus, B. infantis, and B. fragilis enhanced serum tryptophan (precursor of 5-HT) levels and reduced its metabolites. Most of the probiotics tested affected the immune system by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines. Another important pathway through which probiotics may modulate CNS function is intestinal barrier permeability, which is essential for maintaining the immune and nervous systems. Increased intestinal barrier permeability is associated with psychiatric disorders, such as depression and autism, while it can be restored by probiotic formulations of B. longum and L. helveticus, along with improved CNS function.31,41,77
According to the data reviewed, different probiotics exhibited several common effects; however, these effects were strain-dependent and occurred via different pathways at a lower level of the CNS. Thus, more studies are needed for clarify which probiotics target which central biochemical substances and behaviors. In clinical applications, interventions with a probiotics cocktail may have greater effects, because different probiotics may create their effects at the same time through different pathways. However, as yet, such an approach currently lacks clinical evidence.15
Translation of Animal Studies
There are many animal studies about the gut microbiome-brain interaction using germ-free, specific pathogen free (SPF), or gnotobiotic animals, colonization with specific microbiota, probiotic intake, and infections to deliberately alter the GM and to manipulate CNS function.9,29,30,35,37,45,78–82 In humans, we are not able to adapt most of these models for ethical reasons. Ten of the animal studies included in our review used probiotics in animals whose health state had been disturbed by various manipulations, which included antibiotic treatment, gene knockout, inflammation, infection, maternal immune activation, hyperammonemia, and diabetic induction, and depression induced by myocardial infarction. All of the manipulations were aimed at inducing changes in CNS function, including anxiety and depression-like behavior, memory impairment, or ASD-like behaviors. In humans, interfering with the participants’ healthy state is not an option. What is possible is to explore the GM composition, correlating it to certain behaviors, and using probiotics to manipulate the GM-brain interaction. It is also possible to temporarily affect single functions, such as the stress response at the central level or the GM composition by varying the food or drugs used. As yet, evidence from studies using probiotics is confined to animal studies. Validity estimates of probiotic intervention from human studies are still missing and thus need to be carried out.
The translation of behavioral models from animals to humans has both possibilities and limitations. The tests used to measure anxiety in animals, such as the elevate plus/Barnes maze, light/dark box, defensive burying, open field/arms, and step-down, have no equivalents in humans, and neither do tests such as the forced swim and maternal separation for inducing depression and negative mood. In human studies, measurements of anxiety and depression rely primarily on scales such as the HADS, which has accuracy issues due to subjectivity and emotional bias from the participants/patients. Moreover, it remains questionable whether the behavioral tests used in animals do, in fact, adequately reflect the assumed CNS dysfunction (anxiety, depression) in humans and, more specifically, in patients. This leads to a demand for appropriate behavioral tests of anxiety and depression not only for patients with psychiatric disorders but also for the healthy population, and for adequate behavioral measures in animals that match these functions and dysfunctions in humans.
In addition to behavioral measurements, neuroimaging methods may provide insights as to what is altered in the brain that causes behavioral changes after the consumption of probiotics. An emotional faces attention task used in the fMRI study of Tillisch et al63 is one example: the brain response to emotional stimuli that may be related to psychiatric conditions was changed after a 4-week intake of probiotics.
Learning and memory abilities can be tested via numerous paradigms in humans. For spatial memory, there are computerized versions of the Morris water maze (VMWT) and the Blue Velvet Arena (BVA), which is also a variant of the Morris water maze for humans.83–86 For non-spatial memory, object recognition tasks and fear conditioning have been used widely in humans. These memory tasks can be conducted in combination with neuroimaging experiments, such as fMRI and magnetoencephalography (MEG) experiments.
There are also several ways to experimentally induce stress in humans. The Trier Social Stress Test, developed 20 years ago, during which participants are asked to play a role in a job interview, or in performing a public speech, can effectively increase the HPA axis and sympathetic-adrenal-medullary activity.87 Social stress can also be induced using the Cyberball paradigm, during which stress comes from social exclusion and/or ostracism.88,89 Noise as a stressor is easy to manipulate in a laboratory environment by exposing participants to unpleasant sounds so as to induce psychological stress. Cognitive tasks can also stimulate stress responses with the advantage of being able to study the stress level by measuring task performance.90 Other and more physical stressors include cold pressor tasks,91 heat pain,92 and the CO2 challenge test, inducing stress/panic in participants by inhaling carbon dioxide-enriched air.93 All of these have also been shown to be compatible with brain imaging studies.
Conclusions and Indications
We reviewed the effect of probiotics on the central nervous system in randomized controlled trials in animals and humans, and analyzed the possibility of translating animal models to human studies because few human studies have been conducted to date. According to the qualitative analyses of current studies, we can provisionally draw the conclusion that B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were most effective in improving CNS function, including psychiatric disease-associated functions (anxiety, depression, mood, stress response) and memory abilities. Doses between 109 and 1010 CFU and durations of 2 weeks in animals and 4 weeks in humans have shown sufficient effects. Also, translations of animal studies to human studies may be applicable. Human studies can be conducted using the same probiotics and similar experimental paradigms in the emotional and neurocognitive domains. More experimental designs in humans should be developed, and more neuroimaging studies should be conducted rather than using only psychological questionnaires or scales. In addition to studies in healthy populations, clinical studies in patients with mental diseases would be worthwhile, because those with gastrointestinal disorders and psychiatric comorbidities, in general, appear to benefit from probiotic interventions.
Footnotes
Financial support: The research leading to these results has received funding from the People Programme of the European Union’s Seventh Framework Programme under REA grant agreement No. 607652 (NeuroGut).
Conflicts of interest: None.
Author contributions: Paul Enck conceptualized the paper; Huiying Wang reviewed and evaluated the literature; and Huiying Wang, In-Seon Lee, Christoph Braun, and Paul Enck wrote the manuscript.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer AD, Randall HA, Aziz Q. It’s a gut feeling: how the gut microbiota affects the state of mind. J Physiol. 2014;592:2981–2988. doi: 10.1113/jphysiol.2013.270389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keightley PC, Koloski NA, Talley NJ. Pathways in gut-brain communication: evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust N Z J Psychiatry. 2015;49:207–214. doi: 10.1177/0004867415569801. [DOI] [PubMed] [Google Scholar]
- 5.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psycho-tropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 7.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 9.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomova A, Husarova V, Lakatosova S, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Kang DW, Park JG, Ilhan ZE, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 13.Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr. 2011;106:1291–1296. doi: 10.1017/S000711451100287X. [DOI] [PubMed] [Google Scholar]
- 14.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 15.Mazurak N, Broelz E, Storr M, Enck P. Probiotic therapy of the irritable bowel syndrome: why is the evidence still poor and what can be done about it? J Neurogastroenterol Motil. 2015;21:471–485. doi: 10.5056/jnm15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 17.Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252–266. doi: 10.5056/jnm.2011.17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPont HL. Biologic properties and clinical uses of rifaximin. Expert Opin Pharmacother. 2011;12:293–302. doi: 10.1517/14656566.2011.546347. [DOI] [PubMed] [Google Scholar]
- 19.Chichlowski M, Rudolph C. Visceral pain and gastrointestinal microbiome. J Neurogastroenterol Motil. 2015;21:172–181. doi: 10.5056/jnm15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YW, Liu WH, Wu CC, et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016;1631:1–12. doi: 10.1016/j.brainres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Liu WH, Chuang HL, Huang YT, et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res. 2016;298:202–209. doi: 10.1016/j.bbr.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 22.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Sullivan E, Barrett E, Grenham S, et al. BDNF expression in the hippocampus of maternally separated rats: does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes. 2011;2:199–207. doi: 10.3920/BM2011.0015. [DOI] [PubMed] [Google Scholar]
- 25.Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Kwok LY, Wang L, Zhang J, Guo Z, Zhang H. A pilot study on the effect of Lactobacillus casei Zhang on intestinal microbiota parameters in Chinese subjects of different age. Benef Microbes. 2014;5:295–304. doi: 10.3920/BM2013.0047. [DOI] [PubMed] [Google Scholar]
- 29.Smith CJ, Emge JR, Berzins K, et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:G793–G802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Hu X, Liang S, et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes. 2015;6:707–717. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:2046–4053. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. [accessed 22 Sep 2016];The Cochrane Collaboration. 2011 Available from URL: http://handbook.cochrane.org/
- 34.National Collaborating Centre for Methods and Tools. Quality assessment tool for quantitative studies. Hamilton: MaMaster University; 2008. [accessed 22 Sep 2016]. (updated 13 April 2010). Available from URL: http://www.nccmt.ca/resources/search/14. [Google Scholar]
- 35.Bercik P, Verdu EF, Foster JA, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 36.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 37.Ohland CL, Kish L, Bell H, et al. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38:1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Liang S, Wang T, Hu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 40.Singh PK, Chopra K, Kuhad A, Kaur IP. Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: behavioral and biochemical evidences. Neurogastroenterol Motil. 2012;24:366–e170. doi: 10.1111/j.1365-2982.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 41.Arseneault-Bréard J, Rondeau I, Gilbert K, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci China Life Sci. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 43.Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Sun J, Wang F, et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 46.Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 47.Jeong JJ, Kim KA, Ahn YT, et al. Probiotic mixture KF attenuates age-dependent memory deficit and lipidemia in Fischer 344 rats. J Microbiol Biotechnol. 2015;25:1532–1536. doi: 10.4014/jmb.1505.05002. [DOI] [PubMed] [Google Scholar]
- 48.Kantak PA, Bobrow DN, Nyby JG. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG) Behav Pharmacol. 2014;25:71–79. doi: 10.1097/FBP.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 49.D’Mello C, Ronaghan N, Zaheer R, et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci. 2015;35:10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada M, Nishida K, Kataoka-Kato A, et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil. 2016;28:1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- 51.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. doi: 10.1179/1476830515Y.0000000023. Published Online First: 16 Apr 2015. [DOI] [PubMed] [Google Scholar]
- 53.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. doi: 10.4088/PCC.13m01579. Published Online First: 13 Feb 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif SK, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30:430–435. doi: 10.1016/j.nut.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 58.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 59.Reale M, Boscolo P, Bellante V, et al. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br J Nutr. 2012;108:308–314. doi: 10.1017/S0007114511005630. [DOI] [PubMed] [Google Scholar]
- 60.Parracho HMRT, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double blind, placebo controlled, crossover designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics and Prebiotics. 2010;5:69. [Google Scholar]
- 61.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 62.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 63.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. e1–e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sung V, Collett S, de Gooyer T, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr. 2013;167:1150–1157. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 65.Romijn AR, Rucklidge JJ. Systematic review of evidence to support the theory of psychobiotics. Nutr Rev. 2015;73:675–693. doi: 10.1093/nutrit/nuv025. [DOI] [PubMed] [Google Scholar]
- 66.Anabrees J, Indrio F, Paes B, AlFaleh K. Probiotics for infantile colic: a systematic review. BMC Pediatr. 2013;13:186. doi: 10.1186/1471-2431-13-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325–339. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lomasney KW, Cryan JF, Hyland NP. Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. Am J Physiol Gastrointest Liver Physiol. 2014;307:G241–G247. doi: 10.1152/ajpgi.00401.2013. [DOI] [PubMed] [Google Scholar]
- 69.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 71.McFarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis. 2015;60(suppl 2):S85–S90. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- 72.Floch MH, Walker WA, Sanders ME, et al. Recommendations for probiotic use--2015 update: proceedings and consensus opinion. J Clin Gastroenterol. 2015;49(suppl 1):S69–S73. doi: 10.1097/MCG.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 73.Dedovic K, Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatr Dis Treat. 2015;11:1181–1189. doi: 10.2147/NDT.S62289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osborne DM, Pearson-Leary J, McNay EC. The neuroenergetics of stress hormones in the hippocampus and implications for memory. Front Neurosci. 2015;9:164. doi: 10.3389/fnins.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mariga A, Mitre M, Chao MV. Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis. doi: 10.1016/j.nbd.2016.03.009. Published Online First: 22 Mar 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai SJ. Attention-deficit hyperactivity disorder may be associated with decreased central brain-derived neurotrophic factor activity: clinical and therapeutic implications. Med Hypotheses. 2007;68:896–899. doi: 10.1016/j.mehy.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 77.Julio-Pieper M, Bravo JA, Aliaga E, Gotteland M. Review article: intestinal barrier dysfunction and central nervous system disorders – a controversial association. Aliment Pharmacol Ther. 2014;40:1187–1201. doi: 10.1111/apt.12950. [DOI] [PubMed] [Google Scholar]
- 78.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 82.Nishino R, Mikami K, Takahashi H, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25:521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 83.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–190. doi: 10.1016/S0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 84.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/S0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 85.Kalová E, Vlcek K, Jarolímová E, Bures J. Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer’s disease: corresponding results in real space tests and computer tests. Behav Brain Res. 2005;159:175–186. doi: 10.1016/j.bbr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 86.Laczó J, Andel R, Vyhnalek M, et al. Human analogue of the morris water maze for testing subjects at risk of Alzheimer’s disease. Neurodegener Dis. 2010;7:148–152. doi: 10.1159/000289226. [DOI] [PubMed] [Google Scholar]
- 87.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 88.Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–180. doi: 10.3758/BF03192765. [DOI] [PubMed] [Google Scholar]
- 90.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/S0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 93.Vickers K, Jafarpour S, Mofidi A, Rafat B, Woznica A. The 35% carbon dioxide test in stress and panic research: overview of effects and integration of findings. Clin Psychol Rev. 2012;32:153–164. doi: 10.1016/j.cpr.2011.12.004. [DOI] [PubMed] [Google Scholar]