Abstract
Background/Aims
Dai-kenchu-to (DKT), a traditional Japanese herbal medicine, is known to increase gastrointestinal motility and improve ileal function. We tested our hypotheses that (1) pretreatment with DKT would block the colorectal distention-induced visceromotor response in rats, and (2) pretreatment with DKT would attenuate colorectal distention-induced adrenocorticotropic hormone (ACTH) release and anxiety-related behavior.
Methods
Rats were pretreated with vehicle or DKT (300 mg/kg/5 mL, per os). Visceromotor responses were analyzed using electromyography in response to colorectal distention (10, 20, 40, 60, and 80 mmHg for 20 seconds at 3-minutes intervals). Anxiety-related behavior was measured during exposure to an elevated-plus maze after colorectal distention. Plasma ACTH and serum corticosterone levels were measured after exposure to the elevated-plus maze.
Results
Colorectal distention produced robust contractions of the abdominal musculature, graded according to stimulus intensity, in vehicle-treated rats. At 40, 60, and 80 mmHg of colorectal distention, the visceromotor responses of DKT-treated rats was significantly lower than that of vehicle-treated rats. At 80 mmHg, the amplitude was suppressed to approximately one-third in DKT-treated rats, compared with that in vehicle-treated rats. Smooth muscle compliance and the velocity of accommodation to 60 mmHg of stretching did not significantly differ between the vehicle-treated and DKT-treated rats. Similarly, the DKT did not influence colorectal distention-induced ACTH release, corticosterone levels, or anxiety-related behavior in rats.
Conclusions
Our results suggest that DKT attenuates the colorectal distention-induced visceromotor responses, without increasing smooth muscle compliance, ACTH release or anxiety-related behavior in rats.
Keywords: Adrenocorticotropic hormone, Anxiety, Colorectal distention, Dai-kenchu-to, Visceral pain
Introduction
A traditional Japanese herbal medicine Dai-kenchu-to (DKT) is a mixture of Zanthoxyli fructus, Ginseng radix, Zingiberis siccatum rhizome, and Saccharum granorum. In vivo studies have demonstrated that DKT enhances gastrointestinal motility in dogs, mice and rabbits1,2 and is an effective treatment for intestinal adhesions in a rat model.3 In experiments with isolated intestines, DKT induced contractions in rabbit jejunum and guinea pig ileum and colon.4,5 DKT administered directly into the stomach caused phasic contractions through cholinergic and 5-hydroxytryptamine 3 (5-HT3) receptors in the gastric antrum, duodenum, and jejunum in dogs.1 In isolated guinea pig ileum, hydroxy β-sanshool, one of the ingredients of DKT, induced contractions through the release of acetylcholine from intrinsic cholinergic nerves and tachykinins from sensory neurons.6 DKT induces ileal contraction, and this response was suppressed by atropine and a 5-HT4 receptor antagonist.5 Moreover, DKT and one of its active components, [6]-shogaol, elevate intestinal blood flow, which is mainly mediated by calcitonin gene-related peptide (CGRP).7 These results indicate that DKT-induced contractions are likely mediated by acetylcholine release from the ends of cholinergic nerves and that 5-HT3 and 5-HT4 receptors and CGRP seem to be involved in the mechanism.
A 5-HT4 agonist and CGRP stimulated gastrointestinal motility,8,9 whereas the same 5-HT4 agonist and CGRP suppressed colorectal distention-induced visceromotor responses.10–12 Colorectal distention is widely used as a model for visceral stimulation. It is generally accepted that 80 mmHg of colorectal distention is a noxious stimulus; this pressure is perceived as painful by human beings13 and considered noxious in rats.14 Previous reports have suggested that colorectal distention produces visceromotor responses that are quantifiable, reliable, reproducible, and useful for inter-animal and intra-animal studies.14 We previously reported that colorectal distention induces visceromotor responses (VMRs), hippocampal noradrenaline release, adrenocorticotropic hormone (ACTH) release, and anxiety-related behavior in rats.15,16
In human studies, treatment with oral DKT significantly shortened first flatus and bowel movement after colorectal or liver resection.17–20 DKT significantly reduced gas volume and improved functional constipation in post stroke patients.21 On the other hand, a study about female functional constipation patients indicated that there are no effect treatment with oral DKT on colonic transit, rectal sensation, and stool frequency.22 Moreover, the 15 g/day dose was associated with lower rectal first sensation threshold and gas threshold.22 Although the effects of DKT on colonic motility was reported precisely in vivo studies, there are no report about effects of DKT on VMRs, ACTH release, and anxiety-related behavior for visceral pain in animals.
We tested our hypotheses that (1) pretreatment with DKT would block the colorectal distention-induced VMRs in rats, and (2) pretreatment with DKT would attenuate colorectal distention-induced ACTH release and anxiety-related behavior.
Materials and Methods
Animals
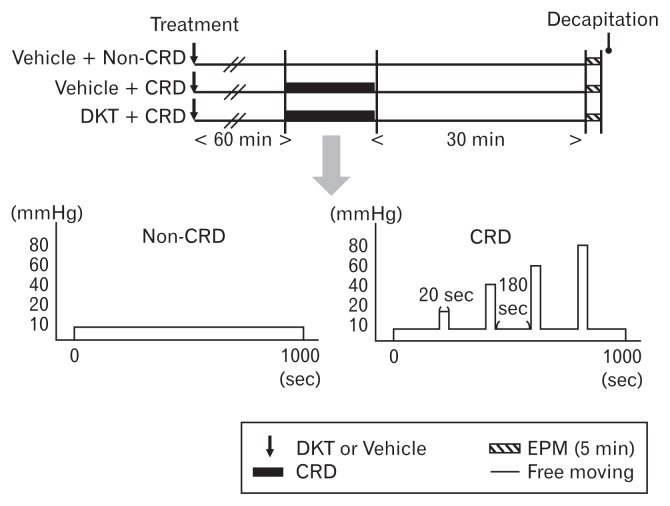
Male Wistar rats (n = 18) weighing 180–210 g were provided by the Charles River Breeding Laboratories Inc. The rats were divided into 3 groups: controls (treated with vehicle and subjected to 10 mmHg of colorectal distention, n = 6), vehicle-treated (treated with vehicle and subjected to 10–80 mmHg of colorectal distention, n = 6), and DKT-treated (treated with DKT and subjected to 10–80 mmHg of colorectal distention, n = 6) (Fig. 1). All rats were housed under controlled illumination (12/12-hour light/dark cycle, starting at 8 AM) and temperature (23 ± 1°C) conditions with free access to food and water. This study was approved by the Ethics Committee of Laboratory Animals, Tohoku University.
Figure 1.
Study protocol. CRD, colorectal distention; DKT, Dai-kenchu-to; EPM, elevated plus-maze.
Drugs
DKT extract powder (extracted in water from a 5:3:2 mixture of Zingiberis siccatum rhizoma, Ginseng radix, and Zanthoxyli fructus) and extract powders of Zanthoxyli fructus, Ginseng radix, and Zingiberis siccatum rhizoma were manufactured by Tsumura & Co (Tokyo, Japan). DKT was prepared by mixing DKT extract powder and Saccharum granorum (Tsumura & Co) at a ratio of 1:8 and dissolving in distilled water. DKT was used at a concentration identical to that of the powdered DKT extract. Distilled water was used as the vehicle.
Surgical Preparation
Rats were deeply anesthetized with pentobarbital sodium (50 mg/kg) administered intraperitoneally. Electrodes (Star Medical, Tokyo, Japan) were stitched into the external oblique musculature to enable the electromyography (EMG) recordings. The electrode leads were tunneled subcutaneously and exteriorized at the nape of the neck for future access. After surgery, the rats were housed separately and allowed to recuperate for at least 2 days before testing.
Colorectal Distention
On the day of the experiment, a 7-cm long polyethylene bag with a diameter of 2.5 cm was passed through the anus of each experimental rat and into the rectum and the distal colon. The end of the bag was positioned 1 cm from the anus and kept in place by taping the bag catheter to the base of the tail. The bag pressure was monitored and controlled using a pressure controller-timing device (Distender Series II; G & J Electronics, Toronto, Ontario, Canada). For the control group, the colon was then distended with 10 mmHg intensity stimulations for 1000 seconds. For the vehicle-treated and DKT-treated groups, the colon was distended with graded (10, 20, 40, 60, and 80 mmHg colorectal distention) intensity stimulations for 20 seconds per stimulation, with an interval of 3 minutes between stimuli.
Measurement of Visceromotor Responses
The EMG recordings were collected and analyzed using software that accompanied the barostat (8 Star, version 6.0–19.2; G&J Electronics; modified by Star Medical, Tokyo, Japan). VMRs to colorectal distention (10–80 mmHg, 20 seconds) were regarded as an increase in EMG activity above the preset voltage threshold.23 The increase in the total number of counts over baseline during distention was taken as the response. Moreover, the EMG activity was rectified, and the increase in the EMG amplitude over the preset voltage threshold was recorded as the response.
Barostat
To further investigate the mechanism by which DKT influences smooth muscle contractility, colorectal compliance was assessed using the automated barostat described above. The velocity of air infusion was 46 mL/sec. The 2 aspects of tone that were assessed were (1) the compliance of the colorectal wall, expressed as the slope of the volume-pressure relationship, and (2) the resistance of the colorectal wall to 60 mmHg of distention stretching, graphed as volume/sec.
Measurement of plasma Adrenocorticotropic Hormone and Serum Corticosterone
Blood was collected in chilled polyethylene tubes with or without 200 μL (200 U) of heparin. After centrifugation at 3000 rpm for 5 minutes, the plasma and serum were stored at −40°C until later assay. Plasma ACTH and serum corticosterone levels were measured using radioimmunoassays.
Measurement of Anxiety-related Behavior
The elevated plus-maze (EPM) was used to measure anxiety-related behavior, as described previously.16 Briefly, normal exploratory behavior favors entry into the closed arms of the maze and temporary entry into the open arms of the maze. Therefore, reduced entry into the open arms and reduced time spent in these arms is thought to indicate anxiety.
Rats were placed in the center of an EPM and facing an enclosed arm of the maze. This 5-minute test was evaluated using a computer-automated measure of arm exploration. Testing was conducted in a quiet and dedicated room. All experiments employed a between-individuals design, in which separate groups of rats were tested in the EPM only once.
Experimental Protocols
DKT (300 mg/kg/5 mL, per os) or the vehicle alone was administered 60 minutes before the colorectal distention. In the control rats, the vehicle was administered 60 minutes before the non-noxious 10 mmHg colorectal distention session. The rats remained in their cages and were not exposed to stress prior to the colorectal distention session. After the colorectal distention experiments, the rats were allowed to recover for 30 minutes. Anxiety-related behavior was evaluated for 5 minutes using the EPM after the recovery period. Blood was sampled by decapitation immediately after the EPM session.
Statistical Methods
All data were expressed as the mean ± standard error (SE). Statistical significance was evaluated using an ANOVA (one-way and two-way, repeated measures) and a post-hoc test. A probability level less than 0.05 was considered statistically significant.
Results
Effects of Dai-Kenchu-To on Colorectal Distention-induced Visceromotor Responses
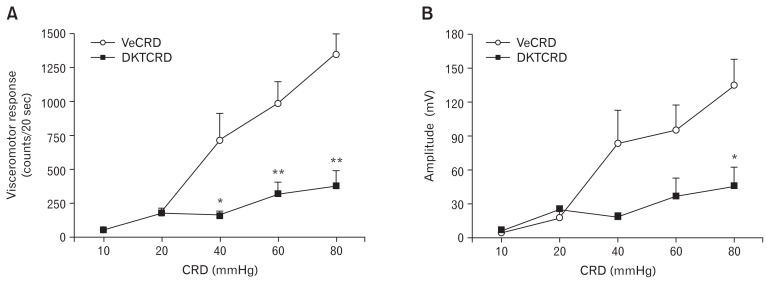
The VMRs (analyzed using a two-way ANOVA) revealed significant group effect (F = 41.5, P < 0.001), stimulus effect (F = 18.9, P < 0.001), and group × stimulation interaction effects (F = 7.9, P < 0.001). Colorectal distention produced robust contractions of the abdominal musculature graded according to the stimulus intensity in vehicle-treated rats (Fig. 2A). At 10 and 20 mmHg of colorectal distention, the VMRs did not significantly differ between the vehicle-treated (56.3 ± 8.2 and 181.8 ± 40.2 counts/20 sec) and the DKT-treated (57.7 ± 18.9 and 180.8 ± 39.5 counts/20 sec) groups, respectively. At 40, 60, and 80 mmHg of colorectal distention, the VMRs in the DKT-treated group were significantly lower than those in the vehicle-treated group (P < 0.05). At 80 mmHg, in particular, the VMR was suppressed to one-third or less in the DKT-treated rats (379.5 ± 110.9 counts/20 sec), compared with that in the vehicle-treated rats (1343.5 ± 159.3 counts/20 sec, P = 0.001). Similarly, the amplitude of the EMG indicated significant group effect (F = 15.6, P < 0.001), stimulus effect (F = 8.9, P < 0.001), and group × stimulation interaction effects (F = 3.6, P = 0.012) (two-way ANOVA). The amplitude over the baseline did not significantly differ between the vehicle-treated and DKT-treated groups at 10 and 20 mmHg of colorectal distention (Fig. 2B). At 80 mmHg, however, the amplitude was suppressed to about one-third in the DKT-treated rats (133.9 ± 24.4 mV) compared with that in the vehicle-treated rats (44.9 ± 16.6 mV, P < 0.05).
Figure 2.
Effects of Dai-kenchu-to (DKT) on colorectal distention (CRD)-induced visceromotor response (VMR). (A) CRD-induced VMR and (B) amplitude over the baseline. Data are expressed as the mean ± SE (n = 6 rats/group). VMR (two-way ANOVA): group effects (F = 41.5, P < 0.001), stimulus effects (F = 18.9, P < 0.001), and group stimulation interactions (F = 7.9, P < 0.001). Amplitude of electromyography: group effect (F = 15.6, P < 0.001), stimulus effect (F = 8.9, P < 0.001), and group × stimulation interaction effects (F = 3.6, P = 0.012). *P < 0.05, **P < 0.01 (vs vehicle). VeCRD, vehicle and colorectal distention; DKTCRD, DKT and CRD.
Effects of Dai-Kenchu-To on Smooth Muscle Compliance and Velocity of Accommodation to 60 mmHg of Stretching
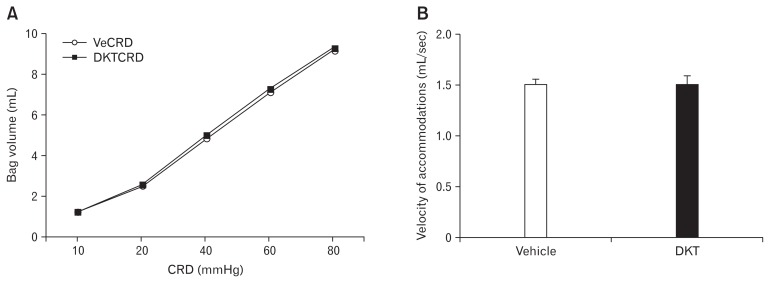
The pressure-volume curves of the smooth muscle were graded according to the stimulus intensity in both experimental treatment groups (Fig. 3A). Furthermore, no significant differences between the vehicle-treated and the DKT-treated groups were observed.
Figure 3.
Effects of Dai-kenchu-to (DKT) on smooth muscle compliance and velocity of accommodation to 60 mmHg of stretching. (A) Pressure-volume curves and (B) velocity of accommodation. Data are expressed as the mean ± SE (n = 6 rats/group). VeCRD, vehicle and colorectal distention; DKTCRD, DKT and colorectal distention.
The values plotted on the x-axis, which represents the velocity of the colorectal wall’s accommodation to 60 mmHg of stretching, were not significantly different between the 2 experimental groups (Fig. 3B).
Effects of Dai-Kenchu-To on Colorectal Distention-induced Plasma Adrenocorticotropic Hormone and Serum Corticosterone Release
The plasma ACTH levels in the vehicle-treated (118.2 ± 28.7 pg/mL) and the DKT-treated (157.9 ± 22.7 pg/mL) groups did not differ from that in the control group treated with 10 mmHg of colorectal distention (160.3 ± 29.1 pg/mL) (Table). Similarly, the serum corticosterone levels in the vehicle-treated (368.6 ± 37.8 pg/mL) and the DKT-treated (382.8 ± 17.3 pg/mL) groups did not differ from that in the control group treated with 10 mmHg of colorectal distention (447.7 ± 31.8 pg/mL) (Table).
Table.
Effects of Dai-Kenchu-To on Colorectal Distention-induced Plasma Adrenocorticotropic Hormone and Serum Corticosterone Release
| Plasma ACTH (pg/mL) | Serum corticosterone (ng/mL) | |
|---|---|---|
| Vehicle + 10 mmHg CRD (n = 5) | 160.3 ± 29.1 | 447.7 ± 31.8 |
| Vehicle + 10–80 mmHg CRD (n = 6) | 118.2 ± 28.7 | 368.6 ± 37.8 |
| DKT + 10–80 mmHg CRD (n = 6) | 157.9 ± 22.7 | 382.8 ± 17.3 |
CRD, colorectal distention; ACTH, adrenocorticotropic hormone; DKT, Dai-kenchu-to. Data are expressed as the mean ± SE (n = 5 or 6 rats/group).
Effects of Dai-Kenchu-To on Colorectal Distention Induced Anxiety-related Behavior
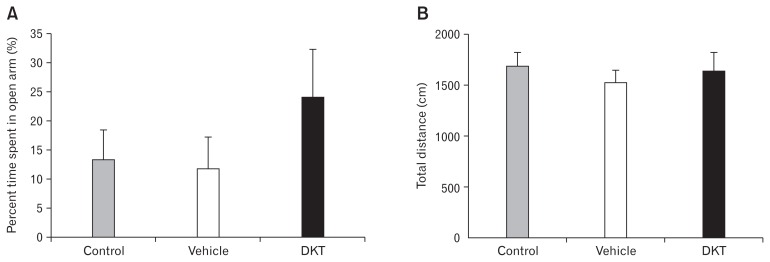
The percent time spent in the open arms of the EPM did not significantly differ among the control (13.5 ± 4.7%), vehicle-treated (11.6 ± 5.7%) or DKT-treated (24.0 ± 8.5%) groups (Fig. 4A). The total distance traveled in the EPM also did not significantly differ among the control (1636.2 ± 126.8 cm), vehicle-treated (1474.5 ± 124.1 cm), or DKT-treated (1580.8 ± 188.8 cm) groups (Fig. 4B).
Figure 4.
Effect of Dai-kenchu-to (DKT) on anxiety-related behavior induced by colorectal distention. (A) Colorectal distention-induced % time spent in open arm and (B) total distance traveled. Data are expressed as the mean ± SE (n = 5–6 rats/group).
Discussion
The present study demonstrated, for the first time, that DKT attenuates colorectal distention-induced VMRs but does not increase smooth muscle compliance or accommodation in rats. This study also suggests that DKT does not influence colorectal distention-induced ACTH release or anxiety-related behavior in rats.
Previous studies have reported that DKT-induced increases in gastrointestinal motility are mediated by acetylcholine release and that 5-HT3 and 5-HT4 receptors as well as CGRP are involved in this mechanism.1–7 The release of 5-HT from mucosal enterochromaffin cells triggers the release of CGRP from intrinsic sensory neurons.24,25 In the human intestine and rat colon, 5-HT stimulates 5-HT4 receptors located on CGRP neurons, whereas in the guinea pig colon, 5-HT binds to both 5-HT4 and 5-HT3 receptors. Both 5-HT and CGRP are released in response to mucosal stimulation, and the release of CGRP is abolished by 5-HT4 antagonists in the human intestine and rat colon.24 5-HT4 receptor agonists and CGRP have also been suggested to have antinociceptive properties.10–12 Tegaserod, a 5-HT4 agonist, increased the pain threshold of awake rats subjected to colorectal distention without modifying rectal compliance.12 Therefore, together with these results, the effect of DKT, which attenuates colorectal distention-induced VMRs, may be mediated through the action of the 5-HT4 receptor and/or CGRP.
In this study, DKT had no effect on colorectal distention-induced ACTH release, corticosterone levels, or anxiety-related behavior. The high levels of ACTH, corticosterone and anxiety-related behavior observed in the control rats may indicate that the insertion of a bag into the colorectum and exposure to the EPM may be sufficient to induce excitation in rats. Gastrointestinal stimulation induces visceral perception and emotion, especially anxiety.26 Acute colorectal distention (80 mmHg for 20 minutes) induced an increase in ACTH secretion and anxiety-related behavior in rats.16 Furthermore, various types of acute stress induce ACTH release and anxiety-related behavior.27–29 These responses are mainly triggered by corticotropin-releasing hormone (CRH) and CRH receptor 1 (CRH-R1). Mice treated with a CRH-R1 antagonist or CRH-R1-deficient mice exhibit a reduction in stress-induced ACTH release and anxiety-related behavior.27–29 Our previous study indicated that acute colorectal distention induced an increase in ACTH release and a decrease in the percent time spent in the open arm of an EPM, and that these changes were blocked by a CRH-R1 antagonist.16 However, in this study, DKT did not attenuate the colorectal distention-induced ACTH release, corticosterone levels, or anxiety-related behavior. These results suggest that the effects of DKT on the VMRs in response to colorectal distention may not involve CRH and CRH-R1. In relation to anxiety-related behavior, previous study indicated that CGRP potentiates anxiety-related behavior.30 If the DKT acts on the colorectum through CGRP release, DKT increases anxiety-related behavior, but the effects were not observed in our study. Also, one previous study indicated that CGRP did not affect anxiety-related behavior estimated by EPM.31 Although previous studies indicated that DKT affects CGRP release and 5-HT4 receptors, which have antinociceptive effects, it has not yet clear whether DKT affects anxiety-related behavior. Our results indicate that DKT might not modulate increased anxiety-related behavior and ACTH release induced by direct colorectal distention.
A recent human study indicated that the 15 g/day dose was associated with lower rectal first sensation threshold and gas threshold.22 Our data indicate that the VMRs and amplitude over the baseline did not significantly differ between the vehicle-treated and DKT-treated groups at 10 and 20 mmHg of colorectal distention. Also, we tested the smooth muscle compliance and velocity of accommodation to 60 mmHg of stretching to clarify whether decrease of VMR for colorectal distention among the DKT group is affected by increase in smooth muscle compliance or not. Our results indicated that decrease of VMR for colorectal distention among DKT group is not affected by increase in smooth muscle compliance or velocity of accommodation. Further studies are needed to clarify the effects and mechanisms of DKT on visceral perception.
The previous studies had the various recovery duration between 0 to 7 days after EMG surgery.32–37 We allowed the rats recovery duration after surgery to be at least 2 days. EMG surgery might induce local inflammation and there is a possibility that inflammation increases VMR for colorectal distention.38 In our study, all rats were estimated under the same conditions and there is less possibility of bias being included for comparison of each group.
In conclusion, our results suggest that DKT attenuates the colorectal distention-induced VMRs in a manner that does not increase smooth muscle compliance, ACTH release or anxiety-related behavior in rats. DKT may be useful for the treatment of functional bowel disorders, especially patients with irritable bowel syndrome who exhibit visceral hypersensitivity.
Footnotes
Financial support: This research was supported by a Grant-in-Aid for Scientific Research from the Ministry of Health, Welfare, and Labor of Japan (No. H13-Chouju-028).
Conflicts of interest: None.
Author contributions: Kumi Nakaya and Shin Fukudo: planning and conducting the study; Kumi Nakaya, Yohko Nagura, Ryoko Hasegawa, and Hitomi Ito: collecting and interpreting data; and Kumi Nakaya and Shin Fukudo: drafting the manuscript.
References
- 1.Shibata C, Sasaki I, Naito H, Ueno T, Matsuno S. The herbal medicine Dai-kenchu-to stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs. Surgery. 1999;126:918–924. doi: 10.1016/S0039-6060(99)70033-4. [DOI] [PubMed] [Google Scholar]
- 2.Jin XL, Shibata C, Naito H, et al. Intraduodenal and intrajejunal administration of the herbal medicine, dai-kenchu-tou, stimulates small intestinal motility via cholinergic receptors in conscious dogs. Dig Dis Sci. 2001;46:1171–1176. doi: 10.1023/A:1010690624187. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa T, Kase Y, Saito K, et al. Effects of Dai-kenchu-to on intestinal obstruction following laparotomy. J Smooth Muscle Res. 1999;35:47–54. doi: 10.1540/jsmr.35.47. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa T, Kase Y, Saito K, et al. Pharmacological studies of the effect of Dai-kenchu-to on spontaneous contraction of isolated rabbit jejunum. J Smooth Muscle Res. 1999;35:55–62. doi: 10.1540/jsmr.35.55. [DOI] [PubMed] [Google Scholar]
- 5.Satoh K, Hayakawa T, Kase Y, et al. Mechanisms for contractile effect of Dai-kenchu-to in isolated guinea pig ileum. Dig Dis Sci. 2001;46:250–256. doi: 10.1023/A:1005636412287. [DOI] [PubMed] [Google Scholar]
- 6.Satoh K, Hashimoto K, Hayakawa T, et al. Mechanism of atropine-resistant contraction induced by Dai-kenchu-to in guinea pig ileum. Jpn J Pharmacol. 2001;86:32–37. doi: 10.1254/jjp.86.32. [DOI] [PubMed] [Google Scholar]
- 7.Murata P, Kase Y, Ishige A, Sasaki H, Kurosawa S, Nakamura T. The herbal medicine Dai-kenchu-to and one of its active components [6]-shogaol increase intestinal blood flow in rats. Life Sci. 2002;70:2061–2070. doi: 10.1016/S0024-3205(01)01552-1. [DOI] [PubMed] [Google Scholar]
- 8.De Ponti F, Crema F, Moro E, Nardelli G, Croci T, Frigo GM. Intestinal motor stimulation by the 5-HT4 receptor agonist ML10302: differential involvement of tachykininergic pathways in the canine small bowel and colon. Neurogastroenterol Motil. 2001;13:543–553. doi: 10.1046/j.1365-2982.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen TN, Schmidt P, Poulsen SS, Holst JJ. Effect of calcitonin gene-related peptide (CGRP) on motility and on the release of substance P, neurokinin A, somatostatin and gastrin in the isolated perfused porcine antrum. Neurogastroenterol Motil. 2001;13:353–359. doi: 10.1046/j.1365-2982.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- 10.Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil. 2002;14:221–227. doi: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 11.Gschossmann JM, Coutinho SV, Miller JC, et al. Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat. Neurogastroenterol Motil. 2001;13:229–236. doi: 10.1046/j.1365-2982.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 12.Coelho AM, Rovira P, Fioamonti J, Bueno L. Antinociceptive properties of HTF919 (tegaserod), a 5-HT4 receptor partial agonist, on colorectal distention in rats. Gastroenterology. 2000;118:A835. doi: 10.1016/S0016-5085(00)85481-5. [DOI] [Google Scholar]
- 13.Ritchie J. Pain from distention of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 15.Saito K, Kanazawa M, Fukudo S. Colorectal distention induces hippocampal noradrenaline release in rats: an in vivo microdialysis study. Brain Res. 2002;947:146–149. doi: 10.1016/S0006-8993(02)03007-X. [DOI] [PubMed] [Google Scholar]
- 16.Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology. 2005;129:1533–1543. doi: 10.1053/j.gastro.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Nishi M, Shimada M, Uchiyama H, et al. The beneficial effects of Kampo medicine Dai-ken-chu-to after hepatic resection: a prospective randomized control study. Hepatogastroenterology. 2012;59:2290–2294. doi: 10.5754/hge10115. [DOI] [PubMed] [Google Scholar]
- 18.Shimada M, Morine Y, Nagano H, et al. Effect of TU-100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi-center, phase III trial (JFMC40-1001) Int J Clin Oncol. 2015;20:95–104. doi: 10.1007/s10147-014-0678-2. [DOI] [PubMed] [Google Scholar]
- 19.Suehiro T, Matsumata T, Shikada Y, Sugimachi K. The effect of the herbal medicines dai-kenchu-to and keishi-bukuryo-gan on bowel movement after colorectal surgery. Hepatogastroenterology. 2005;52:97–100. [PubMed] [Google Scholar]
- 20.Yaegashi M, Otsuka K, Itabashi T, et al. Daikenchuto stimulates colonic motility after laparoscopic-assisted colectomy. Hepatogastroenterology. 2014;61:85–89. [PubMed] [Google Scholar]
- 21.Numata T, Takayama S, Tobita M, et al. Traditional Japanese medicine daikenchuto improves functional constipation in poststroke patients. Evid Based Complement Alternat Med. 2014;2014:231258. doi: 10.1155/2014/231258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iturrino J, Camilleri M, Wong BS, Linker Nord SJ, Burton D, Zinsmeister AR. Randomised clinical trial: the effects of daikenchuto, TU-100, on gastrointestinal and colonic transit, anorectal and bowel function in female patients with functional constipation. Aliment Pharmacol Ther. 2013;37:776–785. doi: 10.1111/apt.12264. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- 24.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270(5 Pt 1):G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 25.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 26.Read NW. Rectal distention: from sensation to feeling. Gastroenterology. 2000;118:972–974. doi: 10.1016/S0016-5085(00)70185-5. [DOI] [PubMed] [Google Scholar]
- 27.Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 28.Weninger SC, Dunn AJ, Muglia LJ, et al. Stress-induced behavior require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GW, Aubry JM, Dellu F, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/S0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 30.Gungor NZ, Pare D. CGRP inhibits neurons of the bed nucleus of the stria terminalis: implications for the regulation of fear and anxiety. J Neurosci. 2014;34:60–65. doi: 10.1523/JNEUROSCI.3473-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schorscher-Petcu A, Austin JS, Mogil JS, Quirion R. Role of central calcitonin gene-related peptide (CGRP) in locomotor and anxiety- and depression-like behaviors in two mouse strains exhibiting a CGRP-dependent difference in thermal pain sensitivity. J Mol Neurosci. 2009;39:125–136. doi: 10.1007/s12031-009-9201-z. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson JK, Greenwood-Van Meerveld B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1080–G1085. doi: 10.1152/ajpgi.00349.2010. [DOI] [PubMed] [Google Scholar]
- 33.Nozu T, Kumei S, Miyagishi S, Takakusaki K, Okumura T. Colorectal distention induces acute and delayed visceral hypersensitivity: role of peripheral corticotropin-releasing factor and interleukin-1 in rats. J Gastroenterol. 2015;50:1153–1161. doi: 10.1007/s00535-015-1070-3. [DOI] [PubMed] [Google Scholar]
- 34.Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Wu X, Cao Z, Chen S, Owyang C, Li Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology. :1732–1740. e3. doi: 10.1053/j.gastro.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Cao Z, Wu X, Chen S, et al. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 37.Kannampalli P, Pochiraju S, Chichlowski M, et al. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol Motil. 2014;26:1694–1704. doi: 10.1111/nmo.12450. [DOI] [PubMed] [Google Scholar]
- 38.Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147–1156. doi: 10.1111/j.1365-2982.2008.01151.x. [DOI] [PubMed] [Google Scholar]