TO THE EDITOR
Diabetes is often associated with digestive disorders including colorectal irritation due to peripheral neuropathy.1,2 Hyperglycemia is thought to be the chief cause of diabetic damage to cells of the peripheral nerves through alterations of Na+/K+ activity.3–5 These alterations aggregate to produce hyper-excitability of nociceptors, a key mechanism in diabetic neuropathy. A recent study, published in the Journal of Neurogastroenterology and Motility “Colonic hypersensitivity and sensitization of voltage-gated sodium channels in primary sensory neurons in rats with diabetes”,6 has observed the induction of streptozotocin (STZ) elevating colonic distension threshold, which is associated by the colonic innervated dorsal root ganglion (DRG) neurons; on which the activities and expressions of NaV1.7 and NaV1.8 are identified as the mechanism underlining diabetes.
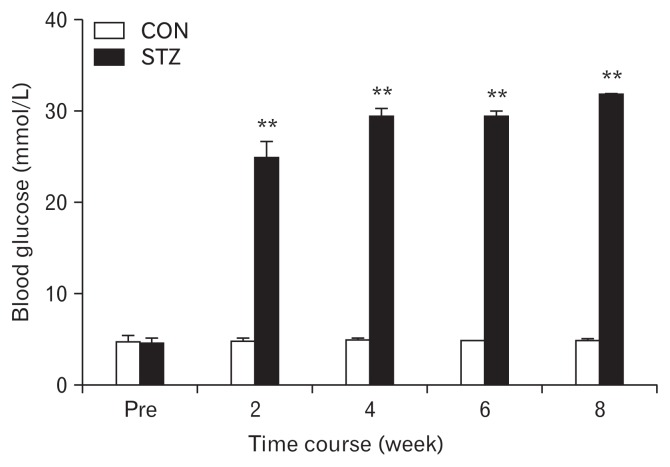
It is an interesting and informative study that intrigues us to invite a discussion in detail. (1) The diabetic model in the study was created by a single small amount of STZ (65 mg/kg) intraperitoneal injection. This method has been well documented by many studies and it has been rarely employed because it is accompanied by serious variability in modeling. Surprisingly, as shown in Figure 1, there is very little variety shown in blood glucose measurements reflecting an identical or similar effect of STZ in all animals. However, there is a lack of pathological analysis.6 An analysis of islet damage under STZ is necessary to establish the modeling validity. (2) The authors evaluated colonic innervated DRG neurons by using DiI labeling cells as shown in Figure 2.6 What is to be expected from the labeling is to know whether STZ changes the amount or percentage of DiI positive cells, in narrowing down the change of NaV1.7 and NaV1.8 of western blots, that is to identify where those are increased from total DRG cells or only DiI positive cells. (3) Although the authors compared the difference of excitability between DiI positive neurons in the 2 groups, a comparison between DiI positive and negative in each group is also important since the neuronal resting membrane potential in DiI positive cells in the control group is − 45.6 ± 0.7 (n = 25), which indicates a depolarization existing in DRG cells without STZ treatment. (4) Resting membrane potential significantly depolarized in STZ (−43.0 ± 0.4, n = 25) indicates a change in K channel, however, there is no information about it. In addition, authors measured the onset and severity of hyper-responsive at week 2 of STZ induction; the other measured parameters including the neuronal hypersensitivity, voltage-gated sodium channel current increase, and expressions of NaV1.7 and NaV1.8 were measured at only week 4; we assumed the outstanding change of distension of threshold at week 2 came from the same source of the change—upregulations of NaV1.7 and NaV1.8. In conclusion, Hu et al6 presents that the peripheral sensory neurons are in frontier in response to very early stage of diabetes through upregulation of NaV1.7 and NaV1.8, hence, hyperexcitability. Whether “peripheral” sodium channels upregulation is one of pathophysiological changes from diabetes, or whether such peripheral sodium channel is a target for control of diabetic visceral pain is extremely expected in next.
Figure 1.
Following a single intraperitoneal injection of streptozotocin (STZ), the blood glucose level was significantly increased. The hyperglycemia persisted for at least another 6 weeks within our observation period of time (n = 8 for both groups, **P < 0.01 compared with control [CON], two-way repeated-measures ANOVA followed by Tukey post hoc test).
Figure 2.
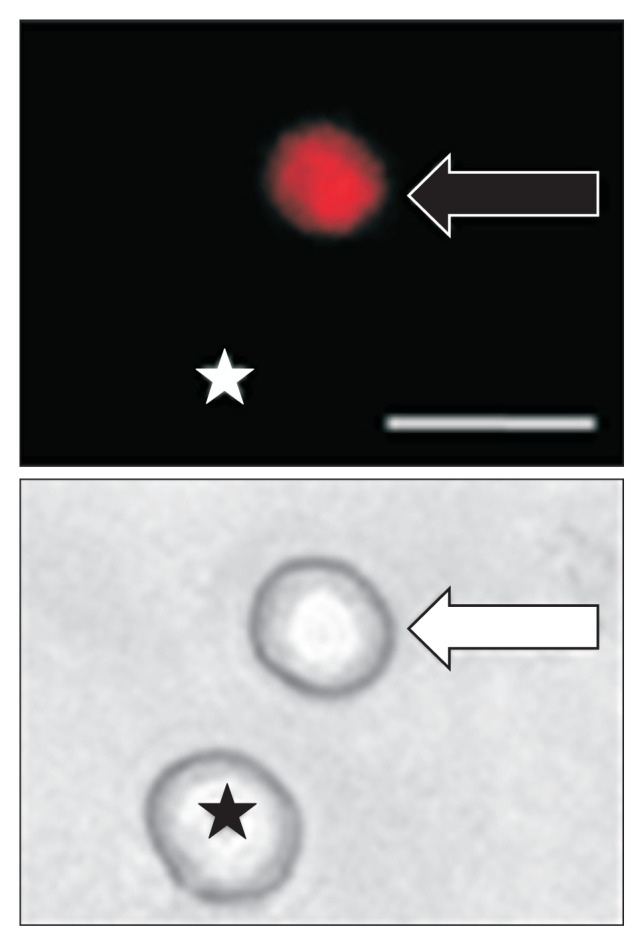
Top: An example of a Dil-labeled dorsal root ganglion (DRG) neuron (arrow). Asterisk indicates the place where a neuron is not labeled by DiI. Bottom: Phase image of the same DRG neuron labeled by DiI is shown on the right (arrow) and the neuron not labeled by DiI is shown on the left (★). Scale bar = 50 μm. Patchclamp recordings were performed on DiI-labeled colon neurons. A total of 25 DiI-labeled neurons from control rats and 25 DiI-labeled neurons from streptozotocin-induced diabetic rats were recorded under current-clamp conditions.
Footnotes
Financial support: This work was supported by the Natural Science Foundation of China grant (No. 81302930). And this work was also supported by Guangxi science foundation grant (No. 2012GXNS-FBA053091).
Conflicts of interest: None.
Author contributions: Jianlin Lv and Mingjie Wang are both equal co-first authors, they wrote the draft; and Meng Xia as corresponding author made revisions and corrections on the manuscript.
References
- 1.Russo A, Botten R, Kong MF, et al. Effects of acute hyperglycemia on anorectal motor and sensory function in diabetes mellitus. Diabet Med. 2004;21:176–182. doi: 10.1111/j.1464-5491.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- 2.Pinna Pintor M, Zara GP, Faletto E, et al. Pudendal neuropathy in diabetic patients with fecal incontinence. Int J Colorectal Dis. 1994;9:105–109. doi: 10.1007/BF00699423. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan AV, Lin CS, Kiernan MC. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain. 2008;131(Pt 5):1209–1216. doi: 10.1093/brain/awn052. [DOI] [PubMed] [Google Scholar]
- 4.Zenker J, Poirot O, de Preux Charles AS, et al. Altered distribution of juxtaparanodal kv1.2 subunits mediates peripheral nerve hyperexcitability in type 2 diabetes mellitus. J Neurosci. 2012;32:7493–7498. doi: 10.1523/JNEUROSCI.0719-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misawa S, Sakurai K, Shibuya K, et al. Neuropathic pain is associated with increased nodal persistent Na+ currents in human diabetic neuropathy. J Peripher Nerv Syst. 2009;14:279–284. doi: 10.1111/j.1529-8027.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Song ZY, Zhang HH, et al. Colonic hypersensitivity and sensitization of voltage-gated sodium channels in primary sensory neurons in rats with diabetes. J Neurogastroenterol Motil. 2016;22:129–140. doi: 10.5056/jnm15091. [DOI] [PMC free article] [PubMed] [Google Scholar]