Abstract
The human circadian timing system is most sensitive to the phase shifting effects of light during the biological nighttime, a time at which humans are most typically asleep. The overlap of sleep with peak sensitivity to the phase shifting effects of light minimizes the effectiveness of using light as a countermeasure to circadian misalignment in humans. Most current light exposure treatments for such misalignment are mostly ineffective due to poor compliance and secondary changes that cause sleep deprivation. Using a 16-day, parallel group design, we examined whether a novel sequence of light flashes delivered during sleep could evoke phase changes in the circadian system without disrupting sleep. Healthy volunteers participated in a two-week circadian stabilization protocol followed by a two-night laboratory stay. During the laboratory session, they were exposed during sleep to either darkness (n=7) or a sequence of 2-msec light flashes given every 30 seconds (n=6) from hours 2–3 after habitual bed time. Changes in circadian timing (phase), micro- and macroarchitecture of sleep were all assessed. Subjects exposed to the flash sequence during sleep exhibited a delay in the timing of their circadian salivary melatonin rhythm as compared to the control dark condition (P<0.05). Confirmation that the flashes penetrated the eyelids is presented by the occurrence of an evoked response in the EEG. Despite the robust effect on circadian timing, there were no large changes in either the amount or spectral content of sleep (P’s>0.30) during the flash stimulus. Exposing sleeping individuals to 0.24 seconds of light spread over an hour shifted the timing of the circadian clock and did so without major alterations to sleep itself. While a greater number of matched subjects and more research will be necessary to ascertain whether there is an effect of these light flashes on sleep, our data suggest that this type of passive phototherapy might be developed as a useful treatment for circadian misalignment in humans.
Keywords: light, circadian, human, phase shift, sleep, electroencephalography
Introduction
The human circadian system controls the timing of most physiological systems including the endocrine, immune, and neurologic. Proper alignment of the circadian system with the external day is important for good health (Vogel et al., 2012). The primary manner by which the internal circadian system remains entrained with the external day is through regular exposure to light and dark (Duffy and Czeisler, 2009). Circadian misalignment can lead to health disruptions from mundane (e.g., jet lag) to serious (e.g., cancer) (Stevens, 2005). A number of sleep disorders (advanced and delayed sleep phase disorder, non-24-sleep-wake disorder, shift work sleep disorder) are also directly attributable to an improper alignment between the circadian system and an individual’s social schedule (Morgenthaler et al., 2007). While bright light is a useful tool for maintaining proper alignment, the human circadian timing system is most sensitive to light during times at which people are normally asleep (St Hilaire et al., 2012). Administration of light during sleep is plausible, given the penetrance of light through closed eyelids (which act as a red-pass filter with approximately twice as much of the longer wavelengths passing through as the shorter wavelengths) (Moseley et al., 1988;Robinson et al., 1991), and preliminary evidence indicates that such treatment is effective in shifting circadian rhythms (Cole et al., 2002;Figueiro and Rea, 2012). Continuous bright light or long duration light pulses delivered during this most sensitive phase would, however, disrupt sleep as these would be likely to either cause or sustain awakenings (Cajochen et al., 2000). We have recently demonstrated that a train of millisecond flashes of light presented to subjects who are awake has the capacity to elicit phase delays of the human circadian system (Zeitzer et al., 2011). Bringing these two streams of evidence together, we examined whether a train of millisecond flashes of light during sleep had the capacity to change the phase of the circadian system and to do so without affecting sleep.
Materials and Methods
The protocol was approved by the Stanford University Institutional Review Board and was conducted under the principles outlined in the Declaration of Helsinki. Written informed consent was received from all participants prior to inclusion in the study. This study was registered with ClinicalTrials.gov #NCT01119365 (http://www.clinicaltrials.gov/ct2/show/NCT01119365).
Participants
Thirteen healthy young (26.9 ± 4.8 years) male (n=6) and female (n=7) volunteers participated in a parallel group 16-day study. Subjects were studied between November 2010 and July 2012. Sample size was calculated a priori based on estimated variance of phase change measures and a minimal detectable difference in phase change of 30 minutes between groups. All volunteers self-reported good physical and mental health, were not depressed (Radloff, 1977), had no evidence of sleep disorders (Buysse et al., 1989) nor did they use any medications that could impact sleep (e.g., antihistamines, antidepressants, benzodiazepine agonists), did not abuse alcohol (Babor et al., 2001), had normal color vision (Ishihara, 2007), were non-smokers, and were of moderate chronotype (Horne and Östberg, 1976). All female subjects began the in-laboratory portion of their stay within five days from the onset of menses (Baker and Driver, 2007).
At-home sleep/circadian stabilization protocol
For two weeks prior to entry into the laboratory, subjects participated in an at-home sleep/circadian stabilization protocol during which they were required to keep a strict sleep schedule. Subjects self-selected bed and wake times that had to be 7–9 hours apart and each bed and wake time had to be within ±30 minutes of this target time. Verification was accomplished using self-reported sleep/wake logs and wrist actigraphy (Ancoli-Israel et al., 2003). Such a schedule is useful for normalizing the amplitude and phase angle of the circadian system (Zeitzer et al., 2011;Jewett et al., 1994;Duffy et al., 1999).
In-lab light exposure protocol
Subjects stayed in the laboratory for a 35-hour protocol starting seven hours after habitual waketime (Figure 1). All events were timed relative to their at-home sleep pattern (Zeitzer et al., 2011). In the laboratory, subjects were exposed to <10 lux during scheduled wake and complete darkness (<0.05 lux) during scheduled sleep. Low illuminance is necessary to unmask the endogenous rhythm of melatonin (Zeitzer et al., 2000). For 9 hours prior to bedtime, subjects lay in bed in a constant, semirecumbent posture (Deacon and Arendt, 1994;Duffy and Dijk, 2002). Instead of receiving dinner, subjects were given hourly equicaloric aliquots of food and isovolumetric (90 mL) water (Mifflin et al., 1990) in order to minimize any possible effects of meals on metabolism and subsequent effects on circadian rhythms. Saliva samples were obtained every 30 minutes, immediately before or midway between snacks, using untreated Salivettes (Sarstedt, Newton NC). Saliva samples were spun and immediately frozen. Prior to bedtime, subjects were fitted with electrodes on their face (bipolar electro-oculogram, chin electromyogram) and scalp (electroencephalogram – EEG C3/C4/O1/O2) (Klem et al., 1999). Sleep data were recorded at a 256 Hz sampling frequency on a polysomnograph (PSG, Siesta, Compumedics, Cary NC) and scored according to standard clinical criteria (Rechtschaffen and Kales, 1968) by a single expert PSG technician blind to group allocation. Sleep data were unavailable from two of the subjects exposed to a dark stimulus due to equipment failure.
Figure 1.
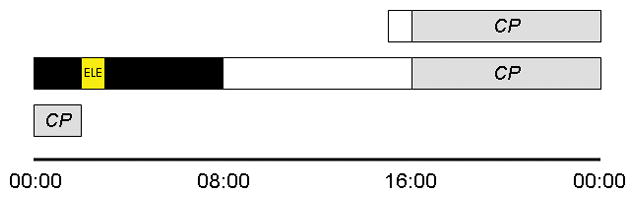
Protocol diagram. A protocol flow for a subject who had habitually slept from 00:00 until 08:00 during the two weeks prior to lab entry is depicted. One hour after entry, subjects had an initial constant posture (CP). For an hour, starting two hours after lights out, subjects were exposed to an experimental light exposure (ELE), consisting of either a flash sequence or darkness, during sleep. Subjects were ambulatory in the dimly lit laboratory until the start of a second CP. Phase change in salivary melatonin phase was determined between CP1 and CP2. Sleep during the ELE was compared with that which occurred during the prior hour on the same night.
From hours 2–3 after bedtime, subjects were exposed to one of two lighting regimes – 60 minutes of darkness (i.e., no change in lighting) (n=7) or 60 minutes of a flash sequence (n=6). The flash sequence consisted of one moderately bright (~2995 lux at corneal level) 2-msec flash of white light delivered every 30 seconds. The flash was generated by a wide spectrum xenon flash bulb (ColorDome, Diagnosys, Lowell MA) placed directly above the subject’s head. During sleep, subjects lay supine, confirmed with a PSG position sensor, with their head between two foam bolsters. The ColorDome was controlled from outside the room and the subject was not intentionally awakened at any point during this procedure. Following the stimulus, subjects were allowed to continue to sleep in darkness for an additional five hours and were awakened into dim light at their habitual wake time. For the first eight hours after wake time on the second day in the laboratory, subjects were ambulatory en suite, after which they got into bed for an 11-hour constant posture protocol as described for Day 1. This second day was used to make a second, post-stimulus phase determination. Unintentionally, the distribution of male and female subjects was not equal across the two study groups. All six of the participants exposed to flashes were female and only one of the seven participants exposed to dark was female. Males have, on average, a circadian period length that is approximately six minutes longer than women (Duffy et al., 2011). As such, we would expect that the group exposed to dark (primarily male) would have a greater change in circadian phase due to their intrinsic period. This would tend to reduce the difference between the dark group and the light-exposed group making a light-induced phase shift more difficult to detect.
Salivary melatonin
Saliva samples were defrosted and assayed for melatonin concentrations using a commercially available enzyme-linked immunosorbent assay (ALPCO, Salem NH) according to manufacturer’s instructions [intra-assay variability (12.6%), interassay variability (22.9%), sensitivity 0.5 pg/mL, per manufacturer]. Single kits were used for each subject. Melatonin phase was specified as the time at which salivary melatonin concentrations rose above a subject-specific threshold, calculated as the average of the first three daytime concentrations plus twice the standard deviation of these values (Voultsios et al., 1997).
Electroencephalography
EEG data were processed for examination of spectral content and for evoked EEG responses associated with the light flashes. For spectral analysis, signals from each cortical electrode (C3-A2, C4-A1, O1-A2, O2-A1) were deconvoluted into component frequencies (PRANA, PhiTools, Chicago IL). Using this method for each 30 s bin, the absolute power was calculated for the frequency bands: delta (0.5–4 Hz), theta (4–7.5 Hz), alpha (8–12.5 Hz), sigma (12.5–14 Hz), beta (14–29 Hz) and gamma (30–40 Hz). Data were converted to relative power by normalizing to the total spectral power. Using automatic detection algorithms, EEG data with artifact were removed from the analyses. Only data from periods of scored NREM were used for power spectral analysis. For examination of evoked responses, we folded the EEG data at 30-second intervals during the one-hour of light stimulus. This was done separately for each of the four derivations (O1-A2, O2-A1, C3-A2, C4-A1). Average waveforms were generated (i.e., each 30-second waveform was the average of 120 30-second intervals). The O1-A2 ERP has data from only five subjects as 60 cycle noise rendered these channels unusable in two subjects. These ERPs were not excluded from the power spectral analysis of sleep.
All data are presented as mean ± SD. To determine the effect of the experimental stimuli on circadian timing, we examined the change in the onset of salivary melatonin onset during the first and second constant posture. To examine the effect of the experimental stimuli on sleep, we compared both EEG power spectral data and sleep staging during the hour of the experimental stimulus with the prior hour of sleep. We also examined EEG power spectral data and sleep staging during the five hours following the experimental stimuli. Within group differences were assessed with paired two-tailed t-tests while between group differences were assessed with unpaired two-tailed t-tests adjusted for scedasticity. When comparing multiple EEG bands, to keep the overall test α′=0.05, individual test α were set to 0.008 incrementing upward to 0.05 for each test, according to the method of Holm-Bonferroni (Holm, 1979).
Results
Timing of the stimuli
In order to examine the effectiveness of brief flashes of light in changing the timing of the human circadian system, we exposed a group of young, healthy volunteers (n=13) to either one hour of darkness or a one-hour sequence of light flashes (a 2-msec, ~2995 lux flash every 30 seconds) during sleep. Both exposures were scheduled to occur approximately four hours after the onset of melatonin secretion, which typically occurs about two hours before habitual bedtime. As the effects of light on the human circadian timing system are dependent on the phase of the circadian cycle during which the light stimulus is presented (St Hilaire et al., 2012), it is important to deliver light at the same phase in all subjects. At the targeted circadian phase, light was expected to delay the phase (timing) of the circadian rhythm (St Hilaire et al., 2012). Of the six subjects exposed to the light flashes, five were clustered at the same phase (beginning 4.3 ± 0.85 hours after melatonin onset, determined retrospectively). One subject (light stimulus started 6.8 hours after melatonin onset) was excluded due to the mistimed light exposure. Of the seven subjects exposed to the control dark stimulus, all were clustered at the same phase (stimulus beginning 4.5 ± 0.78 hours after melatonin onset), which was indistinguishable from the phase at which the flash stimulus was presented (P=0.71, t-test). Thus, subjects exposed in both the control and the flash conditions were given experimental stimuli at the same circadian phase.
Circadian response to the stimuli
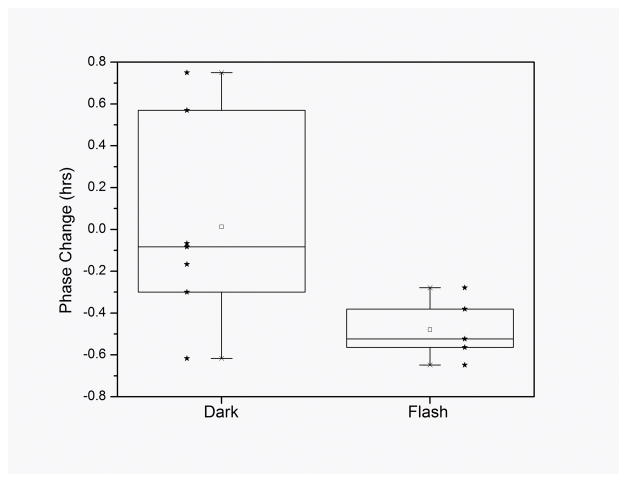
In response to the dark condition (equivalent to no change to regular sleep conditions), subjects exhibited a non-significant 0.01 ± 0.48 hour phase change (advance) of their circadian rhythm of salivary melatonin (P=0.95, paired t-test). In response to the flash stimulus, subjects exhibited a significant −0.48 ± 0.15 hour phase change (delay) in their circadian rhythm (P<0.01, paired t-test). The phase change exhibited by subjects in response to the flash stimulus was significantly different from that exhibited by subjects in response to the control (darkness) stimulus (P<0.05, t-test) (Figure 2). Thus, despite the approximately 90% attenuation of light by the eyelid (Moseley et al., 1988;Robinson et al., 1991), this flash stimulus was able to change the circadian phase in our volunteers while they were asleep.
Figure 2.
Box chart of circadian phase changes (timing of melatonin onset) after exposure to the dark and flash stimuli. There is no significant phase change exhibited under the dark condition (P=0.95, paired t-test), while the flash condition induced a significant 30 minute phase change (P<0.01, paired t-test) that was also significantly different from that observed in the dark condition (P<0.05, t-test). Individual data are shown as stars.
Event-related potential
We used an independent measure to determine whether flashes of light were indeed passing through the eyelids and evoking a change in the brain. We analyzed the EEG for an event-related potential (ERP) occurring at the time of the stimulus. We averaged 30-second windows of the EEG so as to examine the impact that a repeated stimulus might have. In doing so, we detected an ERP in each of the four EEG channels that we recorded (bilateral central leads and occipital leads) (Figure 3). This ERP, a short-lived 0.5 Hz change in the EEG, confirms that the cortex is actually receiving information about the light flashes. We are, therefore, convinced that the flashes that we delivered were able to penetrate the eyelids and impact brain function.
Figure 3.
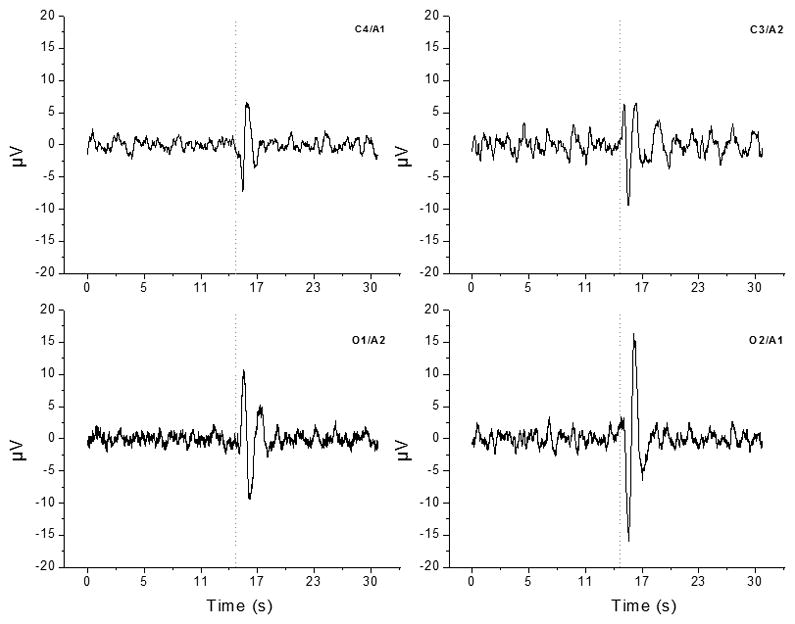
The ERP generated from subjects exposed to the flash sequence during sleep. Each ERP represents the average 30-second EEG waveform during the one-hour exposure. Data were averaged within then between subjects for each of the four derivations (two central – C4 and C3, two occipital – O1 and O2). Data are centered on the timing of the flash (dotted line). Robust changes are observable in both the occipital (primary visual) cortex channels with lesser but still obvious changes in both the central cortical channels, confirming that the flash signal is passing through the eyelids and impacting brain firing patterns.
Effects on sleep
We also examined whether subjects were awake during the flashes or if the flashes caused an alerting response or any other change in sleep. We compared sleep during the hour of exposure to the flashes with sleep the previous hour, during which no flash stimuli were present. There were no differences in the change in wake (10.5 ± 18.0 min, P=0.17, paired t-test), stage N1 (1.07 ± 18.0 min, P=0.88, paired t-test), stage N2 (−8.36 ± 23.5 min, P=0.38, paired t-test), stage N3 (−2.64 ± 9.81 min, P=0.50, paired t-test), or REM (−0.429 ± 6.75 min, P=0.87, paired t-test) sleep between the two hours, nor was there a difference in the number of state transitions (−0.14 ± 13, P=0.98, paired t-test). Thus, from a macroarchitectural level, there were no changes in sleep that could be attributed to the flash stimulus. We then compared the spectral power present in the commonly parsed frequency bands during the hour of flashes to the previous hour. In none of the predefined spectral power bands, delta (10.9 ± 19.9%, P=0.65, paired t-test), theta (44.8 ± 79.1%, P=0.88, paired t-test), alpha (74.4 ± 166%, P=0.30, paired t-test), sigma (65.6 ± 182%, P=0.68, paired t-test), beta (173 ± 309%, P=0.53, paired t-test), gamma (206 ± 383%, P=0.75, paired t-test), was there a difference between the two hours. Thus, from the level of EEG spectrum, there were no changes in sleep that could be attributed to the flash stimulus. Therefore, we observed no direct effect of the flashes on sleep.
Finally, we examined whether the flash stimulus caused a change in sleep that outlived the flashes themselves. We examined sleep during the five hours after experimental intervention, either the flash or dark stimulus, and before lights-on in the morning. This post-stimulus sleep was indistinguishable between the subjects exposed to the flashes and those exposed to darkness. In comparing these five hours in those exposed to dark versus wake, the amounts of wake (43.7 ± 30.0 min vs. 59.3 ± 53.0 min, P=0.58, t-test), N1 (142 ± 28.6 min vs. 148 ± 32.3 min, P=0.75, t-test), N2 (57.9 ± 20.3 min vs. 62.5 ± 21.1 min, P=0.73, t-test), N3 (13.5 ± 12.1 min vs. 1.90 ± 4.25 min, P=0.07, t-test), and REM (43.3 ± 5.90 min vs. 28.3 ± 22.9 min, P=0.22, t-test), as well as the number of transitions (128 ± 10.2 vs. 123 ± 38.0, P=0.79, t-test) were statistically similar between the groups. Relative amounts of delta (55.7 ± 2.29 vs. 46.2 ± 14.9, P=0.23, t-test), theta (11.8 ± 2.37 vs. 16.9 ± 18.4, P=0.57, t-test), alpha (5.52 ± 1.21 vs. 4.64 ± 2.14, P=0.45, t-test), sigma (2.78 ± 1.08 vs. 2.83 ± 0.976, P=0.94, t-test), beta (3.31 ± 0.675 vs. 3.37 ± 0.661, P=0.89, t-test), and gamma (1.10 ± 0.197 vs. 1.73 ± 1.18, P=0.23, t-test) power also were statistically similar between the groups. Thus, not only did the light stimulus not change sleep during the administration of the stimulus, but it did not appear to affect sleep during the five hours after the stimulus, indicating that the flash intervention was able to simultaneously change circadian phase and not impact sleep.
Discussion
We have demonstrated that brief, millisecond flashes of light have the capacity to change circadian phase in people while they are asleep. Given the relatively small sample size and the sex-imbalance in the protocol, it is impossible to rule out an effect of the light on sleep, but we could find no gross objective or subjective changes in sleep that were related to the light exposure during sleep. Light as a therapeutic intervention for circadian-based sleep and medical disorders (“phototherapy”) is most commonly prescribed to occur before normal wake time or after normal bed time (Morgenthaler et al., 2007). While highly efficacious, this type of therapy has poor effectiveness as compliance is low and, when compliance is adhered to, leads to chronic sleep deprivation (Bjorvatn and Pallesen, 2009;Barion and Zee, 2007). Our demonstration of effective light treatment during sleep without gross interference of sleep opens new avenues of therapy as the light treatment is non-intrusive and requires no change in behavior from the recipient. This could lead to novel treatments for highly prevalent disorders such as delayed sleep phase syndrome (adolescents), shift work sleep-wake disorder, and advanced sleep phase syndrome (senescence), or the nuisance of jet lag. As phase delaying light is normally administered in the evening prior to sleep, this type of light could be used to supplement this light exposure (i.e., continue such light exposure into the hours of sleep) or to allow for subjects to go to sleep at an earlier circadian phase, such as might be necessary when adapting for particular shift schedules. A recent study examining the use of light pulses 1000 times longer (2 seconds) has confirmed the possibility of light administration during sleep having a significant impact on circadian physiology without disrupting sleep (Figueiro et al., 2013). It remains to be determined if application of light flashes at all times of the sleep cycle would have equally minimal effects on sleep. Future work on the administration of light flashes during sleep should focus on further confirming these findings and optimization, in terms of spectral content of the flashes (i.e., which photoreceptors are responsible for converting the light flashes to a neural signal), spacing of flashes, and flash duration such that the sequence should be primed to produce the maximal shift with minimal time. Future work should also better address whether there is any impact on sleep and the extent of this impact. Our relatively small sample size did not allow us to explore all possible changes in sleep without the risk of type II error and there are many other analyses that could be used to examine possible changes in sleep. As there is an ever growing number of recognized circadian-related morbidities, this type of passive intervention could be of great societal benefit.
Acknowledgments
The authors wish to thank Mr. Ban Ku, Ms. Chun-Ping Liao, and Mr. Daniel East for help in data collection.
Funding:
This work was supported by the National Heart Lung and Blood Institute (1R01HL108441-01); Air Force Office of Scientific Research (F2-4506); and Department of Veterans Affairs Mental Illness Research, Education, and Clinical Center.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollack CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test, Guidelines for Use in Primary Care. 2 . World Health Organization: Department of Mental Health and Substance Dependence; Geneva: 2001. [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–577. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13:47–60. doi: 10.1016/j.smrv.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk D-J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of alertness in humans. Beh Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Letters. 1994;167:191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A-M, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk D-J, Wright KP, Jr, Czeisler CA. Sex differences in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1010666108. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4:165–177. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk D-J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk D-J, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, Bierman A, Rea MS. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat Sci Sleep. 2013;5:133–141. doi: 10.2147/NSS.S52203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, Rea MS. Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Research Notes. 2012;5:221. doi: 10.1186/1756-0500-5-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Intl J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Ishihara S. Ishihara’s Tests for Colour Deficiency. Kanehara Trading Inc; Tokyo: 2007. [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroencephalography Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Amer J Clin Nutrition. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr, Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley MJ, Bayliss SC, Fielder AR. Light transmission through the human eyelid: in vivo measurement. Ophthalmic Physiol Optics. 1988;8:229–230. doi: 10.1111/j.1475-1313.1988.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. App Psychological Meas. 1977;1:385–401. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Department of Health, Education, and Welfare, Government Printing Office; Washington D.C: 1968. [Google Scholar]
- Robinson J, Bayliss SC, Fielder AR. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 1991;31:1837–1840. doi: 10.1016/0042-6989(91)90031-y. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1h pulse of bright white light. J Physiol. 2012;590:3035–3045. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiol. 2005;16:254–258. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- Vogel M, Braungardt T, Meyer W, Schneider W. The effects of shift work on physical and mental health. J Neural Transm. 2012;119:1121–1132. doi: 10.1007/s00702-012-0800-4. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS ONE. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]