Abstract
Background
Salvia, known as Maryam Goli in the Persian language, is an important genus that includes approximately 900 species in the Lamiaceae family. There are 58 Salvia species growing naturally in Iran, including Salvia chloroleuca Rech. f. and Allen., which grows wild in the northeastern and central parts of the country.
Objectives
This study was designed to determine the chemical composition, in vitro antioxidant activity, and total phenol content of various extracts of S. chloroleuca.
Materials and Methods
Dried aerial parts of the plant were crushed, then sequentially extracted with n-hexane, ethyl acetate, and methanol. The fractions of S. chloroleuca were subjected to silica gel column chromatography and Sephedex LH-20. The antioxidant activities of these extracts were measured by ferric reducing antioxidant power (FRAP), and the total phenolic contents of the extracts were evaluated using Folin-Ciocalteu reagent.
Results
The separation and purification processes were carried out using different chromatographic methods. Structural elucidation was on the basis 1H-NMR and 13C-NMR spectral data, in comparison with that reported in the literature. The isolated compounds were salvigenin (1), luteolin (2), cirsiliol (3), β-sitosterol (4), and daucosterol (5). Ethyl acetate extract displayed the highest level of total antioxidants and total polyphenols compared to the other analyzed extracts (n-hexane and methanol). In the FRAP assay, ethyl acetate extract had the highest (230.4±10.5) FRAP value, followed by methanol (211.4 ± 8.3) and n-hexane (143.4 ± 12.04). Total phenol contents were calculated to be 13.8 ± 0.3, 58.25 ± 0.05, and 43.48 ± 0.38 mg of gallic acid/100 g in the n-hexane, ethyl acetate, and methanol extracts, respectively.
Conclusions
The above-mentioned compounds were isolated for the first time from S. chloroleuca. The antioxidant activity of this plant could be in part related to isolated flavonoids and sterols. The results of this study indicated that S. chloroleuca could be an important dietary source of phenolic compounds with high antioxidant capacity.
Keywords: Lamiaceae, Phytochemical, Antioxidant, Flavonoid, Sterol, Salvia chloroleuca
1. Background
It is well known that free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced in vivo, such as hydroxyl radicals, superoxide anions, and hydrogen peroxide, are extremely reactive and transient chemical species (1). They can damage proteins, DNA, and lipids. It has been reported that imbalances between ROS formation and scavenging systems contributes to the pathogenesis of different conditions, including aging, Alzheimer’s disease, diabetes mellitus, atherosclerosis, and hypertension (2). Antioxidants can inhibit or delay oxidative chain reactions in lipids, proteins, carbohydrates, and DNA (3). Several studies have proposed that natural antioxidants may be less toxic than synthetic ones, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) (4). Salvia is an important genus that includes approximately 900 species in the Lamiaceae family and is known as Maryam Goli in the Persian language. There are 58 Salvia species growing naturally in Iran, 17 of which are endemic. Salvia species have been used in traditional medicine throughout the world as tonics, antirheumatics, antimicrobials, and carminatives (5, 6). It has been speculated that Salvia originated in Afghanistan, although the highest number of species (about 250) are found in Mexico (7). Traditionally, crude extracts of Salvia species have been used for various purposes in foods, drugs, and perfumery (8). Several biological studies have reported considerable anti-inflammatory, antioxidant, antibacterial, and antitumor effects of this genus (9, 10).
Salvia chloroleuca Rech. f. and Allen. is one of Iran’s endemic species. A review of the literature revealed no reports on the non-volatile constituents of the aerial part of S. chloroleuca. The chemical constituents of the essential oil of S. chloroleuca have been investigated, and the main components were α-pinene, β-pinene, β-caryophyllene, 1,8-cineole, and carvacrol (11). The structural elucidations of the isolated compounds were established according to 1H-NMR and 13C-NMR spectral data.
2. Objectives
To our knowledge, there are no studies in the literature on the chemical composition of the non-volatile constituents of the aerial part of S. chloroleuca. Hence, the objective of the present study was to isolate and identify some of the phytochemical constituents of S. chloroleuca. The total phenol content and potential antioxidant activities of the extracts were also evaluated using the ferric reducing antioxidant power (FRAP) method.
3. Materials and Methods
3.1. Plant Materials
The aerial parts of S. chloroleuca were collected from the Shahrestanak region in Tehran province, Iran, in May 2013, and identified by Dr. Y. Ajanii. A voucher specimen (ACECR-244) was deposited at the herbarium of the medicinal plants research center in the faculty of pharmacy at Tehran University of Medical Sciences.
3.2. Extraction and Isolation
The air-dried, powdered aerial parts of S. chloroleuca (2.5 kg) were extracted successively with n-hexane (3 × 9 L), ethyl acetate (3 × 9 L), and methanol (3 × 9 L) by maceration at room temperature. A rotary vacuum evaporator was used to concentrate the solution. The n-hexane portion (8 g) was subjected to silica gel column chromatography (230-400 mesh) with chloroform, chloroform: ethyl acetate (8: 2, 5: 5), and ethyl acetate. After screening with TLC, fractions with similar compositions were pooled in order to yield five combined fractions (A-E). The C fraction (320 mg) was subjected to Sephedex LH-20 column chromatography with methanol to give ten fractions (C1-C10). For more purification, the C5 (17 mg) portion was submitted to Sephedex LH-20 with methanol: ethyl acetate (6: 4) to give pure compound 4 (4 mg). The ethyl acetate (25 g) was subjected to silica gel column chromatography with chloroform, ethyl acetate: methanol (8: 2, 6: 4, 2: 8), and methanol to give six main fractions (A-F). Fraction D (3.3 g) was submitted to the silica gel column with ethyl acetate: methanol (8: 2, 5: 5) and methanol to yield 13 parts (D1 - D13). For more purification, the D1, D6, and D12 parts were subjected to Sephedex LH-20 with methanol: ethyl acetate (9:1) as eluent to give pure compounds 1 (28 mg), 2 (20 mg), and 3 (15 mg), respectively. The methanol extract (30 g) was successively chromatographed on the silica gel column with ethyl acetate, ethyl acetate: methanol (5:5, 2:8), methanol, and water, in order to gain eight fractions (A-H). Fraction G (500 mg) was subjected to silica gel column with ethyl acetate: methanol (5: 5, 1: 9) and methanol to yield ten fractions (G1-G10). G6 (45 mg) was subjected to reverse-phase HPLC using a gradient solvent system consisting of water–methanol (20: 80, 40: 60, 80: 20) and methanol to give compound 5 (7 mg) (12).
3.3. Instruments and Chemicals
The 1H and 13C-NMR spectra were measured on a Bruker DRX-500 spectrometer with tetramethylsilane as an internal standard, and the chemical shifts are given in δ (ppm). Spectrophotometric measurements were performed with a UV-VIS spectrophotometer 2.2. (double-beam) Specord 200 Analytik Jena GmbH, Germany. Silica gel (70 - 230 and 230 - 400 mesh, Merck) was used for column chromatography. Silica TLC was conducted on Merck F254 silica gel plates. The spots were detected by spraying an anisaldehyde-H2SO4 reagent followed by heating (120°C for 5 minutes). HPLC was used on a Knauer model with Vertex (Knauer, Germany) column C18 (250 × 20 mm ID). The UV detector was a PDA, and the injection volume was 2 mL. Ferrous chloride was purchased from Merck (Darmstadt, Germany). Folin-Ciocalteu, gallic acid, and TPTZ (2, 4, 6-tripyridyl-s-triazine) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All solvents and chemicals used in the experiments were of analytical or HPLC grade.
3.4. FRAP Assay
The potential antioxidant capacities of the different extracts were determined with the FRAP assay by measuring their abilities to reduce Fe3+ to Fe2+ (13). Briefly, 100 and 500 µg/mL of each sample were added to 1.5 mL of the freshly prepared FRAP solution, which contained 2.5 mL of 10 mM TPTZ solution in 40 mM Hcl. Next, 2.5 mL of 20 mM Fecl3.6H2O was diluted in 25 mL of 0.3 M sodium acetate buffer (PH = 3.6) and allowed to remain at room temperature for 90 min, then the absorbance of the solution was recorded at 595 nm. The standard curve was prepared using different concentrations of FeSO4.7H2O, and the absorbances were read for the samples. The antioxidant power was calculated as grams of FeSO4.7H2O equivalent per grams of sample.
3.5. Total Phenol Assay
The total phenol concentrations of the extracts were calculated spectrophotometrically using the Folin-Ciocalteu procedure with little modification (14). Lyophilized extract (0.5 mL) was soluted in methanol, then added to 2.5 mL of Folin’s reagent (diluted to 1: 10), and the solution was left at room temperature for 5 minutes. Finally, 2 mL of Na2Co3 (7.5% v/v) was added to the mixture after 90 minutes of incubation. The sample absorbance was read at 760 nm, and the standard curve was prepared using various concentrations of gallic acid as the standard. Total phenol content was expressed as µg of gallic acid equivalent per mg of extract. The data presented are the averages of four measurements.
4. Results
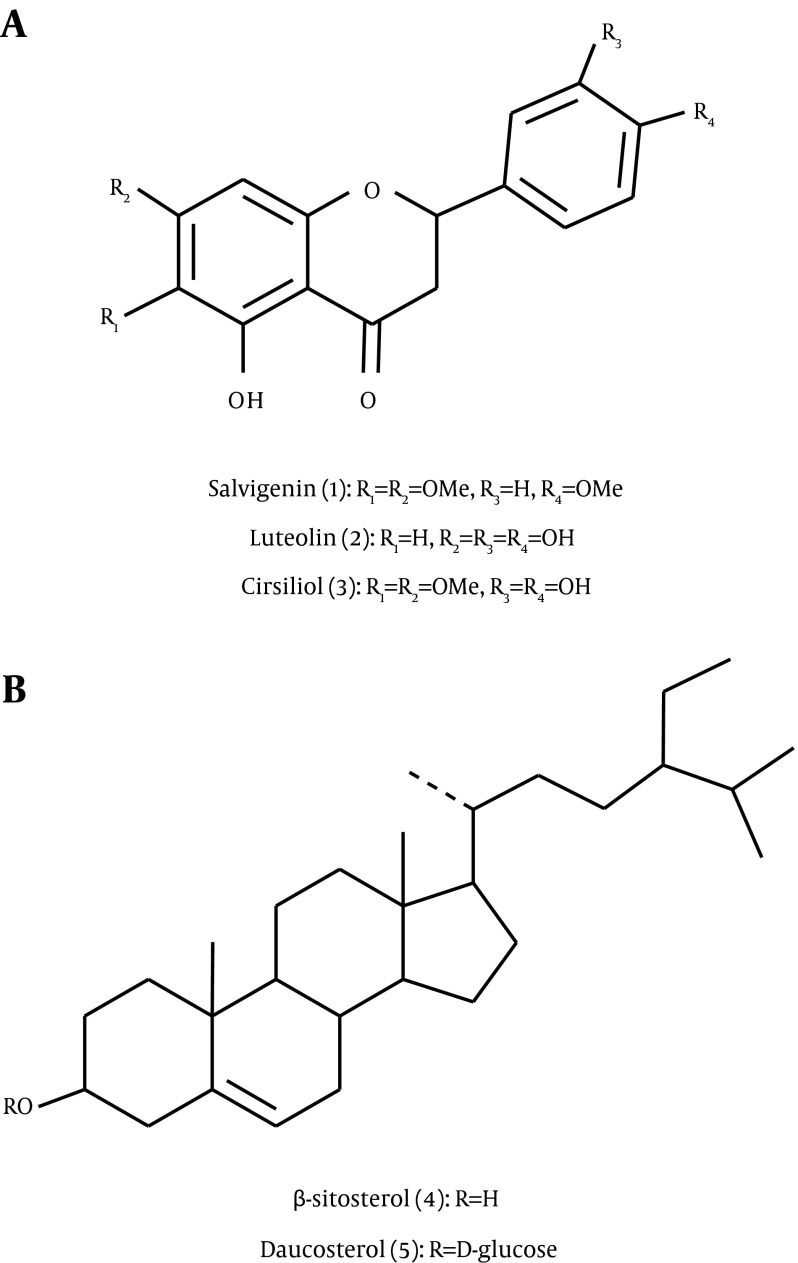
We reported the isolation and structural elucidation of salvigenin (5-hydroxy-4´,6,7-trimethoxy flavone), luteolin (3’,4’,5,7-tetrahydroxyflavone), cirsiliol (5,3,4-trihydroxy 6,7-dimethoxyflavone), β-sitosterol, and daucosterol (Figure 1) on the basis of spectroscopic data. These compounds were isolated and described here for S. chloroleuca for the first time. The structures of the isolated compounds were established mainly by comparisons of their 1H and 13C-NMR spectral data with those reported in the literature, and the results were consistent with our previously published data (15-17). The complete 1H and 13C-NMR analyses of isolated flavonoids (1 to 3) and sterols (4 and 5) are summarized in Tables 1 and 2, respectively.
Figure 1. Structure of Isolated Compounds From the Aerial Part of S. chloroleuca.
Table 1. 1H and 13C -NMR Data of Isolated Flavonoids From the Aerial Part of S. chloroleuca. δ in ppm, J in Hz.
| Position | Salvigenin | Luteolin | Cirsiliol | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 163.5 | 164.4 | 164.6 | |||
| 3 | 6.93 (s) | 103.3 | 6.67 (s) | 103.3 | 6.90 (s) | 103.1 |
| 4 | 182.2 | 182.1 | 182.5 | |||
| 5 | 152.6 | 161.9 | 152.5 | |||
| 6 | 131.8 | 6.19 (d, J = 2.05) | 99.2 | 131.5 | ||
| 7 | 158.6 | 164.4 | 159.0 | |||
| 8 | 6.95 (s) | 91.6 | 6.44 (d, J = 2.05) | 94.2 | 6.74 (s) | 91.8 |
| 9 | 152.6 | 157.7 | 153.0 | |||
| 10 | 105.1 | 104.1 | 105.4 | |||
| 1’ | 122.7 | 122.0 | 121.8 | |||
| 2’ | 8.07 (d, J = 9) | 128.3 | 7.39 (d, J = 2.2) | 113.7 | 7.45 (d, J = 2.5) | 113.9 |
| 3’ | 7.12 (d, J = 9.05) | 114.5 | 146.1 | 146.2 | ||
| 4’ | 162.4 | 149.9 | 150.3 | |||
| 5’ | 7.12 (d, J = 9.05) | 114.5 | 6.88 (d, J = 8,3) | 116.4 | 6.91 (d, J = 2.5) | 116.3 |
| 6’ | 8.07 (d, J = 9) | 128.3 | 7.42 (dd, J = 2.2, J = 8.3) | 119.3 | 7.46 (dd, J = 2.5, J = 8) | 119.4 |
| 5-OH | 12.88 | 12.9 | ||||
| 6-Me | 60.0 | 60.4 | ||||
| 7-Me | 56.4 | 56.8 | ||||
| 4’-Me | 55.6 | |||||
Table 2. 1H and 13C -NMR Data of Isolated Sterols From the Aerial Part of S. chloroleuca. δ in ppm, J in Hz.
| Position | β-sitosterol | Daucosterol | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 37.4 | 37.4 | ||
| 2 | 31.6 | 30.2 | ||
| 3 | 3.52 (m, 1H) | 71.8 | 3.48 (1H, m) | 78.5 |
| 4 | 42.3 | 39.2 | ||
| 5 | 140.9 | 140.9 | ||
| 6 | 5.34 (s, 1H) | 121.9 | 5.33 (m, 1H) | 121.9 |
| 7 | 31.8 | 31.8 | ||
| 8 | 31.8 | 31.8 | ||
| 9 | 50.2 | 50.2 | ||
| 10 | 36.1 | 36.8 | ||
| 11 | 21.1 | 21.1 | ||
| 12 | 39.8 | 39.8 | ||
| 13 | 42.2 | 42.2 | ||
| 14 | 56.7 | 56.8 | ||
| 15 | 24.3 | 24.3 | ||
| 16 | 28.3 | 28.3 | ||
| 17 | 56.1 | 56.1 | ||
| 18 | 0.68 (s, 3H) | 11.8 | 0.65 (s, 3H) | 11.8 |
| 19 | 1.01 (s, 3H) | 19.8 | 1.01 (s, 3H) | 19.3 |
| 20 | 0.92 (d, 3H, J = 6.5) | 36.3 | 36.3 | |
| 21 | 18.8 | 0.91 (d, 3H, J = 6.2) | 18.9 | |
| 22 | 34.1 | 34.1 | ||
| 23 | 26.1 | 26.2 | ||
| 24 | 45.8 | 45.9 | ||
| 25 | 29.1 | 29.2 | ||
| 26 | 0.83 (m, 3H) | 19 | 0.82 (d, 3H, J = 6.6) | 19.0 |
| 27 | 0.84 (m, 3H) | 19.4 | 0.80 (d, 3H, J = 6.6) | 19.8 |
| 28 | 0.84 (m, 2H) | 23.1 | 23.2 | |
| 29 | 0.84 (m, 3H) | 12.0 | 12.0 | |
| G-1 | 4.23 (d, 1H, J = 7.7) | 102.5 | ||
| G-2 | 3.03 (m, 1H) | 75.3 | ||
| G-3 | 3.08 (m, 1H) | 78.6 | ||
| G-4 | 3.05 (m, 1H) | 71.6 | ||
| G-5 | 3.05 (m, 1H) | 78.1 | ||
| G-6 | 3.65(m, 2H) | 62.5 | ||
The total phenolic contents of the aerial parts of S. chloroleuca were measured as 13.8 ± 0.3, 58.25 ± 0.05, and 43.48 ± 0.38 mg gallic acid/100 g for the n-hexane, ethyl acetate, and methanol extracts, respectively. Among all extracts tested, ethyl acetate (230.4 ± 10.5) had the highest FRAP value, followed by methanol (211.4 ± 8.3) and n-hexane (143.4 ± 12.04). FRAP values were reported as the weight (g) of FeSO4 in 100 g of plant extract.
5. Discussion
Column chromatography of the crude extract from the aerial part of S. chloroleuca led to the isolation and identification of three flavonoids (salvigenin, luteolin, and cirsiliol) and two steroids (β-sitosterol and daucosterol). These compounds were isolated for the first time from this plant. There was a significant correlation between total phenolic content and FRAP value, and this result is compatible with previous studies on Salvia (18). Ethyl acetate extract exhibited the highest total phenol and FRAP, which may be due to the existing flavonoids in this portion, while the n-hexane portion had the lowest total phenol and FRAP. The species of Salvia are characterized by 6-hydroxyflavones and 6-methoxyflavones. Luteolin and salvigenin are the major flavones distributed in the extract from the aerial part of Salvia (5). The potential antioxidant activities of the aerial part of Salvia species have previously been demonstrated with various methods (18). The antioxidant ability of polyphenolic compounds is derived from their ability to prevent lipid peroxidation and redox reactions (19). Flavonoids are classes of phenolic compounds that exhibit antioxidant activities (20). Therefore, it is reasonable to assume that the observed antioxidant activity of S. chloroleuca extract in our study could be in part related to its flavonoid content. These phenolic compounds can act as hydrogen donators, reducing agents, and free-radical quenchers (21). It has been demonstrated that flavonoids prevent oxidation of low-density lipoprotein (LDL), and they reduce thrombotic tendencies (22). The consumption of flavonoids in dietary plants may also decrease the risk of death from coronary heart disease in humans (23). Consequently, the antioxidant activities of phenolic compounds in Salvia extract are probably part of the reason they are valuable in the prevention and treatment of heart disease. Luteolin and salvigenin have been used in some countries as food additives to prevent lipid peroxidation (24-26). Salvigenin occurs throughout the Lamiaceae family, especially in the Salvia genus, although it has been identified in other Achillea species. Salvigenin possesses a wide range of biological activities, including antioxidant, antibacterial, antitumor, and immunomodulatory activities; inhibition of the growth of malarial parasites; and vasorelaxant effects in isolated rat aortas (27-29). Cirsiliol has previously been isolated from other Salvia species. It is the most potent inhibitor of arachidonate 5-lipoxygenase, an enzyme responsible for leukotriene biosynthesis (5, 30).
In conclusion, the results of this study indicated that S. chloroleuca could be an important dietary source of phenolic compounds with high antioxidant capacities. With regard to the presence of natural antioxidants with valuable biological activities, such as flavonoids and sterols, it is proposed to evaluate the effect of S. chloroleuca extract on important ailments, including cardiovascular disease and Alzheimer’s.
Acknowledgments
We thank Tehran University of Medical Sciences for financial support. We also thank everyone who helped us in this work.
Footnotes
Authors’ Contribution:Study concept and design, Iraj Salimikia, Hamid Reza Monsef-Esfahani, Ahmad Reza Gohari; analysis and interpretation of data, Ahmad Reza Gohari, Mehrnoosh Salek; drafting of the manuscript, Iraj Salimikia, Ahmad Reza Gohari; critical revision of the manuscript for important intellectual content, Ahmad Reza Gohari, Iraj Salimikia.
Funding/Support:This study was supported by Tehran University of Medical Sciences.
References
- 1.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Nicolson GL, Ash ME. Lipid replacement therapy: a natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2014;1838(6):1657–79. doi: 10.1016/j.bbamem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr. 1990;29(4):273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Foo LY. Polyphenolics of Salvia--a review. Phytochemistry. 2002;59(2):117–40. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 6.Sezik E, Yesilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol. 2001;75(2-3):95–115. doi: 10.1016/s0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 7.Kamatou GP, Makunga NP, Ramogola WP, Viljoen AM. South African Salvia species: a review of biological activities and phytochemistry. J Ethnopharmacol. 2008;119(3):664–72. doi: 10.1016/j.jep.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Karatas H. Antimicrobial activities of the essential oils of four Salvia species from Turkey. J Med Plants Res. 2010;4(12):1238–40. [Google Scholar]
- 9.Alizadeh A. Essential oil constituents, antioxidant and antimicrobial activities of Salvia virgata Jacq. from Iran. J Essential Oil Bearing Plants. 2013;16(2):172–82. [Google Scholar]
- 10.Keshavarz M, Bidmeshkipour A, Mostafaie A, Mansouri K, Mohammadi-Motlagh H. Anti tumor activity of Salvia officinalis is due to its anti-angiogenic, anti-migratory and anti-proliferative effects. Cell Journal. 2011;12(4):477–82. [Google Scholar]
- 11.Yousefzadi M, Sonboli A, Ebrahimi SN, Hashemi SH. Antimicrobial activity of essential oil and major constituents of Salvia chloroleuca. Z Naturforsch C. 2008;63(5-6):337–40. doi: 10.1515/znc-2008-5-605. [DOI] [PubMed] [Google Scholar]
- 12.Salimikia I, Yazdinezhad AR, Golfakhrabadi F, Esfahani HR. In vitro antioxidant and free radical scavenging activity of four Alkanna species growing in Iran. Pharmacognosy Res. 2015;7(1):100–4. doi: 10.4103/0974-8490.147218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239(1):70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem. 2010;58(14):8139–44. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeidnia S, Gohari AR, Ito M, Kiuchi F, Honda G. Bioactive constituents from Dracocephalum subcapitatum (O. Kuntze) Lipsky. Z Naturforsch C. 2005;60(1-2):22–4. doi: 10.1515/znc-2005-1-204. [DOI] [PubMed] [Google Scholar]
- 16.Gohari AR, Saeidnia S, Malmir M, Hadjiakhoondi A, Ajani Y. Flavones and rosmarinic acid from Salvia limbata. Nat Prod Res. 2010;24(20):1902–6. doi: 10.1080/14786411003766912. [DOI] [PubMed] [Google Scholar]
- 17.Saeidnia S, Gohari AR, Malmir M, Moradi-Afrapoli F, Ajani Y. Tryptophan and sterols from Salvia limbata. 2011;1(37):41–7. [Google Scholar]
- 18.Orhan I, Kartal M, Naz Q, Ejaz A, Yilmaz G, Kan Y, et al. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 2007;103(4):1247–54. [Google Scholar]
- 19.Le Marchand L. Cancer preventive effects of flavonoids--a review. Biomed Pharmacother. 2002;56(6):296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 20.Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50(21):6182–7. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 21.Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 22.Asadi S, Ahmadiani A, Esmaeili MA, Sonboli A, Ansari N, Khodagholi F. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: a comparative study. Food Chem Toxicol. 2010;48(5):1341–9. doi: 10.1016/j.fct.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010;140(3):600–4. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 24.Xia Z, Gu J, Ansley DM, Xia F, Yu J. Antioxidant therapy with Salvia miltiorrhiza decreases plasma endothelin-1 and thromboxane B2 after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2003;126(5):1404–10. doi: 10.1016/s0022-5223(03)00970-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhou R, He LF, Li YJ, Shen Y, Chao RB, Du JR. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol. 2012;139(2):440–6. doi: 10.1016/j.jep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Kamatou GP, Van Zyl RL, Davids H, Van Heerden FR, Lourens ACU, Viljoen AM. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. S Afr J Bot. 2008;74(2):238–43. [Google Scholar]
- 27.Si XT, Zhang ML, Shi QW, Kiyota H. Chemical constituents of the plants in the genus Achillea. Chem Biodivers. 2006;3(11):1163–80. doi: 10.1002/cbdv.200690119. [DOI] [PubMed] [Google Scholar]
- 28.Noori S, Hassan ZM, Yaghmaei B, Dolatkhah M. Antitumor and immunomodulatory effects of salvigenin on tumor bearing mice. Cell Immunol. 2013;286(1-2):16–21. doi: 10.1016/j.cellimm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Uydes-Dogan BS, Takir S, Ozdemir O, Kolak U, Topcu G, Ulubelen A. The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol. 2005;43(4):220–6. doi: 10.1016/j.vph.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Schneider I, Bucar F. Lipoxygenase inhibitors from natural plant sources. Part 2: medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother Res. 2005;19(4):263–72. doi: 10.1002/ptr.1604. [DOI] [PubMed] [Google Scholar]