Abstract
Introduction:
In a 24-hour porcine model of liver injury, we showed that fibrinogen supplementation does not downregulate endogenous fibrinogen synthesis. Here we report data from the same study showing the impact of fibrinogen on coagulation variables.
Materials and Methods:
Coagulopathy was induced in 20 German land race pigs by hemodilution and blunt liver injury. Animals randomly received fibrinogen concentrate (100 mg/kg) or saline. Coagulation parameters were assessed and thromboelastometry (ROTEM) was performed.
Results:
Fibrinogen concentrate significantly reduced the prolongations of EXTEM clotting time, EXTEM clot formation time, and prothrombin time induced by hemodilution and liver injury. A decrease in clot strength was also ameliorated. Endogenous thrombin potential was significantly higher in the fibrinogen group than in the control group, 20 minutes (353 ± 24 vs 289 ± 22 nmol/L·min; P < .05) and 100 minutes (315 ± 40 vs 263 ± 38 nmol/L·min; P < .05) after the start of infusion. However, no significant between-group differences were seen in other thrombin generation parameters or in d-dimer or thrombin–antithrombin levels. Fibrinogen–platelet binding was reduced following liver injury, with no significant differences between groups. No significant between-group differences were observed in any parameter at ∼12 and ∼24 hours.
Conclusion:
This study suggests that, in trauma, fibrinogen supplementation may shorten some measurements of the speed of coagulation initiation and produce a short-lived increase in endogenous thrombin potential, potentially through increased clotting substrate availability. Approximately 12 and 24 hours after starting fibrinogen concentrate/saline infusion, all parameters measured in this study were comparable in the 2 study groups.
Keywords: blood coagulation factors, bleeding, hemostasis
Introduction
Fibrinogen supplementation is currently being considered as a therapeutic option for hemostatic management of trauma-related bleeding.1,2 The 2013 update of the European Trauma Guidelines recommend fibrinogen concentrate among the initial procoagulant therapies for patients with massive hemorrhage.3 Fibrinogen concentrate is also recommended in the continuing management of the patient if significant bleeding is accompanied by signs of fibrin deficiency, either from point-of-care viscoelastic methods such as thromboelastometry or from a plasma fibrinogen level of less than 1.5 to 2.0 g/L.3
We investigated the impact of fibrinogen supplementation in a 24-hour porcine model of blunt liver injury. Pigs were used because they provide an established model of trauma,4 enabling improved understanding of the effects of fibrinogen supplementation without the ethical and logistical issues associated with human trials in this clinical setting. The primary findings of our study were that fibrinogen supplementation does not downregulate endogenous fibrinogen synthesis and that there was no significant difference in plasma fibrinogen concentration at 24 hours between animals receiving fibrinogen concentrate and saline.5 The primary publication also reported significantly lower total blood loss in the fibrinogen group, with no signs of thromboembolism. A number of coagulation-related parameters were assessed during the study but not reported in the primary publication. The objective of this secondary analysis was to investigate the absolute and relative effects of fibrinogen on these parameters including thrombin generation, coagulation kinetics, and platelet function. Our hypothesis was that fibrinogen concentrate may have an effect on thrombin generation parameters without affecting thrombin–antithrombin complex (TAT) generation or platelet function parameters.
Materials and Methods
The methods for this study have been reported elsewhere,5 and overlapping sections are reported here only briefly.
Study Approval
This study was conducted according to the Principles of Laboratory Animal Care, as defined by German legislation. Official permission for the study was granted by the Landesamt für Natur, Umwelt und Verbraucherschutz, Recklinghausen, Germany (study number 84-02.04.2011.A254).
Anesthesia
A total of 20 healthy male adult German land race pigs from a disease-free breeding facility (bodyweight 34-41 kg) were included in the study. The sample size was chosen based on previous experience. Before surgery, the animals were kept in ventilated rooms for a minimum period of 5 days to allow acclimatization to their surroundings. The pigs were fasted overnight before the surgical procedure and given unlimited access to water. Anesthesia was initiated by propofol (3 mg/kg intravenously) and maintained with isoflurane (endtidal concentration 1.2%-1.4%) and fentanyl (2 µg·kg−1·h−1). Initial fluid therapy comprised 4 mL·kg−1·h−1 Ringer’s lactate (RL) solution.
Surgical Preparation and Hemodilution
After line placement, a midline laparotomy with splenectomy and cystostomy was performed. A bolus of RL equal to 3 times the weight of the removed spleen was administered. For hemodilution (HD), 60% to 70% of the animal’s blood volume was withdrawn and substituted with RL. Collected blood was processed in an autologous cell saver system.
Liver Injury and Fibrinogen Administration
A standardized grade III blunt liver injury was inflicted using a custom-made instrument.6 Five minutes after injury, all animals received 35 mL/kg RL and at 10 minutes postinjury, the continuous infusion rate was set to 40 mL·kg−1·h−1. In addition, 12 mL/kg washed red blood cells (RBCs) were retransfused.
Animals were randomized to receive saline (control) or 100 mg/kg fibrinogen concentrate (Haemocomplettan, CSL Behring, Marburg, Germany), 20 minutes after injury with an infusion rate of 20 mL/min. Thus, in a 40-kg animal, the fibrinogen dose was 4 g and the duration of administration was 10 minutes. The fibrinogen dose was chosen to achieve a plasma fibrinogen level within the target range of 150 to 200 mg/dL specified in the European guidelines for “Management of bleeding and coagulopathy following major trauma”.3 Two hours following injury, the rate of RL infusion was decreased to 8 mL·kg−1·h−1.
Blood Sampling and Analysis
Blood was collected for analysis before injury (baseline: BL), at the end of HD, immediately before starting infusion of fibrinogen concentrate/saline (t = 0) and at 20, 40, 100, 220, 340, 700, and 1420 minutes after the start of infusion. Partial pressure of oxygen, pH, and carbon dioxide were measured with a blood gas analyzer (ABL725, Radiometer GmbH, Willich, Germany). A standard hematology analyzer (MEK-6108, Nihon Kohden, Tokyo, Japan) was used to measure platelet count. Prothrombin time (PT; reagent: Innovin), activated partial thromboplastin time (aPTT; reagent: Actin FS), and d-dimers (Innovance) were measured using the appropriate reagents and tests from Dade Behring (Marburg, Germany) on an MC 4 plus steel-ball coagulometer (Merlin Medical, Lemgo, Germany). Enzyme-linked immunosorbent assays (ELISAs), specific to porcine fibrinogen (Pig Fibrinogen; ICL Inc, Portland, Oregon) and to human fibrinogen (Human Fibrinogen; Abnova, Heidelberg, Germany), were performed. The levels of TAT were quantified by ELISA (Enzygnost, Dade Behring).
Thromboelastometry and Thrombin Generation Assays
Coagulation was assessed in whole blood and platelet-poor plasma (PPP) using a ROTEM device (Tem International GmbH, Munich, Germany). Clotting time (CT, seconds), clot formation time (CFT, seconds), maximum clot firmness (MCF, mm), α angle (in degrees), and maximum velocity (MaxV, mm/s) were measured in the EXTEM assay. Thrombin generation in plasma was measured using the Calibrated Automated Thrombogram (Thrombinoscope BV, Maastricht, The Netherlands).7 Assays were performed at 37°C and initiated with 1 pmol/L tissue factor in the presence of 4 μmol/L phospholipids. To correct for inner filter effects and substrate consumption, each thrombin generation analysis was calibrated against the fluorescence curve obtained in the same plasma with a fixed amount of calibrator (Thrombin Calibrator; Thrombinoscope BV). Fluorescence was read on a Fluoroscan Ascent fluorometer (Thermo Scientific, Waltham, Massachusetts) equipped with a 390/460 nm filter set, and thrombin generation curves were generated using Thrombinoscope Version 4 software (Thrombinoscope BV). All measurements were performed in duplicate, with each well calibrated to a parallel well with a thrombin calibrator.
Flow Cytometry for Platelet Assessments
Platelet activation tendency was determined as described elsewhere.8 Citrated whole blood was diluted (1:50) in Tyrode’s buffer (150 mmol/L NaCl, 2.5 mmol/L KCl, 12 mmol/L NaHCO3, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 1 mg/mL bovine serum albumin, and 1 mg/mL glucose, pH 7.4). Diluted whole blood (50 µL) was added to 10 μL vehicle or 2-methylthio-adenosine diphosphate (20 μmol/L; Santa Cruz Biotechnology, Santa Cruz, California), 10 μL Alexa Fluor 488-labeled anti-human P-selectin (clone KO.2.5; AbD Serotec, Oxford, United Kingdom), and 10 μL Alexa Fluor 647-conjugated human fibrinogen (1.5 mg/mL; Invitrogen Molecular Probes, Eugene, Oregon). After incubation for 20 minutes at 37°C, samples were fixed with 100 μL CellFix (BD Biosciences, San Jose, California) and stored at 4°C in the dark until analysis. Platelets were identified on the basis of their specific forward scatter and sideward scatter characteristics. Binding of the fluorescent labels to the platelets was assessed using a 3-channel Accuri C6 flow cytometer (BD Biosciences, Ann Arbor, Michigan). Platelet reactivity following adenosine diphosphate (ADP) stimulation was evaluated by calculating the ratio of the mean fluorescence intensity (MFI) of stimulated platelets to the MFI of unstimulated platelets.
Statistical Analysis
GraphPad Prism version 6.0b was used for statistical and graphing purposes (GraphPad Software, La Jolla, California). Differences between groups were analyzed by a 2-way analysis of variance (ANOVA) model, followed by Sidak multiple comparisons test. To investigate the effects of HD, trauma, and fibrinogen supplementation, a repeated-measures model using ANOVA with Tukey post hoc adjustment was conducted with group and time as factors. Normally distributed data are expressed as mean with standard deviation. Statistical tests were performed 2-tailed, and P values < .05 were considered as statistically significant. In all 20 animals, there were no missing data for any of the study parameters.
Results
Baseline Measurements and Impact of Liver Trauma
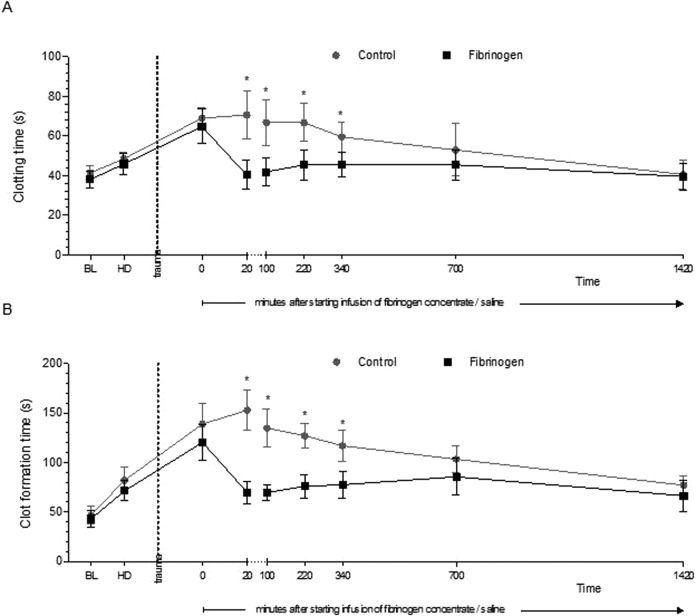
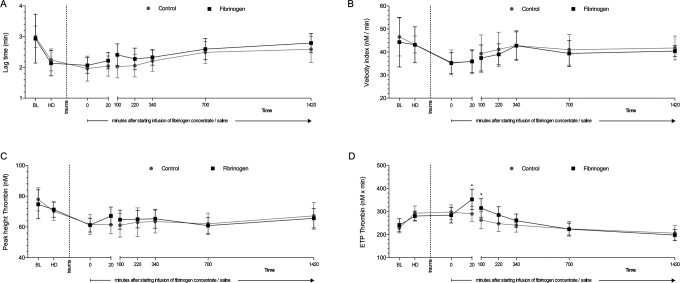
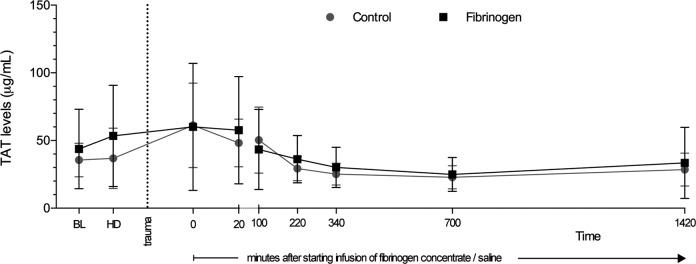
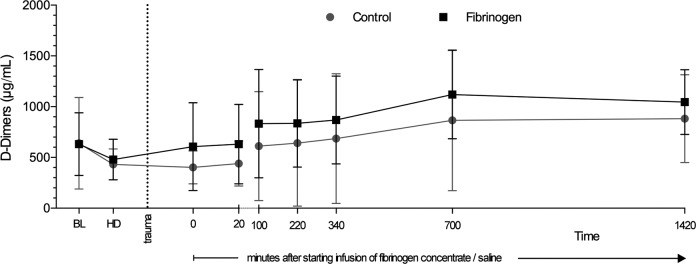
All baseline parameters were comparable in the 2 study groups. Hemodilution involved infusion of similar bolus volumes of RL in each study group (fibrinogen group: 1071 ± 143 mL; control group: 1122 ± 268 mL). The whole-blood thromboelastometry parameters CT and CFT were prolonged following HD and trauma, in comparison with baseline (Figure 1). Whole-blood MaxV was decreased in response to HD and trauma, and there was a small decrease in whole-blood α angle (Table 1). Most of these changes were amplified with PPP, the exception being CT where the change from baseline was similar to both whole blood and PPP (Table 1). In line with the ROTEM data, laboratory coagulation analyses (PT and aPTT) showed prolonged coagulation times following HD and trauma (Table 1). Endogenous thrombin generation (ETP) was increased following HD, although subsequent liver injury had little additional effect (Figure 2). Mean values for ETP (both study groups combined) were 235 ± 25 nmol/L·min at baseline and 291 ± 33 nmol/L·min 20 minutes after injury. Combined HD and liver injury shortened lag time from 3.0 ± 0.6 minutes to 2.0 ± 0.3 minutes. However, all other thrombin generation parameters, including the peak height and velocity index, decreased in response to HD and trauma. Minor changes were observed in TAT levels (small increase) and d-dimer levels (small decrease; Figures 3 and 4). Plasma fibrinogen concentrations, platelet counts, and MCF, reported in the primary publication,5 are included in Table 1 for reference.
Figure 1.
Thromboelastometry parameters prior to and following trauma. A ROTEM EXTEM assay was performed to assess clotting time (A) and clot formation time (B). Data are shown as mean with error bars representing standard deviation (SD); *P < .05 vs control.
Table 1.
Coagulation Parameters During the Study.a
| Pre-injury Baseline | Hemodilution | After Liver Injury and Immediately Before Infusion of Fibrinogen Concentrate/Saline | Time After Starting Infusion of Fibrinogen Concentrate/Saline | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 Minutes | 100 Minutes | 220 Minutes | 340 Minutes | 700 Minutes | 1420 Minutes | ||||
| Platelet count, 103/µL | |||||||||
| Control | 235 ± 29 | 140 ± 23 | 95 ± 14 | 90 ± 14 | 85 ± 11 | 84 ± 14 | 82 ± 17 | 75 ± 16 | 60 ± 10 |
| Fibrinogen | 259 ± 33 | 160 ± 26 | 107 ± 20 | 120 ± 22b | 109 ± 11 | 107 ± 12 | 102 ± 11 | 95 ± 14 | 78 ± 12 |
| PT, seconds | |||||||||
| Control | 9.2 ± 0.7 | 13.0 ± 1.0 | 18.4 ± 1.8 | 20.1 ± 2.0 | 19.7 ± 3.3 | 18.2 ± 4.2 | 17.1 ± 5.0 | 13.3 ± 1.6 | 9.7 ± 2.1 |
| Fibrinogen | 9.3 ± 0.3 | 12.8 ± 1.1 | 17.4 ± 1.2 | 12.1 ± 2.0b | 13.3 ± 3.3b | 13.8 ± 2.8b | 13.2 ± 1.6b | 12.2 ± 1.6 | 9.7 ± 1.0 |
| aPTT, seconds | |||||||||
| Control | 11.0 ± 0.7 | 13.9 ± 1.2 | 18.8 ± 2.2 | 19.0 ± 1.2 | 17.4 ± 1.2 | 17.3 ± 1.4 | 17.1 ± 1.5 | 18.0 ± 2.1 | 18.5 ±3.1 |
| Fibrinogen | 11.0 ± 1.2 | 12.8 ± 1.0 | 18.5 ± 1.5 | 17.0 ± 2.3 | 16.0 ± 1.7 | 15.9 ± 1.7 | 15.7 ± 1.2 | 16.9 ± 1.1 | 17.4 ± 2.1 |
| Fibrinogen concentration: porcine ELISA, mg/dL | |||||||||
| Control | 170 ± 47 | 61 ± 12 | 36 ± 6 | 35 ± 5 | 39 ± 7 | 39 ± 7 | 44 ± 7 | 54 ± 12 | 95 ± 40 |
| Fibrinogen | 159 ± 39 | 49 ± 12 | 40 ± 10 | 53 ± 14 | 54 ± 7 | 54 ± 7 | 51 ± 9 | 57 ± 9 | 90 ± 35 |
| Fibrinogen concentration: human ELISA, mg/dL | |||||||||
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Fibrinogen | 0 ± 0 | 0 ± 0 | 0 ± 0 | 154 ± 10 | 131 ± 10 | 116 ± 15 | 105 ± 16 | 88 ± 16 | 77 ± 18 |
| CT, seconds | |||||||||
| Control | 41 ± 4 | 49 ± 4 | 69 ± 5 | 71 ± 11 | 67 ± 9 | 67 ± 10 | 60 ± 7 | 53 ± 13 | 41 ± 7 |
| Fibrinogen | 38 ± 4 | 46 ± 5 | 65 ± 8 | 40 ± 7b | 42 ± 6b | 45 ± 7b | 46 ± 6b | 45 ± 7 | 40 ± 6 |
| CFT, seconds | |||||||||
| Control | 47 ± 8 | 82 ± 13 | 139 ± 21 | 152 ± 20 | 135 ± 19 | 127 ± 13 | 117 ± 16 | 103 ± 14 | 77 ± 9 |
| Fibrinogen | 43 ± 9 | 71 ± 10 | 128 ± 17 | 70 ± 11b | 70 ± 8b | 76 ± 11b | 78 ± 13b | 86 ± 18 | 67 ± 15 |
| MCF, mm | |||||||||
| Control | 70 ± 4 | 59 ± 5 | 46 ± 2 | 45 ± 4 | 49 ± 4 | 51 ± 3 | 53 ± 3 | 59 ± 2 | 68 ± 2 |
| Fibrinogen | 73 ± 4 | 61 ± 5 | 49 ± 4 | 63 ± 3b | 64 ± 2b | 65 ± 3b | 65 ± 3b | 67 ± 3b | 71 ± 4 |
| α angle, ° | |||||||||
| Control | 81 ± 2 | 72 ± 4 | 63 ± 3 | 62 ± 3 | 65 ± 3 | 66 ± 2 | 67 ± 3 | 67 ± 3 | 78 ± 4 |
| Fibrinogen | 77 ± 4 | 76 ± 2 | 67 ± 4 | 80 ± 1b | 78 ± 2b | 76 ± 4b | 76 ± 3b | 76 ± 4 | 80 ± 5 |
| MaxV, mm/s | |||||||||
| Control | 28 ± 4 | 15 ± 3 | 10 ± 1 | 9 ± 2 | 10 ± 1 | 11 ± 1 | 12 ± 1 | 13 ± 2 | 23 ± 5 |
| Fibrinogen | 30 ± 6 | 19 ± 4 | 15 ± 12 | 24 ± 4b | 20 ± 3b | 19 ± 3b | 19 ± 3b | 19 ± 4b | 28 ± 9 |
| CT PPP, seconds | |||||||||
| Control | 50 ± 7 | 56 ± 11 | 82 ± 27 | 81 ± 18 | 80 ± 15 | 64 ± 11 | 62 ± 11 | 49 ± 6 | 43 ± 9 |
| Fibrinogen | 43 ± 6 | 52 ± 8 | 77 ± 29 | 44 ± 8b | 50 ± 7b | 48 ± 6b | 51 ± 7b | 50 ± 8 | 43 ± 7 |
| CFT PPP, seconds | |||||||||
| Control | 219 ± 127 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | ≥2000 ± 0.0 | 43 ± 9 |
| Fibrinogen | 252 ± 191 | ≥2000 ± 0.0c | ≥2000 ± 0.0c | 256 ± 135c | 391 ± 245c | 390 ± 179c | 346 ± 182c | 309 ± 190c | 101 ± 93 |
| MCF PPP, mm | |||||||||
| Control | 28 ± 4 | 10 ± 3 | 8 ± 4 | 6 ± 2 | 6 ± 2 | 7 ± 2 | 8 ± 2 | 14 ± 3 | 26 ± 3 |
| Fibrinogen | 30 ± 6 | 12 ± 2 | 7 ± 2 | 26 ± 4b | 24 ± 3b | 23 ± 3b | 24 ± 3b | 26 ± 4b | 34 ± 4 |
| α angle PPP, ° | |||||||||
| Control | 79 ± 6 | 34 ± 27 | 16 ± 12 | 11 ± 3 | 12 ± 7 | 16 ± 10 | 19 ± 14 | 56 ± 14 | 80 ± 3 |
| Fibrinogen | 81 ± 2 | 48 ± 22 | 12 ± 4 | 82 ± 1b | 81 ± 1b | 80 ± 2b | 79 ± 2b | 80 ± 2b | 82 ± 4 |
| MaxV PPP, mm/s | |||||||||
| Control | 25 ± 7 | 8 ± 5 | 3 ± 2 | 2 ± 1 | 3 ± 2 | 4 ± 3 | 4 ± 2 | 10 ± 3 | 27 ± 5 |
| Fibrinogen | 27 ± 5 | 9 ± 3 | 3 ± 2 | 31 ± 4b | 27 ± 5b | 24 ± 3b | 23 ± 4 | 24 ± 5b | 33 ± 8 |
Abbreviation: SD, standard deviation; CT, clotting time; CFT, clot formation time; MCF, maximum clot firmness; PT, prothrombin time; aPTT, activated partial thromboplastin time; ELISA, enzyme-linked immunosorbent assay; PPP, platelet-poor plasma.
aData are presented as mean (SD). ROTEM data (CT, CFT, MCF, α-angle, and MaxV) are from the EXTEM assay.
b P < .05 vs control.
c P value for between-group difference not calculated because 2000 was the ceiling level, meaning that limited information was available for data relating to fibrinogen levels above this threshold.
Figure 2.
Thrombin generation in plasma prior to and following trauma. Assays were initiated with 1 pmol/L tissue factor in the presence of 4 μmol/L phospholipids. Parameters of thrombin generation curves are displayed: lag time (A), velocity index (B), peak height (C), and area under the curve (endogenous thrombin potential [ETP]; D). Data are shown as mean with error bars representing standard deviation (SD); *P < .05 vs control.
Figure 3.
Levels of thrombin–antithrombin complex (TAT) in plasma prior to and following trauma. The TAT levels were determined via an enzyme-linked immunosorbent assay. Data are shown as mean with error bars representing standard deviation (SD).
Figure 4.
Levels of d-dimer in plasma prior to and following trauma. The d-dimer levels were determined via an immunoturbidimetric assay. Data are shown as mean with error bars representing standard deviation (SD).
Thromboelastometry and Plasma Coagulation Tests
The EXTEM parameters CT and CFT were initially shortened in response to therapy with fibrinogen, with significantly lower values in the fibrinogen concentrate group versus the control group from 20 to 340 minutes after starting infusion (Table 1 and Figure 1). In the fibrinogen concentrate group, mean values for whole-blood CT and CFT increased gradually over time from 20 minutes until ∼12 hours after the start of infusion. In the control group, these parameters gradually shortened over the whole follow-up period after infusion, with little change immediately after infusion. There were no significant differences between the study groups in whole-blood CT or CFT from ∼12 hours after the start of infusion. The time course of the effects of fibrinogen concentrate on whole-blood EXTEM CT and CFT is similar to that observed with MCF (Table 1). Fibrinogen had a significant effect on all 3 of these parameters within 20 minutes of starting infusion (CT and CFT shortened; MCF increased). Subsequently, there was a gradual reduction over time in the differences between the 2 study groups, with a lack of significant between-group differences at 1420 minutes. The EXTEM CT and CFT results with PPP were generally similar to those with whole blood (Table 1), though ceiling values with CFT made changes over time difficult to detect—particularly in the control group. α angle, for both whole blood and PPP, was increased by fibrinogen to values that were similar to baseline. Injection of saline (control group) had no clear effect on α angle. Similar outcomes were observed with MaxV (Table 1), though the fibrinogen-associated increase in whole-blood MaxV was not enough for the baseline level to be restored. At 24 hours postinjury, no significant differences in any of the plasma coagulation tests or thromboelastometry variables were detected between the treatment and the control groups. Infusion of fibrinogen reduced the prolongation of PT induced by HD and liver injury (Table 1). Conversely, fibrinogen concentrate had no significant effect on aPTT.
Thrombin Generation and Activation of Coagulation
Following administration of fibrinogen concentrate, the total amount of thrombin formed (ETP) was increased, with significantly higher values in the fibrinogen group versus the control group at 20 and 100 minutes after the start of infusion (Figure 2). However, there were no significant between-group differences in ETP after 100 minutes. There were also no significant differences between the 2 groups in rate of thrombin generation (lag time and velocity index) or peak height. The degree of in vivo coagulation activity was evaluated by measuring TAT levels. Results were similar in the 2 treatment groups at all timepoints (Figure 3).
Slightly higher levels of d-dimers were observed at all time points after fibrinogen supplementation. However, the difference did not reach statistical significance at any timepoint (Figure 4). The size of the numerical difference in d-dimers was consistent from the start of infusion of fibrinogen concentrate/saline until 700 minutes thereafter, with a reduction between 700 and 1420 minutes.
Platelet Reactivity
Platelet reactivity was examined by analyzing expression of P-selectin and binding of fibrinogen following stimulation with ADP (Figure 5). The expression of P-selectin did not change significantly over time (Figure 5A). After liver injury, the binding of fibrinogen to platelets following activation was significantly reduced in both groups (Figure 5B). No differences in platelet activation were detected between groups after fibrinogen infusion. However, significantly higher platelet count was evident in the fibrinogen group 20 minutes after starting infusion (Table 1).
Figure 5.
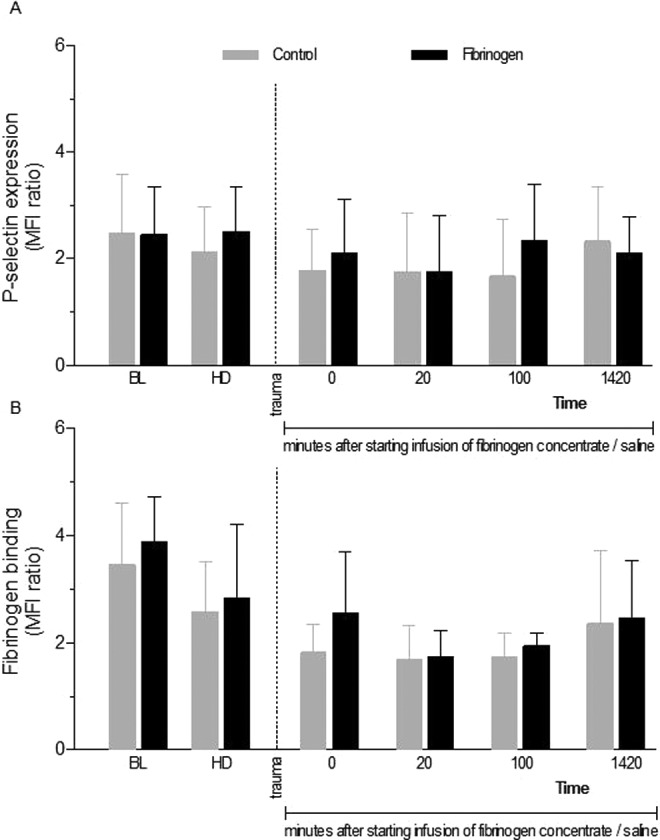
Platelet activity prior to and following trauma. Platelet activity was determined by dilution of whole blood and activation with vehicle or adenosine diphosphate (ADP). P-selectin expression and integrin αIIbβ3 activation were determined by the binding of fluorescein isothiocynanate (FITC)-labeled antibody against P-selectin (A) and AF647-labeled fibrinogen (B). Platelet activation tendency was determined by the ratio of the mean fluorescence intensity (MFI) of ADP- and vehicle-stimulated platelets. Data are presented as mean with error bars representing standard deviation (SD).
Discussion
The beneficial effects of fibrinogen concentrate on coagulation are attributed mainly to its role in forming fibrin as the basis of the clot and increased clot strength through binding of GPIIb/IIIa receptors on platelets.9 The present data indicate that fibrinogen supplementation also affects some measurements relating to the speed of coagulation, and these findings are consistent with previous studies of fibrinogen concentrate in porcine models.10,11
The thromboelastometry variables CT and CFT were shortened by fibrinogen, in both whole blood and PPP, although significant differences versus control were apparent only until 340 minutes after starting infusion of fibrinogen concentrate or saline. Similarly, the prolongation of PT resulting from HD and liver injury was reduced by fibrinogen concentrate, with a significant difference versus control lasting until the 340-minute time point. Prolongation of CT and/or PT, together with clinical signs of bleeding, is sometimes used to indicate a patient’s need for increased thrombin generation (ie, administration of prothrombin complex concentrate [PCC], recombinant activated factor VII, or perhaps therapeutic plasma).12 Data showing that fibrinogen doesn’t influence clot initiation (CT or CFT)13,14 could be interpreted as supporting that approach. Conversely, the present results, in accordance with previous studies,15,16 show that fibrinogen supplementation after HD can shorten CT and CFT. This raises the possibility that prolonged CT and/or PT could be caused by hypofibrinogenemia in a patient with adequate thrombin generation. To avoid risk of unnecessary/inappropriate administration of a thrombin generating agent such as PCC, CT, and/or PT should not be used to determine the need for such products if there is a fibrinogen deficiency.
The latest time at which statistically significant differences were observed between the 2 study groups in any of the study parameters was 340 minutes (∼6 hours) after the start of infusion of fibrinogen concentrate or saline. At this time, CT and CFT were significantly shorter in the fibrinogen concentrate group. The lack of significant between-group differences ∼12 and ∼24 hours after the start of infusion is consistent with previous data showing the effects of fibrinogen concentrate to be relatively short-lived (eg, in previous studies, fibrinogen concentration at 24 hours was not different between patients treated with fibrinogen concentrate or allogeneic blood products).13,17–19 These findings may be explained by the natural rise in endogenous fibrinogen that occurs posttrauma (acute phase reaction); fibrinogen concentrate therapy raises fibrinogen levels by less than is observed during the natural acute phase without fibrinogen therapy. It should also be considered that, in both study groups, fibrinogen consumption is raised by the occurrence of bleeding and trauma.
Most plasma thrombin generation parameters including TAT indicated that fibrinogen concentrate had no significant impact on thrombin generation. However, ETP was significantly increased by fibrinogen infusion. This difference could be explained as follows. Thrombin generation is measured in a cup, using a small 80 µL sample of plasma. Fibrin formation within the sample supports thrombin activity as activated thrombin is protected from the action of antithrombin (AT) by binding to fibrin.20 In contrast, TAT reflects the amount of free thrombin that has already formed in the circulatory system and has been inactivated by AT before the plasma sample was collected for measurement. Increased ETP may reflect an increase in thrombin activity at the wound area resulting from higher fibrinogen plasma concentration, while circulating thrombin is inactivated by AT with little dependence on fibrinogen concentration.21 Further investigation of the relationship between fibrinogen and ETP under different conditions, and the clinical importance of this relationship, is warranted.
Data from this study5 and others13,17–19 have shown that the effect of fibrinogen concentrate on plasma fibrinogen level is short-lived (ie, no persistence of effect at 24 hours). The primary goal of our study was to explain the apparent discrepancy between these observations and the half-life of fibrinogen (2.7-3.6 days in humans)22 which suggests that a more prolonged elevation in plasma levels should occur. As previously reported, there was no decrease in endogenous fibrinogen synthesis, so the explanation could relate to increased fibrinogen consumption or metabolism. Our results suggest a possible increase in the formation of d-dimers following fibrinogen infusion as compared to control animals, although statistically significant between-group differences were not observed. A previous porcine study of fibrinogen supplementation in trauma reported no significant effect on d-dimers.11 On the other hand, a study in coronary artery bypass graft patients showed a statistically significant rise in d-dimer levels among fibrinogen recipients versus controls; the difference was evident at 2 hours but not 24 hours postsurgery.23 Increased d-dimer levels would suggest either increased fibrin formation or increased fibrinolysis; a degree of fibrinolytic activation has been shown to occur in the majority of trauma patients.24 Further experimental investigations using labeled fibrinogen may determine more clearly how fibrinogen is cleared and increase our understanding of how fibrinogen contributes to the hemostatic process in stopping bleeding.
Conventional plasma-based coagulation tests including aPTT and PT are classically used to monitor hemostasis in the perioperative setting. However, these tests provide an incomplete picture of the coagulation process (eg, they are performed in plasma, effectively excluding platelets and RBCs from the assessment; the tests are concluded at an early stage during the clotting process, when only the first fibrin strands have been formed).25,26 Viscoelastic coagulation testing by ROTEM or TEG, performed using whole blood, may provide a better reflection of the in vivo situation.27–29 These methods also provide a shorter turnaround time than conventional laboratory tests.26 Consequently, we would consider viscoelastic coagulation testing as preferable to standard laboratory tests. Several assays are available for viscoelastic assessment. In particular, the FIBTEM assay (ROTEM) or the functional fibrinogen assay (TEG) enable assessment of the fibrin-based clot, and the results may be used to guide fibrinogen supplementation. The FIBTEM assay is not reliable with porcine blood.30 By providing an alternative means of measuring fibrin-based clot strength without a contribution from platelets, performance of the EXTEM assay with PPP can be considered as a substitute for FIBTEM.
We observed that fibrinogen restored EXTEM parameters approximately to baseline levels in both whole blood and PPP. This could be interpreted as a lack of functional deficiency in components of the coagulation system other than fibrinogen—with the caveat that an increase in fibrinogen level above baseline could potentially compensate for deficiencies in other components. This is consistent with previous data showing that fibrinogen is the first coagulation factor to reach critically low levels in major bleeding.31 In addition, our EXTEM data are in agreement with a recent porcine HD study where fibrinogen shortened CT and CFT.16 The effect of fibrinogen on thrombin generation in our study was different from that on EXTEM parameters. Fibrinogen showed no significant effect versus control group on lag time, velocity index, or peak height. Endogenous thrombin generation, which was increased from baseline by HD, increased further in response to fibrinogen but was essentially unaffected by saline (control group). Notably, our finding that ETP was increased versus baseline after HD and trauma reflects the fact that thrombin generation is raised in patients with acute coagulopathy of trauma,32 supporting use of the porcine model. An important finding in our study was that the effects of fibrinogen were short-lived, so that by 24 hours there were no effects on any of the parameters included in our study including ETP. This agrees with the time course of hemostatic effects of fibrinogen concentrate in a randomized controlled trial of patients with cardiovascular disease: lack of difference at 24 hours in ROTEM parameters or standard laboratory tests between patients receiving fibrinogen concentrate or allogeneic blood products.19 The data also concur with a recent trauma study where patients who received fibrinogen concentrate alone for coagulation management had similar values for thrombin generation parameters on days 1 to 7 posttrauma as patients who received no coagulation therapy.33 It is unfortunate that there is no established method for direct measurement of thrombin generation in clinical practice.
In our study, liver injury reduced binding of fibrinogen to platelets. Previous studies have found an association between trauma-induced coagulopathy and impaired platelet function and suggested the occurrence of “exhausted platelet syndrome” as a consequence of prolonged activation.34,35 The lack of effect of fibrinogen supplementation on platelet reactivity was as expected. There was also no change over time in P-selectin activity following ADP stimulation of platelets; this was probably due to the fact that ADP is a weak platelet agonist in terms of granule secretion.
Key limitations of our study were acknowledged in the previous publication.5 Briefly, these included administration of human fibrinogen concentrate to pigs and the need to replicate the study in humans to ensure clinical applicability; induction of HD before the liver injury; and the infliction of injury after administration of anesthetics. In addition, the current study provides no insight regarding optimal dosing of fibrinogen concentrate; a similar study with dose response analysis would be valuable. Derivative parameters from ROTEM assessment are also worthy of future investigation, in the search for a surrogate marker of thrombin generation. MaxV is the only derivative parameter available from this study; time to MaxV and area under the curve should also be studied.
In conclusion, this study indicates that supplementation of fibrinogen may shorten some measurements of the speed of coagulation initiation and produce a short-lived increase in endogenous thrombin potential. However, as reported in the primary publication, no evidence of an increase in thromboembolism was identified.5 No significant differences were observed between the study groups in coagulation variables ∼12 and ∼24 hours after starting infusion of fibrinogen concentrate/saline; this is consistent with previous observations that the effects of fibrinogen supplementation are short-lived.19 Our data suggest that, before using speed of coagulation initiation (eg, EXTEM CT and PT) to guide supplementation of coagulation factors to support thrombin generation in trauma-related bleeding, hypofibrinogenemia or decreased fibrin-based clot firmness should be excluded as a potential cause of prolonged coagulation initiation. However, data from porcine models require confirmation in a clinical setting to draw firm conclusions regarding patients.
Acknowledgments
Meridian HealthComms provided editorial assistance with the manuscript development and this was funded by CSL Behring.
Authors’ Note: The sponsor of this study had no influence on the interpretation of the data.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. C.S. is an employee of CSL Behring and previously received speaker honoraria and research support from Tem International and travel support from Haemoscope Ltd (former manufacturer of TEG). R.R. has received honoraria for lectures and consultancy from CSL Behring (Germany) and Novo Nordisk (Germany). O.G. has received research funding from CSL Behring (Germany), Novo Nordisk (Denmark), Biotest (Germany), Nycomed (Denmark) and consultancy fees from Boehringer Ingelheim (Germany) and Bayer Healthcare (Germany). H.S. has received research funding from Boehringer Ingelheim (Germany) and honoraria for consultancy from Bayer (Germany). The remaining authors declare that they have no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was supported by funding from CSL Behring, Germany to O.G.
References
- 1. Schöchl H, Schlimp CJ, Voelckel W. Potential value of pharmacological protocols in trauma. Curr Opin Anaesthesiol. 2013;26(2):221–229. [DOI] [PubMed] [Google Scholar]
- 2. Sorensen B, Fries D. Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg. 2012;99(suppl 1 ):40–50. [DOI] [PubMed] [Google Scholar]
- 3. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grottke O, Braunschweig T, Spronk HM, et al. Increasing concentrations of prothrombin complex concentrate induce disseminated intravascular coagulation in a pig model of coagulopathy with blunt liver injury. Blood. 2011;118(7):1943–1951. [DOI] [PubMed] [Google Scholar]
- 5. Zentai C, Braunschweig T, Schnabel J, Rose M, Rossaint R, Grottke O. Fibrinogen concentrate does not suppress endogenous fibrinogen synthesis in a 24-hour porcine trauma model. Anesthesiology. 2014;121(4):753–764. [DOI] [PubMed] [Google Scholar]
- 6. Grottke O, Braunschweig T, Philippen B, et al. A new model for blunt liver injuries in the swine. Eur Surg Res. 2010;44(2):65–73. [DOI] [PubMed] [Google Scholar]
- 7. Spronk HM, Dielis AW, De Smedt E, et al. Assessment of thrombin generation II: validation of the calibrated automated thrombogram in platelet-poor plasma in a clinical laboratory. Thromb Haemost. 2008;100(2):362–364. [PubMed] [Google Scholar]
- 8. Krajewski S, Kurz J, Wendel HP, Straub A. Flow cytometry analysis of porcine platelets: optimized methods for best results. Platelets. 2012;23(5):386–394. [DOI] [PubMed] [Google Scholar]
- 9. Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88(3):907–914. [PubMed] [Google Scholar]
- 10. Fries D, Innerhofer P, Reif C, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102(2):347–351. [DOI] [PubMed] [Google Scholar]
- 11. Grottke O, Braunschweig T, Henzler D, Coburn M, Tolba R, Rossaint R. Effects of different fibrinogen concentrations on blood loss and coagulation parameters in a pig model of coagulopathy with blunt liver injury. Crit Care. 2010;14(2):R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levi M, Fries D, Gombotz H, et al. Prevention and treatment of coagulopathy in patients receiving massive transfusions. Vox Sang. 2011;101(2):154–174. [DOI] [PubMed] [Google Scholar]
- 13. Fenger-Eriksen C, Jensen TM, Kristensen BS, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7(5):795–802. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka KA, Taketomi T, Szlam F, Calatzis A, Levy JH. Improved clot formation by combined administration of activated factor VII (NovoSeven) and fibrinogen (Haemocomplettan P). Anesth Analg. 2008;106(3):732–738. [DOI] [PubMed] [Google Scholar]
- 15. Bolliger D, Szlam F, Molinaro RJ, Rahe-Meyer N, Levy JH, Tanaka KA. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: an in vitro model. Br J Anaesth. 2009;102(6):793–799. [DOI] [PubMed] [Google Scholar]
- 16. Schlimp CJ, Solomon C, Keibl C, et al. Recovery of fibrinogen concentrate after intraosseous application is equivalent to the intravenous route in a porcine model of hemodilution. J Trauma Acute Care Surg. 2014;76(5):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karlsson M, Ternstrom L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102(1):137–144. [DOI] [PubMed] [Google Scholar]
- 18. Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102(6):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon C, Hagl C, Rahe-Meyer N. Time course of haemostatic effects of fibrinogen concentrate administration in aortic surgery. Br J Anaesth. 2013;110(6):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86(2):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martini J, Maisch S, Pilshofer L, Streif W, Martini W, Fries D. Fibrinogen concentrate in dilutional coagulopathy: a dose study in pigs. Transfusion. 2014;54(1):149–157. [DOI] [PubMed] [Google Scholar]
- 22. Kreuz W, Meili E, Peter-Salonen K, et al. Pharmacokinetic properties of a pasteurised fibrinogen concentrate. Transfus Apher Sci. 2005;32(3):239–246. [DOI] [PubMed] [Google Scholar]
- 23. Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Skrtic S, Jeppsson A. Prophylactic fibrinogen infusion in cardiac surgery patients: effects on biomarkers of coagulation, fibrinolysis, and platelet function. Clin Appl Thromb Hemost. 2011;17(4):396–404. [DOI] [PubMed] [Google Scholar]
- 24. Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–314. [DOI] [PubMed] [Google Scholar]
- 25. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270–382. [DOI] [PubMed] [Google Scholar]
- 26. Schöchl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davenport R, Khan S. Management of major trauma haemorrhage: treatment priorities and controversies. Br J Haematol. 2011;155(5):537–548. [DOI] [PubMed] [Google Scholar]
- 28. Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65(3):535–543. [DOI] [PubMed] [Google Scholar]
- 29. Tauber H, Innerhofer P, Breitkopf R, et al. Prevalence and impact of abnormal ROTEM(R) assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth. 2011;107(3):378–387. [DOI] [PubMed] [Google Scholar]
- 30. Velik-Salchner C, Schnurer C, Fries D, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb Res. 2006;117(5):597–602. [DOI] [PubMed] [Google Scholar]
- 31. Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81(2):360–365. [DOI] [PubMed] [Google Scholar]
- 32. Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49(12):2652–2660. [DOI] [PubMed] [Google Scholar]
- 33. Schöchl H, Voelckel W, Maegele M, Kirchmair L, Schlimp CJ. Endogenous thrombin potential following hemostatic therapy with 4-factor prothrombin complex concentrate: a 7-day observational study of trauma patients. Crit Care. 2014;18(4): R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon C, Traintinger S, Ziegler B, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2011;106(2):322–330. [DOI] [PubMed] [Google Scholar]
- 35. Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214(5):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]