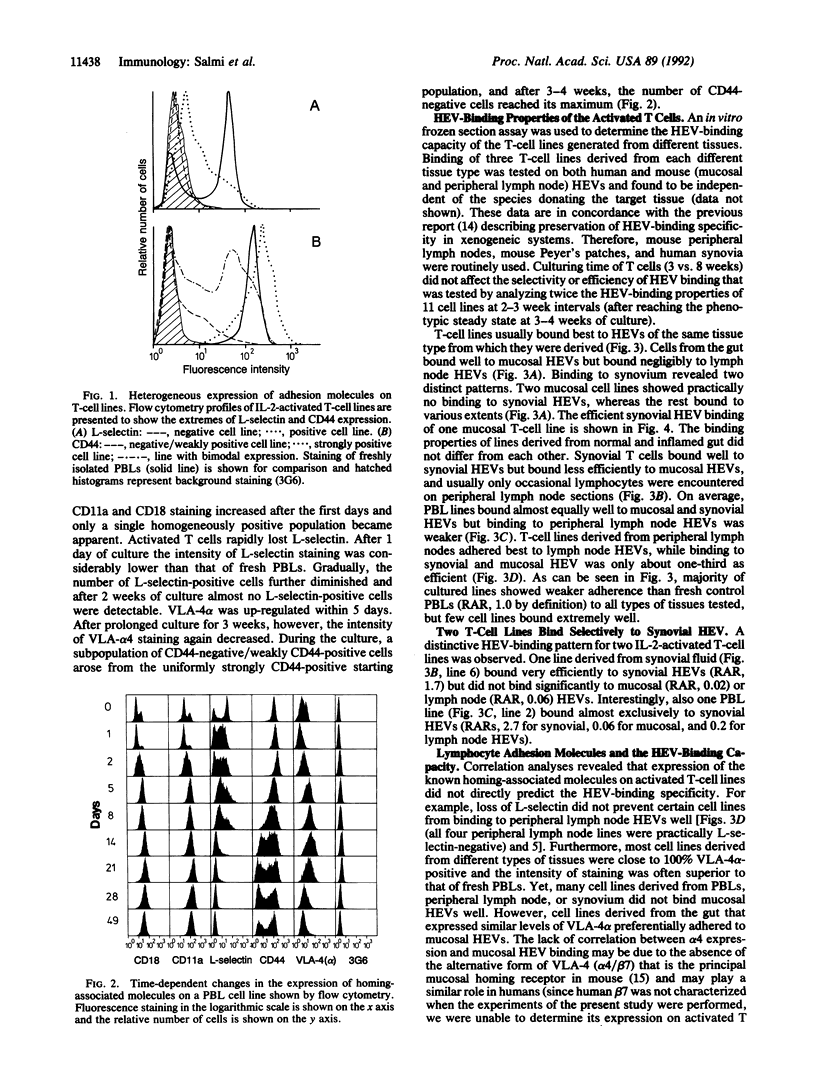
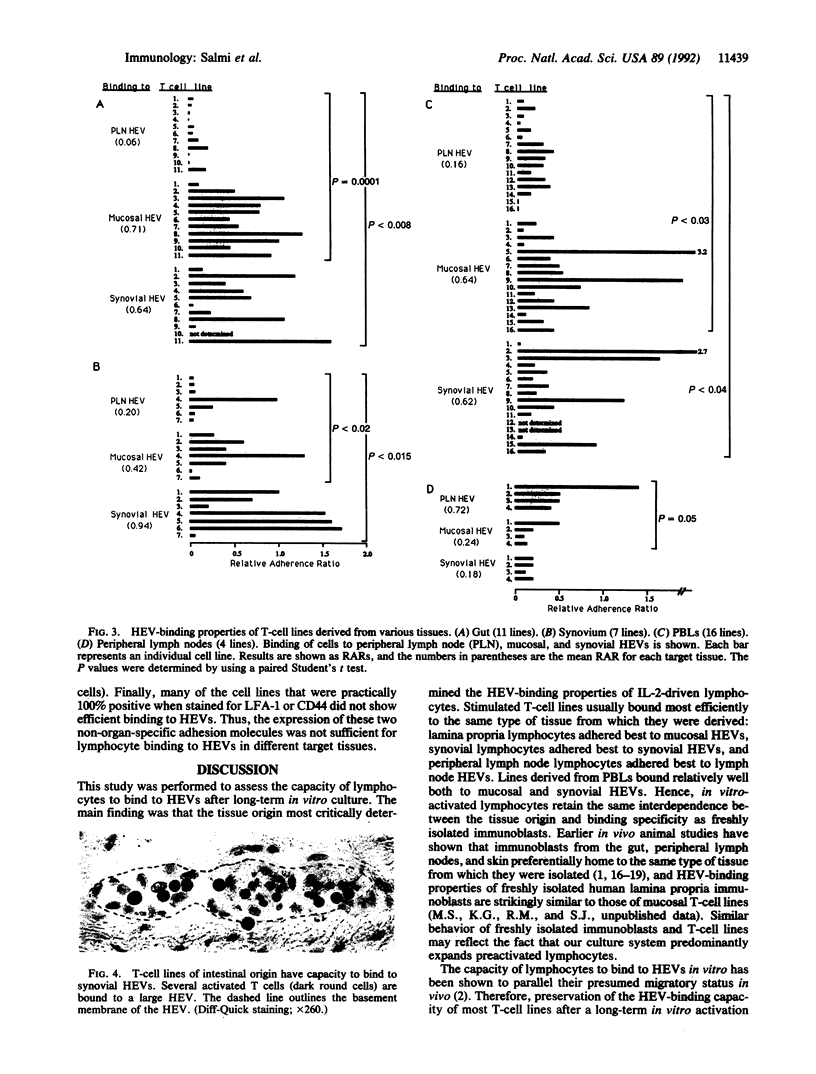
Abstract
The ability of lymphocytes to recognize and bind to high endothelial venules (HEVs) is essential for lymphocyte migration from the blood into lymphoid tissues and into sites of inflammation. Endothelial cell binding capacity also critically determines the clinical usefulness of T-cell lines and clones in immunotherapy. In the present study, interleukin-2-dependent T-cell lines were derived from the blood, lamina propria of the gut, inflamed synovium, synovial fluid, and peripheral lymph nodes. After 3-8 weeks of culture, the expression of homing-associated molecules and binding to mucosal, synovial, and peripheral lymph node HEVs were analyzed. Cell lines derived from the blood and mucosal sites bound significantly better to mucosal and synovial HEVs than to peripheral lymph node HEVs. Three out of seven synovial T-cell lines showed preferential binding to synovial HEVs, whereas the rest bound almost equally well to synovial and mucosal HEVs. T-cell lines from peripheral lymph nodes bound preferentially to lymph node HEVs despite the lack of L-selectin (the peripheral lymph node homing receptor). Expression of the known homing-associated molecules did not predict the HEV-binding specificity of these lines. Importantly, two cell lines bound well to synovial venules, but poorly, if at all, to mucosal or peripheral lymph node HEVs, supporting the concept that synovial-specific HEV recognition mechanisms exist. In conclusion, the tissue origin of T-cell lines critically determines their selectivity for endothelial cell recognition, and besides the known "homing receptors," other molecules may also mediate tissue-specific HEV-binding of interleukin-2-activated T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bookman M. A., Groves E. S., Matis L. A. Expression of MEL-14 antigen is not an absolute requirement for dissemination to lymph nodes after adoptive transfer of murine T lymphocyte clones. J Immunol. 1986 Oct 1;137(7):2107–2114. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Griffith K. D., Read E. J., Carrasquillo J. A., Carter C. S., Yang J. C., Fisher B., Aebersold P., Packard B. S., Yu M. Y., Rosenberg S. A. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst. 1989 Nov 15;81(22):1709–1717. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Vassalli P., McCluskey R. T. The distribution of large dividing lymph node cells in syngeneic recipient rats after intravenous injection. J Exp Med. 1969 Dec 1;130(6):1427–1451. doi: 10.1084/jem.130.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B., Weissman I. L. Integrin molecules involved in lymphocyte homing to Peyer's patches. Immunol Rev. 1989 Apr;108:45–61. doi: 10.1111/j.1600-065x.1989.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Chin W., Hay J. B. The characterization of lymphocytes migrating through chronically inflamed tissues. Immunology. 1982 May;46(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S. T., Butcher E. C. In vitro analysis of the homing properties of human lymphocytes: developmental regulation of functional receptors for high endothelial venules. Blood. 1985 Sep;66(3):577–582. [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Nash G. S., De los Toyos J., MacDermott R. P., Butcher E. C. Human lamina propria lymphocytes bear homing receptors and bind selectively to mucosal lymphoid high endothelium. Eur J Immunol. 1989 Jan;19(1):63–68. doi: 10.1002/eji.1830190111. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Parrott D. M., Bruce R. G. Migration of lymphoblasts to the small intestine. II. Divergent migration of mesenteric and peripheral immunoblasts to sites of inflammation in the mouse. Cell Immunol. 1976 Nov;27(1):36–46. doi: 10.1016/0008-8749(76)90151-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steen P. D., McGregor J. R., Lehman C. M., Samlowski W. E. Changes in homing receptor expression on murine lymphokine-activated killer cells during IL-2 exposure. J Immunol. 1989 Dec 15;143(12):4324–4330. [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Wu N. W., Jalkanen S., Streeter P. R., Butcher E. C. Evolutionary conservation of tissue-specific lymphocyte-endothelial cell recognition mechanisms involved in lymphocyte homing. J Cell Biol. 1988 Nov;107(5):1845–1851. doi: 10.1083/jcb.107.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock T. A., Rosen S. D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]





