Abstract
Anti–aquaporin-4 (AQP4) autoantibody plays a key role in the pathogenesis of neuromyelitis optica (NMO). Studies have shown increased relapse rates in patients with NMO during pregnancy and postpartum. High estrogen levels during pregnancy can increase activation-induced cytidine deaminase expression, which is responsible for immunoglobulin production. Additionally, sex hormones may influence antibody glycosylation, with effects on antibody function. Estrogen decreases apoptosis of self-reactive B cells, through upregulation of antiapoptotic molecules. Furthermore, high estrogen levels during pregnancy can boost B-cell activating factor and type 1 interferon (IFN) production, facilitating development of self-reactive peripheral B cells in association with increased disease activity. Elevated levels of estrogen during pregnancy decrease IFN-γ generation, which causes a shift toward T helper (Th) 2 immunity, thereby propagating NMO pathogenesis. Women with NMO have an elevated rate of pregnancy complications including miscarriage and preeclampsia, which are associated with increased Th17 cells and reduction of T-regulatory cells. These in turn can enhance inflammation in NMO. Increased regulatory natural killer cells (CD56−) during pregnancy can enhance Th2-mediated immunity, thereby increasing inflammation. In the placenta, trophoblasts express AQP4 antigen and are exposed to maternal blood containing anti-AQP4 antibodies. Animal models have shown that anti-AQP4 antibodies can bind to AQP4 antigen in placenta leading to complement deposition and placental necrosis. Reduction of regulatory complements has been associated with placental insufficiency, and it is unclear whether these are altered in NMO. Further studies are required to elucidate the specific mechanisms of disease worsening, as well as the increased rate of complications during pregnancy in women with NMO.
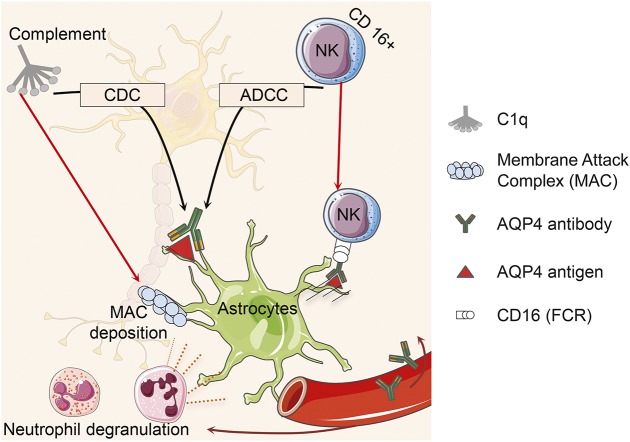
Neuromyelitis optica (NMO) is an inflammatory disease of the CNS1 resulting in astrocyte dysfunction and secondary demyelination. A unique circulating immunoglobulin G (IgG) autoantibody targeting the astrocyte water channel protein aquaporin-4 (AQP4) is an important biomarker of disease, and likely plays a key role in NMO pathogenesis.2 AQP4-IgG binds to AQP4 located on the foot processes of astrocytes. This complex activates complement, resulting in membrane attack complex deposition. The cytokines (e.g., interleukin [IL]–17, IL-8, and granulocyte colony-stimulating factor [G-CSF]) are proposed as a signal for the recruitment of neutrophils and eosinophils into the perivascular spaces. Neutrophil degranulation leads to astrocyte death, which in turn causes oligodendrocyte death and axonal degeneration.1 In the presence of complement, AQP4 antibody antigen complex leads to complement-dependent cytotoxicity (CDC).3 Natural killer cells (NKCs) alone in the absence of complement may also trigger an antibody-dependent cell-mediated cytotoxicity (ADCC) (figure 1).4 Further studies have shown that IL-17 and IL-23 markers and T helper (Th) 17 cells are elevated in the peripheral blood of patients with NMO.5 A T-cell response to AQP4 peptides, particularly p61-80, may play an important role in disease pathogenesis.6 (figure 2).
Figure 1. Aquaporin-4 (AQP4)–immunoglobulin G binds to AQP4 antigen located on the foot processes of astrocytes.
This complex activates complement resulting in membrane attack complexes (MAC) deposition. Neutrophil degranulation leads to astrocyte death, which in turn causes oligodendrocyte death and axonal degeneration. AQP4-antibody-antigen complex leads to complement-dependent cytotoxicity (CDC) in the presence of complement system or antibody-dependent cell-mediated cytotoxicity (ADCC) using cytotoxic CD16+ natural killer (NK) cells.
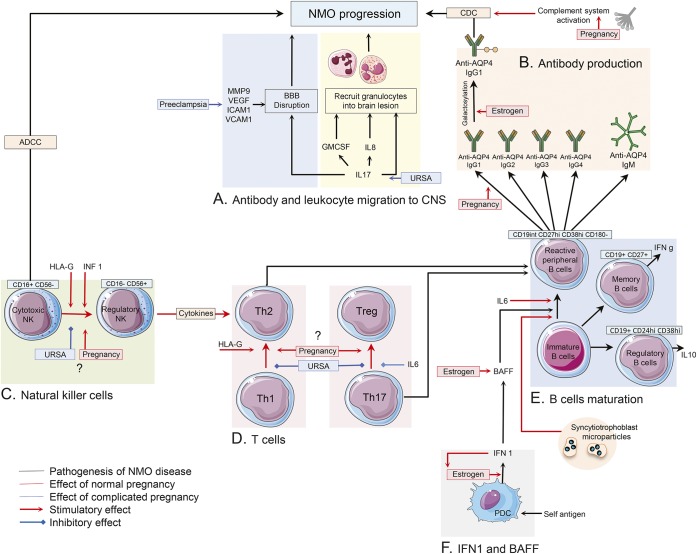
Figure 2. Immune changes in peripheral blood of pregnant patients with NMO.
(A) Interleukin (IL)–17, IL-8, and granulocyte colony-stimulating factor (G-CSF) are proposed as a signal for the recruitment of neutrophils and eosinophil into the perivascular spaces. Matrix metalloproteinase–9 (MMP-9), vascular endothelial growth factor A (VEGF-A), intercellular adhesion molecule–1 (ICAM-1), and vascular cell adhesion molecule–1 (VCAM-1) are considered to have roles in blood–brain barrier (BBB) disruption and lymphocyte recruitment into the CNS of patients with NMO. Serum level of most of these molecules was found to be elevated during preeclampsia. (B) Change in AQP4 antibody classes, subclasses, and glycosylation pattern under influence of sex hormones during and after pregnancy may cause NMO disease progression. (C) During normal pregnancy, NMO could subside due to decreased rate of cytotoxic natural killer cells (NKCs) (CD16+) in peripheral blood, though regulatory NKCs (CD56+) can enhance T-helper (Th2)–mediated immunity and cause NMO disease progression. In patients with unknown recurrent spontaneous abortion (URSA), a shift toward cytotoxic NKCs was reported. Interferon (IFN)–β and human leukocyte antigen G induce a decreased number of cytotoxic NKCs. (D, E) CD19int CD27high CD38high CD180− B-cell plasmablasts are responsible for producing AQP4–immunoglobulin G (IgG) in patients with NMO. IL-6 is important in the survival and activity of these self-reactive peripheral B cells. Increased levels of Th2 immunity, IL-6, B-cell activating factor (BAFF), and IFN 1 in normal pregnancy may cause disease deterioration among patients with NMO, though increased Th17 immunity in complicated pregnancy may have the same effect on NMO progression. Frequency of memory and regulatory B cells and their effects on disease status are required to be investigated in pregnant patients with NMO. (F) Estrogen enhances type 1 IFN responses in plasmacytoid DCs. Type 1 IFNs enhance estrogen signaling in a feed-forward loop. IFN-β treatment worsens the disease course of patients with NMO. Also, IFN-β treatment induces elevated levels of serum BAFF level in patients with NMO. BAFF has a regulatory role in survival and maturation of peripheral B cells. Estrogen boosted BAFF production via stimulation of myeloid cells. ADCC = antibody-dependent cell-mediated cytotoxicity; CDC = complement-dependent cytotoxicity; HLA = human leukocyte antigen; PDC = plasmacytoid dendritic cells.
NMO frequently occurs in female patients with median age at presentation of 39 years, i.e., during the childbearing years.7 A high female to male ratio of NMO prevalence (9:1) indicates that female factors, whether genetic, epigenetic, or hormonal, may play an important role in disease pathogenesis.8 Female gonadal hormones, estrogen and progesterone, rise significantly during pregnancy,9 and decrease during the postpartum period. There is a growing literature demonstrating that pregnancy affects the disease course of NMO, and that NMO may affect the outcome of pregnancy. This suggests that the immunologic changes occurring during pregnancy influence the outcomes of NMO, and may provide novel insights into the immunopathogenesis of disease, as well as insights into the management of NMO in the peripartum period. This article summarizes the effects reported to date of pregnancy on NMO, and discusses the current knowledge of related immunologic mechanisms.
NMO DISEASE COURSE DURING PREGNANCY
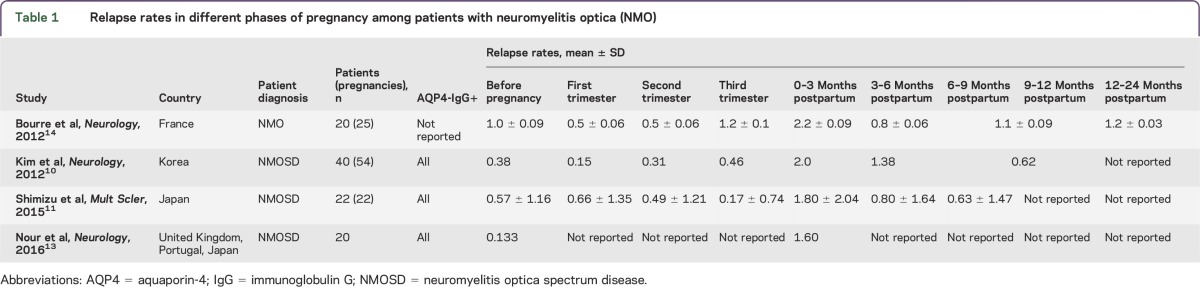
Four NMO studies (table 1) have reported a significant increase in the relapse rate in the immediate postpartum period compared to the relapse rate during pregnancy or prior to pregnancy.10–14 No significant differences in relapse rate were observed between pregnancy trimesters, in any of the studies listed, and unlike multiple sclerosis (MS),15,16 a decrease in relapse rate in the third trimester is not observed.
Table 1.
Relapse rates in different phases of pregnancy among patients with neuromyelitis optica (NMO)
Importantly, given the low prevalence of NMO, the studies above included patients in whom disease started during gestation or the postpartum period. Insufficient numbers of participants as well as including NMO spectrum disease (NMOSD)17 are other limitations of the studies listed.
INFLUENCE OF NMO ON PREGNANCY OUTCOMES
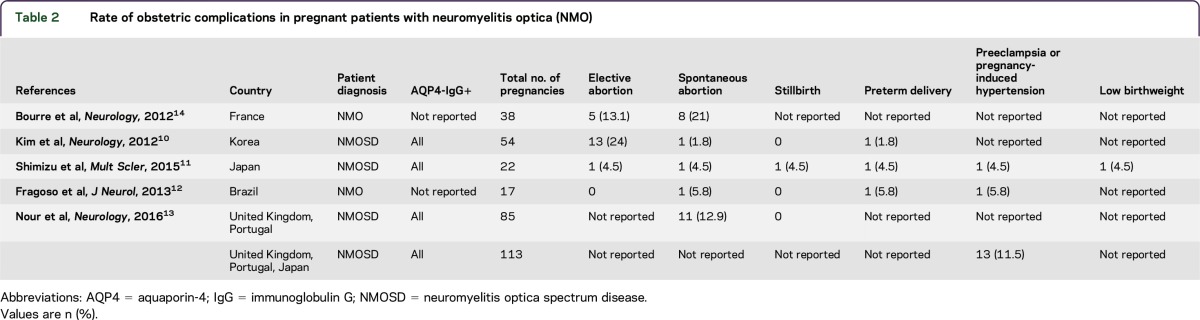
An elevated rate of pregnancy complications in patients with NMO has been noted in several studies18–20 (table 2). For example, of 85 pregnancies reported in 40 patients with NMOSD, 11 pregnancies (12.9%) in 6 participants were terminated by miscarriage (table 2).13 Additionally, an increased rate of miscarriages was reported in pregnancies occurring after NMOSD onset (42.9%) compared to those before disease onset (7.04%). In addition, the rate of preeclampsia was significantly higher in NMO than the general population13 (11.5% vs 3.2% respectively). Bourre et al.14 studied NMO activity during pregnancy of 38 patients with NMO, and found that 8 patients experienced miscarriage and 5 patients had elective abortions. To examine the role and effects of AQP4 antibody on pregnancy-related outcomes, future studies should stratify patients by antibody status. Additional investigations are needed to understand the mechanisms by which pregnancy-related immune changes affect the NMO disease course.
Table 2.
Rate of obstetric complications in pregnant patients with neuromyelitis optica (NMO)
PREGNANCY IN OTHER RELEVANT AUTOIMMUNE DISEASE
Systemic lupus erythematosus (SLE) is a humorally based autoimmune disease with 9:1 female to male ratio.21,22 SLE is known to flare during pregnancy and postpartum.23–26 The rate of obstetric complications like stillbirth, preeclampsia, and preterm delivery are reported to be high in pregnant patients with SLE.23,27–31 In contrast, MS is considered to be a predominantly cell-mediated autoimmune disease, which also presents frequently in women of childbearing age. Unlike SLE and NMO diseases, MS relapse rates decrease during pregnancy but significantly increase postpartum.15,16
IMMUNOLOGY OF PREGNANCY
Th1/Th2/Th17 and T-regulatory (Treg) cells paradigm.
The frequency of Th17 cells within peripheral blood is significantly lower in the third trimester of normal pregnancies compared with nonpregnant individuals,32,33 but it is conversely higher in the decidua, and may play a role in protection against uterine infection.34 In patients with unknown recurrent spontaneous abortion (URSA), the frequency of Th17 cells is increased in both peripheral blood and decidua,34,35 and the frequency and immunosuppressive activity of Treg cells in both decidua and peripheral blood is lower than in pregnant women without URSA.36,37
Immune tolerance at the fetomaternal interface.
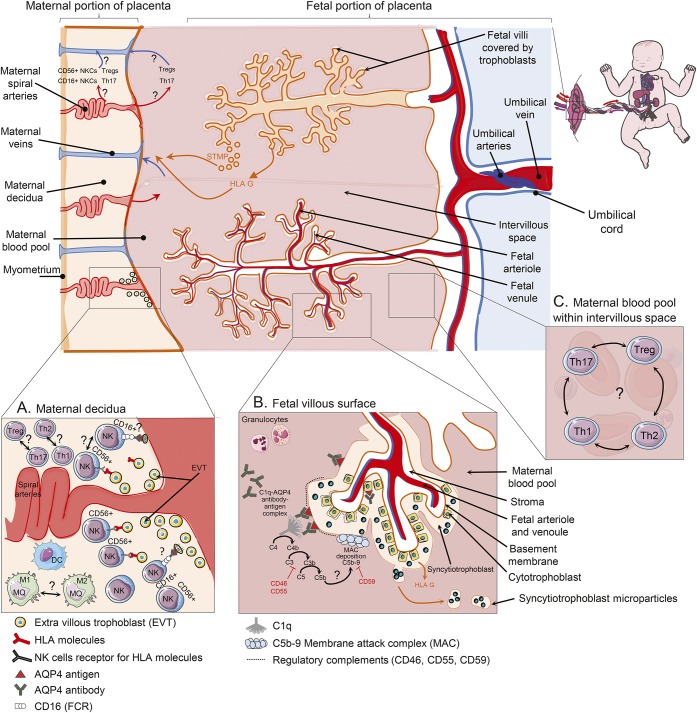
Trophoblasts (including syncytiotrophoblasts and cytotrophoblasts) are the cells set in the boundary between maternal and fetal circulation of the placenta (figure 3B). These cells contain different types of AQP water channels including AQP4 protein, responsible for transferring nutrition from mother to fetus. Syncytiotrophoblasts are made from fusion of cytotrophoblast cells, and are the most exposed fetal cells to maternal circulation (figure 3B). Extravillous trophoblasts are a specialized type of trophoblast, which are able to invade through the uterine wall and destroy the muscular wall of spiral arteries, leading to increase in maternal blood flow into the intervillous space (figure 3A). During gestation, leukocytes infiltrate the endometrium of uterus during the process of decidualization. Decidual leukocytes are composed of nearly 70% NKCs, 20% macrophages, 1% dendritic cells (DCs), and other lymphocytes.38,39
Figure 3. Immune changes in placenta of pregnant patients with NMO.
(A) Extravillous trophoblasts (EVT) invade through the uterine wall and destroy muscular wall of spiral arteries leading to increase in maternal blood flow into the intervillous space. Uterine CD16dim CD56bright natural killer cells (NKCs), which are most frequent leukocyte in decidua, can control the extent of EVT invasion into the uterine myometrium through interactions with major histocompatibility complex (MHC) molecules. Conversely, CD16bright CD56dim are mostly involved in antibody-dependent cell cytotoxicity. EVT cells that express aquaporin-4 (AQP4) antigen in AQP4 antibody–positive patients with NMO may be susceptible to be killed by cytotoxic NKCs within decidua. Furthermore, increased ratio of T-helper (Th) 1/Th2 cells and Th17/T-regulatory (Treg) cells may be involved in pathogenicity of obstetric complications in these patients. (B) Trophoblasts are the cells set in the boundary between maternal and fetal circulation of the placenta. These cells contain AQP4 water channel responsible for transferring nutrition from mother to fetus. Syncytiotrophoblasts are a special type of trophoblast made from fusion of cytotrophoblast cells. Both syncytitrophoblasts and cytotrophoblasts express AQP4 antigen and regulatory complements, though syncytitrophoblast is more exposed to maternal peripheral blood and AQP4 antibody. Binding AQP4 antibody to AQP4 antigen on syncytitrophoblast cells may activate complement cascade and membrane attack complex deposition, which further cause inflammation and necrosis in placenta. The inhibitory role of regulatory complements on complement activity in placenta of patients with neuromyelitis optica (NMO) is not completely clear. Syncytitrophoblast microparticles (STM) are released into maternal circulation during normal repairing processes or injury of trophoblasts. They cause pregnant patients with NMO to be exposed to more AQP4 antigen. Villous trophoblasts are exposed to maternal blood and lack both MHC classes (I and II). They only release an immunoregulatory nonclassical human leukocyte antigen (G) into the maternal circulation. HLA = human leukocyte antigen.
Natural killer cells.
Uterine NKCs have specific function differentiating them from NKCs in the peripheral blood. They are CD16− CD56bright and have predominant immunoregulatory roles. Blood NKCs that express CD16bright CD56dim markers are involved in ADCC. Uterine NKCs control the extent of extravillous trophoblast invasion into the uterine myometrium through interactions with trophoblast major histocompatibility complex (MHC) molecules.40
DCs and macrophages.
The role of DCs in maintaining tolerance during pregnancy seems to vary based on cell maturation. Mature DCs are increased in the deciduae of individuals with repeated miscarriages.41 Macrophages isolated from first trimester decidua are reported to have predominant M2 characteristics and secrete regulatory factors like IL-10 and indoleamine dioxygenase.42
IMMUNOLOGY OF NMO DURING PREGNANCY
AQP4 antibody.
AQP4 antibody classes and subclasses.
AQP4-IgG has been reported in the serum of 68%–91% of patients with NMO.43,44 AQP4-IgG serum level has been reported to rise prior to each relapse and is associated with the disease activity.45 AQP4-IgG1, 2, 3, and 4 were identified respectively in 97.8%, 37.0%, 6.5%, and 6.5% of individuals with anti-AQP4 antibody.46 AQP4–immunoglobulin M (IgM) was found in 10% of AQP4-IgG-positive serum samples. The pathogenic role of AQP4-IgM in the course of NMO disease is unknown.47 Different sex hormones influence the transcription of the activation-induced cytidine deaminase (AID) gene responsible for immunoglobulin mutation and class switching (figure 2A).48 Receptors of sex hormones are expressed in human peripheral leukocytes and play a role in regulating their activity.49,50 Estrogen activates its intracellular receptors, which can move into the cell nucleus and bind to a specific DNA segment called estrogen response elements (EREs). The promoter of the AID gene has a number of these EREs. Accordingly, high estrogen levels increase AID expression, which create more mutations during immunoglobulin production.51,52 Likewise in mouse B cells, estrogen was found to increase IgM to IgG class switching.48 Also, progesterone was reported to have either inhibitory or stimulatory effect on immunoglobulin production in different surveys.53–56 Leukocytes from patients with SLE were treated with 17β-estradiol in vitro, which led to production of anti-dsDNA antibodies, especially IgG type.57 Thus it is relevant to evaluate the amount of AQP4 antibody and immunoglobulin class and subclass changes, which may occur during gestation and puerperium in NMO, where significant sex hormone shifts occur.
AQP4 antibody glycosylation pattern.
Immunoglobulins are glycoproteins and their function is regulated by their glycosylation patterns. Adding sialic acid or galactose to the Fc part of IgG decreases the ability of immunoglobulin to attach complement (needed for CDC) or Fc receptors (needed for ADCC).58 Sex hormones were recently discovered to have an effective role in modifications of Ab glycosylation pattern.59 Moreover, it appears that during pregnancy, especially in the third trimester, more galactose molecules are added to the IgG, but galacotosylation decreases postpartum (figure 2A).60 Enzymatic deglycosylation of anti-AQP4 antibody has been shown to reduce its pathogenicity.e1 Alteration in AQP4-IgG glycosylation pattern and its relationship to disease activity has not yet been explored in pregnant patients with NMO.
B cells.
AQP4 antibody-producing B cells.
Successful treatment of NMO with rituximab demonstrates the importance of B cells in NMO pathogenesis.e2 CD19int CD27high CD38high CD180− B cell plasmablasts are elevated in the peripheral blood of patients with NMO and may be responsible for producing AQP4-IgG. IL-6 production is increased in the blood of patients with NMO and is important in the survival and activity of these self-reactive peripheral B cells (PB) (figure 2B).e3 There is evidence of increased levels of IL-6 in normal pregnancy.e4 Pregnancy complications are accompanied by further increased levels of IL-6.e5 At peripheral tolerance checkpoints, estrogen decreases apoptosis of self-reactive B cells, through upregulation of antiapoptotic molecules like Bcl-2e6,e7 and CD22.e8,e9 Increased survival may be due to ERE found in the bcl-2 gene promoter.e10
Memory B cells.
CD19+CD27+ memory B cells produce proinflammatory cytokines and may exacerbate autoimmunity (figure 2B). Treating patients with NMO with rituximab for 2 years revealed clinical improvement and reduction in CD27+ memory B cells.e11 Rituximab administered 1 week prior to pregnancy decreased CD19+ B cells and improved the clinical course of patients with NMO during gestation.e12 A significant increase in CD19+ B cells was seen in peripheral blood of female patients with MS after high doses of estradiol treatment.e13
Regulatory B cells.
A regulatory B-cell population (CD19+ CD24hi CD38hi B cells) secreting IL-10 was revealed to be protective in inflammatory diseasese14 and their ability to produce IL-10 was decreased in patients with NMO (figure 2B).e15 Estrogen was found to suppress experimental autoimmune encephalomyelitis through enhancing regulatory B in the absence of Treg cells.e16 Further studies are needed to establish the role of different B-cell types on NMO progression during pregnancy.
Cytokines and chemokines.
Serum levels of Th2 cytokines are demonstrated to be higher in normal pregnancy comparing URSA.e4 Serum levels of proinflammatory cytokines including IL-6 and tumor necrosis factor–α (TNF-α), and the chemokines including IL-8, IP-10, and monocyte chemotactic protein 1 (MCP-1) were found to be elevated in preeclampsia.e5 Th2-related cytokines/chemokines, such as IL-1Ra, IL-10, and IL-13, and Th17-related cytokines/chemokines, such as IL-6, IL-8, and G-CSF, are elevated in the CSF analysis of nonpregnant patients with NMO. In addition, IL-6, IL-17, and IL-23 are significantly elevated in the serum of patients with NMO.e17,e18 Despite heterogeneity in articles, secretion of IL8, IL-17, IL-6, IL-10, and TNF-α during gestation was commonly reported to increase in peripheral blood of pregnant patients with SLE.e19–e21 TNF-α was found to be related to the pregnancy loss in patients with antiphospholipid syndrome.e22 Increased disease activity and pregnancy complications among pregnant patients with NMO suggest a dysregulation in the proportion of Th1/Th2/Th17-related inflammatory cytokines.
Interferon (IFN) type 1 (α, β).
Serum levels of type 1 IFN were formerly reported to be higher in patients with NMO.e23 In addition, there is strong evidence that IFN-β treatment worsens the disease course of patients with NMO.e24 Interestingly, estrogen enhances TLR7-mediated type 1 IFN responses in plasmacytoid DCs.e25 Type 1 IFNs enhance estrogen signaling in a feed-forward loop by increasing expression of ER-α70 (figure 2F).e26 In contrast to estrogen, progesterone has inhibitory effect on IFN type 1.e27 Estrogen was found to increase IFN-α expression in SLE animal models.e28 Accordingly, high estrogen level during pregnancy and postpartum may cause change in type 1 IFN level, thereby increasing disease activity of NMO.
B-cell activating factor (BAFF).
BAFF has a regulatory role in survival, maturation, and differentiation of PBs. In animal studies, increased expression of BAFF worsens humorally mediated autoimmunity.e23 In NMO, BAFF levels were found to be significantly higher in both CSF and blood.e29,e30 Treating patients with NMO with rituximab reduces serum BAFF levels accompanied by a significant decrease in anti-AQP4 titers.e31 Estrogen boosted BAFF production via stimulation of murine myeloid cells.e28 A possible increase in BAFF levels during pregnancy may have a significant effect on disease progression among pregnant patients with NMO.
T cells.
Th17 and Treg cells.
Th17 cells and IL-17 are higher in peripheral blood of patients with NMO vs healthy controls.5 Wang et al.35 found an opposite association between frequency of Th17 cells and Tregs in decidua and peripheral blood of URSA patients. They postulated that IL-6 was responsible for regulating Th17/Treg balance (figure 2C). Serum IL-6 is reported to be elevated in the peripheral blood of patients with NMO, which may affect survival of self-reactive B cells,e3 and induce obstetric morbidities. In patients with antiphospholipid syndrome, no significant correlation was found between serum level of IL-6 and obstetric complications.e22
Mice treated with high estradiol dose had decreased expression of IL-17 in Th17 cells.e32 Likewise, high doses of progesterone may inhibit formation of IL-17-producing Th17 cells.e33 Treatment of mice with both estrogen and progesterone could increase Treg cells.e34,e35 Estradiol treatment in experimental autoimmune arthritis reduced the arthritis severity as well as Th17 cells in joints.e36 Thus, Th17 cells within the decidua and blood may play a role in the elevated risk of pregnancy complications13 plus continued disease activity among pregnant patients with NMO. The frequency and balance between Th17 cells and Treg cells, particularly in p61-80 AQP4 specific T cells,6 and their relationship to pregnancy complications or disease activity in pregnant patients with NMO needs to be further explored.
Th17 and Th2 cells.
A slight bias toward Th2 immunity is necessary to preserve fetomaternal tolerance during gestation32 (figure 2C). Treating T cells with low doses of estrogens increases IL-12 levels, thereby activating STAT4 and IFN-γ gene augmentation.e37 Estrogen receptor elements are located in the IFN-γ gene promoter.e38 Estrogen can enhance T-bet expression and increase Th1 immunity.e39 However, high estrogen levels present during pregnancy decrease IFN-γ generation.e40,e41 Likewise, progesterone inhibits expression of IFN-γ in Th1 cells via the progesterone receptor.e42 The frequency and balance between Th1 and Th2 cells in relationship with pregnancy complications and NMO activity needs further characterization.
Innate immune system.
NKCs.
Immune modification during normal pregnancy could also be due to the transformation of cytotoxic CD16+ CD56dim into anti-inflammatory CD16− CD56bright NKCs (figure 2D).e43 Improvement of MS disease16,e44,e45 during pregnancy was postulated to be accompanied by an increase in blood CD16− CD56bright NKCs together with Th2 cytokines augmentation.e46 Therefore, NMO could subside due to decreased rate of cytotoxic NKCs (CD16+) in peripheral blood during pregnancy. Regulatory NKCs (CD56+) can enhance Th2-mediated immunity and cause NMO disease progression (figure 2D). Further studies are required to clarify the role of NKCs in disease progression among pregnant patients with NMO. In patients with URSA, however, a trend toward an increased number of cytotoxic NKCs in both peripheral blood and decidua was found.e47,e48 CD16−CD56bright NKCs regulate the extent of extravillous trophoblasts invasion into uterine myometrium through identifying their MHC molecules (figure 3A).e49 Inadequate trophoblast invasion is known to cause pregnancy complications like preeclampsia, intrauterine growth restriction, and stillbirth.e50 Extravillous trophoblasts that express AQP4 antigen in AQP4 antibody–positive patients with NMO may be susceptible to be killed by cytotoxic NKCs within decidua (figure 3A). Thus it may cause insufficient extravillous trophoblast invasion and subsequent low placenta perfusion in pregnant patients with NMO. Studies about frequency of different NKCs and their function within decidua of pregnant patients with NMO are needed.
Blood–brain barrier (BBB) breakdown.
IL-17 can stimulate production of chemokines including IL-8 and G-CSF, which are responsible for recruiting and stimulating granulocytes into brain lesions.e51 In addition, it can disrupt the BBB, thus allowing anti-AQP4 antibody reach the CNS (figure 2E).e52 Likewise, matrix metalloproteinase–9, vascular endothelial growth factor A, intercellular adhesion molecule–1, and vascular cell adhesion molecule–1 (VCAM-1) are considered to have roles in BBB disruption and lymphocyte recruitment into the CNS of patients with NMO (figure 2E).e53 Despite no significant rise during healthy pregnancy, serum levels of these molecules were found to be elevated during preeclampsia.e54–e56 Thus there may be a significant relationship between disease activity and obstetric morbidities in pregnant patients with NMO due in part to cytokine and chemokine dysregulation.
Miscellaneous factors.
Syncytiotrophoblasts release their microparticles into the maternal circulation, which may cause higher exposure of the mother to AQP4 antigen (figure 2).e57 Villous trophoblasts release an immune regulatory nonclassical human leukocyte antigen (G) into the maternal circulation (figure 3B).e58
INSIGHTS FROM ANIMAL MODELS
In an animal study, injecting AQP4-IgG plus human complement into the peritoneum of pregnant mice resulted in binding antibodies to the placental AQP4. The antibody-antigen complex activated complement system leading to leukocyte infiltration and necrosis.e59 This suggested that the presence of each AQP4-IgG, AQP4 antigen, and complement is necessary for development of inflammation within the placenta.
HISTOPATHOLOGY OF PLACENTA OBTAINED FROM A PREGNANT PATIENT WITH NMO
There are no previously published series regarding histopathology of placenta in pregnant patients with NMO. One case report described a miscarriage in the second trimester of a patient with NMO, declaring that the placenta contained several regions of necrosis and diffuse deposits of membrane attack complexes observed mostly in perivascular areas of syncytiotrophoblasts.18 This raises the possibility of AQP4-antibody-mediated attack on the placenta in women with NMO peripartum.
AQP4-EXPRESSING PERIPHERAL TISSUE IN PATIENTS WITH NMO
Peripheral tissue in patients with NMO.
Besides the CNS, AQP4 antigen is expressed in several peripheral tissues including the kidney, glands, stomach, and skeletal muscle.e60 AQP4 antigen in peripheral organs of patients with NMO binds to the circulating AQP4-IgG without any considerable organ damage.e61 The most probable reasons include high levels of regulatory complements and low levels of AQP4 antigen expression in peripheral tissue.1,e62,e63
Expression of regulatory complements in placenta.
Syncytiotrophoblasts are one of the 3 main types of trophoblasts, which cover the surface of fetal villi, and are in direct contact with the maternal circulation. Syncytiotrophoblasts steadily express all 3 regulatory complements (CD46, CD55, and CD59) during the entire pregnancy period (figure 3B).e64 On the other hand, despite the presence of regulatory complements, complement deposition in the placenta of a normal pregnancy occurs, and is believed to be essential for local infection prevention.e65
Level of AQP4 expression in placenta.
Expression of AQP4 in placenta is high during the second trimester and declines markedly in the third trimester of a normal pregnancy.e66 Nour et al.13 reported miscarriages mostly during early pregnancy, though no significant association between AQP4-IgG titers and pregnancy outcome was observed. Furthermore, placental expression of AQP4 in the setting of elevated estrogens needs to be explored as a trigger for NMO disease, as well as on neonatal outcomes.
DISCUSSION
Recent studies have shown increased NMO disease activity postpartum and either an increase or no suppression intrapartum. In addition, patients with NMO have an increased rate of pregnancy complications. There are several immunologic mechanisms that may influence disease activity and obstetric morbidities in pregnant patients with NMO. High estrogen levels during pregnancy may influence anti-AQP4 antibody type, amount, and glycosylation pattern. Moreover, estrogen can enhance inflammatory mediators to increase survival of self-reactive B cells. A shift toward humoral immunity during normal pregnancy or increased Th17 cells in pregnant patients with NMO may contribute to increased pregnancy complications. A shift from cytotoxic to regulatory NKCs can influence disease activity in different directions. In the placenta, trophoblasts express AQP4 antigen and are highly exposed to maternal circulation. AQP4 antibody in a pregnant animal model was reported to induce placenta insufficiency. Regulatory complements may have an important inhibitory effect in pathogenicity of AQP4 antibody in the placenta.
This review summarizes the current knowledge of the interactions of NMO and pregnancy, along with the current knowledge of the immunopathogenesis of NMO, and potential relationships to the immune changes that occur during pregnancy. Further studies are required to elucidate the roles of pregnancy-related hormones, notably estrogens and progestogens, in the modulation of NMO-specific immune mechanisms. The role of the placenta, which is a source of AQP4 antigen in NMO onset, and pregnancy-related complications need to be further elucidated.
Supplementary Material
ACKNOWLEDGMENT
Figures were produced using Servier Medical Art: servier.com.
GLOSSARY
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AID
activation-induced cytidine deaminase
- AQP4
aquaporin-4
- BAFF
B-cell activating factor
- BBB
blood–brain barrier
- CDC
complement-dependent cytotoxicity
- DC
dendritic cell
- ERE
estrogen response element
- G-CSF
granulocyte colony-stimulating factor
- IFN
interferon
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL
interleukin
- MCP-1
monocyte chemotactic protein 1
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- NKC
natural killer cell
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disease
- PB
peripheral B cells
- SLE
systemic lupus erythematosus
- Th
T-helper
- TNF-α
tumor necrosis factor–α
- Treg
T-regulatory
- URSA
unknown recurrent spontaneous abortion
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Study concept and design: V.D., K.K., T.C. Statistical analysis and interpretation of data: V.D., K.K., T.C. Acquisition of data and interpretation of results: VD V.D., K.K., T.C. Manuscript drafting and revising: V.D., K.K., R.B., T.C.
STUDY FUNDING
No targeted funding.
DISCLOSURE
The authors report no conflicts of interest with respect to this manuscript. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. . A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 3.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 2010;133:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratelade J, Zhang H, Saadoun S, Bennett JL, Papadopoulos MC, Verkman AS. Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol 2012;123:861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang HH, Dai YQ, Qiu W, et al. . Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 2011;18:1313–1317. [DOI] [PubMed] [Google Scholar]
- 6.Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, et al. . Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol 2012;72:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 8.Pandit L, Asgari N, Apiwattanakul M, et al. . Demographic and clinical features of neuromyelitis optica: a review. Mult Scler 2015;21:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012;62:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim W, Kim SH, Nakashima I, et al. . Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology 2012;78:1264–1267. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Fujihara K, Ohashi T, et al. . Pregnancy-related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult Scler Epub 2015 Apr 28. [DOI] [PubMed]
- 12.Fragoso YD, Adoni T, Bichuetti DB, et al. . Neuromyelitis optica and pregnancy. J Neurol 2013;260:2614–2619. [DOI] [PubMed] [Google Scholar]
- 13.Nour MM, Nakashima I, Coutinho E, et al. . Pregnancy outcomes in aquaporin-4–positive neuromyelitis optica spectrum disorder. Neurology 2016;86:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourre B, Marignier R, Zephir H, et al. . Neuromyelitis optica and pregnancy. Neurology 2012;78:875–879. [DOI] [PubMed] [Google Scholar]
- 15.Vukusic S, Hutchinson M, Hours M, et al. . Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 2004;127:1353–1360. [DOI] [PubMed] [Google Scholar]
- 16.Keyhanian K, Davoudi V, Etemadifar M, Amin M. Better prognosis of multiple sclerosis in patients who experienced a full-term pregnancy. Eur Neurol 2012;68:150–155. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuss R, Rommer PS, Bruck W, et al. . A woman with acute myelopathy in pregnancy: case outcome. BMJ 2009;339:b4026. [DOI] [PubMed] [Google Scholar]
- 19.Asgari N, Henriksen TB, Petersen T, Lillevang ST, Weinshenker BG. Pregnancy outcomes in a woman with neuromyelitis optica. Neurology 2014;83:1576–1577. [DOI] [PubMed] [Google Scholar]
- 20.Chang T, Withana M. Gaze palsy, hypogeusia and a probable association with miscarriage of pregnancy: the expanding clinical spectrum of non-opticospinal neuromyelitis optica spectrum disorders: a case report. BMC Res Notes 2015;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simard J, Costenbader KH. What can epidemiology tell us about systemic lupus erythematosus? Int J Clin Pract 2007;61:1170–1180. [DOI] [PubMed] [Google Scholar]
- 22.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2002;16:847–858. [DOI] [PubMed] [Google Scholar]
- 23.Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am 2007;33:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology 2000;39:1014–1019. [DOI] [PubMed] [Google Scholar]
- 25.Urowitz MB, Gladman DD, Farewell VT, Stewart J, McDonald J. Lupus and pregnancy studies. Arthritis Rheum 1993;36:1392–1397. [DOI] [PubMed] [Google Scholar]
- 26.Clowse ME, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum 2005;52:514–521. [DOI] [PubMed] [Google Scholar]
- 27.Clark CA, Spitzer KA, Nadler JN, Laskin CA. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol 2003;30:2127–2132. [PubMed] [Google Scholar]
- 28.Lima F, Buchanan NM, Khamashta MA, Kerslake S, Hughes GR. Obstetric outcome in systemic lupus erythematosus. Semin Arthritis Rheum 1995;25:184–192. [DOI] [PubMed] [Google Scholar]
- 29.Carmona F, Font J, Cervera R, Munoz F, Cararach V, Balasch J. Obstetrical outcome of pregnancy in patients with systemic lupus erythematosus: a study of 60 cases. Eur J Obstet Gynecol Reprod Biol 1999;83:137–142. [DOI] [PubMed] [Google Scholar]
- 30.Cortes-Hernandez J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology 2002;41:643–650. [DOI] [PubMed] [Google Scholar]
- 31.Wong KL, Chan FY, Lee CP. Outcome of pregnancy in patients with systemic lupus erythematosus: a prospective study. Arch Intern Med 1991;151:269–273. [PubMed] [Google Scholar]
- 32.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010;63:601–610. [DOI] [PubMed] [Google Scholar]
- 33.Santner-Nanan B, Peek MJ, Khanam R, et al. . Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 2009;183:7023–7030. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol 2010;64:4–11. [DOI] [PubMed] [Google Scholar]
- 35.Wang WJ, Hao CF, Yi L, et al. . Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 2010;84:164–170. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004;10:347–353. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril 2008;89:656–661. [DOI] [PubMed] [Google Scholar]
- 38.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004;63:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002;2:656–663. [DOI] [PubMed] [Google Scholar]
- 40.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol 2008;77:14–22. [DOI] [PubMed] [Google Scholar]
- 41.Askelund K, Liddell HS, Zanderigo AM, et al. . CD83(+) dendritic cells in the decidua of women with recurrent miscarriage and normal pregnancy. Placenta 2004;25:140–145. [DOI] [PubMed] [Google Scholar]
- 42.Gustafsson C, Mjosberg J, Matussek A, et al. . Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 2008;3:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quek AM, McKeon A, Lennon VA, et al. . Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol 2012;69:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 2010;6:383–392. [DOI] [PubMed] [Google Scholar]
- 46.Isobe N, Yonekawa T, Matsushita T, et al. . Quantitative assays for anti-aquaporin-4 antibody with subclass analysis in neuromyelitis optica. Mult Scler 2012;18:1541–1551. [DOI] [PubMed] [Google Scholar]
- 47.Jarius S, Franciotta D, Bergamaschi R, Wildemann B, Wandinger KP. Immunoglobulin M antibodies to aquaporin-4 in neuromyelitis optica and related disorders. Clin Chem Lab Med 2010;48:659–663. [DOI] [PubMed] [Google Scholar]
- 48.Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. J Exp Med 2009;206:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierdominici M, Maselli A, Colasanti T, et al. . Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett 2010;132:79–85. [DOI] [PubMed] [Google Scholar]
- 50.Sapino A, Cassoni P, Ferrero E, et al. . Estrogen receptor α is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am J Pathol 2003;163:1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karpuzoglu E, Zouali M. The multi-faceted influences of estrogen on lymphocytes: toward novel immuno-interventions strategies for autoimmunity management. Clin Rev Allergy Immunol 2011;40:16–26. [DOI] [PubMed] [Google Scholar]
- 52.Hsu H-C, Wu Y, Yang P, et al. . Overexpression of activation-induced cytidine deaminase in B cells is associated with production of highly pathogenic autoantibodies. J Immunol 2007;178:5357–5365. [DOI] [PubMed] [Google Scholar]
- 53.Roubinian J, Talal N, Siiteri PK, Sadakian JA. Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum 1979;22:1161–1169. [DOI] [PubMed] [Google Scholar]
- 54.Hughes GC, Martin D, Zhang K, et al. . Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum 2009;60:1775–1784. [DOI] [PubMed] [Google Scholar]
- 55.Roubinian J, Talal N, Greenspan J, Goodman J, Siiteri P. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med 1978;147:1568–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pauklin S, Petersen-Mahrt SK. Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol 2009;183:1238–1244. [DOI] [PubMed] [Google Scholar]
- 57.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 1999;103:282–288. [DOI] [PubMed] [Google Scholar]
- 58.Goulabchand R, Vincent T, Batteux F, Eliaou JF, Guilpain P. Impact of autoantibody glycosylation in autoimmune diseases. Autoimmun Rev 2014;13:742–750. [DOI] [PubMed] [Google Scholar]
- 59.Chen G, Wang Y, Qiu L, et al. . Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteomics 2012;75:2824–2834. [DOI] [PubMed] [Google Scholar]
- 60.van de Geijn FE, Wuhrer M, Selman MH, et al. . Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther 2009;11:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.