Abstract:
Open aortic arch replacement is a complex and challenging procedure, especially in post dissection aneurysms and in redo procedures after previous surgery of the ascending aorta or aortic root. We report our experience with the simultaneous selective perfusion of heart, brain, and remaining body to ensure optimal perfusion and to minimize perfusion-related risks during these procedures. We used a specially configured heart–lung machine with a centrifugal pump as arterial pump and an additional roller pump for the selective cerebral perfusion. Initial arterial cannulation is achieved via femoral artery or right axillary artery. After lower body circulatory arrest and selective antegrade cerebral perfusion for the distal arch anastomosis, we started selective lower body perfusion simultaneously to the selective antegrade cerebral perfusion and heart perfusion. Eighteen patients were successfully treated with this perfusion strategy from October 2012 to November 2015. No complications related to the heart–lung machine and the cannulation occurred during the procedures. Mean cardiopulmonary bypass time was 239 ± 33 minutes, the simultaneous selective perfusion of brain, heart, and remaining body lasted 55 ± 23 minutes. One patient suffered temporary neurological deficit that resolved completely during intensive care unit stay. No patient experienced a permanent neurological deficit or end-organ dysfunction. These high-risk procedures require a concept with a special setup of the heart–lung machine. Our perfusion strategy for aortic arch replacement ensures a selective perfusion of heart, brain, and lower body during this complex procedure and we observed excellent outcomes in this small series. This perfusion strategy is also applicable for redo procedures.
Keywords: open aortic arch replacement, cardiopulmonary bypass, cannulation, perfusion strategy, selective antegrade cerebral perfusion, aortic aneurysm, post dissection aneurysm
Aortic arch replacement is a complex and challenging procedure, especially in post dissection aneurysms and in redo procedures after previous surgery of the ascending aorta or aortic root. The selection of cannulation and perfusion strategies, as well as perfusion management during surgery, is critical to ensure end-organ protection and a positive patient outcome after this procedure (1–3). Different surgical techniques have been described for open replacement of the aortic arch (1,2,4–6). In some reported series, they have been associated with significant morbidity and mortality as well as neurological dysfunction–still a major postoperative complication (1,2,6). Redo aortic arch operations are associated with even higher risks than primary procedures. Surgical techniques and results after aortic arch replacement procedures have been well described (7–9). However, although their careful selection is crucial to the success of treatment, the cannulation and perfusion strategies used are specified in only a few publications (10,11). Furthermore, when considering the negative impact of circulatory arrest, one should focus on its effects on both the spinal cord and postoperative end-organ function (12).
We hypothesize that the simultaneous selective perfusion of lower body, heart, and brain shortens organ ischemia time during aortic arch surgery and thus also helps preserving postoperative end-organ function. The use of one pump and branching of the arterial line for uncontrolled simultaneous perfusion of body and brain has been described (11). However, this perfusion strategy might be inadequate. Selective perfusion is feasible with several roller pumps but with the great risk of an air embolism in the extracorporeal circulation system (ECC) (13). We present our cannulation and perfusion strategy for aortic arch procedures which prevents air embolism and enables safe and simultaneous selective perfusion of brain, heart and lower body.
MATERIALS AND METHODS
Patient Monitoring
Intraoperative monitoring involves invasive pressure monitoring of the right radial and left radial or femoral arterial pressure, central venous pressure, and the cardioplegia application and selective antegrade cerebral perfusion (SACP) line pressures. Transesophageal echocardiography is routinely used. A temperature probe is inserted to measure bladder temperature and urine output. Cerebral oxygen saturation via a near infrared spectroscope (NIRS) INVOS (Somanetics, Troy, MI) is measured during all procedures. Spinal fluid drainage and pressure monitoring are added whenever patients need simultaneous descending stent completion.
Extracorporeal Circulation
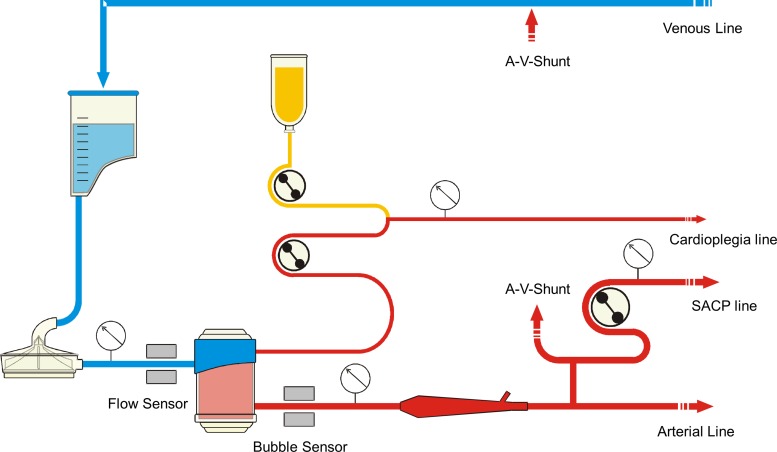
Cardiopulmonary bypass (CPB) is conducted with a specially configured heart–lung machine S5 (Stoeckert, München, Germany) including a centrifugal pump CP5 as an arterial pump instead of a roller pump and an additional roller pump for SACP. A level sensor is used at the reservoir and a bubble sensor is positioned at the arterial line after the oxygenator. For separate perfusion of the heart, we use the cardioplegia pump. The additional pump and cardioplegia pump are connected to the arterial pump using the master-slave setting on the heart–lung machine. The centrifugal pump's flow probe is positioned between the arterial pump and oxygenator. For safety, a pressure transducer is installed in the SACP line and linked to the SACP pump (Figure 1). Temperatures were measured at the venous reservoir inlet, the oxygenator outlet, and at the outlet of the cardioplegia heat exchanger.
Figure 1.
Extracorporeal circulation setup for open aortic arch replacement.
The disposables consist of an open venous reservoir “Synthesis” (Sorin Group, Mirandola, Italy), an additional reservoir “Admicard1800” (Eurosets, Medolla, Italy) for separate filtration of sucker blood, the centrifugal pump head “Revolution” (Sorin Group, Mirandola, Italy), the oxygenator D903 Avant (Sorin Group, Mirandola, Italy) and the dynamic bubble trap (Kardialgut, Axtbrunn, Germany). The tube connecting the additional pump for the SACP branches off from the arterial line after the dynamic bubble trap. To achieve a higher flow in the SACP than normal, we use a 5/16″ tube in the SACP pump to generate a maximum flow of 2.33 L/min. Priming for the circuit consists of 1,000 mL balanced electrolyte solution, 250 mL mannitol 20%, and 15,000 IU heparin.
Perfusion Strategy
Initial arterial cannulation is achieved via right axillary artery with or without a second aortic cannulation. The axillary cannulation line is used for the SACP later. We initiate CPB under full heparinization (300 IU/kg) and after activated clotting time have attained 400 seconds. This duration of activated clotting time is maintained throughout CPB. Cooling is immediately started to drop the patient's temperature to 25°C. Hematocrit is kept between 20% and 25% during circulatory arrest and bilateral SACP, and above 30% after rewarming. Blood gas is measured at 20-minute intervals. We maintain a pump flow of 2.6 L/min/m2 during normothermia and the cooling period. At the beginning of bilateral SACP, a flow of 10 mL/min/kg and pressure of 60 mmHg in the right radial artery are maintained. During bilateral SACP, the flow rate is also adjusted according to the course of NIRS values. pH is managed according to the alpha-stat principle. After the period of SACP and lower body circulatory arrest for the distal arch anastomosis, we start selectively lower body perfusion via the side branch of the arch prosthesis or the femoral artery at a flow of 20–25 mL/min/kg and begin rewarming. After completing the anastomosis of the supra-aortic branches, we lower the flow in the SACP pump and increase the lower body perfusion's flow. To carry out this procedure on a beating heart, we use continuous cardiac perfusion via the cardioplegia pump in an antegrade fashion with the ascending aorta clamped and the perfusion cannula toward the heart. This strategy requires effective left ventricle venting and a competent aortic valve. The selective heart perfusion temperature for this beating heart aortic arch replacement should be kept above 32°C to avoid ventricular fibrillations. In case cardiac arrest is required during the procedure, we use an intermittent infusion of cold-blood cardioplegic solution for myocardial protection.
Surgical Procedure
The spectrum of procedures facilitated by the described perfusion strategy includes conventional, open prosthetic replacement of the complete aortic arch as first-time, or redo-procedure for both dissected and non-dissected aortic pathologies, with or without placement of a “frozen elephant trunk” as step one of a staged thoracic aortic repair.
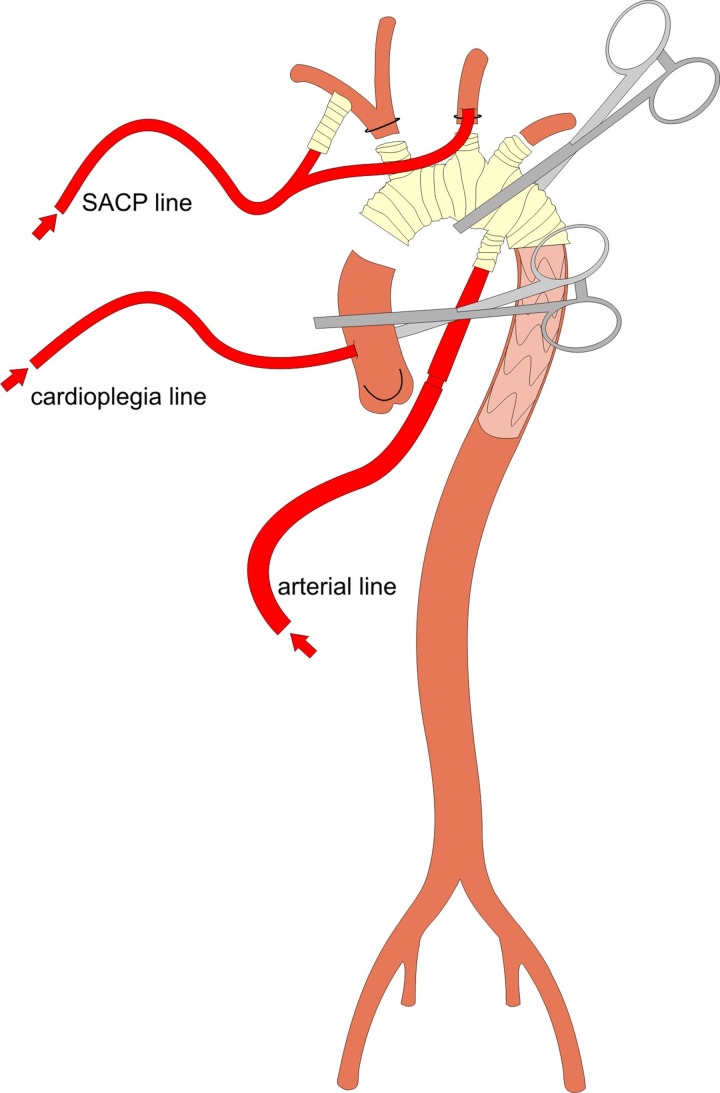
The right axillary artery is dissected free in the infraclavicular groove and an 8-mm dacron graft is anastomosed for arterial cannulation. The surgical access is a conventional median sternotomy. Extracorporeal circulation is commenced and cooling to 25°C body core temperature is then initiated. After the extensive preparation and mobilization of the aortic arch, all three native supra-aortic branches are visualized. Moderate hypothermic circulatory arrest (HCA) of the lower body is achieved, the aortic arch is opened and the left common carotid artery is then selectively cannulated for bilateral SACP. A prefabricated hybrid stent-graft and conventional vascular prosthesis (Thoraflex™ Hybrid Prosthesis [Vascutek Ltd., Glasgow, Scotland, UK]) is used as frozen elephant trunk for complete aortic arch replacement. After the implantation of the hybrid stent graft through the open aortic arch into the proximal descending thoracic aorta, the distal anastomosis to the prosthesis is performed with continuous 2–0 Prolene suture including felt pledgets. The distal arch anastomosis might be proximalized by surgical closure of the left subclavian artery (LSA) and anastomosing the arch prosthesis to the arch region between LSA and left carotid artery. The LSA is connected to the prosthesis using the respective branch or left-sided carotid-subclavian bypass was implanted before the arch procedure. The side branch of the arch prosthesis is cannulated, lower body perfusion initiated with the arterial pump after clamping the Dacron prosthesis. The line of the SACP pump is connected to the SACP cannulas (Figure 2). Anastomoses of the supra-aortic branches are performed next, and thereafter, the proximal graft-to-graft anastomosis is completed (Figure 3).
Figure 2.
Cannulation for simultaneous perfusion of heart, brain and lower body after lower body circulatory arrest for open aortic replacement.
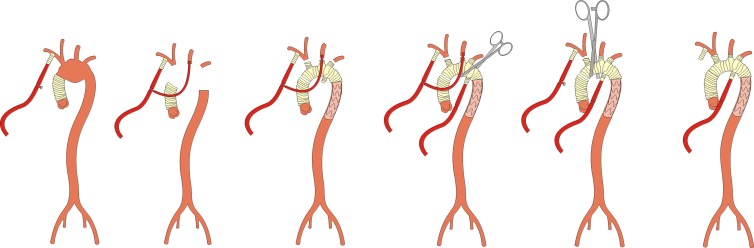
Figure 3.
Bilateral SACP and subsequent perfusion of lower body for frozen elephant trunk procedure with a trifurcated vascular graft (Thoraflex™ hybrid prosthesis, Vascutek Ltd., Glasgow, Scotland, UK) as a reoperation after initial ascending aortic replacement.
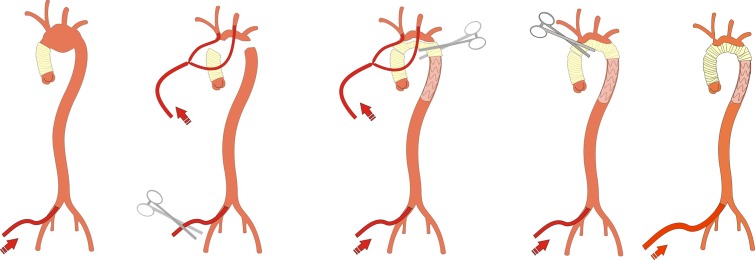
This procedure can be performed using different arch prostheses without supra-aortic branches and the insula technique for reimplantation of the supra-aortic branches and femoral perfusion, as well (Figure 4).
Figure 4.
Combined femoral and bilateral SACP for aortic arch replacement procedure using the insula technique and antegrade stent-graft implantation as a reoperation after initial ascending aortic replacement.
RESULTS
Eighteen patients were successfully treated with this perfusion strategy from October 2012 to November 2015. It was applied in 13 patients during arch replacement as the first procedure. Seven patients received a replacement of the ascending aorta, one patient received aortic valve replacement; another patient received two coronary artery bypass grafts, too. Five patients received aortic arch replacement as a redo procedure after replacement of the ascending aorta. Mean age was 63.9 ± 10.9 years. Twelve patients (66%) were men. Detailed patient data are listed in Table 1.
Table 1.
Patient data.
| Number of patients | 18 |
| Age (years) | 63.9 ± 10.9 |
| Men | 12 (66%) |
| Aortic arch aneurysm | 11 |
| Aneurysm of aortic arch and descending aorta | 3 |
| Acute type A dissection | 1 |
| Type B dissection | 3 |
| Previous surgical approach (acute type A dissection) | 6 |
| Time since previous surgical approach (month) | 53 ± 56 (6–148) |
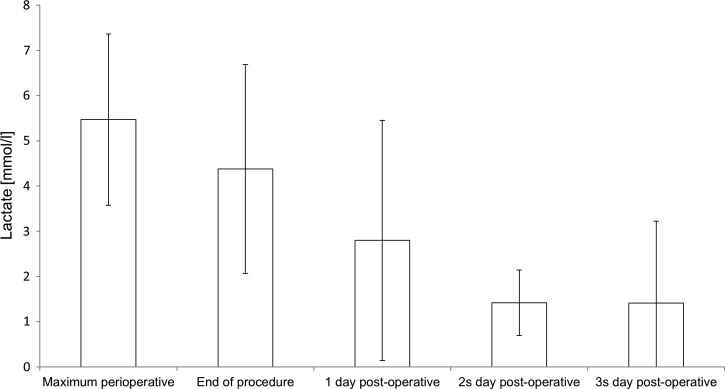
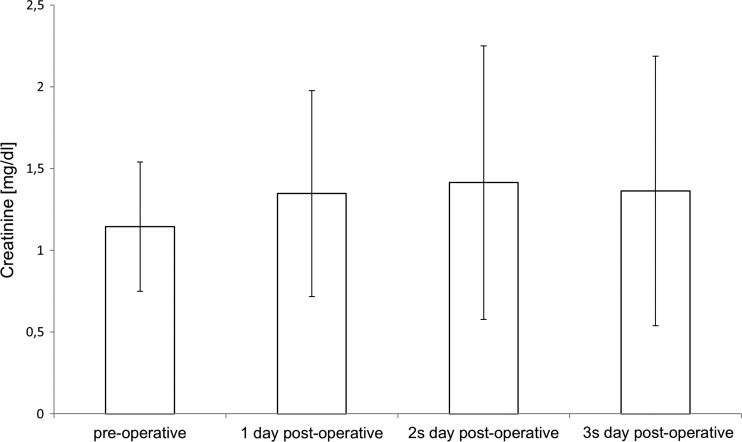
A Thoraflex™ hybrid prosthesis was used in 16 patients, while 1 patient underwent a stent-graft implantation and aortic arch replacement via the insula technique and another patient underwent aortic arch replacement with a branched prosthesis without stent graft (Plexus™ Prosthesis [Vascutek Ltd., Glasgow, Scotland, UK]). A bilateral SACP with moderate hypothermia and subsequent simultaneous selective perfusion of heart, brain, and body was used in all 18 patients with no problems. No complications related to the heart–lung machine or cannulation occurred during the procedures. Mean CPB time was 239 ± 33 minutes, the simultaneous selective perfusion of brain, heart, and remaining body was 55 ± 23 minutes. A typical pump-flow course during CPB is illustrated in Figure 5. Minimum body temperature during HCA was 25°C. Intraoperative details are shown in Table 2. Postoperative mechanical ventilation time was 30 ± 17 hours. Detailed NIRS data are shown in Table 3. One patient required re-exploration for 15 days after the procedure caused by bleeding. Three patients suffered a transient delirium consisting of confusion and agitation that resolved completely within 24 hours. One patient suffered a temporary neurological deficit focused on a sensibility disorder in the left arm that resolved completely during intensive care unit (ICU) stay. Postoperative data are shown in Table 4. No patient experienced a permanent neurological deficit. Two patients were in the RIFLE stadium “Risk” postoperatively. In all other patients, the creatinine level increased less than 1.5 times compared to the baseline. No patient required dialysis. Creatinine and lactate levels are listed in Figures 6 and 7.
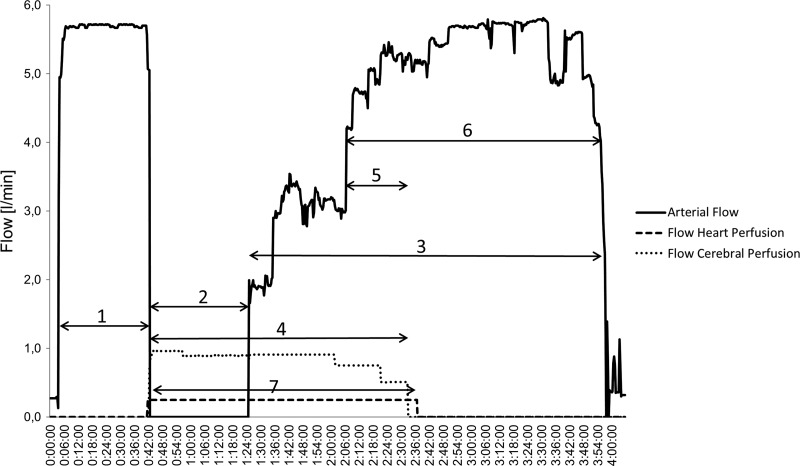
Figure 5.
Course of flow during aortic arch replacement. 1, perfusion of the whole body and cooling; 2, selective bilateral cerebral perfusion and lower body circulatory arrest; 3, post ischemic lower body perfusion and rewarming; 4, SACP; 5, anastomosis of Truncus; 6, completion of the operation and rewarming with full pump flow; 7, selective heart perfusion.
Table 2.
Perioperative data.
| Initial arterial cannulation via right axillary artery | n = 16 |
| Initial arterial cannulation via femoral artery | n = 2 |
| Cardiopulmonary bypass time (minutes) | 239 ± 33 |
| Selective antegrade cerebral perfusion and lower body circulatory arrest (minutes) | 60 ± 11 |
| Selective antegrade cerebral perfusion and lower body perfusion (minutes) | 55 ± 23 |
| Cross-clamp time (minutes) | 128 ± 40 |
| Flow selective antegrade cerebral perfusion (mL/min) | 700–1,400 |
| Lowest body temperature (°C) | 25.0 |
Table 3.
NIRS values.
| Left hemisphere | Right hemisphere | |
|---|---|---|
| Reference value (%) | 60.4 ± 9.3 | 58.2 ± 9.7 |
| Mean NIRS value during cooling (%) | 65.6 ± 3.6 | 66.4 ± 3.6 |
| Mean NIRS value during bilateral SACP (%) | 62.8 ± 8.7 | 65.1 ± 9.3 |
| Mean NIRS value during rewarming (%) | 63.6 ± 6.4 | 64.6 ± 5.8 |
| Time below reference value (minutes) | 16 | 11 |
Table 4.
Postoperative data.
| ICU stay (days) | 5.7 ± 4.1 |
| Postoperative mechanical ventilation time (hours) | 30 ± 17 |
| Hospital stay (days) | 23.7 ± 4.2 |
| Transient delirium | 3 |
| Temporary neurological deficit | 1 |
| Permanent neurological deficit | 0 |
| Acute renal failure | 0 |
| Re-exploration caused by bleeding | 1 |
Figure 6.
Peri- and postoperative lactate.
Figure 7.
Pre- and postoperative creatinine levels.
DISCUSSION
Results of aortic arch replacement surgery have improved over the years. The key to a successful outcome in open aortic arch replacement is the combination of an adapted cannulation and perfusion technique to minimize circulatory arrest and organ ischemia.
To achieve these goals, we successfully used a customized setup of the heart–lung machine. In standard cardiac surgery procedures, no difference in patient outcomes between roller and centrifugal pump was reported (14), but we found the centrifugal pump's characteristics to be advantageous for this perfusion strategy. One can also use a roller pump as the arterial pump in this perfusion approach (13). However, the acute risk of air embolism in the ECC system arises with that system when the two slave pumps operate faster than the arterial pump and thereby generate a negative pressure at the oxygenator outlet (15). The arterial roller pump has to be readjusted each time during a flow change in the cardioplegia or SACP pump as well. Unlike a roller pump, the centrifugal pump's speed need not be altered when changing the flow rate in the cardioplegia or SACP pump. The nonocclusive centrifugal pump prevents negative pressure in the perfusion system and thereby prevents air embolism, especially at high flow rates in the slave pumps too. The additional roller pump for the SACP permits accurate control and monitoring of the flow rate, which enables us to respond, e.g., to decreasing NIRS values during SACP.
In contrast to other perfusion strategies, using a single centrifugal pump and a dividing arterial line (11) or two roller pumps (10), we can regulate and control pump flow independently of each other. Also, using dividing arterial lines and adjustment of flows using flow restricting devices may induce hemolysis. Opting to use two roller pumps for selective heart and cerebral perfusion, and the centrifugal pump as an arterial pump and for lower body perfusion, enables us perfusing the heart, brain, and lower body entirely independently of each other. To improve the safety of these perfusion techniques, further improvements in the configuration features of the heart–lung machine would be necessary. Limiting the flow rates of the slave-pumps in relation to the arterial pump's flow rate would substantially reduce the risk of air embolism in the perfusion system.
In addition, speaking of cannulation strategies, the arterial cannula does not interfere with the surgeon's view of the surgical field. The cannulas need not be switched, and only the perfusion limb on the arch prosthesis must be cannulated and connected with the arterial line and the SACP line has to be connected with the SACP cannulas.
Due to the fact that we do not use a separate heat exchanger for the SACP, we cannot set separate temperatures for perfusion of the lower body and the SACP. If we start to rewarm the lower body, we automatically warm up the head as well. Since the anastomosis of the left carotid artery or the insula is usually performed during rewarming already, the team needs to ensure adequate left-sided NIRS during the phase of only unilateral SACP through the right axillary cannulation.
Our cannulation and perfusion strategy for aortic arch replacement ensures safe perfusion during this complex procedure, and it is applicable for redo procedures.
The use of SACP under mild hypothermia and parallel perfusion of the lower body for aortic arch replacement without stent-graft implantation in the descending aorta has been described (16). During antegrade stent-graft implantation and distal anastomosis, circulatory arrest of the lower body is unavoidable due to the bloodless surgical field. We prefer moderate hypothermia and SACP during lower body circulatory arrest for antegrade stent-graft implantation in the descending aorta and distal anastomosis, as this enables us to treat an aneurysm or descending aorta dissection during the same procedure.
Limitations
The number of patients that underwent the aforementioned perfusion strategy is still low. Furthermore, we did not have a control group to compare our present results with a perfusion strategy without simultaneous selective perfusion of brain, heart and body. Critique might arise from adding more complexity to an already complex operation. We admit that for perfusionist and surgeon, best possible preparation and communication are advisable dealing with more perfusion and pressure lines as usual.
CONCLUSION
Despite the considerable complexity of this surgical technique (for both aortic arch replacement and a redo procedure), the heart, brain and lower body perfusion strategy with separate pumps is feasible and safe as we observed excellent outcomes in this small series. We have found the frozen elephant trunk procedure using the Thoraflex™ hybrid prosthesis to be a well-suited tool for aortic arch replacement and for treating a descending aneurysm in a single procedure.
REFERENCES
- 1. de la Cruz K. I., Coselli J. S., LeMaire S. A.. Open aortic arch replacement: A technical odyssey. J Extra Corpor Technol. 2012;44:42–7. [PMC free article] [PubMed] [Google Scholar]
- 2. Leontyev S., Borger M. A., Etz C. D., et al. Experience with the conventional and frozen elephant trunk techniques: A single-centre study. Eur J Cardiothorac Surg. 2013;44:1076–82. [DOI] [PubMed] [Google Scholar]
- 3. De Paulis R., Czerny M., Weltert L., et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch. Eur J Cardiothorac Surg. 2015;47:917–23. [DOI] [PubMed] [Google Scholar]
- 4. Kondoh H., Taniguchi K., Funatsu T., et al. Total arch replacement with long elephant trunk anastomosed at the base of the innominate artery: A single-center longitudinal experience. Eur J Cardiothorac Surg. 2012;42:840–8. [DOI] [PubMed] [Google Scholar]
- 5. Numata S., Tsutsumi Y., Monta O., et al. Aortic arch repair with antegrade selective cerebral perfusion using mild to moderate hypothermia of more than 28°C. Ann Thorac Surg. 2012;94:90–5. [DOI] [PubMed] [Google Scholar]
- 6. Czerny M., König T., Reineke D., et al. Total surgical aortic arch replacement as a safe strategy to treat complex multisegmental proximal thoracic aortic pathology. Interact Cardiovasc Thorac Surg. 2013;17:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ius F., Fleissner F., Pichlmaier M., et al. Total aortic arch replacement with the frozen elephant trunk technique: 10-year follow-up single-centre experience. Eur J Cardiothorac Surg. 2013;44:949–57. [DOI] [PubMed] [Google Scholar]
- 8. Kuki S., Taniguchi K., Masai T., et al. A novel modification of elephant trunk technique using a single four-branched arch graft for extensive thoracic aortic aneurysm. Eur J Cardiothorac Surg. 2000;18:246–8. [DOI] [PubMed] [Google Scholar]
- 9. Moz M., Misfeld M., Leontyev S., et al. Aortic arch reoperation in a single centre: Early and late results in 57 consecutive patients. Eur J Cardiothorac Surg. 2013;44:e82–6. [DOI] [PubMed] [Google Scholar]
- 10. Nappi G., Maresca L., Torella M., et al. Body perfusion in surgery of the aortic arch. Tex Heart Inst J. 2007;34:23–9. [PMC free article] [PubMed] [Google Scholar]
- 11. Iwata K., Shimazaki Y., Sakamoto T., et al. Aortic arch surgery with a single centrifugal pump for selective cerebral perfusion and systemic circulation. Artif Organs. 2010;34:E22–5. [DOI] [PubMed] [Google Scholar]
- 12. Shrestha M., Bachet J., Bavaria J., et al. Current status and recommendations for use of the frozen elephant trunk technique: A position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–69. [DOI] [PubMed] [Google Scholar]
- 13. Haldenwang P. L., Klein T., Neef K., et al. Evaluation of the use of lower body perfusion at 28°C in aortic arch surgery. Eur J Cardiothorac Surg. 2012;41:e100–8. [DOI] [PubMed] [Google Scholar]
- 14. Parolari A., Alamanni F., Naliato M., et al. Adult cardiac surgery outcomes: Role of the pump type. Eur J Cardiothorac Surg. 2000;18:575–82. [DOI] [PubMed] [Google Scholar]
- 15. Kayser K. L. Pulsatile perfusion problems. Ann Thorac Surg. 1979;27:284–5. [DOI] [PubMed] [Google Scholar]
- 16. Luehr M., Bachet J., Mohr F. W., et al. Modern temperature management in aortic arch surgery: The dilemma of moderate hypothermia. Eur J Cardiothorac Surg. 2013;45:27–39. [DOI] [PubMed] [Google Scholar]