Abstract
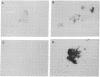
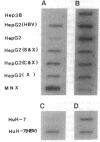
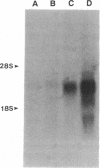
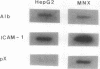
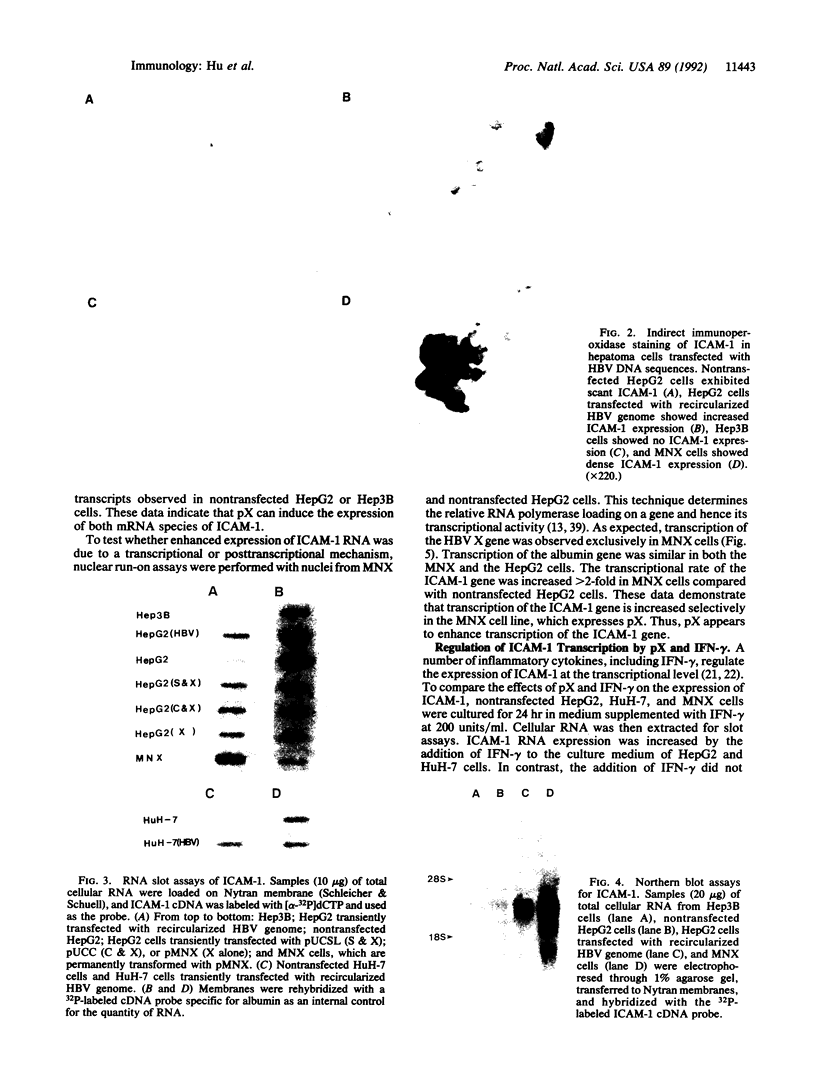
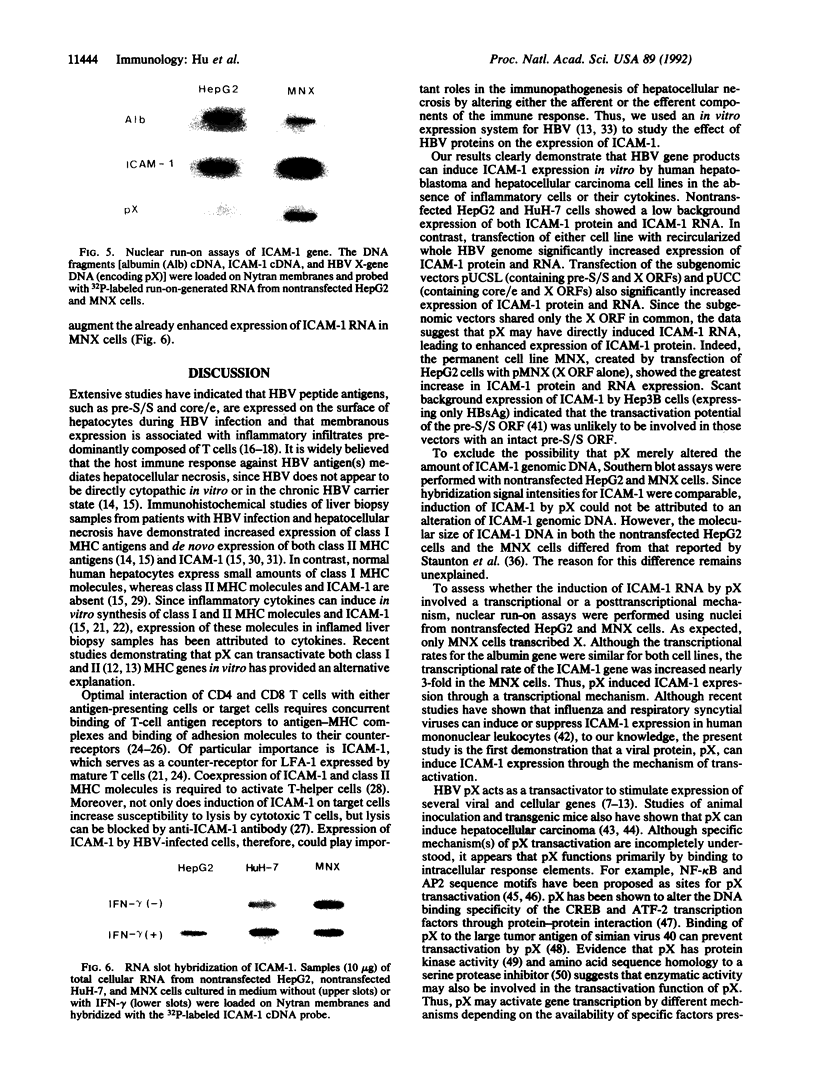
Intercellular adhesion molecule 1 (ICAM-1), a counter-receptor for lymphocyte function-associated antigen 1 on T cells, is critically important to a wide variety of adhesion-dependent leukocyte functions, including antigen presentation and target cell lysis. ICAM-1 expression by hepatocytes is increased in areas of inflammation and necrosis during chronic hepatitis B. Whether induction of ICAM-1 is due to the effect of inflammatory cytokines or involves a direct effect of the hepatitis B virus (HBV) remains unknown. In the present study, transfection of the HBV genome into human hepatoma cell lines resulted in enhanced expression of ICAM-1 protein and RNA in the absence of inflammation. Results of subgenomic transfections indicated that the HBV X protein (pX) induced ICAM-1 expression. Nuclear run-on assays showed that pX induced the ICAM-1 gene by increasing its rate of transcription. Although both pX and interferon gamma induced transcription of ICAM-1, addition of interferon gamma to cells expressing pX did not show an additive or synergistic effect. These results indicate that pX can directly regulate expression of ICAM-1 and may participate in the immunopathogenesis of HBV infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S. K., Kalvakolanu D. V., Sen G. C. Gene induction by interferons: functional complementation between trans-acting factors induced by alpha interferon and gamma interferon. Mol Cell Biol. 1990 Oct;10(10):5055–5063. doi: 10.1128/mcb.10.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselmann W. H., Meyer M., Kekulé A. S., Lauer U., Hofschneider P. H., Koshy R. A trans-activator function is generated by integration of hepatitis B virus preS/S sequences in human hepatocellular carcinoma DNA. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2970–2974. doi: 10.1073/pnas.87.8.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano P., Berchtold C., Casero R. A., Jr A simplification of the nuclear run-off transcription assay. Biotechniques. 1989 Oct;7(9):942–944. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L. Two-way signalling through the LFA-1 lymphocyte adhesion receptor. Bioessays. 1990 Sep;12(9):421–427. doi: 10.1002/bies.950120905. [DOI] [PubMed] [Google Scholar]
- Eggink H. F., Houthoff H. J., Huitema S., Wolters G., Poppema S., Gips C. H. Cellular and humoral immune reactions in chronic active liver disease. II. Lymphocyte subsets and viral antigens in liver biopsies of patients with acute and chronic hepatitis B. Clin Exp Immunol. 1984 Apr;56(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H., Dusheiko G. M., Schafer D. F., Jones E. A., Micetich K. C., Young R. C., Costa J. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med. 1982 Apr;96(4):447–449. doi: 10.7326/0003-4819-96-4-447. [DOI] [PubMed] [Google Scholar]
- Hu K. Q., Hao L. J., Will H. A preliminary study on localization of HBxAg in liver tissue of patients with chronic liver disease and its significance. Chin Med J (Engl) 1988 Sep;101(9):671–674. [PubMed] [Google Scholar]
- Hu K. Q., Hao L. J., Zhang Y. Y., Wang Y. K. Intrahepatic expression of pre-S proteins of hepatitis B virus and its possible relation to liver cell necrosis. Am J Gastroenterol. 1989 Dec;84(12):1538–1542. [PubMed] [Google Scholar]
- Hu K. Q., Siddiqui A. Regulation of the hepatitis B virus gene expression by the enhancer element I. Virology. 1991 Apr;181(2):721–726. doi: 10.1016/0042-6822(91)90906-r. [DOI] [PubMed] [Google Scholar]
- Hu K. Q., Vierling J. M., Siddiqui A. Trans-activation of HLA-DR gene by hepatitis B virus X gene product. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7140–7144. doi: 10.1073/pnas.87.18.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvakolanu D. V., Bandyopadhyay S. K., Harter M. L., Sen G. C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins: block in transcriptional complex formation. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. M., Koike K., Saito I., Miyamura T., Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991 May 23;351(6324):317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Maguire H. F., Hoeffler J. P., Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991 May 10;252(5007):842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Malizia G., Dino O., Pisa R., Caltagirone M., Giannuoli G., Di Marco V., Aragona E., Calabrese A., Raiata F., Craxi A. Expression of leukocyte adhesion molecules in the liver of patients with chronic hepatitis B virus infection. Gastroenterology. 1991 Mar;100(3):749–755. doi: 10.1016/0016-5085(91)80021-z. [DOI] [PubMed] [Google Scholar]
- Mondelli M. U., Manns M., Ferrari C. Does the immune response play a role in the pathogenesis of chronic liver disease? Arch Pathol Lab Med. 1988 May;112(5):489–497. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Peters M., Vierling J., Gershwin M. E., Milich D., Chisari F. V., Hoofnagle J. H. Immunology and the liver. Hepatology. 1991 May;13(5):977–994. [PubMed] [Google Scholar]
- Salkind A. R., Nichols J. E., Roberts N. J., Jr Suppressed expression of ICAM-1 and LFA-1 and abrogation of leukocyte collaboration after exposure of human mononuclear leukocytes to respiratory syncytial virus in vitro. Comparison with exposure to influenza virus. J Clin Invest. 1991 Aug;88(2):505–511. doi: 10.1172/JCI115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S. Mutual functional antagonism of the simian virus 40 T antigen and the hepatitis B virus trans activator. J Virol. 1991 May;65(5):2351–2356. doi: 10.1128/jvi.65.5.2351-2356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata Y., Kawada M., Fujiki Y., Sano H., Oda M., Yaginuma K., Kobayashi M., Koike K. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn J Cancer Res. 1989 Jul;80(7):617–621. doi: 10.1111/j.1349-7006.1989.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Gaynor R., Srinivasan A., Mapoles J., Farr R. W. trans-activation of viral enhancers including long terminal repeat of the human immunodeficiency virus by the hepatitis B virus X protein. Virology. 1989 Apr;169(2):479–484. doi: 10.1016/0042-6822(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Jameel S., Mapoles J. Expression of the hepatitis B virus X gene in mammalian cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2513–2517. doi: 10.1073/pnas.84.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Thomas J. A. Cellular expression of lymphocyte function associated antigens and the intercellular adhesion molecule-1 in normal tissue. J Clin Pathol. 1990 Nov;43(11):893–900. doi: 10.1136/jcp.43.11.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Takada S., Koike K. X protein of hepatitis B virus resembles a serine protease inhibitor. Jpn J Cancer Res. 1990 Dec;81(12):1191–1194. doi: 10.1111/j.1349-7006.1990.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Hepatic expression of intercellular adhesion molecule-1 (ICAM-1) in viral hepatitis B. Hepatology. 1990 Jul;12(1):148–154. doi: 10.1002/hep.1840120123. [DOI] [PubMed] [Google Scholar]
- Wang W. L., London W. T., Lega L., Feitelson M. A. HBxAg in the liver from carrier patients with chronic hepatitis and cirrhosis. Hepatology. 1991 Jul;14(1):29–37. doi: 10.1002/hep.1840140106. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Zhou Z. Y., Judd A., Cartwright C. A., Robinson W. S. The hepatitis B virus-encoded transcriptional trans-activator hbx appears to be a novel protein serine/threonine kinase. Cell. 1990 Nov 16;63(4):687–695. doi: 10.1016/0092-8674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Zahm P., Hofschneider P. H., Koshy R. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene. 1988 Aug;3(2):169–177. [PubMed] [Google Scholar]
- Zhou D. X., Taraboulos A., Ou J. H., Yen T. S. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990 Aug;64(8):4025–4028. doi: 10.1128/jvi.64.8.4025-4028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]