Abstract
BACKGROUND: In 1992, non-onchocercal uveitis caused 9% of blindness, 8% of visual impairment, and 11% of uniocular blindness among patients visiting an eye hospital in Sierra Leone, west Africa. The aim of this study was to determine the aetiology of uveitis in this population. METHODS: General and ophthalmic examination complemented by serum and aqueous humour analyses for various infectious agents was performed for 93 uveitis patients and compared with serum (n = 100) and aqueous humour (n = 9) analysis of endemic controls. RESULTS: At the initial examination, 45 patients (48%) proved to be severely visually handicapped. After clinical and laboratory analyses, an aetiological diagnosis was established for 49 patients (52%). Toxoplasma gondii was the most important cause of uveitis (40/93; 43%). Anti-toxoplasma IgM antibodies were detected in serum samples of seven of 93 patients (8%) compared with one of 100 controls (1%, p < 0.05). At least six patients (15%) with ocular toxoplasmosis had acquired the disease postnatally. Antibodies against Treponema pallidum were detected in 18 of 92 patients (20%) and in 21 controls (21%). Other causes of uveitis were varicella zoster virus (one patient), herpes simplex virus (two patients), and HLA-B27 positive acute anterior uveitis with ankylosing spondylitis (one patient), while one patient had presumed HTLV-I uveitis. CONCLUSIONS: In a hospital population in Sierra Leone, west Africa, uveitis was associated with severe visual handicap and infectious diseases. Toxoplasmosis proved to be the most important cause of the uveitis. Although the distribution of congenital versus acquired toxoplasmosis in this population could not be determined, the results indicate an important role of postnatally acquired disease. The results further suggested minor roles for HIV, tuberculosis, toxocariasis, and sarcoidosis as causes of uveitis in this population.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayanru J. O. The problem of uveitis in Bendel State of Nigeria: experience in Benin City. Br J Ophthalmol. 1977 Oct;61(10):655–659. doi: 10.1136/bjo.61.10.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson M. W., Takafuji E. T., Lemon S. M., Greenup R. L., Sulzer A. J. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982 Sep 9;307(11):666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- Bloch-Michel E., Nussenblatt R. B. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987 Feb 15;103(2):234–235. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- Chesterton J. R., Perkins E. S. Ocular toxoplasmosis among Negro immigrants in London. Br J Ophthalmol. 1967 Sep;51(9):617–621. doi: 10.1136/bjo.51.9.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussaix E., Cerqueti P. M., Pontet F., Bloch-Michel E. New approaches to the detection of locally produced antiviral antibodies in the aqueous of patients with endogenous uveitis. Ophthalmologica. 1987;194(2-3):145–149. doi: 10.1159/000309752. [DOI] [PubMed] [Google Scholar]
- GOLDMANN H., WITMER R. Antikörper im Kammerwasser. Ophthalmologica. 1954 Apr-May;127(4-5):323–330. doi: 10.1159/000301976. [DOI] [PubMed] [Google Scholar]
- Glasner P. D., Silveira C., Kruszon-Moran D., Martins M. C., Burnier Júnior M., Silveira S., Camargo M. E., Nussenblatt R. B., Kaslow R. A., Belfort Júnior R. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992 Aug 15;114(2):136–144. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- Henderly D. E., Genstler A. J., Smith R. E., Rao N. A. Changing patterns of uveitis. Am J Ophthalmol. 1987 Feb 15;103(2):131–136. doi: 10.1016/s0002-9394(14)74217-5. [DOI] [PubMed] [Google Scholar]
- Kotake S., Kimura K., Yoshikawa K., Sasamoto Y., Matsuda A., Nishikawa T., Fujii N., Matsuda H. Polymerase chain reaction for the detection of Mycobacterium tuberculosis in ocular tuberculosis. Am J Ophthalmol. 1994 Jun 15;117(6):805–806. doi: 10.1016/s0002-9394(14)70328-9. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Tajima K., Watanabe T., Yamaguchi K. Human T lymphotropic virus type 1 uveitis. Br J Ophthalmol. 1994 Feb;78(2):149–154. doi: 10.1136/bjo.78.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoo F. L., Hack C. E., Oosting J., Stilma J. S., Kijlstra A. Neutrophil activation in ivermectin-treated onchocerciasis patients. Clin Exp Immunol. 1993 Nov;94(2):330–333. doi: 10.1111/j.1365-2249.1993.tb03452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B. The natural history of uveitis. Int Ophthalmol. 1990 Oct;14(5-6):303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Perkins E. S. Epidemiology of uveitis. Trans Ophthalmol Soc U K. 1976 Apr;96(1):105–107. [PubMed] [Google Scholar]
- Rehder J. R., Burnier M. B., Jr, Pavesio C. E., Kim M. K., Rigueiro M., Petrilli A. M., Belfort R., Jr Acute unilateral toxoplasmic iridocyclitis in an AIDS patient. Am J Ophthalmol. 1988 Dec 15;106(6):740–741. doi: 10.1016/0002-9394(88)90712-x. [DOI] [PubMed] [Google Scholar]
- Ronday M. J., Luyendijk L., Baarsma G. S., Bollemeijer J. G., Van der Lelij A., Rothova A. Presumed acquired ocular toxoplasmosis. Arch Ophthalmol. 1995 Dec;113(12):1524–1529. doi: 10.1001/archopht.1995.01100120054009. [DOI] [PubMed] [Google Scholar]
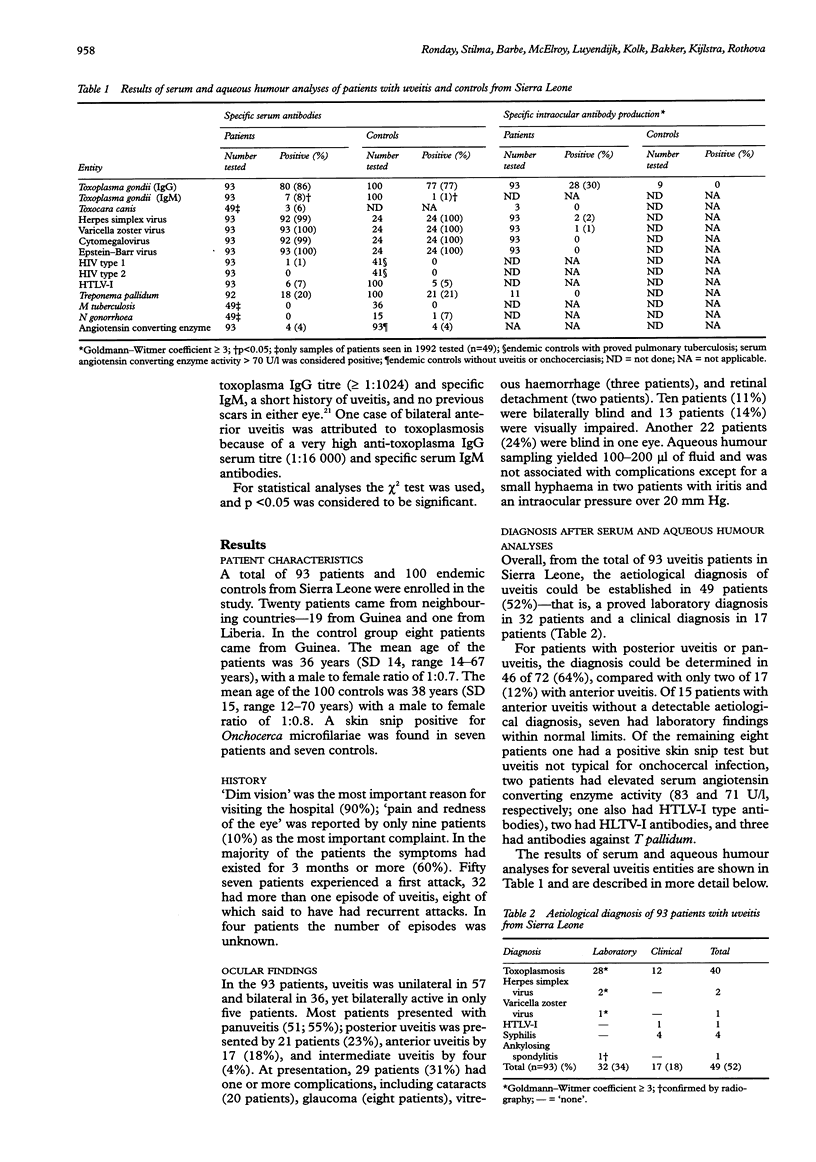
- Ronday M. J., Stilma J. S., Barbe R. F., Kijlstra A., Rothova A. Blindness from uveitis in a hospital population in Sierra Leone. Br J Ophthalmol. 1994 Sep;78(9):690–693. doi: 10.1136/bjo.78.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova A., Buitenhuis H. J., Meenken C., Brinkman C. J., Linssen A., Alberts C., Luyendijk L., Kijlstra A. Uveitis and systemic disease. Br J Ophthalmol. 1992 Mar;76(3):137–141. doi: 10.1136/bjo.76.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdy P. R., Lapworth R., Bird R. Angiotensin-converting enzyme and its clinical significance--a review. J Clin Pathol. 1983 Aug;36(8):938–947. doi: 10.1136/jcp.36.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbara K. F., al Kaff A. S., Fadel T. Ocular manifestations of endemic syphilis (bejel). Ophthalmology. 1989 Jul;96(7):1087–1091. doi: 10.1016/s0161-6420(89)32781-3. [DOI] [PubMed] [Google Scholar]
- Touboul J. P., Le Hoang P., Fontaine M., Wechsler B., Cabane J., Godeau P., Schuller E. Uvéites au cours de la syphilis acquise. J Fr Ophtalmol. 1985;8(4):321–331. [PubMed] [Google Scholar]
- Van der Lelij A., Doekes G., Hwan B. S., Vetter J. C., Rietveld E., Stilma J. S., Kijlstra A. Humoral autoimmune response against S-antigen and IRBP in ocular onchocerciasis. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1374–1380. [PubMed] [Google Scholar]
- Verbon A., Weverling G. J., Kuijper S., Speelman P., Jansen H. M., Kolk A. H. Evaluation of different tests for the serodiagnosis of tuberculosis and the use of likelihood ratios in serology. Am Rev Respir Dis. 1993 Aug;148(2):378–384. doi: 10.1164/ajrccm/148.2.378. [DOI] [PubMed] [Google Scholar]
- Young H., Low A. C. Serological diagnosis of gonorrhoea: detection of antibodies to gonococcal pili by enzyme-linked immunosorbent assay. Med Lab Sci. 1981 Jan;38(1):41–47. [PubMed] [Google Scholar]
- de Boer J. H., Luyendijk L., Rothova A., Baarsma G. S., de Jong P. T., Bollemeijer J. G., Rademakers A. J., Van der Lelij A., Zaal M. J., Kijlstra A. Detection of intraocular antibody production to herpesviruses in acute retinal necrosis syndrome. Am J Ophthalmol. 1994 Feb 15;117(2):201–210. doi: 10.1016/s0002-9394(14)73077-6. [DOI] [PubMed] [Google Scholar]
- de Boer J. H., Luyendijk L., Rothova A., Kijlstra A. Analysis of ocular fluids for local antibody production in uveitis. Br J Ophthalmol. 1995 Jun;79(6):610–616. doi: 10.1136/bjo.79.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Knapen F., van Leusden J., Polderman A. M., Franchimont J. H. Visceral larva migrans: examinations by means of enzyme-linked immunosorbent assay of human sera for antibodies to excretory-secretory antigens of the second-stage larvae of Toxocara canis. Z Parasitenkd. 1983;69(1):113–118. doi: 10.1007/BF00934015. [DOI] [PubMed] [Google Scholar]



