Abstract
Disinhibition is an important symptom in neurodegenerative diseases. However, the clinico-anatomical underpinnings remain controversial. We explored the anatomical correlates of disinhibition in neurodegenerative disease using the perspective of grey and white matter imaging. Disinhibition was assessed with a neuropsychological test and a caregiver information-based clinical rating scale in 21 patients with prefrontal syndromes due to behavioural variant frontotemporal dementia (n = 12) or progressive supranuclear palsy (n = 9), and healthy controls (n = 25). Cortical thickness was assessed using the Freesurfer software on 3T MRI data. The integrity of selected white matter tracts was determined by the fractional anisotropy (FA) from Diffusion Tensor Imaging. Disinhibition correlated with the cortical thickness of the right parahippocampal gyrus, right orbitofrontal cortex and right insula and the FA of the right uncinate fasciculus and right anterior cingulum. Notably, no relationship was seen with the thickness of ventromedial prefrontal cortex. Our results support an associative model of inhibitory control, distributed in a medial temporal lobe-insular-orbitofrontal network, connected by the intercommunicating white matter tracts. This reconciles some of the divergences among previous studies, but also questions the current conceptualisation of the “prefrontal” syndrome and the central role attributed to the ventromedial prefrontal cortex in inhibitory control.
Introduction
Disinhibition is considered a cardinal feature in the prefrontal syndrome, [1–3]. More specifically, the classical clinico-anatomical correlate of disinhibition is the orbitofrontal cortex (OFC) [1–3]. This appears to apply also to neurodegenerative diseases, where disinhibition is associated with OFC atrophy [4–6]. However, a number of studies in these conditions imply considerably larger areas involved in behavioural inhibition including the temporal lobe, amygdala, hippocampus, caudate, insula and other parts of the prefrontal cortex (PFC) than the OFC [7–10]. Studies not finding any correlate to the OFC, have questioned the “OFC model” in its entirety [9]. The studies mentioned above have all correlated disinhibition to imaging of the grey matter, while similar approaches using white matter neuroimaging with Diffusion Tensor Imaging (DTI) are just emerging [11]. DTI is a method that gives the opportunity to describe the anatomy of white matter fibre bundles in vivo and to quantify changes in these tracts as a consequence of pathology [12]. In the context of correlative neuroanatomy, this enables a structural connectivity approach as a supplement to the cortical locationalist approach [13], which potentially can yield better models. In some conditions, the white matter imaging perspective has been able to literally bridge apparent discrepancies in assessments of cortical function. Psychopathy, for example, has been alternately associated with aberrations in the OFC and/or the right anterior temporal lobe, which may be reconciled by the findings of loss of altered diffusion properties of the white matter fibre bundle connecting these regions, i.e. the uncinate fasciculus [14].
In the current study we used a mixed cohort of patients with prefrontal syndromes with a diagnosis of either behavioural variant frontotemporal dementia (bvFTD) or progressive supranuclear palsy (PSP). These disorders were chosen since they have overlapping cognitive and behavioural deficits, with a variable extent of prefrontal syndrome severity [15–19], and are biologically similar with overlapping topography, neuropathology and genetics [20–22]. In bvFTD atrophy predominantly affects the prefrontal (including OFC), anterior temporal and insular cortex [23] with corresponding affliction of the basal ganglia [24], with the emergence of corresponding symptoms: apathy, disinhibition, dysphasia, loss of insight and executive deficits [25]. In PSP, the pathology always affects the brainstem and basal ganglia and to a variable extent the prefrontal cortex, resulting in a prefrontal-subcortical syndrome [6, 16, 22]. This is dominated by apathy but includes disinhibited behaviours, albeit being less frequent [15, 26]. As bvFTD/PSP represent a continuum of neurodegeneration of the prefrontal-subcortical systems corresponding to a varying degree of disinhibition, a mixed bvFTD/PSP cohort is favourable compared with the bvFTD and Alzheimer´s disease (AD) cohorts (two considerably more diverse disorders) when used in correlational studies of neurodegenerative disease [11, 27].
The primary aim of this study was to explore the neuroanatomical basis of disinhibition. To this purpose, measurements of cortical thickness and white matter integrity of fibre bundles leading to/from the OFC were correlated with results of a neuropsychological test of disinhibition and quantifications of behavioural disturbance using caregiver-based information. We hypothesised that by examining the correlation of behavioural measures to grey and white matter, we could provide an explanation for the conflicting results in previous grey matter only correlation studies. Also, direct comparisons of grey and white matter atrophy between the two prefrontal syndromes in our cohort (bvFTD and PSP) are rare and as such constituted a secondary aim of the study.
Methods
Participants
Participants were from the Lund Prospective Frontotemporal Dementia Study (LUPROFS), a longitudinal study of patients with any of the frontotemporal dementia spectrum disorders. Diagnostic workup included clinical examination, caregiver history with rating of behavioural disturbances and general disease severity, standardized neurological examination, MRI according to study protocol, and a comprehensive neuropsychological assessment. Selection criteria for the present study was a possible, probable or definite diagnosis of bvFTD or PSP according to FTDC [28] or NINDS criteria [29], respectively, completed MRI and availability of the Hayling neuropsychological test (described below) [30], which was part of an extended neuropsychological test protocol. Among the bvFTD patients (n = 12), 9 had probable and 3 definite bvFTD (genetic mutation carriers). Cerebrospinal fluid (CSF) was analysed for Alzheimer´s Disease (AD) core biomarkers (amyloid-ß 1–42, tau and p-tau) and a CSF biomarker profile indicative of AD [31], was used as an exclusion criterion [28]. Of the PSP patients (n = 9), 1 had possible and 8 probable PSP [29]. All patients except one were right-handed. Healthy controls (n = 25) underwent clinical interview and examination, including neuropsychological testing and MRI. Demographic and clinical data on the subjects are presented in Table 1.
Table 1. Demographic and clinical data of subjects.
| HC | bvFTD | PSP | Across | bvFTD vs. HC | PSP vs. HC | bvFTD vs. PSP | |
|---|---|---|---|---|---|---|---|
| Number | 25 | 12 | 9 | - | - | - | - |
| Sex | 13M 12F | 5M 7F | 3M 6F | n.s. | - | - | - |
| Age | 68 (35–82) | 71.5 (57–77) | 67 (58–76) | n.s. | - | - | - |
| Mini Mental Status Examination | 30 (29–30) | 26 (21–30) | 28 (24–30) | p < 0.001 | p < 0.001 | p = 0.004 | n.s. |
| Education | 14 (8–14) | 9 (7–14) | 11 (7–16) | n.s. | - | - | - |
| Frontotemporal Lobar Degeneration modified Clinical Dementia Rating scale | n.a. | 8.5 (2–15) | 4 (0.5–10.5) | - | - | - | n.s. |
| Duration | n.a. | 2.5 (1–12) | 4 (2–6) | - | - | - | n.s. |
| Frontal Behavioural Inventory1-10 | n.a. | 14 (6–21) | 7 (2–17) | - | - | - | n.s. |
| Frontal Behavioural Inventory12-22 | n.a. | 9 (1–20) | 5 (1–7) | - | - | - | p = 0.012 |
| The Hayling test | 4.5 (0–20) | 23 (5–72) | 7 (1–56) | p = 0.001 | p < 0.001 | n.s. | n.s. |
Data are median values, with range. Age: age at examination in years, bvFTD: behavioural variant frontotemporal dementia, Duration: disease duration (in years), Education: education (in years), Frontal Behavioural Inventory1-10 and Frontal Behavioural Inventory12-22: sum of items 1–10 and 12–22, respectively, The Hayling test: total error score on the Hayling test, HC: healthy controls, n.a: not applicable, n.s.: not significant, PSP: progressive supranuclear palsy.
Ethics statement
All participating subjects were informed of the study content in both oral and written form. Informed consent was taken in written form from the study subjects. If there was a doubt that a study subject had a compromised capacity to consent, informed consent was taken from the study subject and the spouse, or the spouse only. Importantly, regardless from whom informed consent was taken, the study subject always retained the right to decline or interrupt participation at any time. No formal assessment of the patients´ capacity to consent was performed. This procedure for informed consent, as well as all other aspects of the study, are according to the Helsinki declaration and were approved by the Regional Ethical Review Board, Lund, Sweden (Permit number 617/2008).
Neuropsychological measure of disinhibition
The Hayling Sentence Completion Test, often referred to as just the Hayling test, is designed to measure response initiation and inhibition of response by a sentence completion task [30]. In the first part of the test, subjects are asked to complete a sentence with an appropriate word. For instance, the examiner reads aloud “The rich child attended a private…”, where “school” is a correct response. In the second part of the test, subjects are asked to complete a sentence with a word that is not at all appropriate, thus requiring inhibiting of an automatic response. An example from this part of the test is: “London is a very lively…”, where “city” is an incorrect answer, but “banana”, “pet”, or “shoe” are examples of correct answers. Errors in the second part are recorded and form a total error score, in addition to time to complete for the first and the second parts, which together are transformed into an overall score. The construct validity of the Hayling test has been examined in a limited number of studies. In comparison with other tests of executive functions, the Hayling test has shown positive correlations with the Six Elements test and subsequent time in the Tower of London test in psychiatric patients with clinical difficulties of inhibition [32, 33]. In healthy controls, there is a positive correlation between the Hayling test and the Stroop test regarding initiation whereas no association was found regarding the inhibitory components [34, 35], indicating that these tests measure a different dimension of inhibitory capacity. The Hayling test has been used previously for patients with bvFTD [11, 36], PSP [37] and appears to be more discriminative in bvFTD vs. AD compared with the more commonly used Stroop test [38]. The latter findings also support that the Hayling test does indeed reflect a different aspect of inhibition than the Stroop test. Patients with bvFTD generally show worse performance than controls on the Hayling test overall scaled score, second part scaled score, and the total error score (i.e. cumulative errors from the second part of the test) [11, 36]. For the current study the total error score was chosen, since this appears to be the most specific for bvFTD [11, 36].
Behavioural and clinical severity rating
As a measure of behavioural inhibition in everyday life we used the Frontal Behavioural Inventory (FBI) [39]. The FBI is a rating scale based on a caregiver interview, administered by a health professional, with 24 items designed to capture core symptoms of bvFTD. It is widely used in studies on dementia and is more specific for “prefrontal” disturbances than more general neurobehavioural rating scales such as the Neuropsychiatric Inventory (NPI) [40]. The severity of single items is scored on a scale from 0 to 3 (not present, mild/occasional, moderate and severe/most of the time). The FBI is divided into two parts, where FBI items 1–10 represent negative symptoms such as apathy, emotional flatness, and personal neglect, and FBI item 12–22 represent positive symptoms with diminished inhibition such as perseverations, excessive jocularity, poor judgement, inappropriateness, impulsivity and hyperorality. For the current study, we used the sum of items 1–10 (FBI1-10) and the sum of items 12–22 (FBI12-22). For rating general disease severity we employed the sum of boxes in the Frontotemporal Lobar Degeneration modified Clinical Dementia Rating (FTLD-CDR) [41].
MRI acquisition
MRI was performed using a Philips Achieva 3T scanner equipped with an eight-channel head coil. DTI data were acquired using a single-shot spin echo sequence with EPI using 48 diffusion encoding directions, a diffusion-weighting factor (b) of 800 s/mm2, voxel size 2x2x2 mm3, TR 7881 ms, and TE 90 ms. Motion and eddy current correction of the data was performed with ElastiX [42], by affine registration of the diffusion-weighted images to the first non-diffusion weighted image in the protocol [43]. The DTI protocol was followed by a T1-weighted 3D volumetric sequence with TR 8.3 ms, TE 3.84 ms, FOV 256x256x175 and a voxel size of 1x1x1 mm3.
Cortical thickness analysis
Modeling and volumetric estimations of cortical brain regions were performed on structural T1 images using the Freesufer image analysis package version 5.3 (http://surfer.nmr.mgh.harvard.edu/). This automated tool performs imaging intensity normalization, removal of non-brain tissues, segmentation of cortical and subcortical brain regions into white and grey matter, spherical surface-based intersubject registration, which is based on the cortical surface curvature (sulci and gyri), and, finally, an automated parcellation of the cortical surface. Quality control of the Freesurfer output was done by visual inspection. When errors were noted the first approach was to edit these errors and, if errors remained after rerun of the edited output, the images were discarded from further analysis. The Query Design Estimate Contrast (QDEC) tool was used to do a general linear model (GLM) analysis at each vertex of the cortical surface. The dependent variable was cortical thickness. In the group comparisons, no nuisance variables were entered in to the model as groups were balanced with regards to gender and age. The results of the GLM analysis were corrected for multiple comparisons at the cluster level using the Monte Carlo method for p-cluster at p < 0.01 (z-vertex 2.0). In the correlation analysis between cortical thickness and clinical and neuropsychological data, age was entered as a nuisance variable and corrections for multiple comparisons were at the p < 0.01 (z-vertex 1.3) level.
Tractography and postprocessing
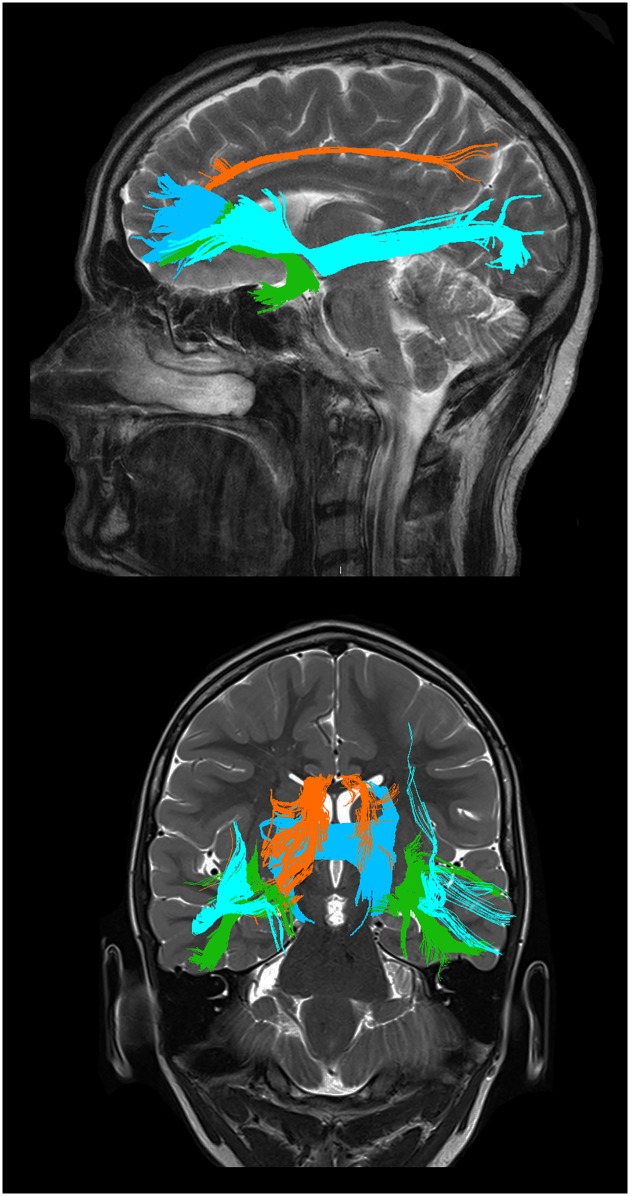
Deterministic tractography was performed with an FA threshold of 0.2, an angular threshold of 45 degrees and no length threshold using the diffusion toolkit and TrackVis. ROIs manually drawn on FA maps were employed to dissect four pathways: the inferior frontooccipital fasciculus (IFOF), uncinate fasciculus (UF), anterior cingulum (aCi) and the forceps minor (Fig 1). For the IFOF, UF and aCi ROIs, methods described by Catani and co-workers were used [44]. Briefly, these consists of placing a ROI in the extreme capsule, which is linked with an occipital ROI to dissect the IFOF and linked with a ROI in the anterior temporal lobe to dissect the UF, respectively. For the cingulum, a single ROI covers the whole tract from its appearance ventral to the genu of the corpus callosum to its temporal terminations. We modified this approach to only include the anterior part of the cingulum, ending the ROI dorsally at the first slice where the corpus callosum appears continuous on axial slices. For the forceps minor we used two parasagittal ROIs over the anterior corpus callosum with a fixed dorsal extension, a procedure that will include the rostrum of the corpus callosum together with the ventral half of the genu, or area 1 and ventral half area 2 according to Witelson [45]. In all tractography procedures (except for the corpus callosum) a midsagittal ROI was placed to exclude commissural fibres. When apparent artefacts were generated, an additional exclusion ROI was drawn to exclude these. An attempt was made to dissect the anterior commissure but this resulted in low reliability. We also attempted an isolated subgenual parcellation of the cingulum. However, this resulted in a high proportion of either missing tracts or tracts composed of very few streamlines. All image analysis was done blinded for diagnosis. Intra- and inter-rater reliability (authors KL vs. KL and AFS vs. KL) for the tracking procedures were assessed on five patients and five controls using the volume of the tract in millilitres as the quantitative measure. In DTI there is a choice between different parameters, which appear to reflect different biological phenomena [46]. Since our primary questions were relationships between symptoms and integrity of different tracts, we chose the fractional anisotropy (FA) as our primary parameter. To corroborate our findings we used mean diffusivity (MD), which is closely related to axial and radial diffusivity (MD = [axial+radial diffusivity]/2), and less sensitive for partial volume effects in some circumstances [47], as a secondary parameter.
Fig 1. Graphical representations of the tracts studied.
Sagittal (upper) and coronal (lower) view. The uncinate fasciculus (green), the anterior cingulum (orange), the inferior frontooccipital fasciculus (light blue) and the forceps minor (dark blue). Tracts are from TrackVis, overlaid on high resolution images for illustrative purposes.
Statistical analysis
Demographic, cognitive, behavioural and DTI parameters of tracts were compared across groups by the use of the Kruskal-Wallis test. Differences between group pairs were assessed with the Mann-Whitney U test. Distribution of gender was assessed by the Fisher´s Exact test. To examine the relationships between neuropsychological test results, behavioural data and DTI variables of tracts, we applied linear regression models adjusted with age. Model assumptions were checked and the logarithm of the dependent variable was used if needed to achieve normality and/or other model assumptions. The reliability of the tracking procedures was calculated using the intraclass correlations coefficient (ICC), single measures, and absolute agreement. Based on the effect sizes in previous group comparisons and correlative studies [48–50], the power of the current study (n = 21) is sufficient. The statistical analyses were performed in SPSS Statistics 21 for Mac (IBM Corporation, Somers, NY, USA). p values less than 0.05 were considered as statistically significant.
Results
Subject characteristics
Clinical and demographic characteristics of the patients and healthy controls are presented in Table 1. There were no statistically significant differences in age, education or sex distribution between the three groups. Also, there were no statistically significant differences in symptom duration or global cognitive level as measured with the FTLD-CDR or Mini Mental State Examination (MMSE) between patients with bvFTD and PSP. Patients with bvFTD had higher total FBI and FBI12-22 symptom score compared with PSP, but not FBI1-10 symptom scores, although the latter was borderline statistically significant (p = 0.054). The Hayling total error score showed a robust difference between bvFTD patients and controls, while PSP patients had values closer to controls. Although median total error score was higher in the bvFTD than the PSP group, this did not reach statistical significance (p = 0.055). All behavioural and neuropsychological measures (i.e. FTLD-CDR, FBI, the latter including subscores, and Hayling) showed considerable overlap in range, supporting the use of a combined bvFTD and PSP cohort.
Correlations with the Hayling test
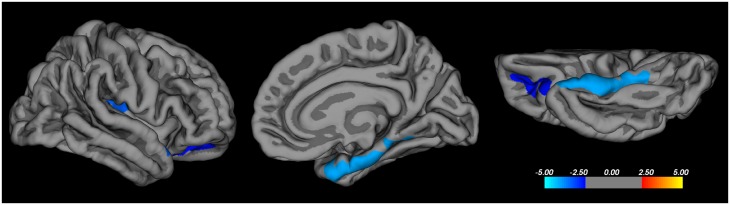
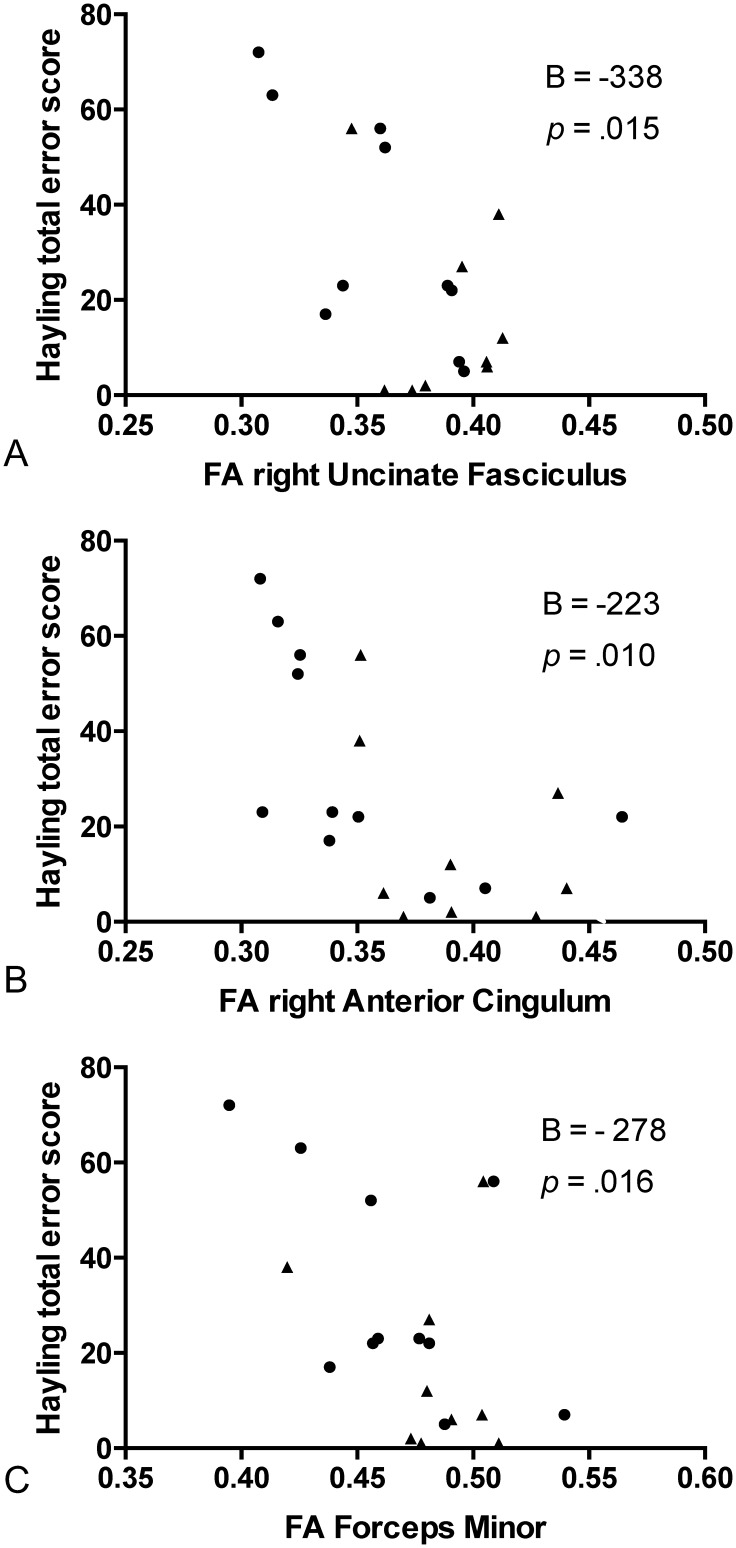
In the combined patient cohort (bvFTD and PSP) there was a significant correlation between the Hayling total error score with cortical thickness in the following regions, confined to the right hemisphere: the OFC (excluding the ventromedial prefrontal cortex- vmPFC), the parahippocampal gyrus and the posterior insula (Fig 2). For white matter tracts, the linear regression model with Hayling total error score as the dependent and FA of the tract as the independent variable, was significant for the right UF (adjusted R2 = 0.55, unstandardized B = -338 [95% CI -601, -75], p = 0.015), right aCi (adjusted R2 = 0.56, B = -223 [95% CI -388, -60], p = 0.010), and forceps minor (adjusted R2 = 0.54, B = -278 [95% CI -496, -59], p = 0.016) (Fig 3), but not for any other tract. Age was a significant covariate in all models. All significant models were re-run with MD instead of FA as the DTI parameter of the tract, with the same models and parameters reaching statistical significance. The models were also re-run with gender as a covariate, which did not contribute significantly to the models.
Fig 2. Cortical thickness and Hayling error score correlations.
Correlations between cortical thickness and total error score on the Hayling test in patients with behavioural variant frontotemporal dementia and progressive supranuclear palsy using Freesurfer. Correction for multiple comparisons was made using the Monte Carlo method at the cluster level, at p < 0.01 (z-vertex 1.3). Age was entered as a nuisance variable. Coloured areas represent significant negative correlations, with the scale bar representing p on a logarithmic scale. Only the right hemisphere is shown, from lateral, medial and inferior views. No regions with significant correlation with cortical thickness were detected in the left hemisphere.
Fig 3. Fractional anisotropy and Hayling error score correlations.
Scatter plots of total error score on the Hayling test and fractional anisotropy (FA) of tracts in patients with behavioural variant frontotemporal dementia (dots) and progressive supranuclear palsy (triangles), for A: the right uncinate fasciculus, B: the right anterior cingulum, and C: the forceps minor. B (adjusted) and p are derived from the linear regression model, with age as covariate.
Correlations with the Frontal Behavioural Inventory
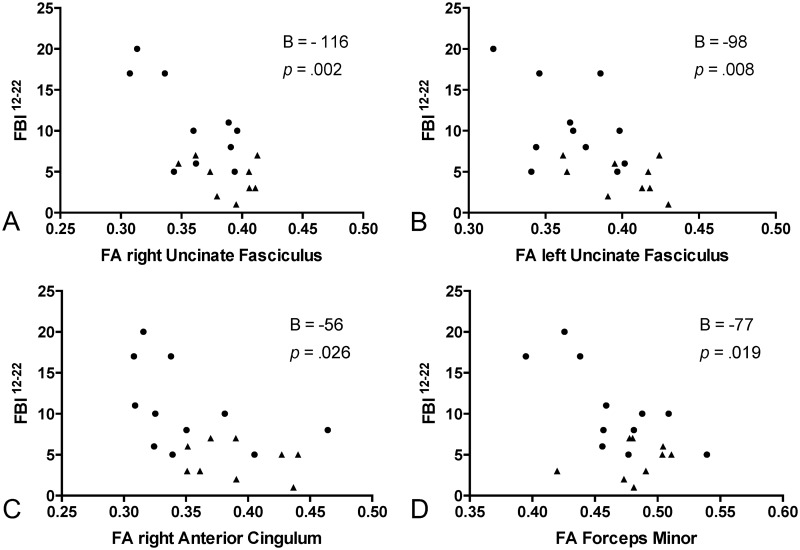
In the linear regression model with the FBI12-22 of the combined patient cohort as the dependent variable, a statistically highly significant effect was seen for the right UF (adjusted R2 = 0.44, unstandardized B = -116 [95% CI -182, -50], p = 0.002), left UF (adjusted R2 = 0.319, B = -98 [95% CI -167, -29], p = 0.008), the right aCi (adjusted R2 = 0.23, B = -56 [95% CI -105, -7.6], p = 0.026) and forceps minor (adjusted R2 = 0.26, B = -77, [95% CI -139, -14], p = 0.019) (Fig 4). Age was not a significant covariate in the models. All significant models were re-run with MD instead of FA as the DTI parameter of the tract and showed the same results except for the right aCi, in which the MD model was not significant. The models were also rerun with gender as an additional covariate, which did not contribute significantly to the models. To examine to what extent the correlations were specific for symptoms of the FBI12-22, we computed the same analyses with FBI1-10 as the dependent variable. These analyses showed no significant effect of the DTI variables on FBI1-10 scores, but in several cases a significant effect of age. In the analysis of correlations between FBI scores and cortical thickness no significant correlation was seen with FBI12-22 nor FBI1-10 in any region.
Fig 4. Frontal Behavioural Inventory and FA of tracts.
Scatter plots of Frontal Behavioural Inventory composite score of items 12–22 (FBI12-22) and fractional anisotropy (FA) of tracts in patients with behavioural variant frontotemporal dementia (bvFTD) (dots) and progressive supranuclear palsy (PSP) (triangles), for A: the right uncinate fasciculus, B: the left uncinate fasciculus, C: the anterior cingulum and D: the forceps minor. B (adjusted) and p are derived from the linear regression model, with age as covariate.
Group comparisons of cortical thickness
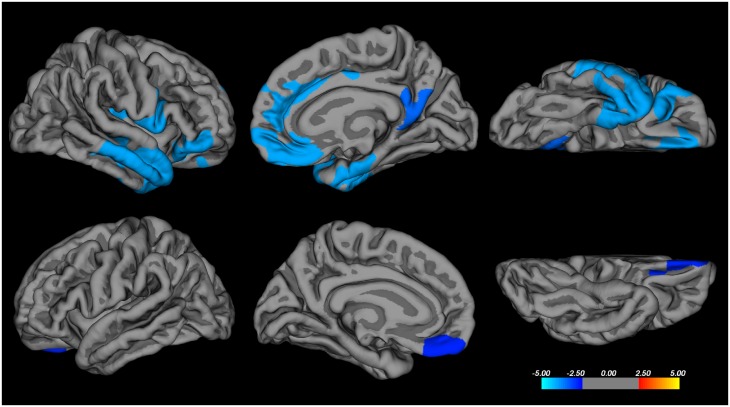
Compared with controls bvFTD patients showed widespread cortical thinning, bilaterally, with right hemispheric dominance (Fig 5). Thinning was significant (in the right hemisphere) in the medial prefrontal cortex (extending to the precuneus), the OFC (including the vmPFC), the inferior frontal gyrus/frontal operculum, and the insula. Regions of cortical thinning also included the temporal pole, extending to the middle and inferior temporal gyrus and parahippocampal gyrus. In the left hemisphere, cortical thinning was confined to the medial OFC/vmPFC. The PSP patients did not show any cortical thinning compared with controls, nor was there any statistically significant difference between patients with bvFTD and PSP. One patient with bvFTD had to be excluded from the analysis of cortical thickness because of incorrect parcellation.
Fig 5. Cortical thinning in bvFTD patients.
Results of the comparison of cortical thickness between patients with behavioural variant frontotemporal dementia and healthy controls. Results are from the Freesurfer analysis, results of the general linear model analysis were corrected for multiple comparisons at the cluster level using the Monte Carlo method for p-cluster at p < 0.01 (z-vertex 2.0). No nuisance variables were entered into the model. Coloured areas represent areas of significant differences, where warmer colours represent cortical thickening and cooler colours cortical thinning. Scale bar represents p on a logarithmic scale. The upper row is the right hemisphere, the lower row is left hemisphere, from lateral, medial and inferior views.
Group comparisons of white matter integrity
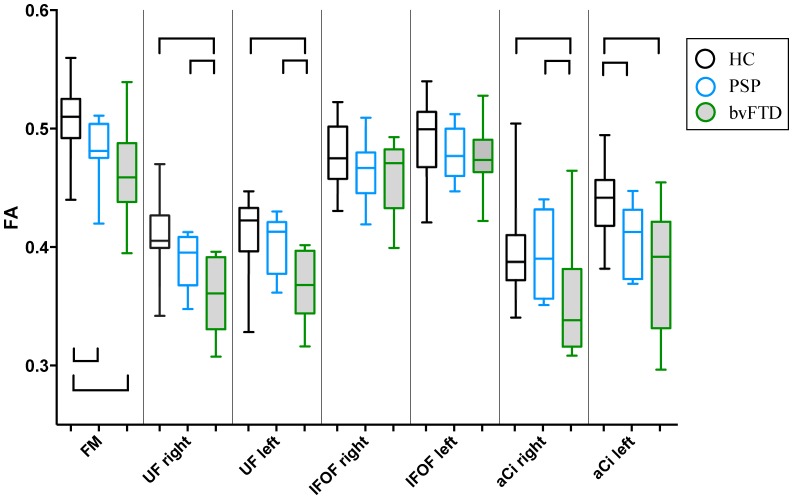
Boxplots of FA values of the tracts are shown in Fig 6. All tracts except the IFOF showed statistically significant differences across all three groups. Patients with bvFTD showed a robust and statistically significant lowering of FA in all tracts except the IFOF compared with healthy controls, whereas significant differences between PSP and controls were confined to the forceps minor and the left aCi. Comparisons between the two patient groups showed no difference in the forceps minor, IFOF or the left aCi, but lower FA in bvFTD in the right aCi and right and left UF compared with PSP. Values of mean diffusivity (MD) displayed a similar but reversed pattern (i.e. MD bvFTD>PSP>controls) (S1 Fig), the difference being that MD showed a statistical significant difference between bvFTD and controls also for the IFOF, there were no differences between PSP and controls, and bvFTD and PSP patients displayed statistically significant differences in the left aCi as well. Intra-raterreliability ranged from 0.93 to 0.99 and inter-raterreliability ranged from 0.89 to 0.98, in agreement with other centres using deterministic tractography with manually traced ROIs [51]. One patient with bvFTD had to be excluded from the DTI analysis because of movement during the scan.
Fig 6. Boxplots of FA values of tracts studied.
HC: healthy controls, PSP: progressive supranuclear palsy, bvFTD: behavioural variant frontotemporal dementia. FA: fractional anisotropy. FM: forceps minor, UF: uncinate fasciculus, IFOF: inferior frontooccipital fasciculus, aCi: anterior cingulum. Rh: right hemisphere and lh: left hemisphere. Boxes represent 25th and 75th percentile with median, whiskers minimum and maximum value. Staples represent statistically significant differences in between group pairs, at p < 0.05, uncorrected for multiple comparisons.
Discussion
The primary purpose of this study was to explore the clinico-anatomical correlates of disinhibition, adding the perspective of white matter to the more common grey matter imaging. The main finding of our study is that in the prefrontal syndrome disinhibition is related to the integrity of an axis between the right temporal-, insular- and orbitofrontal cortex, and their main interconnecting white matter structures, the right uncinate fasciculus and right cingulum. As such, our results have consequences for understanding the clinical anatomy of behavioural control. The secondary aim of the study, the comparison of the two syndromes bvFTD and PSP, will also be discussed in the following.
Correlates of disinhibition in the present study
The second part of the Hayling test is specifically designed to tap inhibitory function [30] and thus to be a specific marker of disinhibition. In our study the total error score on the Hayling test correlated with white matter involvement of the right aCi, right UF, and forceps minor, but not the other tracts studied. This pattern is very similar to that in a combined neurodegenerative (bvFTD + AD) correlational study using VBM analysis of DTI and the Hayling test [11], with the difference that our findings were unilateral in the right hemisphere. The Hayling test has an embedded verbal component, but in the present study no association was seen with left hemispheric language areas. This is in agreement with a previous study of a bvFTD cohort [11], where verbal function did not influence results on the Hayling test. To quantify lack of inhibitory control outside the test setting, we chose a composite symptom score, FBI12-22. All FBI12-22 items include an aspect of inhibitory control, but the items and thus the composite score are likely to depend on other cerebral functions and regions as well. Eating disturbances, social cognition and aberrant motor behaviour are all symptoms of bvFTD and map into the OFC, medial PFC, insula and anterior temporal lobe [5, 52, 53]. However, the same tracts that correlated with the FBI12-22 symptom score correlated with the Hayling test (with the addition of the left UF), a considerably more “pure” disinhibition measure, which shows that our composite symptom score has a validity for the purpose of quantifying disinhibition in real life. The Hayling total error score correlated to reduced cortical thickness in the parahippocampal gyrus, OFC and insula, all in the right hemisphere, which, importantly, constitute a subset of the regions involved in our bvFTD patients. Taken together, our grey and white matter findings point to a right hemispheric medial temporal lobe (presumably including amygdala and hippocampus)-insular-orbitofrontal cortex network, areas which all are interconnected by the uncinate fasciculus and the cingulum [2].
Consequences for the neuroanatomy of disinhibition
Measures of disinhibition in bvFTD, PSP and other neurodegenerative disorders tend to map into the OFC [5–8, 10, 11, 49, 54], which in the standard model of behavioural neurology is critical for behavioural inhibition [1, 3]. However, the studies are heterogeneous and several studies show a relationship to a considerably wider set of regions, mainly in the right hemisphere: the hippocampus/amygdala [9, 54], caudate/nucleus accumbens [9, 54], insula [8, 11], anterior cingulate cortex/medial prefrontal cortex [10, 54], inferior frontal gyrus [8, 10, 48, 49], and the temporal lobe [8, 10, 11, 54, 55] and other studies have shown both disinhibition and apathy correlating with the OFC [7, 8]. In healthy subjects the results are more consistent and inhibition (commonly assessed experimentally with motor inhibition) is related to function of the inferior frontal gyrus [56]. This has also emerged in FTD (see above) but shown to be secondary to OFC involvement [49]. In vivo white matter correlational studies are more recent, but point to the UF pathway for behavioural regulation both in acquired and developmental disorders [11, 14]. Comprehensively, we believe that the results of the present study taken together with the existing literature point to a right hemispherical medial temporal-insular-OFC axis for behavioural control, with the UF and Ci as the main structural linkage.
Interestingly, we did not find a correlation of disinhibition with cortical thickness of the vmPFC. Care should be taken when interpreting statistically non-significant findings as negative but the vmPFC did not correlate at a permissive, post-hoc p < 0.05 analysis, and was affected in our bvFTD cohort. This finding is in contrast to the majority of studies in bvFTD and PSP cited above, with the notable exception of the study by Zamboni et al [9]. Results from their study and our current agree with the models based on lesion and animal work [57–59], according to which the vmPFC is a critical hub for emotional decision making and/or evaluation of hedonistic experience, but not for inhibitory control per se, and extend these models to the context of neurodegenerative disease.
Our conclusion raises the broader question of why inhibitory control is anatomically “distributed” in such a manner. One explanation, using a locationalist view, is that not only behavioural but also neuropsychological measures of disinhibition are dependent on other functions in addition to inhibitory control which are localised in other cortical regions, communicate by the white matter pathways, and will “contaminate” the neuroimaging results. As an example, social cognition is coupled to the anterior temporal lobe [60], medial PFC is coupled to mentalisation and decision making [61], the amygdala to emotional processing [62] and the medial temporal lobe to contextual information [63]. An alternative explanation is an associative network model, where the inhibitory function itself relies on a distributed network with several nodes, i.e. parts of the network that each contribute to but are not individually critical for its function [13, 64]. We believe that the results of the present study, where behavioural and neuropsychological measures of disinhibition correlate with distributed right hemispheric cortical areas and their interconnections is more in line with the latter model. This we believe could explain some of the divergences among previous studies. Such a model however does question the concept of a “prefrontal” syndrome as currently conceptualized [65].
Grey and white matter affliction in bvFTD vs. PSP
The regions affected by cortical atrophy among the bvFTD patients in our study (medial prefrontal, orbitofrontal, inferior frontal, insular and temporal cortex) are consistent with the expected pattern in bvFTD [23], although our patients showed a right-sided dominance. The involvement of major associative and commissural tracts leading to/from these areas [2, 14, 66], i.e. the aCi, UF and forceps minor, is also in accordance with previous studies on bvFTD in which these are the most consistently affected tracts [50, 67–69].
In the PSP patients we did not find any statistically significant cortical thinning compared with controls, which was contrary to expectations since the PSP patients had prefrontal symptomatology (“Richardson´s syndrome”). In PSP these symptoms are related to both subcortical and prefrontal cortical affliction [6, 22, 70, 71]. It is possible that measuring cortical thinning is a less sensitive method than those used in the other studies, or an issue of power. On the other hand, the pattern of tract involvement (forceps minor and left aCi) is in accordance with previous DTI tractographical studies of PSP [72–74].
The symptomatology of our patients confirmed that it is the FBI “positive” symptoms (FBI12-22) that, from a behavioural perspective, best separates PSP and bvFTD, while the “negative” symptom (FBI1-10) burden is more similar [15, 17, 26]. Adding to the topographical overlap of the conditions, with a variable extent of prefrontal and subcortical atrophy [6, 20–23, 70, 71], we believe this supports the use of a combined disease cohort of bvFTD and PSP patients when exploring the underlying neuroanatomy of disinhibition. Lagarde et al. [71] have made the only previous direct grey matter atrophy comparison between bvFTD and PSP and did not find any differences when using their a priori threshold, as in this study. However, using a post-hoc, more permissive threshold they showed greater atrophy in dorsolateral, medial and orbital prefrontal cortex in bvFTD compared with PSP. Similarly, when we used a more permissive multiple comparison cut-off (p < 0.05) we did indeed find a cortical thinning in bvFTD compared with PSP, in the OFC (including the vmPFC) and anterior temporal lobe (S2 Fig). To our knowledge our study is the first direct DTI comparison between bvFTD and PSP using DTI for comparing white matter integrity in the two disorders. Comparison between the two disease groups showed lower FA in bvFTD in the right aCi and the UF bilaterally compared with PSP. This itself is an indication that the phenotype distinguishing bvFTD from PSP (that is the “positive” FBI12-22 symptomatology) is related to the involvement of these tracts.
Methodological discussion
From a clinico-anatomic-correlation standpoint, it is important to note that all tracts we studied, although they constitute anatomically discrete fibre systems, are composed of subcomponents which likely have different functions and possibly different involvement in the diseases studied. For example, the least affected tract in the bvFTD group, the IFOF, is composed of several subcomponents [75] that are not separated in the present study. Another limitation of the DTI method employed is that fibres that cross or”kiss” other fibres will be excluded which limits the extents of the tracts in a non-anatomical fashion [12]. Also, the current DTI resolution means that DTI parameters of tracts will be contaminated from adjoining or intermingling tracts. An example of this problem is in the orbitofrontal terminations of the UF and IFOF, which will be intermingled [2]. Future studies with methods that enable more detailed tract parcellations may overcome some of these problems [12]. The imaging methods chosen for the current study do not visualize the cortico-striatal-thalamic loop. Considering its role in inhibitory control, and (as noted previously) its affliction in bvFTD and PSP, both grey and white matter imaging of this system would constitute an important improvement.
A broader methodological aspect of this study lies in the use of the clinico-anatomical-correlation method in neurodegenerative diseases. Neurodegenerative diseases, through the regional selectivity of their respective pathological processes, probably reflect entire large-scale functional-structural networks [76]. This could pose a limitation, making it difficult to disentangle separated functions in an afflicted network even with a hodological approach [77].
Conclusion
In conclusion, we show that disinhibition is related to the integrity of a right hemispheric medial temporal-insular-orbitofrontal network, interconnected by the right uncinate fasciculus and right cingulum. These results support a frontotemporal network model of disinhibition, and question the concept of a purely prefrontal syndrome as currently formulated. Also, our study questions the role of atrophy of the ventromedial prefrontal cortex as the major cause for loss of inhibitory control in neurodegenerative disease.
Supporting Information
HC: healthy controls, PSP: progressive supranuclear palsy, bvFTD: behavioural variant frontotemporal dementia. MD: mean diffusivity. FM: forceps minor, UF: uncinate fasciculus, IFOF: inferior frontooccipital fasciculus, aCi: anterior cingulum. Rh: right hemisphere and lh: left hemisphere. Boxes represent 25th and 75th percentiles with median, whiskers minimum and maximum value. Staples represent statistically significant differences between paired groups, at p < 0.05, uncorrected for multiple comparisons.
(EPS)
Results of the post-hoc comparison of cortical thickness between patients with behavioural variant frontotemporal dementia (bvFTD) and progressive supranuclear palsy (PSP) using Freesurfer. Results of the general linear model analysis were corrected for multiple comparisons at the cluster level using the Monte Carlo method for p-cluster at p < 0.05 (z-vertex 2.0). No nuisance variables were entered into the model. Coloured areas represent areas of significant differences, where warmer colours represent cortical thickening and cooler colours cortical thinning. Scale bar represents p on a logarithmic scale. Only the right hemisphere is shown, from lateral, medial and inferior views. No regions with significant correlation with cortical thickness were detected in the left hemisphere.
(EPS)
Acknowledgments
Our thanks to the patients and their families for their willingness to participate in the study, Karin Nilsson for work in patient recruitment, Helene Jacobsson for statistical support, Fredrik Skåtar for graphical support, and Kristofer Tilderkvist for data extraction.
Data Availability
All relevant data are within the paper and its Supporting Information Files.
Funding Statement
The work was supported by government funding of clinical research within NHS Sweden (ALF), The Basal Ganglia Disorders Linneaus Consortium (BAGADILICO), MultiPark, The Fromma Foundation, The Swedish Alzheimer Foundation, The Thureus´s Foundation, The Konsul Thure Carlssons Minne Foundation, The Trolle-Wachtmeister Foundation for Medical Research, The Märta Lundqvist Foundation, and the Swedish Research Council Grant No 521-210-3034. The financial sponsors did not have any role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the article for publication.
References
- 1.Fuster J. The Prefrontal Cortex. 4th ed London: Academic Press; 2009. 10.1016/B978-008045046-9.01118-9 [DOI] [Google Scholar]
- 2.Catani M, Thiebaut de Schotten M. Atlas of Human Brain Connections. 1st ed New York: Oxford University Press; 2012. 10.1093/med/9780199541164.001.0001 [DOI] [Google Scholar]
- 3.Mesulam M. Behavioral Neuroanatomy: Large-Scale Networks, Association Cortex, Frontal Syndromes, the Limbic System, and Hemispheric specializations In: Mesulam M, editor. Principle of Behavioural and Cognitive Neurology. 2nd ed New York: Oxford University Press; 2000. p. 1–91. [Google Scholar]
- 4.Miller B, Boeve B. The Behavioral Neurology of Dementia. 1st ed New York: Cambridge University Press; 2009. 10.1017/CBO9780511581410 [DOI] [Google Scholar]
- 5.Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain: a journal of neurology. 2005;128:2612–25. 10.1093/brain/awh628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain: a journal of neurology. 2005;128:1259–66. 10.1093/brain/awh508 [DOI] [PubMed] [Google Scholar]
- 7.Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dementia and geriatric cognitive disorders. 2006;21:373–9. 10.1159/000091898 [DOI] [PubMed] [Google Scholar]
- 8.Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dementia and geriatric cognitive disorders. 2009;27:96–104. 10.1159/000194658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71:736–42. 10.1212/01.wnl.0000324920.96835.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borroni B, Grassi M, Premi E, Gazzina S, Alberici A, Cosseddu M, et al. Neuroanatomical correlates of behavioural phenotypes in behavioural variant of frontotemporal dementia. Behavioural brain research. 2012;235:124–9. 10.1016/j.bbr.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain: a journal of neurology. 2011;134:2502–12. 10.1093/brain/awr173 [DOI] [PubMed] [Google Scholar]
- 12.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44:936–52. 10.1016/j.cortex.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Catani M, Dell'acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48:1262–87. 10.1016/j.cortex.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain: a journal of neurology. 2013;136:1692–707. 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bak TH, Crawford LM, Berrios G, Hodges JR. Behavioural symptoms in progressive supranuclear palsy and frontotemporal dementia. Journal of neurology, neurosurgery, and psychiatry. 2010;81:1057–9. 10.1136/jnnp.2008.157974 [DOI] [PubMed] [Google Scholar]
- 16.Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC. Frontal presentation in progressive supranuclear palsy. Neurology. 2007;69:723–9. 10.1212/01.wnl.0000267643.24870.26 [DOI] [PubMed] [Google Scholar]
- 17.Kobylecki C, Jones M, Thompson JC, Richardson AM, Neary D, Mann DM, et al. Cognitive-behavioural features of progressive supranuclear palsy syndrome overlap with frontotemporal dementia. Journal of neurology. 2015;262:916–22. 10.1007/s00415-015-7657-z [DOI] [PubMed] [Google Scholar]
- 18.Stamelou M, Diehl-Schmid J, Hapfelmeier A, Kontaxopoulou D, Stefanis L, Oertel WH, et al. The frontal assessment battery is not useful to discriminate progressive supranuclear palsy from frontotemporal dementias. Parkinsonism & related disorders. 2015;21:1264–8. 10.1016/j.parkreldis.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 19.O'Keeffe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, et al. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain: a journal of neurology. 2007;130:753–64. 10.1093/brain/awl367 [DOI] [PubMed] [Google Scholar]
- 20.Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dementia and geriatric cognitive disorders. 2004;17:282–6. 10.1159/000077155 [DOI] [PubMed] [Google Scholar]
- 21.Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Annals of neurology. 2008;64:4–14. 10.1002/ana.21426 [DOI] [PubMed] [Google Scholar]
- 22.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet neurology. 2009;8:270–9. 10.1016/S1474-4422(09)70042-0 [DOI] [PubMed] [Google Scholar]
- 23.Schroeter ML, Laird AR, Chwiesko C, Deuschl C, Schneider E, Bzdok D, et al. Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses—The case of behavioral variant frontotemporal dementia. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;57C:22–37. 10.1016/j.cortex.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macfarlane MD, Jakabek D, Walterfang M, Vestberg S, Velakoulis D, Wilkes FA, et al. Striatal Atrophy in the Behavioural Variant of Frontotemporal Dementia: Correlation with Diagnosis, Negative Symptoms and Disease Severity. PloS one. 2015;10:e0129692 10.1371/journal.pone.0129692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet neurology. 2011;10:162–72. 10.1016/S1474-4422(10)70299-4 [DOI] [PubMed] [Google Scholar]
- 26.Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996;47:1184–9. 10.1212/WNL.47.5.1184 [DOI] [PubMed] [Google Scholar]
- 27.Irish M, Hornberger M, El Wahsh S, Lam BY, Lah S, Miller L, et al. Grey and white matter correlates of recent and remote autobiographical memory retrieval—insights from the dementias. PloS one. 2014;9:e113081 10.1371/journal.pone.0113081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: a journal of neurology. 2011;134:2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- 30.Burgess P, Shallice T. The Hayling and Brixton tests. 1st ed Thurston Suffolk: Pearson; 1997. [Google Scholar]
- 31.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature reviews Neurology. 2010;6:131–44. 10.1038/nrneurol.2010.4 [DOI] [PubMed] [Google Scholar]
- 32.Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J Abnorm Child Psychol. 2000;28:403–14. [DOI] [PubMed] [Google Scholar]
- 33.Marczewski P VdL M, Laroi F. Further investigation of the Supervisory Attentional System in schizophrenia: Planning, inhibition, and rule abstraction. Cognitive Neuropsychiatry. 2001;6:175–92. 10.1080/13546800042000115 [DOI] [Google Scholar]
- 34.de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20:206–14. 10.1037/0894-4105.20.2.206 [DOI] [PubMed] [Google Scholar]
- 35.Jantscher S W U, Schmoeger M, Mueller C, Auff E. Validation of the Hayling Sentence Completion Test- German version and Stroop- test. European Psychiatry. 2011;26:420 10.1016/S0924-9338(11)72128-9 [DOI] [Google Scholar]
- 36.Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges JR. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer's disease. Dementia and geriatric cognitive disorders. 2010;30:547–52. 10.1159/000321670 [DOI] [PubMed] [Google Scholar]
- 37.Ghosh BC, Carpenter RH, Rowe JB. A longitudinal study of motor, oculomotor and cognitive function in progressive supranuclear palsy. PloS one. 2013;8:e74486 10.1371/journal.pone.0074486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Callaghan C, Hodges JR, Hornberger M. Inhibitory dysfunction in frontotemporal dementia: a review. Alzheimer disease and associated disorders. 2013;27:102–8. 10.1097/WAD.0b013e318265bbc9 [DOI] [PubMed] [Google Scholar]
- 39.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1997;24:29–36. 10.1017/S0317167100021053 [DOI] [PubMed] [Google Scholar]
- 40.Mathias JL, Morphett K. Neurobehavioral differences between Alzheimer's disease and frontotemporal dementia: a meta-analysis. Journal of clinical and experimental neuropsychology. 2010;32:682–98. 10.1080/13803390903427414 [DOI] [PubMed] [Google Scholar]
- 41.Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain: a journal of neurology. 2008;131:2957–68. 10.1093/brain/awn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity-based medical image registration. IEEE transactions on medical imaging. 2010;29:196–205. 10.1109/TMI.2009.2035616 [DOI] [PubMed] [Google Scholar]
- 43.Nilsson M, Szczepankiewicz F, van Westen D, Hansson O. Extrapolation-Based References Improve Motion and Eddy-Current Correction of High B-Value DWI Data: Application in Parkinson's Disease Dementia. PloS one. 2015;10:e0141825 10.1371/journal.pone.0141825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44:1105–32. 10.1016/j.cortex.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 45.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain: a journal of neurology. 1989;112 (Pt 3):799–835. [DOI] [PubMed] [Google Scholar]
- 46.Johansen-Berg H, Behrens TEJ. Diffusion MRI: from quantitative measurement to in-vivo neuroanatomy. 1st ed London: Academic; 2009. [Google Scholar]
- 47.Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage. 2011;55:1566–76. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- 48.Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain: a journal of neurology. 2015;138:1961–75. 10.1093/brain/awv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Callaghan C, Naismith SL, Hodges JR, Lewis SJ, Hornberger M. Fronto-striatal atrophy correlates of inhibitory dysfunction in Parkinson's disease versus behavioural variant frontotemporal dementia. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1833–43. 10.1016/j.cortex.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 50.Santillo AF, Martensson J, Lindberg O, Nilsson M, Manzouri A, Landqvist Waldo M, et al. Diffusion tensor tractography versus volumetric imaging in the diagnosis of behavioral variant frontotemporal dementia. PloS one. 2013;8:e66932 10.1371/journal.pone.0066932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–44. 10.1016/j.neuroimage.2007.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, et al. Oops! Resolving social dilemmas in frontotemporal dementia. Journal of neurology, neurosurgery, and psychiatry. 2007;78:457–60. 10.1136/jnnp.2006.098228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. 10.1212/01.wnl.0000277461.06713.23 [DOI] [PubMed] [Google Scholar]
- 54.Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Annals of neurology. 2005;57:216–25. 10.1002/ana.20365 [DOI] [PubMed] [Google Scholar]
- 55.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–203. 10.1212/01.WNL.0000091868.28557.B8 [DOI] [PubMed] [Google Scholar]
- 56.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–28. 10.1177/1073858407299288 [DOI] [PubMed] [Google Scholar]
- 57.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- 58.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature reviews Neuroscience. 2005;6:691–702. 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- 59.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews Neuroscience. 2009;10:885–92. 10.1038/nrn2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Social cognitive and affective neuroscience. 2013;8:123–33. 10.1093/scan/nss119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews Neuroscience. 2006;7:268–77. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- 62.Gupta R, Koscik TR, Bechara A, Tranel D. The amygdala and decision-making. Neuropsychologia. 2011;49:760–6. 10.1016/j.neuropsychologia.2010.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78:1354–62. 10.1212/WNL.0b013e3182518375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mesulam M. The evolving landscape of human cortical connectivity: facts and inferences. NeuroImage. 2012;62:2182–9. 10.1016/j.neuroimage.2011.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huey ED, Zahn R, Grafman J. "[H]E is no more a person now but a whole climate of opinion" (Auden, 1940). Cortex; a journal devoted to the study of the nervous system and behavior. 2007;43:1097–8; discussion 116–21. 10.1016/S0010-9452(08)70710-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1175–90. 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex. 2012;22:2705–14. 10.1093/cercor/bhr288 [DOI] [PubMed] [Google Scholar]
- 68.Mahoney CJ, Ridgway GR, Malone IB, Downey LE, Beck J, Kinnunen KM, et al. Profiles of white matter tract pathology in frontotemporal dementia. Human brain mapping. 2014. 10.1002/hbm.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain: a journal of neurology. 2009;132:2579–92. 10.1093/brain/awp071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiu WZ, Papma JM, de Koning I, Donker Kaat L, Seelaar H, Reijs AE, et al. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. Journal of neurology, neurosurgery, and psychiatry. 2012;83:910–5. 10.1136/jnnp-2011-302035 [DOI] [PubMed] [Google Scholar]
- 71.Lagarde J, Valabregue R, Corvol JC, Pineau F, Le Ber I, Vidailhet M, et al. Are frontal cognitive and atrophy patterns different in PSP and bvFTD? A comparative neuropsychological and VBM study. PloS one. 2013;8:e80353 10.1371/journal.pone.0080353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canu E, Agosta F, Baglio F, Galantucci S, Nemni R, Filippi M. Diffusion tensor magnetic resonance imaging tractography in progressive supranuclear palsy. Movement disorders: official journal of the Movement Disorder Society. 2011;26:1752–5. 10.1002/mds.23739 [DOI] [PubMed] [Google Scholar]
- 73.Surova Y, Szczepankiewicz F, Latt J, Nilsson M, Eriksson B, Leemans A, et al. Assessment of global and regional diffusion changes along white matter tracts in parkinsonian disorders by MR tractography. PloS one. 2013;8:e66022 10.1371/journal.pone.0066022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kvickstrom P, Eriksson B, van Westen D, Latt J, Elfgren C, Nilsson C. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC neurology. 2011;11:13 10.1186/1471-2377-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain structure & function. 2013;218:21–37. 10.1007/s00429-011-0372-3 [DOI] [PubMed] [Google Scholar]
- 76.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartolomeo P. The quest for the 'critical lesion site' in cognitive deficits: problems and perspectives. Cortex; a journal devoted to the study of the nervous system and behavior. 2011;47:1010–2. 10.1016/j.cortex.2010.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HC: healthy controls, PSP: progressive supranuclear palsy, bvFTD: behavioural variant frontotemporal dementia. MD: mean diffusivity. FM: forceps minor, UF: uncinate fasciculus, IFOF: inferior frontooccipital fasciculus, aCi: anterior cingulum. Rh: right hemisphere and lh: left hemisphere. Boxes represent 25th and 75th percentiles with median, whiskers minimum and maximum value. Staples represent statistically significant differences between paired groups, at p < 0.05, uncorrected for multiple comparisons.
(EPS)
Results of the post-hoc comparison of cortical thickness between patients with behavioural variant frontotemporal dementia (bvFTD) and progressive supranuclear palsy (PSP) using Freesurfer. Results of the general linear model analysis were corrected for multiple comparisons at the cluster level using the Monte Carlo method for p-cluster at p < 0.05 (z-vertex 2.0). No nuisance variables were entered into the model. Coloured areas represent areas of significant differences, where warmer colours represent cortical thickening and cooler colours cortical thinning. Scale bar represents p on a logarithmic scale. Only the right hemisphere is shown, from lateral, medial and inferior views. No regions with significant correlation with cortical thickness were detected in the left hemisphere.
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information Files.